Abstract
Benthic copepods (Harpacticoida) are key members of the meiofauna community, and potentially important conveyers of energy from primary producers to higher trophic levels. However, little is known on their capability for trophic upgrading of food quality (essential fatty acids). Therefore, Platychelipus littoralis copepods were subjected to famine (3 days) and subsequent refeeding (6 days) on high (Thalassiosira weissflogii) and low (Dunaliella tertiolecta) quality food at 4, 15 and 24 °C, and their resilience for recovery of structural and storage fatty acids was determined. Additionally, stable isotope probing of fatty acids gave insight into the copepods’ ability to synthesize ARA (20:4ω6), EPA (20:5ω3) and DHA (22:6ω3) from low quality food under different temperatures. High intra-specific variability (among copepod replicates) in fatty acid composition and 13C enrichment was observed when copepods were exposed to heat (24 °C) and food quality stress, and operated therefore as an indicator of environmental stress. Synthesis of the essential fatty acids ARA, EPA and DHA from dietary precursors increased with temperature. However, despite the capability for synthesis, no fatty acid accumulation was observed, which suggested substantial fatty acid turnover, especially under heat stress. Moreover, synthesis rates were not sufficient to restore the ω3 pools and ensure survival, at least for the duration of the experiment. Therefore, the question rises whether copepods of this local P. littoralis population will be able to cope with the reduced dietary supply of essential ω3 fatty acids, as predicted under global warming, given that the physiological need for these essential compounds likely increases with temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Estuaries are among the most productive, marine ecosystems of the world, however, their associated phytoplankton and microphytobenthos show a high degree of spatial and temporal heterogeneity (Underwood and Kromkamp 1999). Consequently, first-level consumers have to deal with pulsed and variable food supplies. Microalgal–meiofaunal trophic relationships are vital for understanding the mechanisms that affect the trophic energy flow in benthic food webs, yet this relationship is very complex (Pinckney et al. 2003; Cibic et al. 2009) and may depend on food availability both in terms of quantity and quality. In particular, the issue of occasional food limitation periods by meiofauna has been addressed (Blanchard 1991; Montagna et al. 1995; Pinckney et al. 2003) and the answer seems to be habitat-dependent (beach sand, salt marshes or mud flats).
Benthic copepods (Copepoda, Harpacticoida) are key members of the meiofauna community (Hicks and Coull 1983), and therefore, potentially important conveyers of energy from primary producers to higher trophic levels. In aquatic ecosystems, lipids, and particularly their fatty acid (FA) building blocks, are the most energy-rich compounds (Parrish 2013). Moreover, FA are key descriptors of food quality in aquatic environments and represent a potentially important link between primary producers and consumers (Bell et al. 2007). In particular, the essential FA (EFA), arachidonic acid (ARA, 20:4ω6), eicosapentaenoic acid (EPA, 20:5ω3) and docosahexaenoic acid (DHA, 22:6ω3), are considered important drivers of ecosystem health and stability (Parrish 2013).
Despite the interesting trophic position of benthic copepods as first-level consumers, little is known on their lipid (FA) metabolism. In particular, to what extent benthic copepods are able to recover from famine as indicated by their storage (reserve) and membrane (structural) FAs, remains unresolved and is the focus of this study. Ambient temperature and nutritional quality of food sources can influence the rate at which energy reserves are restored (Koussoroplis et al. 2014). Moreover, temperature modulated the use of storage FA by the harpacticoid Platychelipus littoralis, i.e., FA depletion and mobilization (Werbrouck et al. 2016a). Therefore, temperature dependency likely applies to the reverse process, the recovery of membrane and storage FA. P. littoralis is a temperate, intertidal copepod species, and therefore experimental temperatures were 4, 15 °C, part of the natural temperature range, and 24 °C, representing a summer extreme. The dominant photoautotrophs in estuarine and salt marsh sediments are diatoms and chlorophytes, in addition to cyanobacteria (Pinckney et al. 2003) and were thus used as experimental food sources. In particular, the diatom Thalassiosira weissflogii and the chlorophyte Dunaliella tertiolecta represent two extremes in the food quality spectrum as indicated by their FA profile. D. tertiolecta is dominated by 18:3ω3, a polyunsaturated FA (PUFA, i.e., FA with >1 double bond), and 18:4ω3 is typically the longest and most unsaturated FA detected (Thor et al. 2007). In contrast, T. weissflogii is characterized by the presence of EPA and DHA (St. John et al. 2001), which both belong to the HUFA class (highly unsaturated FA, i.e., FA ≥20 carbon atoms and ≥3 double bonds). To monitor the flow of carbon from food sources to first-level consumers, the primary producers were isotopically labeled using H13CO3 −. Tracing stable isotopes in the consumer is a powerful tool in animal physiological ecology as they can be used to reconstruct diets, to trace movements, to assess physiological condition and to determine the fate of assimilated nutrients within an animal (Gannes et al. 1997). This set-up is allowed to resolve the following questions:
-
1.
Does the harpacticoid resume feeding after famine (bulk 13C uptake) and how does the uptake rate vary under different temperature and food regimes?
-
2.
How are storage FA affected by famine and subsequent refeeding?
-
3.
How does copepods’ fitness evolve in terms of survival, individual carbon content and membrane FA content and composition?
Starvation followed by a refeeding period on a low quality food source (D. tertiolecta), further allowed to investigate the harpacticoids’ FA bioconversion capability to ARA (20:4ω6), EPA (20:5ω3) and DHA (22:6ω3). To this purpose, D. tertiolecta is a most suitable ‘precursor’ diet as it is devoid of HUFA, but contains substantial amounts of their precursors 18:2ω6 and 18:3ω3 (Thor et al. 2007). We hypothesize that preceding starvation will stimulate compensatory biochemical pathways as P. littoralis is likely not habituated to this particular feeding regime (3 days of famine) and foreign food (a chlorophyte mono diet). In particular, 3 days of food deprivation under 15 °C depleted the EPA and DHA levels substantially, without increasing the mortality (Werbrouck et al. 2016a). Potentially, this physiological shortage drives EFA synthesis during the refeeding process. Virtually all PUFA originate from primary producers but can be modified (elongated or desaturated) as they pass up the food chain (Bell and Tocher 2009). Although marine invertebrates tend to have greater capacities for FA modification than higher animals (Iverson 2009; Kelly and Scheibling 2012), little is known about the pathways of PUFA conversion and metabolism in the trophic levels between primary producers and fish that are largely filled by invertebrates (Monroig et al. 2013).
Calanoid copepods are best studied among the zooplankton and are recognized for their unusual ability to produce large amounts of long-chain monounsaturated fatty alcohols (the wax ester of 20:1ω11, 20:1ω9, 22:1ω11, 22:1ω9) as part of their primary storage fats (Iverson 2009). For harpacticoid copepods, several studies highlighted their capacity for ω3-HUFA production, although all based on indirect evidence. This included increased relative abundance of EPA and DHA in the copepod compared to its diet (Nanton and Castell 1998) and similar ω3 HUFA levels in newly hatched offspring, regardless of the presence of these HUFA in the diet of the parental copepods (Norsker and Støttrup 1994). Unraveling metabolic pathways in copepods might have interesting applications in aquaculture, especially considering the superiority of copepods for the larviculture of marine fish. Currently, rotifers and brine shrimps (Artemia) are used as feed for fish larvae; however, they need to be enriched in FA to support full development (van der Meeren et al. 2008; Karlsen et al. 2015). Direct evidence of EFA synthesis by a harpacticoid was presented in De Troch et al. (2012), using 13C enriched food sources and subsequent compound-specific carbon isotope analysis (CSIA) of the FA. FA-SIA combines stable isotope and FA analyses as it determines the C isotopic composition of individual FA in the consumer (Budge et al. 2008). Therefore, it is a powerful technique for estimating the diet’s contribution to the consumer’s FA profile to reveal the presence of certain bioconversion pathways. It is now well-established that a HUFA-deficient diet may drive bioconversion (Iverson 2009; De Troch et al. 2012). However, less is known regarding the effect of ambient temperature, to our knowledge only addressed in Nanton and Castell (1999), despite the pressing need for a better understanding of global warming alterations. Concerning the latter, consumers may suffer from direct temperature effects on their physiology, i.e., homeoviscous adaptation (Hazel 1984) and rearrangement of the energy budget due to increased maintenance costs (Sokolova et al. 2012). In addition, global warming potentially induces indirect negative effects through altered food characteristics, such as changes in algal biochemical composition, in timing and in composition of the algal assemblage (Søreide et al. 2010; Galloway and Winder 2015; Charette and Derry 2016; Hixson and Arts 2016). Consequently, the consumer’s capability for producing the EFA may be critical to cope with the upcoming global warming. Therefore, the current study also targeted the following objectives:
-
1.
Is the harpacticoid P. littoralis able to convert dietary precursors to ARA, EPA and DHA?
-
2.
Where are these synthesized products predominantly incorporated, in membrane or storage lipids?
-
3.
Is EFA synthesis a temperature-mediated process?
Materials and methods
Algae culturing: copepod collection
Thalassiosira weissflogii (Bacillariophyceae) and D. tertiolecta (Chlorophyceae) were obtained from the Marine Algal Culture Centre—Göteborg University (strain GUMACC123) and the Aquaculture lab—Ghent University, respectively. Both algae were non-axenically cultured in tissue bottles using filtered (0.5 µm) and autoclaved seawater (salinity: 30), supplemented with Walne’s medium and a vitamin mix solution (Walne 1970). They were incubated at 20 ± 1 °C in climate rooms under a 14:10 h light–dark period (receiving 25–50 µmol photons m−2 s−1). Additionally, the T. weissflogii culture was supplemented with a silicate solution. Both algae were labeled with 13C by adding 16.8 mg NaH13CO3 (99%, 13C, Cambridge Isotope Laboratories) per 100 mL of culture medium. The 13C labeled supernatants was discarded from the cultures by means of centrifugation. Subsequently, the algal cultures were washed twice with filtered (0.5 µm) and autoclaved seawater (salinity: 30) to achieve complete removal of the 13C labeled medium. Equal volumes of the algal concentrate were distributed in Eppendorf tubes and stored at −80 °C for subsequent copepod feeding. In parallel, triplicates were stored at −80 °C for later lipid fractionation (FA analyses), SI analysis, carbon content and dry weight determination. Concerning the latter, samples were filtered (Whatman GF/F), dried overnight at 60 °C and stored 4 h in a desiccator prior to weighing. The algal carbon content supplied at each feeding event was considered well above the food limitation levels, i.e., 0.46 ± 0.03 and 0.53 ± 0.01 mg C for T. weissflogii and D. tertiolecta, respectively. The atomic %13C of T. weissflogii and D. tertiolecta were altered to 36.9 ± 0.2% 13C and 36.7 ± 0.2% 13C, respectively. At the start of the experiment, estimated T. weissflogii and D. tertiolecta cell lengths were 14 ± 1 and 7 ± 1 µm, respectively (inverted microscope Zeiss Axiovert 40C).
Harpacticoid copepods (P. littoralis Brady 1880, family Laophontidae) (~0.9 mm length) were collected from the top sediment layer (Nov 2015, 14 ± 1 °C) in a small intertidal muddy creek at the Paulina intertidal flat (Westerschelde estuary, 51°21′N, 3°43′E, SW Netherlands). P. littoralis individuals were extracted alive using sediment decantation. Under a wild M5 binocular, adult specimens were randomly collected with a glass pasteur pipette. Triplicate samples of field-collected copepods were stored at −80 °C for later bulk stable isotope (13C) analysis (20 ind. sample−1) and lipid fractionation (FA analysis) (100 ind. sample−1). All copepods were kept overnight in glass jars with some sediment aliquots at 15 ± 1 °C prior to the start of the experiment.
Experimental set-up
Experimental units consisted of Petri dishes (surface area = 26.4 cm2, 20 mL) with artificial seawater (Instant Ocean synthetic salt, salinity: 25, filtered over 0.2 µm Millipore filters), and contained minimum 120 copepods each. Prior to the feeding experiment, the copepods were starved for 3 days at 15 ± 1 °C under a 12:12 h light–dark regime (25–50 µmol photons m−2 s−1) to deplete their FA levels substantially (Werbrouck et al. 2016a). After one day of food deprivation, copepods were transferred to new Petri dishes with (acclimated) artificial seawater to remove their fecal pellets. After two additional days of famine, the copepods from three replicate units were sorted; 20 copepods from each unit were picked randomly for bulk stable isotope (13C) analysis. The remaining copepods (min. 100 ind. sample−1) were stored for lipid fractionation and FA extractions. In the refeeding experiment, copepods were offered pre-thawed, 13C enriched D. tertiolecta or T. weissflogii cells, and each food treatment was triplicated at 4, 15 and 24 ± 1 °C. To keep the nutritional characteristics of the algae constant and limit succession, the old algal cells were removed by washing the copepods daily over a 100-µm sieve. Subsequently, the copepods were placed in new Petri dishes with acclimated, artificial seawater and supplied with freshly thawed 13C enriched algae. After six feeding days, copepod mortality was assessed in each unit. The copepods were transferred to artificial seawater and starved for 6 h at their experimental temperature. This allowed gut clearance before they were sorted for bulk stable isotope analysis and lipid (FA) analysis (scheme in Supplemental Material).
Bulk stable isotope analysis
Carbon stable isotope ratios and carbon content of copepods and algae were determined in three biological replicates for each treatment, with an isotope ratio mass spectrometer (type Europa Integra) at the Davis Stable Isotope Facility (University of California, USA). Stable isotope ratios are expressed in the δ notation with Vienna Pee Dee Belemnite (VPDB) as reference standard and expressed in units per thousand (‰), according to the standard formula \(\delta^{13} {\text{C }} = \left[ {\left( {R_{\text{sample}} /R_{\text{VPDB}} } \right) - 1} \right] \, \times \, 10^{3}\), where R is 13C/12C and R VPDB = 0.01118.
However, the % 13C values were used to estimate the fraction (f) of copepod carbon derived from the 13C-labeled diet, i.e., T. weissflogii and D. tertiolecta:
where a13C cop, treatment, a13C cop, starved, a13C not-enriched food, a13C natural food are the isotopic compositions of copepods fed 13C enriched food, starved copepods, and enriched and not-enriched food, respectively. Multiplication of this fraction with the mean copepod carbon content results in the amount of assimilated algal carbon per individual copepod.
Lipid extraction and fractionation, FA derivatization
All lipids of copepods were extracted with a modified Bligh and Dyer extraction (Findlay et al. 1989). Subsequently, the total lipid extract was fractionated on a silicic acid solid phase extraction column (Merck) into different polarity classes by sequential elution with chloroform (neutral lipids), acetone (glycolipids) and methanol (polar lipids) (Christie 1989). To allow gas chromatographic separation, FAs of the lipid fractions were derivatized to fatty acid methyl esters (FAME). Phospholipid fatty acids (PLFA) of the methanol fraction were methylated using a mild alkaline methanolysis as in Boschker et al. (1999), while glyco- and neutral lipid fatty acids (NLFA) of the acetone and chloroform fractions, respectively, were derivatized using a modified method after Abdulkadir and Tsuchiya (2008). Here, the boron trifluoride-methanol reagent was replaced by a 2.5% of H2SO4-methanol solution, since the BF3-methanol can cause artifacts or loss of PUFA (Eder 1995). FAME of 19:0 (Sigma-Aldrich) was added as internal standard. Samples were concentrated to 200 µl hexane before analysis with a gas chromatograph (Hewlet Packard 6890 N) coupled to a mass spectrometer (HP 5973) as in De Troch et al. (2012). The samples were run in splitless mode injecting 1 µl at an injector temperature of 250 °C using an HP88 column (Agilent J&W; Agilent). The FAME were identified by comparing the retention times and mass spectra with authentic standards and mass spectral libraries (WILEY, own library) and analyzed using MSD ChemStation software (Agilent Technologies). Quantification of individual FAME was accomplished using a component FAME 37 and BAME mix (Bacterial Acid Methyl Esters) (Sigma-Aldrich) and completed with individual FAME standards (Larodan). Shorthand FA notations of the form A:BωX were used, where A represents the number of carbon atoms, B gives the number of double bonds, and X gives the position of the first double bond counting from the terminal methyl group. Algal and copepod FA contents are expressed per mass dry weight (DW) and per individual, respectively.
Compound-specific stable isotope analysis of FA
The 13C enrichments of the EFA (ARA, EPA and DHA) were determined on FAME extracts derived from polar and neutral lipid fractions of the starved copepods and the copepods refed with 13C labeled D. tertiolecta at 4, 15 and 24 °C. The FAME extracts were analyzed using gas chromatography combustion isotope ratio mass spectrometry (GC-c-IRMS) consisting of a Trace GC Ultra, coupled to a DeltaplusXP continuous flow stable isotope ratio mass spectrometer by a GC combustion III interface (all Thermo Scientific, Bremen, Germany). Chromatographic separation was done using a BPX5 column (30 m × 0.25 mm × 0.5 µm; SG9) with a He flow rate of 1.2 mL min−1, column kept at 50 °C for 1.5 min, followed by a ramp at 50 °C min−1 to 190 °C, then a second ramp at 3 °C min−1 to 290 °C. Injection was done with a large volume PTV injector, heated from 40 to 325 °C in 23 s during the injection phase. Each extract was run in duplicate on the GC-c-IRMS. The calculations are specified for EPA herein. Similar calculations were performed for ARA and DHA.
The fraction (f) of membrane-EPA derived from the 13C-labeled food was computed according to
where a13C membrane-EPA, T (°C) and a13C membrane-EPA, starved are the isotopic compositions of membrane-associated EPA in copepods fed D. tertiolecta at a particular temperature and in copepods starved for 3 days, respectively. a13C enriched DT and a13C not-enriched DT are the isotopic compositions of 13C enriched and not-enriched D. tertiolecta. Multiplication of this fraction with the amount (C) of membrane-associated EPA per copepod, observed at a particular temperature, results in the amount of synthesized EPA associated with the membrane lipids of copepods exposed to a particular temperature:
Similar calculations are performed for storage-associated EPA:
Subsequently, the amounts of synthesized EPA in both lipid pools for a particular temperature treatment are summed:
Eventually, the total amount of synthesized EPA by the copepods under a particular temperature is standardized for the amount of carbon assimilation (D. tertiolecta):
Statistical analyses
The following variables were subjected to one- and two-way ANOVA tests: survival, copepod carbon content, carbon assimilation, membrane and storage FA content, DHA/EPA ratio, and also the EPA and DHA content associated with each polar (membrane) and neutral (storage) lipid fraction (IBM SPSS Statistics Version 22). One-way tests compared the levels of each variable in copepods from the field, after starvation and from each temperature-diet treatment. Two-way ANOVA tests investigated the impact of temperature (3 factor levels) and diet (2 factor levels). In case of significant differences, Tukey’s HSD post hoc tests were performed to detect pairwise differences, using 95% confidence limits. Prior to ANOVA, Levene’s test was used to check the assumption of homoscedasticity. When the assumption of homoscedasticity was not met after log transformation, non-parametric tests (PERMANOVA) were performed using Primer 6 Version 6.1.11 and 1.0.1 (Clarke and Gorley 2006). To assess if isotopic enrichment was significant, isotopic composition of treated samples (copepods refed with 13C enriched D. tertiolecta) were compared with the respective non-enriched (starved) copepod samples, using one sample t tests (SPSS). The relationship between the incubation temperatures and the amounts of synthesized ARA, EPA, DHA was investigated by performing a Spearman’s rho correlation analysis (1-tailed) (SPSS). Standard deviation (SD) was chosen as measure of variability.
Prior to the multivariate statistics, relative FA data of T. weissflogii, D. tertiolecta, and membrane and storage lipids were arcsine square root transformed to meet the assumptions for normality and homogeneity of variance. Subsequently, a non-metric multidimensional scaling method (nMDS) (Bray–Curtis similarity) ordered the samples in a low-dimensional space (Primer 6 software). Next, a two-way ANOSIM test revealed the effects of temperature and food on the membrane and storage FA composition. A subsequent SIMPER analysis identified the FA contributing most to the observed differences. The FA profiles of T. weissflogii and D. tertiolecta were compared using a one-way ANOSIM test, also followed by a SIMPER analysis. Glycolipids in copepods were excluded from the analyses as their relative contribution to the overall FA pool appeared smallest (average of 10%).
Results
Diet characterization
The carbon contents of T. weissflogii and D. tertiolecta were 0.52 ± 0.17 and 0.37 ± 0.09 mg C mg dry wt−1, respectively. Although T. weissflogii and D. tertiolecta were characterized by a similar total FA content, i.e., 38.6 ± 2.3 and 36.7 ± 1.1 µg FA mg dry weight−1, respectively, different FA profiles were observed due to 18:3ω3, EPA and 16:3ω4 (Appendix Table 3).
Feeding experiment
Survival–carbon content–assimilation
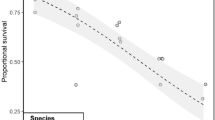
Both temperature and diet affected survival [pseudo-F(2,15) = 8.9 and pseudo-F(1,16) = 16.1, both P < 0.01, no interaction] (Fig. 1). T. weissflogii (99 ± 2%) supported higher survival than D. tertiolecta (95 ± 4%). Survival at 4 °C (99 ± 1%) was higher compared to 24 °C (94 ± 5%) (pairwise P < 0.01).
Starvation reduced the carbon content from 1.9 ± 0.1 µg C ind.−1 (field) to 1.5 ± 0.1 µg C ind.−1 However, copepods refed with T. weissflogii recovered successfully as their carbon content approached the original values (1.9 ± 0.2 µg C ind.−1), in contrast to copepods offered a D. tertiolecta diet (1.6 ± 0.1 µg C ind.−1) (Fig. 1) [pseudo-F(1,16) = 7.8, P < 0.05, no temperature or interaction effect].
The δ 13C values of field-collected and starved copepods did not differ significantly, i.e., −15.5 ± 0.05‰ (1.0887% 13C) and −15.2 ± 0.3‰ (1.0891% 13C), respectively. Refeeding with a 13C labeled feed increased the atomic 13C % with a minimum of 2 ± 0.04% (4 °C with D. tertiolecta) and even up to 8 ± 1% (15 °C with T. weissflogii). Temperature, diet [F(2,15) = 81.1 and F(1,16) = 329.1, both P < 0.001] and their interaction [F(2,12) = 9.3, P < 0.01] influenced the amount of assimilated carbon by the copepods (Fig. 2). For each temperature, the copepods assimilated more carbon when T. weissflogii was offered compared to D. tertiolecta (Tukey HSD all P < 0.05). In the T. weissflogii treatments, the highest assimilation was observed at 15 °C (0.36 ± 0.02 µg C) compared to 4 °C (0.19 ± 0.03 µg C) and 24 °C (0.23 ± 0.02 µg C) (Tukey HSD all P < 0.001). On a D. tertiolecta diet, the lowest assimilation appeared at 4 °C (0.04 ± 0.005 µg C) compared to 15 °C (0.15 ± 0.01 µg C) and 24 °C (0.11 ± 0.03 µg C) (Tukey HSD both P < 0.01) (Fig. 2).
Membrane and storage FA content
Starvation reduced the individual membrane FA content to ± 64% of the field level [F(7,16) = 15.1, P < 0.001; Tukey HSD P < 0.01] (Fig. 3a). Refeeding at 24 °C lowered the membrane FA content further regardless of the offered diet, i.e., to 36% of the field level [F(2,15) = 10.1, P < 0.01 for temperature, diet and interaction were not significant]. The copepods did not manage to recover their membrane FA content to pre-starvation levels (Tukey HSD all P < 0.001), although the decline at 4 °C and 15 °C with a T. weissflogii diet (65–57% of the field level) appeared smaller compared to D. tertiolecta (50–46%).
a Membrane and storage fatty acid content, b EPA (20:5ω3) and c DHA (22:6ω3) (ng ind.−1) of P. littoralis prior (field), after 3 days of starvation (starved) and after 6 days of incubation with D. tertiolecta and T. weissflogii at 4, 15 and 24 °C. Mean + 1 SD for storage and glycolipids (n = 3), mean − 1 SD for membrane lipids (n = 3)
Three days of famine roughly halved the storage FA content, i.e., from 54 ± 12 to 31 ± 3.3 ng FA ind.−1 [F(7,16) = 22.4, P < 0.001; Tukey HSD P < 0.01] (Fig. 3a). Temperature, diet and their interaction, all influenced the storage FA recovery [F(2,15) = 8.2, F(1,16) = 24.4 and F(2,12) = 7.6, all P < 0.01]. When T. weissflogii was offered, the storage FA content was highest at 4 °C compared to 15 and 24 °C (Tukey HSD both P < 0.01), while no significant differences among temperature incubations appeared when copepods were offered D. tertiolecta. The effect of diet emerged only at 4 °C as indicated by the higher storage FA content when copepods were fed with T. weissflogii (Tukey HSD P < 0.01). Similar as for the membrane FA content, a refeeding period of 6 days did not restore the storage FA content.
Membrane- and storage-associated EPA and DHA content
Famine reduced the membrane- and storage-associated EPA content roughly by half [F(7,16) = 28.2 and F(7,16) = 25.5, Tukey HSD both P < 0.01] (Fig. 3b), but not the membrane- and storage-associated DHA content (Fig. 3c). The membrane-associated EPA and DHA content further declined after refeeding at 24 °C, regardless of the diet (Tukey HSD all P < 0.05). Refeeding the copepods with T. weissflogii at 4 °C did not change the storage-associated EPA and DHA content significantly as compared to the levels in starved copepods.
Both temperature and diet affected the membrane-associated EPA content [F(2,15) = 19.6 and F(1,16) = 14.9, both P < 0.01, no interaction]. More specifically, a T. weissflogii diet resulted in a higher EPA content in the copepods’ membranes compared to D. tertiolecta, i.e., 9.5 ± 3.5 ng EPA ind.−1 compared to 6.7 ± 1.9 ng EPA ind.−1, respectively. Furthermore, the membrane-associated EPA content decreased with rising temperature on both diets (Tukey HSD all P < 0.05).
Only temperature affected the DHA content in the copepods’ membranes [F(2,15) = 8.2, P < 0.01], i.e., exposure to 4 °C (15.0 ± 2.6 ng DHA per ind.) resulted in a higher concentration compared to 24 °C (9.3 ± 2.7 ng DHA per ind.) (Tukey HSD P < 0.01). Temperature, diet and their interaction affected both the storage-associated EPA [F(2,15) = 13.1, F(1,16) = 38.2 and F(2,12) = 7.8, all P < 0.01] and DHA content [F(2,15) = 10.7, F(1,16) = 28.6 and F(2,12) = 7.9, all P < 0.01], revealing the same response pattern as the total storage FA content.
Membrane and storage FA composition
The copepods’ membrane and storage FA composition (Table 1a, b) grouped according to ambient temperature and offered diet as indicated by the nMDS plots (Fig. 4a, b), and the significance of this grouping was confirmed by subsequent ANOSIM tests. In particular, two-way ANOSIM tests for the effects of temperature and diet on the FA composition have resulted in R = 0.93 (P < 0.001) and R = 1 (P < 0.001) for membrane lipids, and R = 0.745 (P < 0.001) and R = 0.73 (P < 0.004) for storage lipids, respectively. Table 2 summarizes the average dissimilarity % among the temperature and diet groups for membrane and storage lipids, and the FA contributing most to the observed differences (SIMPER analysis). The stronger impact of food on the membrane FA composition was further reflected by the high pairwise dissimilarity percentage (39.9%) compared to the storage FA composition (14.7%).
Starvation increased the original DHA/EPA ratio in the membrane lipids from 1.1 ± 0.1 to 1.6 ± 0.1 [F(7,16) = 20.3, P < 0.001, Tukey HSD P < 0.001] (Table 1a). Both temperature and food affected the ratio [F(2,15) = 16.0 and F(1,16) = 40.0, both p < 0.001, no interaction]. Recovery on a D. tertiolecta or T. weissflogii diet resulted in DHA/EPA ratios of 1.8 ± 0.2 and 1.4 ± 0.2, respectively. Furthermore, the highest ratio was observed at 24 °C (1.8 ± 0.2) versus 4 °C (1.4 ± 0.2) and 15 °C (1.5 ± 0.2) (Tukey HSD both P < 0.01).
Compound-specific stable isotope analysis of FA
Significant isotopic enrichment of ARA, EPA and DHA, associated with membrane and/or storage lipids, was found in the treated samples (copepods refed with 13C enriched D. tertiolecta) compared with the respective starved samples (all P < 0.001) (Fig. 5). However, compared to concentration measurements, higher FA concentration are needed for accurate 13C determination. Therefore, 13C of DHA in the storage lipids at 4 °C could not be determined and only one of three replicates of the 15 and 24 °C incubations had the required concentration (Fig. 5b). Synthesis of ARA (r = 0.949, P < 0.001), EPA (r = 0.632, P < 0.05) and DHA (r = 0.738, P < 0.05) all increased with temperature (Fig. 6).
δ 13C values (‰ + 1 SD, n = 3) of a EPA and DHA in the membrane lipids, b EPA and DHA in the storage lipids and c ARA in the membrane lipids of P. littoralis, starved (3 days) prior to refeeding with 13C enriched D. tertiolecta at 4, 15 and 24 °C. *, DHA concentration was only sufficient in one replicate to allow reliable 13C determination
Discussion
Assimilation
Famine strongly impacted the copepods based on their decreased carbon (20%) and FA (36%) content. Nevertheless, the copepods resumed feeding on both diets based on the observed assimilation. Assimilation of T. weissflogii increased the copepod carbon content almost to the original field levels and likely supported higher copepod survival. The formation of new biomass as metabolic components, body structure, and reproductive tissues is a key process underlying organismal fitness (Frost et al. 2005).
Temperature impact on the energy acquisition (feeding rate, digestive and assimilation efficiency), incorporation (biomolecule synthesis, allocation and storage) and release (respiration, egestion and excretion) (Frost et al. 2005) may all have contributed to the observed differences in assimilation in the current study. Assimilation peaked at 15 °C, which likely approaches the optimum growth temperature of this temperate, intertidal harpacticoid copepod, evoking the maximum rate of performance within its thermal performance curve (Schulte 2015).
Although the current assimilation estimates proved their value for relative comparison among temperatures, extrapolation to field estimates requires caution as they result from manipulated laboratory experiments. In particular, daily stress of the copepods due to experimental handling with a sieve at each feeding event and the absence of a sediment matrix may underestimate the assimilation. For example, Cnudde et al. (2012) suggested that the presence of sediment stimulated the diatom assimilation by P. littoralis, likely due to its epibenthic lifestyle as this is not necessarily the case for all harpacticoid species (De Troch et al. 2006). Also, the lack of a sticky diatom biofilm, i.e., a mucilaginous extracellular polymer matrix, may affect the trophic interaction with the consumer (Decho and Lopez 1993).
Storage FAs
In contrast to the regained carbon content, no recovery of the storage FA was observed, even when refed with the high quality food. T. weissflogii supported complete copepod development (Koski et al. 2008) and stimulated the build-up of storage FA in Acartia copepods (Werbrouck et al. 2016c). Moreover, Arctic Calanus species increased their lipid content on a T. weissflogii diet, while their carbon content remained almost constant during feeding (Graeve et al. 2005). Rather the opposite appeared in the current study as the copepods increased their individual carbon content, while no alteration or rather a decline in storage FA was observed. A refeeding period of 6 days appeared too short for copepods to recover from severe nutritional stress (3 days of famine) as suggested by their FA profile.
Recovery is not only a matter of resuming feeding but also of digestion (Tiselius 1998). Potentially, assimilated dietary FA were catabolized to match the energy demand for catch-up biosynthesis of gut cells and digestive enzymes during recovery. In fact, starved copepods reintroduced to food have been showing elevated clearance rates (Tiselius 1998) and augmented respiration rates, transiently rising above the respiration rate of constantly feeding copepods (Thor 2003), both interpreted as a ‘hunger response’. As the duration of the hunger response depended on the duration of the preceding starvation period (Tiselius 1998; Thor 2003), a refeeding period of 6 days was possibly not sufficient for P. littoralis to accomplish full recovery and additional storage accumulation.
The lack of FA build-up, however, does not imply a reduced activity or FA turnover, i.e., new FA replacing older ones, thereby altering the FA composition. In particular, FA turnover is thought to vary with life stage, but depends also on food availability and water temperature (Brett et al. 2009). When copepods were offered a high quality diet, the divergence of their storage FA composition from the post-starvation one was mainly driven by temperature through its effect on the copepods’ assimilation. In particular, assimilation of high quality food (dietary FA) peaked at 15 °C, and accordingly coincided with the strongest alterations in storage FA composition. On a low quality diet, changes in storage FA composition were driven both by temperature and continued food stress, manifested as a high variability in storage FA composition among replicates (herein ‘intra-specific variability’), especially under heat stress. The link between the pools of storage and membrane FA and their different function in the copepod, may explain these observations. In particular, the membrane FA composition is usually rather stable, given reasonably constant environmental conditions and diet, as membrane FA play a major role in maintaining the structure and function of cellular biomembranes (Tocher 2003). In particular, DHA (32%), EPA (29%), 16:0 (15%) and 18:0 (5%) (see also Albers et al. 1996), dominated the membranes of field-collected P. littoralis specimens. In contrast, a stable FA composition is less of a constraint for the reserves, and consequently the storage lipids may act as FA providers for the synthesis of membrane phospholipids (Desvilettes et al. 1997; Bergé and Barnathan 2005) or provide replacer FA for membrane remodeling (Girón-Calle et al. 1997). Restructuring the lipid composition of biological membranes in response to changing temperatures is a major strategy by which ectotherms maintain vital physiological functions of membranes (Martin-Creuzburg et al. 2012).
Therefore, the high intra-specific variability in FA composition can be interpreted as a biochemical indicator of environmental stress, appearing first at the level of the storage FA, and only later at the level of membrane FA. When copepods were exposed to the combined impact of low food quality and heat exposure, their storage FA pool lost their buffering role which resulted in increased intra-specific variability in membrane FA composition. Possibly, this induced copepod mortality.
Membrane FAs
Strikingly, copepods did not recuperate at the level of their membrane FA content to any extent, despite a famine-induced reduction of 40%. Moreover, a further decline was observed during the refeeding period. EPA and DHA are important compounds of phospholipids, which in turn, constitute the main building blocks of membranes (Kidd 2007). Consequently, shortage of dietary EFA may have prevented the synthesis of new membrane, despite the capability of the copepod for EPA and DHA synthesis (see following section ‘Capability for FA bioconversion’).
The increased DHA/EPA ratio in the membranes of food-deprived copepods, may be the result of preferential retention of DHA (see also Werbrouck et al. 2016a) and the need to metabolize EPA. Although both are considered EFA, their essentiality may result from different physiological roles, i.e., a functional or structural role. In particular, EPA acts as a precursor of eicosanoids, a group of biologically active molecules, serving as messengers in the central nervous system and acting as signaling molecules to control inflammation and immunity (Arts and Kohler 2009). Possibly, DHA has a primarily structural role as it provided the optimal physical environment in retinal membranes for light-activated rhodopsin to initiate the visual signal (Hulbert et al. 2014). Moreover, DHA catabolism requires peroxisomal and mitochondrial β-oxidation while EPA can be readily β-oxidized as has been shown in rats (Madsen et al., 1999). During the refeeding process, the DHA/EPA ratio appeared very responsive to temperature and diet. Maximum environmental stress, evoked by heat and low quality food, induced the highest DHA/EPA ratio (1.9 ± 0.2) in the copepod’s membranes, almost double compared with the ratio in field-collected copepods (1.1 ± 0.1).
The lack of membrane FA recovery, despite the available dietary EFA in T. weissflogii, was more peculiar, and the resulting scenario appeared little better, as the post-starvation levels were maintained, except under heat stress conditions. Potentially, a longer refeeding period on high quality food would have allowed increasing the membrane FA content. Regardless of the diet, the membrane FA content was strongly reduced at 24 °C (see also Werbrouck et al. 2016b) and might be the result of thermal (oxidative) stress.
Capability for FA conversion
The harpacticoid was capable of ARA, EPA and DHA synthesis through conversion of dietary precursors and this activity was clearly temperature-driven, with the strongest dependency observed for ARA followed by DHA and EPA. Renault et al. (2002) suggested that during recovery, the resynthesis rates of all metabolites increase with temperature. Synthesized EFA were primarily incorporated into the membranes, as reflected by the increased 13C enrichment, and only to a limited extent into the storage lipids. This illustrates that EFA function exclusively at the level of the cell membranes (Kidd 2007). Although occurring in smaller amounts, ARA may be an equally important FA as it also operates as a precursor for eicosanoids. In particular, ARA and EPA act in concert by controlling the opposite physiological responses, evoking inflammatory and rather anti-inflammatory effects (Arts and Kohler 2009; Parrish 2009). Although eicosanoids are normal physiological products, extreme stress may trigger elevated eicosanoid biosynthesis (Arts and Kohler 2009). Therefore, heat stress may have driven the increased synthesis of their precursors, ARA and EPA in the appropriate proportions.
Most animals can biosynthesize saturated FA and the common monounsaturated FA de novo (Arts et al. 2001). The synthesis of unsaturated FA usually starts with the insertion of the first double bond near the middle of the molecule in all organisms. Plants normally introduce a second double bond between the existing position and the terminal methyl group, while animals insert double bonds between an existing double bond and the carboxyl end of the molecule (Parrish 2009), and therefore synthesize fewer and simpler FA (Iverson 2009). This restricted biochemical capability, combined with the requirement for FA with the first double bond in the ω3 or ω6 position for optimal functioning, lies at the base of the essentiality of ω3 and ω6 FA (Parrish 2009). The extent, to which a given species can convert one ω3 FA to another, or one ω6 FA to another, leads to degrees of essentiality (Parrish 2009). Animals can elongate both endogenously and exogenously produced FA to some extent, but this is generally limited, and both the de novo biosynthesis and FA modification (elongation–desaturation) are inhibited by diets containing adequate or excess lipid, and long-chain PUFA (Iverson 2009). Reflecting its economic importance, most FA research to date dealt with fish/shellfish and fewer studies have focused on other invertebrates (Arts et al. 2001). In contrast to freshwater ecosystems, where several fish species are able to synthesize longer chain PUFA through a series of elongations and desaturations (Tocher 2003), marine fish seem to require pre-formed HUFA (Sargent et al. 1993). Consequently, organisms occupying the lower trophic levels in marine ecosystems are assigned a pivotal role as HUFA providers. EPA and DHA are very abundant in the marine environment, originating mainly from diatoms and flagellates, respectively, whence they are transmitted intact via zooplankton to fish (Tocher 2003).
The paradox of increasing EFA synthesis with temperature, yet decreasing EFA concentrations in both lipid pools, can be explained by heat-induced oxidative stress. In particular, increased EFA synthesis and incorporation into the membranes can be interpreted as an attempt to replace damaged HUFA and guarantee continued membrane function. Turnover processes of de- and re-acylation, with respect to both head groups and fatty acyl chains of phospholipids, are important in the organism’s adaptation to environmental changes (Tocher 2003). Heat stress may induce oxidative stress in marine organisms, which is reflected by the production and accumulation of reactive oxygen species (ROS) (Lesser 2006). Although essential for membrane function, PUFA are very susceptible to attack by ROS (Mazière et al. 1999), and the resulting oxidative damage to PUFA in membrane phospholipids can have serious consequences for cell membrane structure and fluidity, with potential pathological effects on cells and tissues (Tocher 2003). Oxidative stress, associated with heat exposure, was likely the driver of increased EFA synthesis and incorporation into the membranes, as an attempt to replace damaged HUFA and guarantee continued membrane function. Furthermore, the presence of oxidative stress in the high temperature treatments was suggested by the loss of the copepods’ red pigmentation (pers. obs). Astaxanthin, a powerful antioxidant among carotenoids, may protect copepods from different sources of oxidative stress (McNulty et al. 2008; Schneider et al. 2016). This antioxidant can be esterified with storage FA and accordingly, free astaxanthin can be incorporated in cell membranes where it efficiently reduces lipid peroxidation, while preserving membrane structure (Schneider et al. 2016). Despite the initial presence of potential antioxidants, high copepod survival was not maintained under heat exposure.
Ecological implications
Previous work reported the relative increase of EPA in the membranes of cold-exposed invertebrates (Hall et al. 2002; Schlechtriem et al. 2006) and the accumulation of EPA and DHA in cold-exposed and winter-active ectotherms has led to the suggestion of their important structural or functional role in cold acclimation or adaptation (Farkas 1979; Martin-Creuzburg et al. 2012). Also, the harpacticoid P. littoralis increased its relative EPA concentration with approximately 4% at 4 °C compared to 24 °C, regardless of the diet. Furthermore, the synthesis of EPA seemed to prevail over DHA under cold conditions. A priori, EPA requirement, and thus synthesis was expected to be highest after cold exposure, however, synthesis increased with rising temperature. Although EPA potentially plays an important structural or functional role in cold acclimatization, our results suggest that part of the previously reported cold-induced EPA accumulation could be attributed to the reduced turnover rates at low temperature. Moreover, increased functional activity, for example, EPA acting as a precursor for eicosanoids (Arts and Kohler 2009) would imply its disappearance from the membranes, rather than its retention.
In conclusion, the harpacticoid P. littoralis was capable of synthesizing ARA, EPA and DHA from dietary precursors under the three temperature regimes, yet no increase in their respective concentration was observed. The detritus-rich, and therefore, EFA-limited environments typically inhabited by harpacticoid copepods have been linked with their capability for EFA synthesis (Anderson and Pond 2000). In view of their EFA-depleted diet, it is plausible that evolutionary pressures have led to harpacticoid copepods developing the capability to synthesize these EFA (Bell et al. 2007). However, the temperate, epibenthic harpacticoid P. littoralis populates a very productive intertidal mudflat, with chl a estimates ranging annually from 18 to 229 mg m−2 (Sahan et al. 2007). Moreover, the biochemical pathways that animals are capable of performing are not necessarily the same as their propensity for using them (Iverson 2009). The lack of substantial EFA accumulation, combined with the increased mortality suggests that ARA, EPA and DHA are still essential dietary compounds for this harpacticoid copepod, at least for a post-starvation period of 6 days. Therefore, it remains doubtful whether this local P. littoralis population will be able to adapt to the impacts of global warming. Their capability for essential ω3 synthesis may compensate for the reduced dietary supply of essential ω3 FA, as predicted under global warming (Hixson and Arts 2016). However, whether this will meet their increased physiological need for these essential compounds at higher temperatures remains unanswered.
References
Abdulkadir S, Tsuchiya M (2008) One-step method for quantitative and qualitative analysis of fatty acids in marine animal samples. J Exp Mar Biol Ecol 354:1–8
Albers CS, Kattner G, Hagen W (1996) The compositions of wax esters, triacylglycerols and phospholipids in Arctic and Antarctic copepods: evidence of energetic adaptations. Mar Chem 55:347–358
Anderson TR, Pond DW (2000) Stoichiometric theory extended to micronutrients: comparison of the roles of essential fatty acids, carbon, and nitrogen in the nutrition of marine copepods. Limnol Oceanogr 45:1162–1167
Arts MT, Kohler CC (2009) Health and condition in fish: the influence of lipids on membrane competency and immune response. In: Arts MT, Brett MT, Kainz MJ (eds) Lipids in aquatic ecosystems. Springer, New York, pp 237–255
Arts MT, Ackman RG, Holub BJ (2001) “Essential fatty acids” in aquatic ecosystems: a crucial link between diet and human health and evolution. Can J Fish Aquat Sci 58:122–137
Bell MV, Tocher DR (2009) Biosynthesis of polyunsaturated fatty acids in aquatic ecosystems: general pathways and new directions. In: Arts MT, Brett MT, Kainz MJ (eds) Lipids in aquatic ecosystems. Springer, New York, pp 211–236
Bell MV, Dick JR, Anderson TR, Pond DW (2007) Application of liposome and stable isotope tracer techniques to study polyunsaturated fatty acid biosynthesis in marine zooplankton. J Plankton Res 29:417–422
Bergé JP, Barnathan G (2005) Fatty acids from lipids of marine organisms: molecular biodiversity, roles of biomarkers, biologically active compounds, and economical aspects. Adv Biochem Eng Biotechnol 96:1–77
Blanchard G (1991) Measurement of meiofauna grazing rates on microphytobenthos: is primary production a limiting factor? J Exp Mar Biol Ecol 147:37–46
Boschker HTS, de Brouwer JFC, Cappenberg TE (1999) The contribution of macrophyte-derived organic matter to microbial biomass in salt-marsh sediments: stable carbon isotope analysis of microbial biomarkers. Limnol Oceanogr 44:309–319
Brett MT, Müller-Navarra DC, Persson J (2009) Crustacean zooplankton fatty acid composition. In: Arts MT, Brett MT, Kainz MJ (eds) Lipids in aquatic ecosystems. Springer, New York, pp 115–146
Budge SM, Wooller MJ, Springer AM, Iverson SJ, McRoy CP, Divoky GJ (2008) Tracing carbon flow in an arctic marine food web using fatty acid-stable isotope analysis. Oecologia 157:117–129
Charette C, Derry AM (2016) Climate alters intraspecific variation in copepod effect traits through pond food webs. Ecology 97:1239–1250
Christie WW (1989) Gas chromatography and lipids: a practical guide, 1st edn. The Oily Press Ltd, Ayr
Cibic T, Blasutto O, Bettoso N (2009) Microalgal–meiofaunal interactions in a sublittoral site of the Gulf of Trieste (northern Adriatic Sea, Italy): a three-year study. J Exp Mar Biol Ecol 370:144–154
Clarke KR, Gorley RN (2006) Primer v6: user manual/tutorial. PRIMER-E, Plymouth
Cnudde C, Moens T, Willems A, De Troch M (2012) Substrate-dependent bacterivory by intertidal benthic copepods. Mar Biol 160:327–341
De Troch M, Houthoofd L, Chepurnov V, Vanreusel A (2006) Does sediment grain size affect diatom grazing by harpacticoid copepods? Mar Environ Res 61:265–277
De Troch M, Boeckx P, Cnudde C, Van Gansbeke D, Vanreusel A, Vincx M, Caramujo MJ (2012) Bioconversion of fatty acids at the basis of marine food webs: insights from a compound-specific stable isotope analysis. Mar Ecol Prog Ser 465:53–67
Decho AW, Lopez GR (1993) Exopolymer microenvironments of microbial flora: Multiple and interactive effects on trophic relationships. Limnol Oceanogr 38:1633–1645
Desvilettes Ch, Bourdier G, Breton JCh (1997) On the occurrence of a possible bioconversion of linolenic acid into docosahexaenoic acid by the copepod Eucyclops serrulatus fed on microalgae. J Plankton Res 19:273–278
Eder K (1995) Gas Chromatographic analysis of fatty acid methyl esters. J Chromatogr B 671:113–131
Farkas T (1979) Adaptation of fatty acid compositions to temperature—a study on planktonic crustaceans. Comp Biochem Physiol B 64:71–76
Findlay RH, King GM, Watling L (1989) Efficacy of phospholipid analysis in determining microbial biomass in sediments. Appl Environ Microbiol 55:2888–2893
Frost PC, Evans-White MA, Finkel ZV, Jensen TC, Matzek V (2005) Are you what you eat? Physiological constraints on organismal stoichiometry in an elementally imbalanced world. Oikos 109:18–28
Galloway AWE, Winder M (2015) Partitioning of the relative importance of phylogeny and environmental conditions on phytoplankton fatty acids. PLoS One. doi:10.1371/journal.pone.0130053
Gannes LZ, O’Brien DM, Martínez del Rio C (1997) Stable isotopes in animal ecology: assumptions, caveats and a call for more laboratory experiments. Ecology 78:1271–1276
Girón-Calle J, Schmid PC, Schmid HHO (1997) Effects of oxidative stress on glycerolipid acyl turnover in rat hepatocytes. Lipids 32:917–923
Graeve M, Albers C, Kattner G (2005) Assimilation and biosynthesis of lipids in Arctic Calanus species based on feeding experiments with a 13C labelled diatom. J Exp Mar Biol Ecol 317:109–125
Hall JM, Parrish CC, Thompson RJ (2002) Eicosapentaenoic acid regulates scallop (Placopecten magellanicus) membrane fluidity in response to cold. Biol Bull 202:201–203
Hazel JR (1984) Effects of temperature on the structure and metabolism of cell membranes in fish. Am J Physiol 246:460–470
Hicks GRF, Coull BC (1983) The ecology of marine meiobenthic harpacticoid copepods. Oceanogr Mar Biol Annu Rev 21:67–175
Hixson SM, Arts MT (2016) Climate warming is predicted to reduce omega-3, long-chain, polyunsaturated fatty acid production in phytoplankton. Glob Change Biol 22:2744–2755
Hulbert AJ, Kelly MA, Abbott SK (2014) Polyunsaturated fats, membrane lipids and animal longevity. J Comp Physiol B 184:149–166
Iverson SJ (2009) Tracing aquatic food webs using fatty acids. In: Arts MT, Brett MT, Kainz MJ (eds) Lipids in aquatic ecosystems. Springer, New York, pp 281–307
Karlsen Ø, van der Meeren T, Rønnestad I, Mangor-Jensen A, Galloway TF, Kjørsvik E, Hamre K (2015) Copepods enhance nutritional status, growth and development in Atlantic cod (Gadus morhua L.) larvae—can we identify the underlying factors? PeerJ 3:1–27
Kelly JR, Scheibling RE (2012) Fatty acids as dietary tracers in benthic food webs. Mar Ecol Prog Ser 446:1–22
Kidd PM (2007) Omega-3 DHA and EPA for cognition, behavior, and mood: clinical findings and structural- functional synergies with cell membrane phospholipids. Altern Med Rev 12:207–227
Koski M, Wichard T, Jónasdóttir SH (2008) “Good” and “bad” diatoms: development, growth and juvenile mortality of the copepod Temora longicornis on diatom diets. Mar Biol 154:719–734
Koussoroplis AM, Nussbaumer J, Arts MT, Guschina IA, Kainz MJ (2014) Famine and feast in a common freshwater calanoid: effects of diet and temperature on fatty acid dynamics of Eudiaptomus gracilis. Limnol Oceanogr 59:947–958
Lesser MP (2006) Oxidative stress in marine environments: Biochemistry and physical ecology. Annu Rev Physiol 68:253–278
Madsen L, Rustan AC, Vaagenes H, Berge K, Dyrøy E, Berge RK (1999) Eicosapentaenoic and docosahexaenoic acid affect mitochondrial and peroxisomal fatty acid oxidation in relation to substrate preference. Lipids 34:951–963
Martin-Creuzburg D, Wacker A, Ziese C, Kainz MJ (2012) Dietary lipid quality affects temperature-mediated reaction norms of a freshwater key herbivore. Oecologia 168:901–912
Mazière C, Conte MA, Degonville J, Ali D, Mazière JC (1999) Cellular enrichment with polyunsaturated fatty acids induces an oxidative stress and activates the transcription factors AP1 and NFκB. Biochem Biophys Res Commun 265:116–122
McNulty H, Jacob RF, Mason RP (2008) Biologic activity of carotenoids related to distinct membrane physicochemical interactions. Am J Cardiol 101:S20–S29
Monroig O, Tocher DR, Navarro JC (2013) Biosynthesis of polyunsaturated fatty acids in marine invertebrates: recent advances in molecular mechanisms. Mar Drugs 11:3998–4018
Montagna PA, Blanchard GF, Dinet A (1995) Effect of production and biomass of intertidal microphytobenthos on meiofaunal grazing rates. J Exp Mar Biol Ecol 185:149–165
Nanton DA, Castell JD (1998) The effects of dietary fatty acids on the fatty acid composition of the harpacticoid copepod, Tisbe sp., for use as a live food for marine fish larvae. Aquaculture 163:251–261
Nanton DA, Castell JD (1999) The effects of temperature and dietary fatty acids on the fatty acid composition of harpacticoid copepods, for use as a live food for marine fish larvae. Aquaculture 175:167–181
Norsker NH, Støttrup JG (1994) The importance of dietary HUFAs for fecundity and HUFA content in the harpacticoid, Tisbe holothuriae Humes. Aquaculture 125:155–166
Parrish CC (2009) Essential fatty acids in aquatic food webs. In: Arts MT, Brett MT, Kainz MJ (eds) Lipids in aquatic ecosystems. Springer, New York, pp 309–326
Parrish CC (2013) Lipids in marine ecosystems. ISRN Oceanogr 1–16
Pinckney JL, Carman KR, Lumsden SE, Hymel SN (2003) Microalgal-meiofaunal trophic relationships in muddy intertidal estuarine sediments. Aquat Microb Ecol 31:99–108
Renault D, Hervant F, Vernon P (2002) Comparative study of the metabolic responses during food shortage and subsequent recovery at different temperatures in the adult lesser mealworm, Alphitobius diaperinus (Coleoptera: Tenebrionidae). Physiol Entomol 27:291–301
Sahan E, Sabbe K, Creach V, Hernandez-Raquet G, Vyverman W, Stal LJ, Muyzer G (2007) Community structure and seasonal dynamics of diatom biofilms and associated grazers in intertidal mudflats. Aquat Microb Ecol 47:253–266
Sargent JR, Bell JG, Bell MV, Henderson RJ (1993) The metabolism of phospholipids and polyunsaturated fatty acids in fish. In: Lahlou B, Vitiello P (eds) Aquaculture: Fundamental and Applied Research. American Geophysical Union, Washington, DC, pp 103–124
Schlechtriem C, Arts MT, Zellmer ID (2006) Effect of temperature on the fatty acid composition and temporal trajectories of fatty acids in fasting Daphnia pulex (Crustacea, Cladocera). Lipids 41:397–400
Schneider T, Grosbois G, Vincent WF, Rautio M (2016) Carotenoid accumulation in copepods is related to lipid metabolism and reproduction rather than to UV-protection. Limnol Oceanogr 61:1201–1213
Schulte PM (2015) The effects of temperature on aerobic metabolism: towards a mechanistic understanding of the responses of ectotherms to a changing environment. J Exp Biol 218:1856–1866
Sokolova IM, Frederich M, Bagwe R, Lannig G, Sukhotin AA (2012) Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar Environ Res 79:1–15
Søreide JE, Leu E, Berge J, Graeve M, Falk-Petersen S (2010) Timing of blooms, algal food quality and Calanus glacialis reproduction and growth in a changing Arctic. Glob Chang Biol 16:3154–3163
St. John MA, Clemmesen C, Lund T, Köster T (2001) Diatom production in the marine environment: implications for larval fish growth and condition. ICES J Mar Sci 58:1106–1113
Thor P (2003) Elevated respiration rates of the neritic copepod Acartia tonsa during recovery from starvation. J Exp Mar Biol Ecol 283:133–143
Thor P, Koski M, Tang KW, Jónasdóttir SH (2007) Supplemental effects of diet mixing on absorption of ingested organic carbon in the marine copepod Acartia tonsa. Mar Ecol Prog Ser 331:131–138
Tiselius P (1998) Short term feeding responses to starvation in three species of small calanoid copepods. Mar Ecol Prog Ser 168:119–126
Tocher D (2003) Metabolism and functions of lipids and fatty acids in teleost fish. Rev Fish Sci 11:107–184
Underwood GJC, Kromkamp J (1999) Primary production by phytoplankton and microphytobenthos in estuaries. Adv Ecol Res 29:93–153
van der Meeren T, Olsen RE, Hamre K, Fyhn HJ (2008) Biochemical composition of copepods for evaluation of feed quality in production of juvenile marine fish. Aquaculture 274:375–397
Walne PR (1970) Studies on the food value of nineteen genera of algae to juvenile bivalves of the genera Ostrea, Crassostrea, Mercenaria, Mytilus. Fish Investig 26:1–62
Werbrouck E, Van Gansbeke D, Vanreusel A, De Troch M (2016a) Temperature affects the use of storage fatty acids as energy source in a benthic copepod (Platychelipus littoralis, Harpacticoida). PLoS One 11:1–16
Werbrouck E, Van Gansbeke D, Vanreusel A, Mensens C, De Troch M (2016b) Temperature-induced changes in fatty acid dynamics of the intertidal grazer Platychelipus littoralis (Crustacea, Copepoda, Harpacticoida): insights from a short-term feeding experiment. J Therm Biol 57:44–53
Werbrouck E, Tiselius P, Van Gansbeke D, Cervin G, Vanreusel A, De Troch M (2016c) Temperature impact on the trophic transfer of fatty acids in the congeneric copepods Acartia tonsa and Acartia clausi. J Sea Res 112:41–48
Acknowledgements
We thank Prof. Dr. Tom Moens for his advice in the set-up of the experiment and for sharing his insights in the interpretation of the data. We also express our gratitude to the Laboratory of Aquaculture and Artemia Reference Center (UGent) and to the Department of Biological and Environmental Sciences (University of Gothenburg) for providing us D. tertiolecta and the T. weissflogii strains, respectively. The first author acknowledges funding from the Research Foundation of Flanders (No. FWO12/ASP/319). This research was further financed by Ghent University (BOF-GOA 01GA1911W). The FA analysis was supported by the Research Foundation of Flanders (Grant 31523814—Fatty acids as dietary tracers in benthic food webs) awarded to the last author. We thank two reviewers for their helpful comments that improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest, that consent was obtained from all participants of the study, that all animals have been sampled and/or treated according to the national legislation and that all required permissions have been obtained.
Additional information
Responsible Editor: A. E. Todgham.
Reviewed by P. Schnurr and an undisclosed expert.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Rights and permissions
About this article
Cite this article
Werbrouck, E., Bodé, S., Van Gansbeke, D. et al. Fatty acid recovery after starvation: insights into the fatty acid conversion capabilities of a benthic copepod (Copepoda, Harpacticoida). Mar Biol 164, 151 (2017). https://doi.org/10.1007/s00227-017-3181-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-017-3181-2