Abstract
Copepods serve as a major link in marine food webs, bridging the energy transfer from primary producers to higher trophic levels. Oceanic warming is linked to reduced concentrations of essential fatty acids (FA) in phytoplankton, namely eicosapentaenoic acid (EPA, 20:5ω3) and docosahexaenoic acid (DHA, 22:6ω3), and it remains largely unknown if copepods have the capacity to endure. The calanoid Temora longicornis and the harpacticoid Platychelipus littoralis were chosen to analyse their FA and biosynthesis activity in response to a long-chain polyunsaturated FA (LC-PUFA) deficient diet (Dunaliella tertiolecta) along a temperature gradient. Copepods were fed D. tertiolecta labelled with the stable isotope carbon-13 (13C) to quantify carbon assimilation into their total FA and de novo EPA and DHA biosynthesis after 6 days incubated at 11, 14, 17, 20 and 23 °C. The calanoid had increased mortality with warming, whereas the harpacticoid exhibited high survival across the thermal gradient. After the incubation, P. littoralis assimilated minimal amounts of dietary carbon into its total FA in comparison to T. longicornis. T. longicornis depleted their field EPA and DHA stores more rapidly, whereas P. littoralis maintained its relative storage of EPA and DHA and absolute concentrations of DHA. T. longicornis displayed higher fractions of de novo EPA and DHA biosynthesis than P. littoralis at all temperatures, with the exception of DHA at 23 °C. Within our experimental incubation period both species were unable to meaningfully upgrade the LC-PUFA deficient algae to biosynthesize de novo EPA and DHA as a relevant source for higher trophic levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Record temperature increases and large fluctuations are undisputedly becoming more ordinary and frequent in marine ecosystems (Stenseth et al. 2002) pressuring the adaptive and acclimatization limits of organisms who have limited motility. These temperature changes can restructure the base of complex marine food webs notably through range-shifts (Beaugrand et al. 2002; McGinty et al. 2021), changes in reproductive timing (Daase et al. 2013), abundances and size (Garzke et al. 2015), and via the modification of individuals’ fatty acids (FA) (Garzke et al. 2016). This adjustment of primary producer food quality, specifically the predicted reduction of omega(ω)-3 FA with warming, can have major implications on the availability of these important essential FA (EFA) (Hixson and Arts 2016; Colombo et al. 2020). EFA (e.g. eicosapentaenoic acid (EPA): 20:5ω3, docosahexaenoic acid (DHA): 22:6ω3) are critical for growth and survival and cannot be produced de novo by marine invertebrates in the considerable amounts required (Bell et al. 2007). However, through the recent development of detailed molecular and isotope tracing methods many metazoans have been shown to contain the critical enzymes with the capacity to perform biosynthetic pathways producing ω-3 FA (Kabeya et al. 2018, 2021). Calanoids were often believed to have poor biosynthesis capacities (Moreno et al. 1979; Bell et al. 2007), more recent research suggest that at least some species have the ability to produce long-chain polyunsaturated FA (LC-PUFA) from precursors in ecologically relevant quantities (Nielsen et al. 2019). Cyclopoid, calanoid, and harpacticoid copepod species were shown to possess these biosynthesis capabilities (De Troch et al. 2012; Nielsen et al. 2020), that intensified under warming pressures (Werbrouck et al. 2017; Helenius et al. 2020). LC-PUFA are defined by a FA chain length of 20 or more carbon units (Ratnayake and Galli 2009). LC-PUFA biosynthesis is enabled by a series of enzymes including fatty acyl desaturases, which introduce a double bond in the FA carbon chain, and elongases, that elongate very long-chain FA by introducing two additional carbon atoms (Bell and Tocher 2009; Monroig and Kabeya 2018). While front-end desaturases and elongases are present throughout copepod orders (Nielsen et al. 2019; Lee et al. 2020; Kabeya et al. 2021), methyl-end desaturases—enabling biosynthesis of monounsaturated FA (MUFA) towards LC-PUFA—have recently been detected in at least harpacticoid, cyclopoid and siphonostomatoid copepods, completely revising the current assumptions on global de novo LC-PUFA production within aquatic food webs (Kabeya et al. 2018). This ability for biosynthesis has been proposed to be a potential adaptive mechanism to overcome reduced dietary LC-PUFA availability (Nielsen et al. 2020); however, the triggers/circumstances for biosynthesis and the extent to which individuals can offset these deficiencies remains unknown.
Copepods are a dominant group of zooplankton and play an important role due to their high lipid concentrations in comparison to primary producers (Kattner and Hagen 2009), providing higher trophic levels with an energetic food source. In marine intertidal sediments the order Harpacticoida dominates, due to high inputs of detritus and nutrients stimulating microphytobenthos growth, and availability of benthic microbial communities (Meyer 1994; Cnudde et al. 2015). Harpacticoids are a lipid-rich dietary item for demersal and juvenile fish species (Gee 1987; Coull 1990), and can enrich sediment with organic matter, promoting biogeochemical cycling processes (Stock et al. 2014). Comparatively, in the pelagic environment the order Calanoida is the major group within the zooplankton community, serving as prey-items for (larval) fish (Beaugrand et al. 2003; Turner 2004), seabirds (Frederiksen et al. 2013; Bertram et al. 2017), and whales (Cronin et al. 2017). Apart from direct consumption, they also contribute to the detrital food web through the microbial remineralization (Lampitt et al. 1990), and to the biological carbon pump (Jónasdóttir et al. 2015). Although morphologically distinct, these two orders fill a similarly critical niche in energy transfer, within their respective oceanic realms, and will face analogous warming pressures.
Global sea surface temperatures (SSTs) are expected to rise between 1.2 and 3.47 °C by 2100 as per Shared Socioeconomic Pathway (SSP) scenarios 2.6 and 8.5, respectively (Kwiatkowski et al. 2020). Since zooplankton have a relatively short generational time (< 1 year) and are poikilothermic, the population dynamics and energetics tied to environmental warming are meaningful (Hays et al. 2005; Richardson 2008). This environmental pressure can have an effect on both the organism itself and the algae they consume. Hence, assessing the effects of dietary LC-PUFA provision along a temperature gradient in these important primary consumers is relevant to understand future climate effects on the marine food web. Both temperature and food quality have been shown to be the stressors with the largest impact on individual FA composition (Deschutter et al. 2019), thereby impacting energy flow changes for higher trophic levels. A methodology being used to quantify the transfer of FA incorporation and biosynthesis in a consumer is compound-specific stable isotope analysis (CSIA). By labelling the food source with the stable isotope carbon-13 (13C), we are able to track the percent of algae-derived FA under experimental conditions, and understand differential incorporation or modification/biosynthesis processes for each FA (Twining et al. 2020). These data are resolved via gas chromatography combustion-isotope ratio mass spectrometry (GC-c-IRMS), allowing us to ascertain the 13C/12C ratio of individual FA found within the copepod consumer. Accordingly, the amount of FA in the consumer, derived from the isotopically labelled food source can be determined. As LC-PUFA are absent in the chlorophyte Dunaliella tertiolecta, this alga was selected. As such, the LC-PUFA with D. tertiolecta derived carbon in the copepod consumer can be used to assess LC-PUFA biosynthesis during the lab incubation (de novo). This method is a proposed alternative FA tracer method to liposomes (Bell et al. 2007).
The objective of this study was to measure the effects of a LC-PUFA deficient diet on the FA composition, incorporation and de novo biosynthesis in two copepod species of different orders along a temperature gradient. Using 7-day lab treatments, we evaluated the temperature-specific response of carbon incorporation in consumer FA under LC-PUFA deficient conditions between Platychelipus littoralis, a benthic harpacticoid species with known temperature-dependent biosynthesis capabilities (Werbrouck et al. 2017), and the calanoid Temora longicornis, the dominant zooplankton species in the southern North Sea (Semmouri et al. 2021), with as of yet unknown biosynthesis capabilities.
Materials and methods
Sampling and experimental design
The calanoid copepod T. longicornis (Müller 1785) were collected from the Belgian part of the North Sea (BPNS), on the research vessel (RV) Simon Stevin on 15th February 2021 at sampling station 330 (51°25′ 995″ N, 2°48′41.5″ E) in the coastal waters near Ostend. Copepods were collected using a vertically towed WP2 net (57 cm diameter, 200 µm mesh size), towed from bottom to surface (SST: 4.8 °C, 32.997 PSU, 0 µg L−1 chlorophyll a). Individuals were transported and held in 35 L vessels, containing natural seawater obtained from the sampling station. The harpacticoid copepod P. littoralis (Brady, 1880) were obtained during low tide from the Paulina intertidal mudflat, Westerscheldt estuary, Netherlands (51°21′ 24″ N, 3° 42′ 51″E) on 9 March 2021. The top sediment layer was sampled (5.45 °C, 21.55 PSU), and individuals were isolated by sieving through a 250 µm mesh. Copepods (CV/CVI) were randomly selected under a Wild Heerbrugg M5 stereomicroscope (length P. littoralis: ~ 0.9 mm (Werbrouck et al. 2017), T. longicornis: 1.39 ± 0.27 mm (Semmouri 2022)). To characterize the FA profile of individuals in the field, quadruplicates of 50 copepods were sampled and stored at – 80 °C after allowing gut clearance for 12 h in autoclaved filtered natural seawater (FNSW). Following identification to species level, individuals (n = 60: T. longicornis, n = 70: P. littoralis) were placed directly in 1 L glass jars of autoclaved FNSW with aeration for 12 h at 11 °C to allow gut clearance before addition of the food. Based on previous laboratory experiments (e.g., Werbrouck et al. 2017), no aeration was added to the P. littoralis jars to not disturb their benthic lifestyle as they could not hide in any sediment in the experimental unit.
The chlorophyte, D. tertiolecta (Butcher 1959), was obtained from the Laboratory of Aquaculture & Artemia Reference Center at Ghent University, and cultured at 15 °C in autoclaved FNSW with NutriBloom Plus. D. tertiolecta cultures were isotopically labelled with 16.8 mg NaH13CO3 stock solution per 100 mL of growth medium (De Troch et al. 2012; Werbrouck et al. 2017), and grown in climate rooms (15 °C, 12:12 h light:dark, 17–46 µmol photons m−2 s−1) for 10 days. Cell concentrations were monitored with a Beckman Coulter counter Multisizer 3. Prior to addition in experimental units, D. tertiolecta cultures were centrifuged, the supernatant containing the 13C label and nutrients was removed, then D. tertiolecta was resuspended in autoclaved FNSW. This was repeated twice to inhibit further algal growth (De Troch et al. 2012; Werbrouck et al. 2017). Quadruplicate 10 mL samples of D. tertiolecta were taken for FA analysis in pre-combusted glass vials, and for total carbon analysis by filtering 25 mL onto Whatman GF/F filter, both stored at – 80 °C. Algae concentrations were measured approximately 12 h after addition to the experimental units and after 6 days.
Four replicates of glass jars filled with 1 L of autoclaved FNSW per species (P. littoralis, 70 ind. unit−1; T. longicornis, 60 ind. unit−1) were placed at each of the five temperature treatments (11, 14, 17, 20, 23 °C), controlled Lovibond TC-175 incubators (temperature control ± 1 °C). Experimental units (total n = 40) were fed ad libitum (20 000–45 000 cells mL−1, 0.248–1.098 mg carbon L−1) with the prepared 13C-labelled D. tertiolecta (above) for 6 days under a 12:12 h light:dark regime. These units were acclimated from 11 °C to their treatment temperature at a rate of 2 °C h−1. To assess potential algae growth throughout the experiments, quadruplicate experimental units containing only D. tertiolecta were placed in the 14 °C incubator for the duration of the experiment. No increase in cell concentration was reported in these samples (Fig. S1), hereafter we assume algae growth was successfully inhibited. On day 6 individuals were sieved on a 38 µm mesh and living individuals were transferred to autoclaved FNSW to allow gut clearance for 24 h. After this period, surviving individuals were transferred to glass vials and stored at – 80 °C prior to FA analysis.
Total fatty acid extraction, quantification and CSIA
An internal standard (FA 19:0, 5 µg) was added to the freeze-dried samples, then FA methyl esters (FAME) were prepared via a direct transesterification procedure with 2.5% (v:v) sulfuric acid in methanol as described by De Troch et al. (2012) to achieve total FA analysis. FAME were extracted twice with hexane. Composition analysis of FA was carried out using a gas chromatograph (GC) (HP 7890B, Agilent Technologies, Diegem, Belgium) equipped with a flame ionization detector (FID) and connected to an Agilent 5977A Mass Selective (MS) Detector (Agilent Technologies). The GC was further equipped with a PTV injector (CIS-4, Gerstel, Mülheim an der Ruhr, Germany). A 60 m × 0.25 mm × 0.20 μm film thickness HP88 fused-silica capillary column (Agilent Technologies) was used for the GC analysis, at a constant Helium flow rate (2 mL min−1). The injection sample volume was 2 μL, and the oven temperature program was set as described in Boyen et al. (2020). FAME were analysed with the GC–MS prior to CSIA due to the higher total FA profile resolution and detection capabilities. The signal obtained with the FID detector was used to generate quantitative data of all compounds (MassHunter Quantitative Analysis Software, Agilent Technologies). Chromatogram peaks were identified based on their retention times, the external standards (Supelco 37 Component FAME Mix, Sigma-Aldrich) and the mass spectra. Quantification of FAME was based on the FID area of the internal standard (19:0), and the conversion of peak areas to the amount of the FA by a theoretical response factor for each FA (Ackman and Sipos 1964; Wolff et al. 1995).
To assess the 13C within the FA, FAME from all treatments and field samples were analysed by capillary gas chromatography combustion-isotope ratio mass spectrometry (GC-c-IRMS) at the Isotope Bioscience Laboratory (ISOFYS), Ghent University. The GC-c-IRMS system consisted of a Trace GC 1310 equipped with a PTV injector and a VF23-MS column (length = 60 m, ID = 0.25 mm, film = 0.25 µm), connected to combustion/pyrolysis unit (GC-ISOLINK) where the FAME are converted to CO2. The FAME is let by an automated open split system (Conflo IV) to an IRMS detector (DeltaV advantage, Thermo Scientific, Bremen Germany). 13C abundance was calibrated using the F8-3 mix of Arndt Schimmelman. Typical precision of 13C abundance is within 0.0005%. The GC-c-IRMS was not able to determine the position of the unsaturation in the carbon-20 chain (20:1), therefore its full notation is not indicated in the figures and tables reported in the results and supplementary information.
CSIA calculations
During GC-c-IRMS analysis the analytes are converted to CO2 to be analysed by the IRMS detector where m/z 44, 45 and 46 are recorded simultaneously by three detectors. From the ratio of these three traces the a13C can be determined with high precision. The peak area (PA) of the individual FA can be used to also assess the FA content ([FA]). Commonly, in not artificially 13C enriched material this is done using the combined peak area of the three mass traces. However, due to the high 13C enrichments and the different amplifications of the detectors, the [FA] per copepod was determined as follows:
with PAX,FAME and PAX,IS being the peak area at m/z = x of the FAME of interest and of the internal standard (IS), respectively, a13CIS the 13C abundance in the IS (1.08%), mIS the mass of the C19:0-FAME added (50 µg), MFA and MIS the molar mass of the FA of interest and of the IS (312.54 g∙mol−1), respectively, nCFA and nCIS indicating the number of carbons in the FA of interest and in IS (20), and N being the number of copepods in the extracted sample.
The GC-c-IRMS measurements delivers the 13C abundance of the individual FAME (a13CFAME). To obtain the a13C of the corresponding FA (a13CFA), the measured a13CFAME must be corrected for the contribution of the methyl (a13CMeOH), added during derivatization to FAME:
The fraction of carbon assimilated (fC assi) in consumer FA derived from the 13C-labelled D. tertiolecta can be computed as:
with a13CFA-exp. and a13CFA-control representing the a13CFA of the specific FA in copepods fed with 13C-labelled D. tertiolecta and control copepod (directly collected on field site), respectively, a13Clabelled DUNA and a13Cfield food (1.08%) indicating the bulk a13C of the 13C-labelled D. tertiolecta and of the food prior to incubation, respectively (adapted from Werbrouck et al. 2017). The bulk 13C of the labelled D. tertiolecta, was not measured due to instrumental limitations to measure very high enrichments, therefore the a13Clabelled DUNA was estimated using the a13CFA of 18:3ω3 (46.45%) found in the calanoid copepod samples. This value was used as a proxy due to the high concentration of 18:3ω3 in D. tertiolecta (Thor et al. 2007), and high uptake by T. longicornis. Finally, the absolute amount of FA derived from the carbon assimilated of the 13C-labelled D. tertiolecta ([FA]C assi) could be computed as follows:
For FA already present in D. tertiolecta (SFA, MUFA and PUFA < 20 carbon units), we assume that FA derived from the labelled feed in the copepods are a combination of direct unaltered incorporation, biosynthesis and conversion. LC-PUFA (ARA, EPA and DHA) are not present in D. tertiolecta, therefore LC-PUFA derived from the labelled feed in the copepod are the result of biosynthesis from dietary obtained FA precursors (see Supplementary Information, Table S1). The carbon assimilation from the algae into the total sum of all measured FA (TFA) relative to the absolute concentrations was additionally calculated.
Statistical analysis
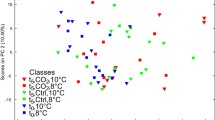
All statistical analyses and visualizations were conducted in R, version 4.1.1 (R Core Team 2021). Intra-specific cell concentrations of D. tertiolecta between day 1 and 6 were compared using a Bonferroni corrected multiple pairwise t-test. No increase of algae concentrations during the experimental treatment was detected, therefore algae growth inhibition was considered successful (Fig. S1). Relative percent FA composition data were analysed using non-parametric multidimensional scaling (nMDS), Bray–Curtis dissimilarity, on cube-root transformed data. A permutational analysis of variance (PERMANOVA) was conducted based on groups determined by hierarchical clustering. To discriminate which FA were contributing the most to these differences, a similarity percentages test (SIMPER) was conducted.
A quasi-binomial logistic generalized linear model (GLM) was used to model proportional copepod survival along temperature, considering species identity as a factor and weighted by the number of copepods in each sample, to account for an overdispersion of the data estimated by the ratio of the residuals deviance and the degrees of freedom (Haman 2020). Multiple comparisons of type Tukey were applied to the survival GLM, using the package ‘multcomp’ to determine significant differences considering species and temperature (Hothorn et al. 2008) (Table S2). Generalized additive models (GAM) were used to evaluate the significance of the non-linear relationship of the relative carbon assimilation into the TFA (Cassi TFA−1) and the fraction of carbon assimilation into specific FA along a temperature gradient between species using the package ‘mgcv’ (Wood 2011). Non-parametric smoothers (s) by restricted maximum likelihood were applied to the temperature effects (T) by species identity (S), considering species as a factor: Cassi TFA−1 ~ f(S) + s(T, by = S). If these data violated homogeneity assumptions evaluated by the dispersion of the residuals versus fitted values, due to zero-inflation, a gamma distribution family was assumed with a log-link function (Zuur et al. 2009) (Table S3). Due to high mortality the FA data from two T. longicornis replicates at 23 °C have been omitted. Model selection was done on the basis of the Akaike Information Criterion (AIC) and ANOVA. The significance of the smooth terms are reported, and explained deviance is listed on the GAM as it is considered as a generalized measurement of goodness of fit, rather than R2-values (Wood 2011). Carbon assimilation per FA is listed in the supplementary information (Fig. S2, Table S4, Table S5), note some models could not be reliably interpreted for FA with numerous undetected values and were omitted from this analysis (i.e., 15:0, 16:2ω4, 18:1ω9, 20:1). Due to size differences between species, the fraction of de novo FA was modelled rather than the absolute amount.
Results
Survival and diet characterization
The proportional survival could be predicted from the interaction between temperature (T) exposure and species (S) identity (GLM, P = 0.003) (Fig. 1). Accordingly, the effect of temperature on survival was species specific. The harpacticoid, P. littoralis, survival was not significantly different (94.8 ± 4.1%) across all temperature treatments (GLM Tukey, 11:23 °C, P = 0.999) (Table S2). In contrast, the survival of the calanoid, T. longicornis, decreased with at higher temperature treatments, ranging from 83.8 ± 8.3% at 11 °C to 22.5 ± 14.7% at 23 °C (GLM Tukey, P < 0.01) (Table S2).
Generalized linear model of proportional survival (ranging from 0 to 1, indicating complete mortality or survival of individuals within experimental units, respectively) for Platychelipus littoralis and Temora longicornis along a temperature gradient (11, 14, 17, 20, 23 °C). The shaded lines around the mean (dashed) per species, represent the 95% confidence interval
Interspecific comparison of FA composition
In T. longicornis FA 16:0, 20:5ω3 (EPA), and 22:6ω3 (DHA) were the most abundant, comprising of > 70% of the total FA composition (Table S6). Comparatively, in P. littoralis FA 16:0, 16:1ω5, 16:1ω7, EPA, and DHA were the most abundant, corresponding to > 70% of the total FA composition (Table S6). There were significant differences in FA composition between all temperature treatments and field samples among species (PERMANOVA, P = 0.012). However, hierarchical clustering and nMDS visualization of the data showed these did not fall into natural groups, therefore only significant differences (PERMANOVA, P = 0.001) between broad field and experimental species groups were considered (Fig. S3). Looking only at field samples between species, the FA that contributed the most to the differences are EPA, 14:0, 16:1ω5, DHA, and 16:1ω7 (Table S7). FA EPA, 14:0 and DHA were present in higher relative concentrations in field T. longicornis than in field P. littoralis (Table S6). Notably in field samples, P. littoralis contained 7.7% DHA whereas T. longicornis contained 29% DHA. Accordingly, these same FA also contributed largely to the differences between the temperature incubation treatments per species, in addition to 18:3ω3, which was the dominant FA in the algal feed (D. tertiolecta, Table S1), 24:1ω9 and 18:1ω7 (Table S7).
Interestingly, there was no significant difference between the relative amounts of EPA in P. littoralis in the field and experimental samples, regardless of temperature treatment (Kruskal–Wallis, P = 0.96), whereas they decrease in T. longicornis (Kruskal–Wallis, P = 0.031) (Table S6). Between field and the incubated samples absolute EPA concentrations (ng ind.−1) were significantly lower for both P. littoralis (Kruskal–Wallis, P < 0.001) (Fig. 2a) and T. longicornis (ANOVA, P = 0.002) (Fig. 2b, Table S8). There was no difference between the relative amount of DHA in P. littoralis’ field and experimental incubation samples, with the exception of treatments at 17 (Pairwise t-test, P = 0.042) and 23 °C (Pairwise t-test, P = 0.018), similarly when considering the absolute DHA values, with an exception at 20 °C (Pairwise Wilcox test, P = 0.024) (Fig. 2c). In T. longicornis, relative DHA values increased between field and experimental samples for temperatures 11 (Pairwise t-test, P = 0.042), 14 (Pairwise t-test, P = 0.004), 17 (Pairwise t-test, P = 0.012), and 20 °C (Pairwise t-test, P = 0.013) with the exception of the treatment at 23 °C (Pairwise t-test, P = 0.22). However, when considering T. longicornis absolute DHA values, they were significantly lower in temperature treatments 14, 17, 20 °C than the field samples and the 11 °C treatment (ANOVA, P = 0.007) (Fig. 2d). Therefore, with the exception of DHA in P. littoralis, EPA and DHA concentrations decrease in incubated samples fed D. tertiolecta after 6 days in comparison to the field (Fig. 2).
Mean absolute a–b EPA (20:5ω3) and (c-d) DHA (22:6ω3) amounts (ng) per individual ± standard error in P. littoralis and T. longicornis samples from the field and in the incubated temperature treatments (11, 14, 17, 20 and 23 °C) after day 6. Visualization is divided per FA and species, note the differences of scale between panels. Pairwise post-hoc tests were performed for all temperature treatments against natural reference (‘Field’) group (n = 4) per FA and species after finding significant difference of global means, means sharing a letter are not significantly different (P > 0.05). Note, T. longicornis 23 °C treatment significance should be interpreted with caution due to high mortality (low number of individuals per sample), and low sample size (n = 2)
Carbon assimilated into total fatty acids
The total carbon assimilated by P. littoralis during the experimental incubation, indicated by carbon assimilation derived from 13C-labelled D. tertiolecta, into the TFA (Cassi TFA−1) varied with increasing temperature (Fig. 3a). Carbon assimilated into the total consumer FA decreased from 0.45 ± 0.21% at 11 °C to 0.28 ± 0.07% at 20 °C, then increased to 1.29 ± 0.32% at 23 °C (GAM, P < 0.001). Comparatively, T. longicornis displayed a significantly higher relative carbon assimilation than P. littoralis with a mean value of 12.08 ± 4.62%, with no significant effect of temperature (GAM, P = 0.34) (Fig. 3b). Overall, T. longicornis displayed higher carbon assimilation into it’s FA pool than P. littoralis per individual, derived from D. tertiolecta in 6 days. Similar patterns were observed in carbon assimilation per SFA (14:0, 16:0, 18:0), MUFA (16:1ω9, 18:1ω11), and short-chain PUFA (18:3ω3) (Fig. S2, Table S4).
Fraction of the total fatty acid carbon derived from the labelled D. tertiolecta (Cassi TFA−1) after 6 days in a P. littoralis and b T. longicornis along an experimental temperature gradient. Data displayed are untransformed and separated by species factor. The shaded lines around the mean (dashed line), coloured per species, represent the 95% confidence interval. Note the difference of scale between the two panels
Comparison of de novo production of EPA and DHA between species
T. longicornis displayed higher de novo production of both EPA and DHA than P. littoralis with an exception at 23 °C (Table S4). T. longicornis EPA production ranged from 0.226 ± 0.117 to 0.030 ± 0.060 ng ind.−1 at 11 to 23 °C, respectively (Table S5). In comparison, EPA production in P. littoralis ranged from 0.015 ± 0.011 to 0.033 ± 0.008 ng ind.−1 at 11 to 23 °C, respectively. Lower DHA than EPA production was observed ranging from 0.161 ± 0.043 to 0.012 ± 0.025 and from 0.009 ± 0.001 to 0.030 ± 0.019 ng ind.−1 in the temperature range from 11 to 23 °C in T. longicornis and P. littoralis, respectively.
There is a significant effect of temperature on the fraction of de novo EPA in T. longicornis derived from the D. tertiolecta after 6 days (GAM, P < 0.001, explained deviance = 91.6%) (Fig. 4a, Table S3). Comparatively, there is no relationship between temperature and fraction of EPA produced by P. littoralis with a mean value of 0.023 ± 0.007 ng ind.−1 (no significant difference between treatments) (GAM, P = 0.935, explained deviance = 91.6%). Despite showing significant relationships for both species for the fraction of de novo DHA with temperature, due to the numerous undetected values resulting from low concentrations, this model output should be interpreted with caution (Fig. 4b, Table S3). This is reflected in a low proportion of the variance and deviance explained (R2 = 0.219, 68.0%).
Discussion
The aim of this study was to discern the effects of a poor quality diet on the FA composition, and evaluate the potential for LC-PUFA biosynthesis after 6 days in two copepod species along a temperature gradient. To compensate for poor quality food, organisms can increase ingestion rates (Malzahn and Boersma 2012), and their carbon incorporation efficiency (Gulati and Demott 1997). By choosing a labelled algal diet absent of LC-PUFA, we were able to discern the effect of poor food quality on assimilation and thereby what was retained by the individuals. Furthermore, we could evaluate the potential of LC-PUFA biosynthesis from labelled precursor compounds consumed within the duration of the experiment. T. longicornis displayed higher overall carbon assimilation than P. littoralis, and maintained this across all temperature treatments, likely to compensate for the high metabolic costs associated with the LC-PUFA deficient diet and warming pressure. Since the calanoid carbon assimilation did not significantly vary across the temperature range, this indicates that either this process is not regulated (i.e., independent of temperature), or the experimental stress was heightened enough at 11 °C to induce maximum ingestion rates, assumed from carbon assimilation, to compensate for the temperature and diet stressors. In comparison, P. littoralis has relatively low assimilation rates, only increasing at the highest temperature treatment (23 °C). This—in conjunction with the retention of the relative field LC-PUFA concentrations—may indicate that P. littoralis does not require to increase assimilation to meet their metabolic demands until the extreme of 23 °C. The carbon assimilation rate in P. littoralis ranges from 0.075 to 0.214% day−1 at 11 and 23 °C, respectively, whereas it is on average 2.014% day−1 for T. longicornis. This suggests that P. littoralis increased assimilation of the labelled D. tertiolecta as temperatures increased, whereas T. longicornis maintained the same uptake throughout. The variation of T. longicornis carbon assimilation per TFA increased with temperature, whereas for P. littoralis replicates were quite similar. This increased variability between replicates is recognized as a biochemical indicator of environmental stress (Werbrouck et al. 2017).
Higher observed assimilation rates have been recorded in Antarctic calanoid species Calanoides acutus and Calanus propinquus of 3.1 and 3.9% day−1, respectively, when fed on a diatom diet under natural temperature conditions (Graeve et al. 2020). The small herbivorous arctic calanoid Pseudocalanus minutus has demonstrated a more similar carbon assimilation rate to T. longicornis of 2.6% day−1, while the cyclopoid Oithona similis has a carbon assimilation rate more similar to P. littoralis at 0.5% day−1, when fed on a diatom dinoflagellate mixture at 4 °C (Boissonnot et al. 2016). These studies reporting the assimilation efficiency have been conducted under ambient sampling temperature and with higher food quality (presence of LC-PUFA), therefore individuals may have increased their uptake in response to these favourable conditions. Reduced ingestion of carbon in T. longicornis fed on D. tertiolecta has been previously recorded (Arendt et al. 2005). We suggest that the combined stress of a LC-PUFA deficient diet and temperature resulted in reduced rates of carbon assimilation for the two species investigated in this study in comparison to those reported in the literature. The low assimilation rates recorded in P. littoralis may be due to the preferential use or ability to retain its own relative lipid content rather than utilization of the poor external food source.
Although T. longicornis exhibits higher carbon incorporation into its TFA than P. littoralis, this does not restore the absolute FA concentrations to the levels observed in the field samples. There was a considerable reduction of EPA concentrations between field and experimental samples for both T. longicornis (~ 60% reduced) and P. littoralis (~ 32% reduced) (Fig. 2). A similar reduction of 33% was observed in T. longicornis DHA concentrations between field and experimental samples. For T. longicornis DHA concentrations have been shown to be an important factor contributing to reproductive success (Arendt et al. 2005). Comparatively, P. littoralis was able to retain the same DHA concentrations between the field and the temperature exposed samples after 6 days. This retention of DHA in P. littoralis fed a LC-PUFA deficient diet was similarly recorded by Boyen et al. (2020), where this species was exposed to + 3 °C warming conditions for four additional days to this study. Considering the low fractions of EPA and DHA derived from D. tertiolecta in both T. longicornis (< 0.001–0.002) and P. littoralis (< 0.001–0.001) (Fig. 4, Table S4), under the experimental conditions described T. longicornis was not able to biosynthesize EPA and DHA to restore their ω3-stores. This is in accordance with the observed poor ability of calanoids to biosynthesize LC-PUFA (Bell et al. 2007). The minor amounts of biosynthesized EPA and DHA by T. longicornis are presumed to be derived from FA precursors modified via front-end desaturases and elongase genes present in calanoids (Monroig and Kabeya 2018). Monitoring the differential gene expression of these aforementioned biosynthesis genes can help elucidate the specific pathways utilized (Nielsen et al. 2019). Previously, under LC-PUFA deficient conditions P. littoralis has been shown to have high assimilation of carbon into their EPA and DHA, thus demonstrating strong biosynthesis capabilities (0.088 ng DHA ind.−1) (Werbrouck et al. 2017). However, this was still not sufficient to recover the individuals DHA stores (Werbrouck et al. 2017). Under our experimental conditions we observed the opposite, rather P. littoralis was able to retain its DHA stores while at the same time exhibited poor biosynthesis abilities (0.023 ng DHA ind.−1), and lower than T. longicornis. Both species exhibited a lower fraction of EPA and DHA (Fig. 4) derived from the labelled food in comparison to tropical cyclopoid Apocyclops royi (EPA: 1.64%, DHA: 2.35%) and calanoid Pseudodiaptomus annandalei (EPA: 0.55%, DHA: 3.09%) fed D. tertiolecta after 48 h (Nielsen et al. 2020). Nielsen et al. (2020) proposes that the biosynthesis pathways are more active in these two brackish species since they naturally inhabit a PUFA-poor environment. Thus, the life-history feeding conditions and field PUFA availability may contribute to the copepods ability to utilize these pathways. We suggest that since DHA levels were maintained, the need for additional de novo biosynthesis was not necessary for P. littoralis. Alternatively, P. littoralis could maintain its DHA concentrations through the conversion of EPA, previously obtained in the field, to DHA (Monroig and Kabeya 2018), rather than utilizing FA obtained from the labelled experimental feed. However, as we cannot trace the modification of the FA acquired in the field with CSIA, we suggest monitoring via a gene-specific approach, once it is confirmed that biosynthesis pathways are being employed, or over multiple generations.
The species-specific response may be attributed to the difference of functional traits resulting from the unique stressors in their respective natural environments. Our results indicate that the harpacticoid, P. littoralis, appears to be more suited to more variable conditions than the calanoid T. longicornis. The differing lifestyles of pelagic calanoids and benthic harpacticoids can be linked to the abiotic stressors faced, energy demands throughout their lifespan, prey types encountered, and frequency of feeding periods. Since P. littoralis occupies the benthos, this species is more sedentary and expends less energy in comparison to T. longicornis’ vertical movement throughout the water column (Hays et al. 2001). In the harsher intertidal conditions that the harpacticoids inhabit, the temperature change can be more stochastic, ranging between 4 and 22 °C throughout the year (Sahan et al. 2007). Comparatively, calanoids in the pelagic environment experience temperature buffering effects from the water column, with short-term temperature change occurring at a slower rate than in the exposed mudflats. Large-scale poleward calanoid population displacements have also been noted, linked closely to temperature changes (Beaugrand et al. 2002), in relation to their planktonic nature. This justifies P. littoralis’ short-term eurythermic survival response, i.e., ability to tolerate a wide temperature range, compared to T. longicornis’ more stenothermic survival response.
Field T. longicornis contained higher amounts of both DHA and EPA than P. littoralis, the ratios of 16:1ω7/16:0 < 1 and EPA/DHA < 1 indicated a non-diatom dominated diet, and the higher amounts of 18:4ω3 suggested a dinoflagellate abundant diet (Kelly and Scheibling 2012). Harpacticoids exhibit differences in the way they take up food, due to their contact with the sediment, they are able to ingest microphytobenthos indirectly through the consumption of bacteria or ciliates (Cnudde et al. 2015). As such, P. littoralis from the field contained higher relative amounts of 16:1ω7 and 16:1ω5, suggesting herbivorous feeding on microphytobenthos (Graeve et al. 1994), and bacterial markers (18:1ω7, 17:1) (Conway and McDowell Capuzzo 1991; Kelly and Scheibling 2012). Therefore, the energy stores in which these individuals entered the experiment are contrasting as are their FA requirements for optimal functioning, which may be attributed to the differences in their available prey field and life histories. The overall outcome of this study suggests that based on their FA dynamics, P. littoralis has a greater potential for resilience than T. longicornis under more extreme conditions due to the higher variability in their natural environment.
As 18:3ω3 (ALA) is present in high quantities in D. tertiolecta, this implies we are unable to quantify copepod biosynthesis of MUFA (i.e., 18:1ω9) into short-chain PUFA (i.e., linoleic acid (LA, 18:2ω6), ALA) by methyl-end desaturases (Kabeya et al. 2021). Therefore, if you wish to follow this specific pathway, baker’s yeast could be used as an alternative to D. tertiolecta, as it contains no or very little ALA and no other LC-PUFA (Payne and Rippingale 2000; Nielsen et al. 2020). Additionally, synthesis of FA from acetyl coenzyme A (2C) is possible and can be monitored via the gene expression of specific enzymes in the FA synthase pathway (Tarrant et al. 2016). In future harpacticoid studies we suggest monitoring the triggers resulting in high assimilation prior to investigating biosynthesis regulation. Potentially seasonality and field FA composition may play a role in regulating P. littoralis biosynthesis pathways, as these were the primary difference between our study and Werbrouck et al. (2017). Separation of the lipid classes into non-polar and polar fractions prior to CSIA may be interesting to understand how individuals regulate their membrane versus storage lipids during the experimental treatment (Parrish 2013; Werbrouck et al. 2017). Measures of fitness, such as reproductive success, should be considered to quantify the significance of EPA and DHA loss and production.
Conclusions
This study fills the gap of the knowledge of the FA response and biosynthesis capabilities in two species of copepods under the same experimental temperature conditions. P. littoralis did not assimilate dietary carbon readily, and thus had a LC-PUFA biosynthesis rate that is lower than what is found in other copepods (Boissonnot et al. 2016; Graeve et al. 2020). T. longicornis displayed higher fractions of de novo biosynthesis of EPA and DHA than P. littoralis at all temperatures, with the exception of DHA at 23 °C. This temperature was the most stressful for the calanoid displaying a higher mortality with warming. Comparatively, the harpacticoid was eurythermal, with survival independent of temperature.
Although there may be a reduction in absolute ω3 LC-PUFA availability in primary producers (Hixson and Arts 2016; Colombo et al. 2020; Holm et al. 2022), it is important to consider that complete absence, as in our experiment, is not a realistic scenario. Despite the fact that T. longicornis demonstrated higher de novo production, albeit not in sufficient amounts, individuals depleted their field EPA and DHA stores more rapidly. This indicates that T. longicornis is not able to biosynthesize EPA and DHA at a rate necessary for basic metabolic functioning. Conversely, P. littoralis has maintained its relative storage of EPA and DHA and absolute concentrations of DHA, suggesting these extremes are within their coping capacity. Under the stressors imposed, P. littoralis has a greater potential for resilience when faced with extreme temperature conditions than T. longicornis. Within our experimental incubation both species were unable to meaningfully upgrade the LC-PUFA deficient algae to biosynthesize de novo EPA and DHA as a relevant source for higher trophic levels.
Data availability
The dataset produced from this experimental study is included in the electronic supplementary material.
References
Ackman RG, Sipos JC (1964) Application of specific response factors in the gas chromatographic analysis of methyl esters of fatty acids with flame ionization detectors. J Am Oil Chem Soc 41:377–378. https://doi.org/10.1007/BF02654818
Arendt KE, Jónasdóttir SH, Hansen PJ, Gärtner S (2005) Effects of dietary fatty acids on the reproductive success of the calanoid copepod Temora longicornis. Mar Biol. https://doi.org/10.1007/s00227-004-1457-9
Beaugrand G, Reid PC, Ibañez F, Lindley JA, Edwards M (2002) Reorganization of North Atlantic marine copepod biodiversity and climate. Science 296:1692–1694. https://doi.org/10.1126/science.1071329
Beaugrand G, Brander KM, Alistair Lindley J, Souissi S, Reid PC (2003) Plankton effect on cod recruitment in the North Sea. Nature 426:661–664. https://doi.org/10.1038/nature02164
Bell MV, Dick JR, Anderson TR, Pond DW (2007) Application of liposome and stable isotope tracer techniques to study polyunsaturated fatty acid biosynthesis in marine zooplankton. J Plankton Res 29:417–422. https://doi.org/10.1093/plankt/fbm025
Bell MV, Tocher DR (2009) Biosynthesis of polyunsaturated fatty acids in aquatic ecosystems: general pathways and new directions. In: Kainz M, Brett MT, Arts MT (eds) Lipids in Aquatic Ecosystems. Springer, New York, pp 211–236
Bertram DF, Mackas DL, Welch DW, Boyd WS, Ryder JL, Galbraith M, Hedd A, Morgan K, O’Hara PD (2017) Variation in zooplankton prey distribution determines marine foraging distributions of breeding Cassin’s Auklet. Deep Sea Res Part I 129:32–40. https://doi.org/10.1016/j.dsr.2017.09.004
Boissonnot L, Niehoff B, Hagen W, Søreide JE, Graeve M (2016) Lipid turnover reflects life-cycle strategies of small-sized Arctic copepods. J Plankton Res. https://doi.org/10.1093/plankt/fbw076
Cnudde C, Moens T, Werbrouck E, Lepoint G, Van Gansbeke D, De Troch M (2015) Trophodynamics of estuarine intertidal harpacticoid copepods based on stable isotope composition and fatty acid profiles. Mar Ecol Prog Ser 524:225–239. https://doi.org/10.3354/meps11161
Colombo SM, Rodgers TFM, Diamond ML, Bazinet RP, Arts MT (2020) Projected declines in global DHA availability for human consumption as a result of global warming. Ambio 49:865–880. https://doi.org/10.1007/s13280-019-01234-6
Conway N, McDowell Capuzzo J (1991) Incorporation and utilization of bacterial lipids in theSolemya velum symbiosis. Mar Biol 108:277–291. https://doi.org/10.1007/BF01344343
Coull BC (1990) Are members of the Meiofauna food for higher trophic levels? Trans Am Microsc Soc. https://doi.org/10.2307/3226794
Cronin TW, Fasick JI, Schweikert LE, Johnsen S, Kezmoh LJ, Baumgartner MF (2017) Coping with copepods: do right whales ( Eubalaena glacialis ) forage visually in dark waters? Philos Trans the Royal Soc Biol Sci 372:20160067–20160067. https://doi.org/10.1098/rstb.2016.0067
Daase M, Falk-Petersen S, Varpe Ø, Darnis G, Søreide JE, Wold A, Leu E, Berge J, Philippe B, Fortier L (2013) Timing of reproductive events in the marine copepod Calanus glacialis: A pan-Arctic perspective. Can J Fish Aquat Sci 70:871–884. https://doi.org/10.1139/cjfas-2012-0401
De Troch M, Boeckx P, Cnudde C, Van Gansbeke D, Vanreusel A, Vincx M, Caramujo MJ (2012) Bioconversion of fatty acids at the basis of marine food webs: Insights from a compound-specific stable isotope analysis. Mar Ecol Prog Ser. https://doi.org/10.3354/meps09920
Deschutter Y, De Schamphelaere K, Everaert G, Mensens C, De Troch M (2019) Seasonal and spatial fatty acid profiling of the calanoid copepods Temora longicornis and Acartia clausi linked to environmental stressors in the North Sea. Mar Environ Res. https://doi.org/10.1016/j.marenvres.2018.12.008
Frederiksen M, Anker-Nilssen T, Beaugrand G, Wanless S (2013) Climate, copepods and seabirds in the boreal Northeast Atlantic - current state and future outlook. Glob Change Biol. https://doi.org/10.1111/gcb.12072
Garzke J, Ismar SMH, Sommer U (2015) Climate change affects low trophic level marine consumers: warming decreases copepod size and abundance. Oecologia 177:849–860. https://doi.org/10.1007/s00442-014-3130-4
Garzke J, Hansen T, Ismar SMH, Sommer U (2016) Combined effects of ocean warming and acidification on copepod abundance, body size and fatty acid content. PLoS One. https://doi.org/10.1371/journal.pone.0155952
Gee JM (1987) Impact of epibenthic predation on estuarine intertidal harpacticoid copepod populations. Mar Biol. https://doi.org/10.1007/BF00397967
Graeve M, Kattner G, Hagen W (1994) Diet-induced changes in the fatty acid composition of Arctic herbivorous copepods: Experimental evidence of trophic markers. J Exp Mar Biol Ecol 182:97–110. https://doi.org/10.1016/0022-0981(94)90213-5
Graeve M, Boissonnot L, Niehoff B, Hagen W, Kattner G (2020) Assimilation and turnover rates of lipid compounds in dominant Antarctic copepods fed with 13 C-enriched diatoms. Philos Trans Royal Soc Biol Sci 375:20190647–20190647. https://doi.org/10.1098/rstb.2019.0647
Gulati R, Demott W (1997) The role of food quality for zooplankton: remarks on the state-of-the-art, perspectives and priorities. Freshw Biol 38:753–768
Haman J (2020) Quasibinomial model in R glm(). In: Random Effect. https://randomeffect.net/post/2020/10/12/quasi-binomial-in-r-glm/.
Hays GC, Kennedy H, Frost BW (2001) Individual variability in diel vertical migration of a marine copepod: Why some individuals remain at depth when others migrate. Limnol Oceanogr 46:2050–2054. https://doi.org/10.4319/lo.2001.46.8.2050
Hays G, Richardson A, Robinson C (2005) Climate change and marine plankton. Trends Ecol Evol 20:337–344. https://doi.org/10.1016/j.tree.2005.03.004
Helenius L, Budge SM, Nadeau H, Johnson CL (2020) Ambient temperature and algal prey type affect essential fatty acid incorporation and trophic upgrading in a herbivorous marine copepod. Philos Trans Royal Soc Biol Sci. https://doi.org/10.1098/rstb.2020.0039
Hixson SM, Arts MT (2016) Climate warming is predicted to reduce omega-3, long-chain, polyunsaturated fatty acid production in phytoplankton. Glob Change Biol. https://doi.org/10.1111/gcb.13295
Holm HC, Fredricks HF, Bent SM, Lowenstein DP, Ossolinski JE, Becker KW, Johnson WM, Schrage K, Van Mooy BAS (2022) Global ocean lipidomes show a universal relationship between temperature and lipid unsaturation. Science 376:1487–1491. https://doi.org/10.1126/science.abn7455
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Jónasdóttir SH, Visser AW, Richardson K, Heath MR (2015) Seasonal copepod lipid pump promotes carbon sequestration in the deep North Atlantic. Proc Natl Acad Sci 112:12122–12126. https://doi.org/10.1073/pnas.1512110112
Kabeya N, Fonseca MM, Ferrier DEK, Navarro JC, Bay LK, Francis DS, Tocher DR, Filipe L, Castro C, Monroig Ó (2018) Genes for de novo biosynthesis of omega-3 polyunsaturated fatty acids are widespread in animals. Sci Adv. https://doi.org/10.1126/sciadv.aar6849
Kabeya N, Ogino M, Ushio H, Haga Y, Satoh S, Navarro JC, Monroig Ó (2021) A complete enzymatic capacity for biosynthesis of docosahexaenoic acid (DHA, 22: 6n–3) exists in the marine Harpacticoida copepod Tigriopus californicus. Open Biol. https://doi.org/10.1098/rsob.200402
Kattner G, Hagen W (2009) Lipids in marine copepods: latitudinal characteristics and perspective to global warming. Lipids in Aquatic Ecosystems. Springer, New York, pp 257–280
Kelly J, Scheibling R (2012) Fatty acids as dietary tracers in benthic food webs. Mar Ecol Prog Ser 446:1–22. https://doi.org/10.3354/meps09559
Kwiatkowski L, Torres O, Bopp L, Aumont O, Chamberlain M, Christian JR, Dunne JP, Gehlen M, Ilyina T, John JG, Lenton A, Li H, Lovenduski NS, Orr JC, Palmieri J, Santana-Falcón Y, Schwinger J, Séférian R, Stock CA, Tagliabue A, Takano Y, Tjiputra J, Toyama K, Tsujino H, Watanabe M, Yamamoto A, Yool A, Ziehn T (2020) Twenty-first century ocean warming, acidification, deoxygenation, and upper-ocean nutrient and primary production decline from CMIP6 model projections. Biogeosciences 17:3439–3470. https://doi.org/10.5194/bg-17-3439-2020
Lampitt RS, Noji T, Von Bodungen B (1990) What happens to zooplankton faecal pellets? Implications for material flux. Springer-Verlag, Berlin
Lee MC, Choi BS, Kim MS, Yoon DS, Park JC, Kim S, Lee JS (2020) An improved genome assembly and annotation of the Antarctic copepod Tigriopus kingsejongensis and comparison of fatty acid metabolism between T. kingsejongensis and the temperate copepod T. japonicus. Compar Biochem Physiol Part D Genom Proteom. https://doi.org/10.1016/j.cbd.2020.100703
Malzahn AM, Boersma M (2012) Effects of poor food quality on copepod growth are dose dependent and non-reversible. Oikos 121:1408–1416. https://doi.org/10.1111/j.1600-0706.2011.20186.x
McGinty N, Barton AD, Record NR, Finkel ZV, Johns DG, Stock CA, Irwin AJ (2021) Anthropogenic climate change impacts on copepod trait biogeography. Glob Change Biol 27:1431–1442. https://doi.org/10.1111/gcb.15499
Meyer JL (1994) The microbial loop in flowing waters. Microb Ecol. https://doi.org/10.1007/BF00166808
Monroig Ó, Kabeya N (2018) Desaturases and elongases involved in polyunsaturated fatty acid biosynthesis in aquatic invertebrates: a comprehensive review. Fish Sci 84:911–928. https://doi.org/10.1007/s12562-018-1254-x
Moreno VJ, De Moreno JEA, Brenner RR (1979) Fatty acid metabolism in the calanoid copepodParacalanus parvus: 1. Polyunsaturated fatty acids. Lipids 14:313–317. https://doi.org/10.1007/BF02533413
Nielsen BLH, Gøtterup L, Jørgensen TS, Hansen BW, Hansen LH, Mortensen J, Jepsen PM (2019) n-3 PUFA biosynthesis by the copepod Apocyclops royi documented using fatty acid profile analysis and gene expression analysis. Biol Open. https://doi.org/10.1242/bio.038331
Nielsen BLH, van Someren GH, Rayner TA, Hansen BW (2020) Biochemical adaptation by the tropical copepods apocyclops royi and pseudodiaptomus annandalei to a pufa-poor brackish water habitat. Mar Ecol Prog Ser 655:77–89. https://doi.org/10.3354/meps13536
Parrish CC (2013) Lipids in marine ecosystems. ISRN Oceanograp 2013:1–16. https://doi.org/10.5402/2013/604045
Payne MF, Rippingale RJ (2000) Evaluation of diets for culture of the calanoid copepod Gladioferens imparipes. Aquaculture 187:85–96. https://doi.org/10.1016/S0044-8486(99)00391-9
R Core Team (2021) R: A language and environment for statistical computing.
Ratnayake WMN, Galli C (2009) Fat and fatty acid terminology, methods of analysis and fat digestion and metabolism: a background review paper. Ann Nutr Metab 55:8–43. https://doi.org/10.1159/000228994
Richardson AJ (2008) In hot water: Zooplankton and climate change
Sahan E, Sabbe K, Creach V, Hernandez-Raquet G, Vyverman W, Stal LJ, Muyzer G (2007) Community structure and seasonal dynamics of diatom biofilms and associated grazers in intertidal mudflats. Aquat Microb Ecol. https://doi.org/10.3354/ame047253
Semmouri I, De Schamphelaere KAC, Willemse S, Vandegehuchte MB, Janssen CR, Asselman J (2021) Metabarcoding reveals hidden species and improves identification of marine zooplankton communities in the North Sea. ICES J Mar Sci 78:3411–3427. https://doi.org/10.1093/icesjms/fsaa256
Semmouri I (2022) Temporal effects of chemical and physical stressors on marine zooplankton: a molecular approach. Ghent University, Faculty of Bioscience Engineering
Stenseth NC, Mysterud A, Ottersen G, Hurrell JW, Chan K-S, Lima M (2002) Ecological effects of climate fluctuations. Science 297:1292–1296. https://doi.org/10.1126/science.1071281
Stock W, Heylen K, Sabbe K, Willems A, De Troch M (2014) Interactions between benthic copepods, bacteria and diatoms promote nitrogen retention in intertidal marine sediments. PLoS ONE. https://doi.org/10.1371/journal.pone.0111001
Tarrant AM, Baumgartner MF, Lysiak NSJ, Altin D, Størseth TR, Hansen BH (2016) Transcriptional profiling of metabolic transitions during development and diapause preparation in the copepod Calanus finmarchicus. Integr Comp Biol 56:1157–1169. https://doi.org/10.1093/icb/icw060
Thor P, Koski M, Tang K, Jónasdóttir S (2007) Supplemental effects of diet mixing on absorption of ingested organic carbon in the marine copepod Acartia tonsa. Mar Ecol Prog Ser 331:131–138. https://doi.org/10.3354/meps331131
Turner JT (2004) The importance of small planktonic copepods and their roles in pelagic marine food webs
Twining CW, Taipale SJ, Ruess L, Bec A, Martin-Creuzburg D, Kainz MJ (2020) Stable isotopes of fatty acids: current and future perspectives for advancing trophic ecology. Philos Trans R Soc B 375:20190641. https://doi.org/10.1098/rstb.2019.0641
Werbrouck E, Bodé S, Van Gansbeke D, Vanreusel A, De Troch M (2017) Fatty acid recovery after starvation: insights into the fatty acid conversion capabilities of a benthic copepod (Copepoda, Harpacticoida). Marine Biol https://doi.org/10.1007/s00227-017-3181-2
Wolff RL, Bayard CC, Fabien RJ (1995) Evaluation of sequential methods for the determination of butterfat fatty acid composition with emphasis on trans -18:1 acids. Application to the study of seasonal variations in french butters. J Am Oil Chem Soc 72:1471–1483. https://doi.org/10.1007/BF02577840
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J Royal Statist Soc Series B Statist Methodol. https://doi.org/10.1111/j.1467-9868.2010.00749.x
Acknowledgements
We thank Dr. Bruno Vlaeminck, Marine Biology Research Group, Ghent University, for assisting with the fatty acid extraction and analysis. We also thank the anonymous reviewers for their constructive insights which improved this manuscript.
Funding
This research was supported with infrastructure funded by EMBRC Belgium - FWO international research infrastructure I001621N. RS is backed by a Bijzonder Onderzoeksfonds, Special Research Fund PhD grant – BOF, from Ghent University (BOF21/DOC/228), and JB by a fundamental research PhD grant from the Research Foundation Flanders – FWO (11E2320N).
Author information
Authors and Affiliations
Contributions
RS, JB, IS, MDT conceptualized this study. RS, JB, IS conducted the experiments. SB performed the GC-c-IRMS measurements. RS, JB, SB performed the calculations. RS performed the statistical analysis and prepared the first manuscript draft. All authors contributed to the interpretation of the results and manuscript revisions, and consent to the publication of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interest to disclose.
Ethics approval
No approval of research ethics committees was required to accomplish the goals of this study because experimental work was conducted with an unregulated invertebrate species.
Additional information
Responsible Editor: C. Meunier.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sahota, R., Boyen, J., Semmouri, I. et al. An inter-order comparison of copepod fatty acid composition and biosynthesis in response to a long-chain PUFA deficient diet along a temperature gradient. Mar Biol 169, 133 (2022). https://doi.org/10.1007/s00227-022-04121-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-022-04121-z