Abstract
Pocillopora acuta, formerly synonymized with P. damicornis, is an ecologically important reef-building coral that exhibits mixed reproductive modes, geographic variation in clonality, and conflicting reports of population genetic structure. Using 16 polymorphic microsatellite loci, this study examined clonality, genetic differentiation, and connectivity of genetically identified P. acuta (n = 428) in the Bolinao–Anda Reef Complex (BARC), Philippines, characterized by varying levels of wave exposure. Estimates of clonal richness indicate that the populations are largely derived from asexual reproduction, more likely via dispersal of ameiotic larvae. Clonal richness, population density, and mean colony size vary with wave exposure, suggesting the potential influence of local-scale disturbance on clonality, reproductive mode, and population structure. Populations in low-energy environments were characterized by greater colony density, larger colonies, and a greater proportion of clones compared to high-energy environments. Despite evidence for realized clonal dispersal of P. acuta extending up to 22 km, significant genetic differentiation among BARC populations reveals restricted gene flow at small spatial scales. Moreover, genetic differentiation is more pronounced when considering the spatial distribution of clones (FST including clones = 0.059; FST excluding clones = 0.028), suggesting that (1) asexually produced propagules are likely retained locally and across-site settlement is not as common; and (2) sexually derived propagules may have broader scales of dispersal. This study reexamines the population genetics of this often-problematic coral and underlines the importance of contextualizing site and species biology in designing or enhancing management towards the maintenance of functional genetic diversity and pathways of connectivity among populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over an evolutionary history of at least 200 million years, reef-building corals have maintained complex life history strategies including symbiosis, clonality, and highly diverse and flexible reproductive strategies (Baird et al. 2009; Harrison 2011; Stambler 2011; Stella et al. 2011). These life history strategies have persisted and likely contributed to the survival of reef-building corals through profound environmental changes over geologic time and across highly heterogeneous coral reef environments (Harrison and Wallace 1990; Richmond and Hunter 1990; Carlon 1999; Baird et al. 2009). While many corals can be easily placed into simple categories of recognized reproductive traits (i.e., asexual or sexual, gonochoric or hermaphroditic, external or internal fertilization), several coral taxa have been shown to employ flexible and mixed modes of reproduction (Ayre and Resing 1986; Baird et al. 2009; Combosch and Vollmer 2013). Mixed modes of reproduction can provide the flexibility for genotypes locally adapted to favorable environments to be rapidly multiplied through clonal propagules, or for the mixing of gametes to provide the genetic novelty necessary for colonizing new habitats (Williams 1975; Jackson 1986). Maintaining flexible reproductive modes allows population persistence in environments that are unpredictable or have widely fluctuating conditions.
Physical disturbances such as storms or strong wave action can be an important driving factor for genetic diversity and distribution of reef-building corals (Coffroth and Lasker 1998; Foster et al. 2013; Williams et al. 2014). High levels of disturbance impedes the successful recruitment of coral larvae or may drastically increase post-settlement mortality in early-stage recruits (Crabbe et al. 2002; Williams et al. 2008; Crabbe 2012). Disturbance can also influence patterns of clonality within existing populations (i.e., the relative proportion of asexual and sexual recruits) (Hunter 1993; Karlson et al. 1996; Coffroth and Lasker 1998; Foster et al. 2013). Classic models of clonal propagation dynamics suggest a relationship between genotypic diversity and physical disturbance where genotypic diversity is low (high clonality) in stable environments (Williams 1975), and high (low clonality) in disturbed environments (Connell 1978; Sebens and Thorne 1985). A study by Coffroth and Lasker (1998) on a clonal gorgonian suggested that the influence of disturbance on clonal diversity additionally depends on how a coral taxon’s reproductive strategy relies on disturbance for production and propagation. With this paradigm, intermediate-level disturbance and clonality would be expected to be strongly linked for species that have “disturbance-sensitive” propagation (e.g., fragmentation), while clonal diversity in species that undergo “disturbance-insensitive” propagation (e.g., brooded larvae) will instead be secondarily shaped by the effects of disturbance on recruitment rates, habitat availability, and the longevity of recruits.
Patterns of genetic diversity and distribution among populations can indicate the likely mode of reproduction and dispersal capabilities of an organism (Stoddart 1984a, b; van Oppen and Gates 2006; Hellberg 2007). As sessile benthic taxa with a dispersive larval phase, corals may be informative models for estimating dispersal capabilities of other marine organisms with similar life histories, although connectivity can be highly variable and sensitive to life history traits (Toonen et al. 2011). Signatures of gene flow inferred from the distribution of alleles among populations can be effective proxies for estimating migration, contemporary shifts in demography, and possible ecological and selective influences of changing environmental conditions. Moreover, determining the relevant spatial scales of dispersal and recruitment, especially in disturbed environments, can provide insights into the adaptive capacity, resilience, and maintenance of coral populations which are important for developing science-based management strategies (Palumbi 2003; Jones et al. 2009).
The stony coral Pocillopora damicornis (Scleractinia: Pocilloporidae) is a shallow-water reef-building species widely distributed across the tropical Indo-Pacific (Veron and Pichon 1976) and is one of the more extensively studied scleractinians in terms of its biology. In light of species delineation using molecular approaches, P. damicornis was revealed to be a polyphyletic group of morphologically overlapping lineages that employ varied albeit sometimes combined modes of reproduction (Flot et al. 2008; Souter 2010; Schmidt-Roach et al. 2013), that have implications for dispersal range and population structure. Taxonomic boundaries within the species complex were subsequently revised into five genetically distinct congeners, including the formerly synonymized P. acuta (Schmidt-Roach et al. 2014b). Following this, several studies reevaluated species occurrences and distributions through species-focused characterizations (Kitano et al. 2015; Mayfield et al. 2015, 2017; Gélin et al. 2017a; Johnston et al. 2017; Poquita-Du et al. 2017; De Palmas et al. 2018; Chiazzari et al. 2019). In the Philippines, not only was the prevalence of P. acuta revealed among colonies morphologically identified as P. damicornis, but also the absence of P. damicornis sensu stricto was shown as well (Torres and Ravago-Gotanco 2018).
Pocillopora acuta (congruent to P. damicornis type β sensu Schmidt-Roach et al. (2013) or type 5 sensu Pinzón et al. 2013) is usually distinguished from its congeners through its elongate and finely branched coral morph with “acute” branch tips, but which sometimes still overlaps with P. verrucosa morphology due to plasticity (Torres and Ravago-Gotanco 2018). It has been shown to employ mixed reproductive strategies, with direct evidence of broadcast spawning of gametes and planulation of both clonal and sexual larvae (Schmidt-Roach et al. 2014a; Nakajima et al. 2018; Oury et al. 2019; Smith et al. 2019). Population studies have shown predominantly asexual contributions for P. acuta populations in Western Australia (Thomas et al. 2014) and Réunion Island (Gélin et al. 2017b), while sexual modes of reproduction exhibit greater contributions to populations in the Great Barrier Reef based on low proportions of clones for newly settled juvenile and adults (< 7%; Torda et al. 2013b, c) and in Madagascar based on high genotypic diversity of adult colonies (Gélin et al. 2018). Even in genetically identified populations, P. acuta exhibits wide variation across its range. It is therefore essential to evaluate the spatial range of this species’ larval dispersal with the intent of eliminating any artifact of sampling a highly cryptic species, assessing the relative importance of reproductive modes, and integrating locality-specific environmental factors. These factors will prove to be important in understanding the life history of this coral and in identifying distinct genetic units useful for management and conservation.
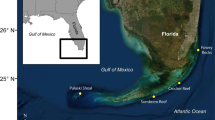
The goal of this study was to characterize the clonality, population structure, and connectivity of P. acuta (as identified by RFLP assays and sequencing of the mitochondrial ORF region sensu Torda et al. 2013a; Torres and Ravago-Gotanco 2018) within the Bolinao–Anda Reef Complex (BARC), a reef system of ecological and economic importance located at the northern tip of the Lingayen Gulf in northwestern Philippines (Fig. 1). BARC experiences various levels of wave energies (Villanoy et al. 2013) based on Relative Exposure Indices (REIs) computed for all Philippine coastal municipalities using the NOAA Wave Exposure Model (Malhotra and Fonseca 2007). Incorporating the effects of wind, geographic configuration, and bathymetry, the REI in the municipality of Bolinao was shown to be higher (REI = 3920; medium) than in Anda (REI = 747; low), an island municipality that is situated within the Lingayen Gulf and sheltered from stronger wave energies (Fig. 1c). The wave exposure variability in BARC provides an opportunity to characterize patterns of genetic diversity and differentiation along a gradient of disturbance, within fine spatial scales in a reef system. Genotypic diversity and genet distribution were evaluated using polymorphic short tandem repeats or microsatellites to estimate the relative contribution of sexual and clonal modes of reproduction to the maintenance of P. acuta populations in the region. Genetic differentiation and both demographic and genetic connectivity among populations were also assessed to provide insights on the realized dispersal of P. acuta and infer the ecologically relevant spatial scales of dispersal for this reef-building species.
Maps showing study sites and REI (Relative Exposure Indices from Villanoy et al. 2013) computed for all municipalities across the a Philippines, b Lingayen Gulf, and c the Bolinao–Anda Reef Complex. d A segment of the reef site in Caniogan
Methods
Study sites and sampling
Populations of Pocillopora acuta in the Bolinao–Anda Reef Complex were assessed in this study. Four reef sites at 3–5 m depths, separated by distances of 5–22 km, were chosen to represent reefs adjacent to the municipalities of Bolinao (Malilnep/MLP, Lucero/LCR) and Anda (Cangaluyan/CLY, Caniogan/CNG; Fig. 1c, Table 1). Each site was represented by an exhaustively sampled 400 m2 transect, set up by fixing a 40 m line and intersecting with one or two movable 10 m lines that may be repositioned along the longer axis during collection (Fig. 1d). Identified adult colonies (> 5 cm diameter, n = 451) of P. damicornis sensu lato (Veron and Pichon 1976) were photographed and mapped within the transect. A 2–4 cm fragment was clipped from the center of each P. damicornis s.l. colony, preserved in salt-saturated DMSO buffer (Gaither et al. 2011), and stored at 4 °C. A putative outgroup population of P. damicornis s.l. (n = 37) haphazardly sampled in a reef site in Gonzaga, Cagayan, located ~ 325 km northeast of the selected sites, was also examined.
Species identification and microsatellite genotyping
Genetic identification of P. acuta was performed (Torres and Ravago-Gotanco 2018) using a rapid PCR–RFLP assay developed by Torda et al. (2013a) and sequencing of the ORF to ensure single-species analyses.
Microsatellite markers developed for P. damicornis were tested for amplification in P. acuta. Sixteen loci exhibiting robust amplification and polymorphism were used for genotyping: PV2, PV5, PV6, PV7 (Magalon et al. 2004); Pd2-001, Pd3-002, Pd2-003, Pd3-004, Pd3-005, Pd2-006, Pd2-007, Pd3-008, Pd3-009 (Starger et al. 2008); Pd4, Pd11, and Pd13 (Torda et al. 2013a) (Online Resource 1). Forward primers for all loci were labeled at the 5′-end with either of four fluorophores (6-FAM, VIC, PET, or NED) for allelic detection. Each colony was genotyped by amplification of loci in three multiplex PCR panels. Multiplex PCR was performed in a final volume of 10 μL, consisting of 1–2 μL DNA template, 0.2 μM each primer, and 1 × QIAGEN Multiplex PCR Master Mix, following a thermal cycling protocol of 95 °C for 15 min, 30 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 90 s, and extension at 72 °C for 90 s, with a final extension at 72 °C for 10 min.
Amplified fragments (1–2 μL of PCR product) were pooled with GeneScan™ 500 LIZ™ size standard and Hi-Di™ formamide (Applied Biosystems) to a final volume of 10 μL. Fragment analysis was performed on an ABI3730xl sequencer (Applied Biosystems) at the Philippine Genome Center core sequencing facility (University of the Philippines Diliman). Fragment sizes were manually checked in Geneious Prime v2020.0.3 using the Microsatellite Plug-in v1.4.6 (Kearse et al. 2012). Reamplification was performed for loci and individuals with missing data. Individuals with missing data after two rounds of repeat amplification were removed from further analyses. A subset of the samples (n = 20) were randomly selected, re-amplified, and rescored to check for the accuracy of allele calling. The multilocus dataset was checked for genotyping errors and evidence of stuttering, large allele drop-outs, and null alleles using Micro-checker v2.2.3 (Van Oosterhout et al. 2004).
Clonal richness and clonal structure
We use the term ‘genet’ to refer to genetically identical colonies descended from a single zygote, and ‘ramet’ as physiologically distinct colonies with no interconnecting tissue or skeleton that belong to the same genet (Harper 1977). Genets, or colonies sharing distinct multilocus genotypes (MLGs) were diagnosed using GenClone v2.0 (Arnaud-Haond and Belkhir 2007). To minimize overestimation of unique MLGs due to scoring errors, samples under MLG pairs exhibiting small allelic differences were rechecked and rescored, if necessary. The probability that ramets sharing identical MLGs may result from distinct sexual reproductive events (psex; Arnaud-Haond et al. 2007) was examined using GenClone, with psex estimated for all pairwise sample combinations under a unique MLG.
Indices for clonal richness, heterogeneity, evenness, and spatial components of clonality were computed for each site using GenClone. Clonal richness (R) was computed as the ratio of the number of genets (i.e., distinct MLGs; NG) and total number of individuals (N) examined, R = (NG − 1)/(N − 1) (Dorken and Eckert 2001). The relative contribution of asexual and sexual reproduction was estimated as described by (Baums et al. 2006) as the ratio of observed genotypic diversity (GO; Stoddart and Taylor 1988) and expected genotypic diversity (GE), where GE is the total number of individuals genotyped per site. GO/GE has a maximum of 1 in a solely sexual population, and a value of 0 in a population dominated by a single genet. Clonal heterogeneity was estimated as the probability that two randomly selected individuals from a population belong to distinct genets, using Simpson’s complement, D* (Pielou 1969). Equitability in the distribution of clonal membership among MLGs was estimated using the Shannon–Wiener evenness index (V′H″), also known as Pielou’s evenness (Pielou 1975). For all BARC sites where geographic coordinates of individual colonies are available, the spatial distribution of genotypes within each population were estimated using the aggregation index (AC) and “edge effect” (EE) (Arnaud-Haond et al. 2007), with statistical significance tested against a null hypothesis of random distribution generated using 1000 permutations (Pinzón et al. 2012). The maximum distance of clonal dispersal was estimated as the clonal subrange (CR; Alberto et al. 2005). Analyses of clonal richness and structure (excluding estimation of spatial components) considering all four BARC sites as a single population were also conducted. Spatial autocorrelation analyses were also performed using RClone v1.0.2 (Bailleul et al. 2016) for R (R v3.6.3, R Core Team 2020) on the all-ramet dataset, to test for the signature of clonal spatial aggregation using the kinship estimator coefficient of Loiselle (FIJ) as a genetic relatedness statistic (Loiselle et al. 1995). Regression analysis of FIJ against the mean geographic distance, over 10 equally spaced distance classes, and the spatial autocorrelation profile was estimated using the Sp statistic (Vekemans and Hardy 2004), with significance tested against a null distribution generated using 104 permutations.
Population genetic diversity, differentiation, and connectivity
Estimators of genetic diversity and differentiation were calculated at the ramet (all colonies) and genet levels (clone-corrected dataset, retaining only one representative for each MLG per site). Descriptive statistics (allelic richness, allele frequencies, observed and expected heterozygosity) were calculated using GenClone. Deviations from Hardy–Weinberg and linkage equilibria were assessed for each population using Genepop v4.7.5 (Raymond and Rousset 1995; Rousset 2008). Exact tests for Hardy–Weinberg equilibrium (HWE) were performed for each locus using 104 permutations and quantified using the inbreeding coefficient (FIS). Linkage disequilibrium (LD) for all pairwise combinations of loci within each population were tested using a log-likelihood ratio statistic (G-test) with 104 permutations. HWE and LD p values were adjusted using a Bonferroni correction (Rice 1989).
Multiple approaches were used to examine genetic differentiation and their spatial patterns. Genetic differentiation was estimated using FST (Weir and Cockerham 1984), and a standardized GST to adjust for high levels of polymorphism observed in microsatellite markers (\(G_{{{\text{ST}}}}^{\prime }\); Hedrick 2005). Global genetic differentiation indices as well as population pairwise matrices of FST and \(G_{{{\text{ST}}}}^{\prime }\) were calculated using the diffCalc function in the R package ‘diveRsity’ v1.9.90 (Keenan et al. 2013). Statistical significance was evaluated by calculating 95% confidence intervals (CI95%) by bootstrap resampling with 1,000 replicates. Patterns of geographical structure were examined using a discriminant analysis of principal components (DAPC) to perform multivariate analysis of genotype data, available in the R package ‘adegenet’ v2.1.2 (Jombart et al. 2010). DAPC was used to recover patterns of genetic structure which seeks to maximize variation between groups by first performing a principal component analysis (PCA), where sampling sites was used as priors for grouping, followed by a discriminant analysis (DA) using the PCA factors as variables. We also used the spatially explicit model-based individual clustering program Geneland v4.9.0 (Guillot et al. 2005) on the clone-corrected dataset to infer the number of populations (genetically distinct clusters), assign individuals to clusters, and map the spatial locations of genetic discontinuities between clusters. Twenty replicates of the spatial model in Geneland were run through the R command line, using the correlated allele frequency model with recommended starting parameters: number of clusters (K) set to vary from 1 to 10, with 106 MCMC iterations, thinning every 100 iterations, burn-in of 200, maximum rate of the Poisson process fixed to the number of samples, and maximum number of nuclei in the tessellation process fixed to 3 times the number of samples. The modal K (most likely number of K clusters) was determined from these initial runs and used to perform 20 replicate runs at a fixed K using the same parameters. The run with the highest posterior probability was selected to assign individuals to clusters and map spatial discontinuities. Geneland assumes that populations are spatially organized across a contiguous environment and that the algorithm does not integrate oceanographic or land barriers in its approximations, which are primary factors to population structuring. Analysis of molecular variance (AMOVA) was performed to evaluate the significance of hierarchical components of genetic variance under hypothesized scenarios of population structure (Weir and Cockerham 1984; Excoffier et al. 1992) based on groupings inferred from FST, DAPC, and Geneland analyses.
Genetic connectivity was also examined by estimating directional gene flow between samples using the DivMigrate online package (Keenan et al. 2013; Sundqvist et al. 2016). A relative migration network was constructed using GST as index of differentiation with an arbitrary filter threshold set at 0.25 to retain informative connectivity values.
Results
Size-frequency distributions differ across BARC sites
Geographic variability in coral colony size and number was observed (Table 1). Bolinao sites MLP and LCR, located at the northern to northwestern sides of the reef system had significantly fewer and smaller colonies than the eastern sites CLY and CNG of Anda (ANOVA, F (1, 424) = 146.045, P < 0.001). There was no difference in colony size and number between Bolinao sites (ANOVA, F (1, 110) = 0.144, P = 0.705). Between the Anda sites, however, ANOVA revealed significant difference in size and frequency (F (1, 312) = 104.11, P < 0.001).
Clonal richness, heterogeneity, and clonal structure
Of 451 P. damicornis s.l. samples collected; 431 individuals were genetically identified as Pocillopora acuta while the rest were identified as P. verrucosa. A total of 428 P. acuta samples from five sites were successfully genotyped at 16 loci. Replication of genotyping for a subset of the samples yielded generally concordant scores at all loci, except for four individuals that did not produce amplicons at one locus (Pd3-009), indicating overall robustness of genotyping (98% consistency in total number of allele calls). All loci were polymorphic, with the number of alleles per locus ranging from 3 for Pd2-003 to 16 for Pd2-007, PV2 and Pd2-005. Allelic richness per population ranged from 2 for Pd2-03 to 12.89 for Pd2-005 (Online Resource 1). Over the 428 ramets with complete multilocus genotype data, 196 distinct MLGs were detected. A very small proportion of pairwise MLGs exhibited allelic differences of five mutational steps or less (0.72%, 138 pairwise comparisons), and the minimum observed number of pairwise allelic differences between MLGs was 2 (13 pairwise combinations). The hypothesis that colonies with identical MLGs are products of multiple events of sexual reproduction was rejected in all cases, as all psex values were below a suggested threshold of 0.01 (all psex < 2.95 × 10–5) (Arnaud-Haond et al. 2007). Genotype accumulation curves show that a minimum of 10 loci can recover all 196 genotypes across all sites, and a minimum of 3–8 loci is adequate to recover all MLGs in a site (25–71 MLGs, GON and CLY, respectively) (Online Resource 2). Hence, identical genotypes are also unlikely to be due to inadequate resolving power of the number of loci used. Consequently, all 196 MLGs were considered different multilocus lineages (MLLs; Arnaud-Haond et al. 2007), although the term MLG is used throughout this manuscript for consistency.
Of 196 MLGs, 59 were represented by more than one ramet, with 50 MLGs shared within sites (private MLGs, n = 202 colonies) and 9 MLGs shared between sites (shared MLGs, n = 89 colonies) (Fig. 2, Online Resource 3). The remaining 137 MLGs consisted of a single ramet (singleton MLGs). All shared MLGs, here designated A through I, were only found within BARC. There were no shared MLGs between BARC and GON. Of the 9 shared MLGs, 6 were shared among reefs separated by distances of 5–13 km: between MLP and CNG (B), MLP and CLY (C and E), LCR and MLP (F and H), LCR and CLY (I). Three MLGs were shared among reefs separated by a maximum distance of 22 km: 2 among LCR, CLY and CNG (A and D), and 1 between LCR and CNG (G).
Mapping P. acuta colonies sampled at four sites in the Bolinao–Anda Reef Complex (n = 428). Each symbol represents a colony. Colonies are identified according to their multilocus genotypes (MLGs), and whether these MLGs are singletons (occurring in only one colony), private (occurring in more than one colony, but only within a site), and shared (occurring in more than one colony, across two or more sites). The size of the symbol corresponds to colony diameter (cm)
Exhaustively sampled populations of P. acuta at four BARC locations exhibit varying levels of clonal input (Table 1). The proportion of genets in each site i.e., clonal richness (R), was lowest in CNG (R = 0.333; closer to a monoclonal stand) and highest in MLP (R = 0.605; greater proportion of distinct MLGs). The relative contribution of asexual and sexual reproduction measured using the GO/GE estimator revealed greater contribution of asexual reproduction in Anda populations CLY and CNG (GO/GE = 0.058 and 0.142), compared to Bolinao populations LCR and MLP (GO/GE = 0.300 and 0.428). Clonal heterogeneity estimated by Simpson’s complement unbiased index (D*) as the probability of sampling different MLGs reveals decreasing D* values southward from MLP (D* = 0.964) down to CNG (D* = 0.887; Table 1). Anda populations CLY and CNG exhibit a higher proportion of asexual reproduction, and lower clonal evenness (V′H″) relative to the Bolinao populations MLP and LCR. In CNG, a single MLG accounts for 31.1% of the population (n = 46 ramets), while three MLGs account for 44.6% of the population and singletons account for 29.7% of the population (Fig. 2, Online Resource 3). In CLY, the two most abundant MLGs account for 25.2% of the population (n = 23 + 13 ramets), while singletons account for 39.1%. In contrast, the most abundant MLGs in MLP and LCR account only for 15.38 and 13.1% of the population, respectively, while singletons account for 43.6 and 26.2% of the population. Interestingly, clonal input (the proportion of asexual reproduction), clonal heterogeneity, and clonal evenness appear to be correlated with colony size and level of disturbance due to wave exposure (Fig. 3). At sites with higher REI (MLP and LCR), colonies are smaller, there is a greater contribution of sexual reproduction (higher GO/GE), clonal heterogeneity is higher, and a more even distribution of MLGs (evenness) is observed. At sites with lower levels of REI (CLY, CNG), colonies are larger on average, asexual reproduction is more predominant (lower GO/GE), clonal heterogeneity is lower, and the population is dominated by ramets from a few MLGs (lower evenness).
Spatial analysis of MLG distribution was performed for all BARC sites. The clonal subrange (CR), which corresponds to the maximum distance between ramets within the same MLG is higher in more clonal populations CNG and CLY, ranging from 38–40 m, than less clonal populations LCR and MLP, with a shorter CR of 29–33 m (Table 1). The aggregation coefficient AC did not reveal significant deviation from a null hypothesis of random spatial distribution of MLGs (P < 0.05; Table 1, Online Resource 4). Spatial autocorrelation analysis also did not reveal any pattern of greater relatedness among geographically close MLGs, as Sp statistics for all-ramet data for the four BARC populations were non-significant (P < 0.05). Edge effect index EE for all BARC populations were not significant, indicating no bias in distribution of unique or rare MLGs at the periphery of the sampling area.
Population genetic structure of P. acuta
To evaluate the independence of microsatellite loci, tests for deviation from linkage disequilibrium (LD) were performed on the clone-corrected dataset (n = 207 colonies). Of the 600 pairwise locus tests across 5 populations, 368 tests (61% of tests) were significant at the 0.05 level, even after Bonferroni correction. Of these, 13 pairwise combinations involving 3 loci exhibited LD at all 5 populations: Pd3-005 (with PV2, PV5, Pd3-004, Pd3-009, Pd11, Pd13); Pd13 (with Pd2-007, Pd3-004, Pd3-009, PV2, Pd3-004), and Pd3-004 (with PV5, Pd3-009). Consequently, Pd3-005, Pd13, and Pd3-004 were excluded, resulting in a dataset with 13 loci. Excluding the 3 loci resulted in a reduction in the overall proportion of significant tests for LD (70 of 390 tests, 17.9% of combinations). None of the locus combinations exhibited LD at all 5 populations; therefore, physical linkage between loci is unlikely. Consequently, the 13 loci were considered independent. Excluding clonal replicates, null alleles were detected in 30 out of 80 locus-population combinations, with 3 loci exhibiting significant frequencies of null alleles at all 5 populations (PV5, Pd2-006, and Pd11; 95% Confidence Interval (CI95%) lower bound > 0). Deviations from HWE were detected in 29 out of 65 tests, of which 2 loci were not at HWE at all 5 populations (Pd3-002 and Pd11). All 29 locus-population tests deviating from HWE had FIS values > 0 (heterozygote deficit), possibly due to the influence of null alleles. To examine the effect of including loci with significant null allele frequencies on estimates of genetic differentiation, we used the package FreeNA (Chapuis and Estoup 2007) to calculate corrected FST values (excluding null alleles). There was no significant difference between corrected and uncorrected FST values (FST corrected = 0.0627, FST uncorrected = 0.0659; paired t test P > 0.05), suggesting that null alleles have a limited effect on genetic differentiation. Hence, all 13 loci were used for analyses of genetic differentiation and structure.
Analyzing all ramets, all four BARC populations were significantly different from each other (FST = 0.0592, \(G_{{{\text{ST}}}}^{\prime }\) = 0.140). Greater levels of differentiation are observed when the geographic scale is expanded to include GON (FST = 0.0659, \(G_{{{\text{ST}}}}^{\prime }\) = 0.1761). Analyzing genets only (clone-corrected data, n = 207 individuals) yielded lower, but still significant values of FST and \(G_{{{\text{ST}}}}^{\prime }\) for all 5 populations (FST = 0.0279, \(G_{{{\text{ST}}}}^{\prime }\) = 0.1032), as well as for BARC populations only (FST = 0.0186, \(G_{{{\text{ST}}}}^{\prime }\) = 0.1640).
The DAPC of clone-corrected data including a priori sampling sites reveals ordination of all populations into four broad clusters (Fig. 4a). The first discriminant axis which accounts for 53.91% of the total variance, separates GON from the rest of the BARC sites (Fig. 4c). The divergence of GON from the rest of the BARC clusters is in agreement with pairwise FST values showing all BARC-GON comparisons having the highest values table-wide (Table 2). The second discriminant axis, accounting for 20.71% of the variance revealed a separation of BARC populations into three groups: LCR, MLP–CLY, and CNG. Similar ordination patterns were seen in a DAPC for BARC populations only (Fig. 4b).
Scatterplots showing genetic clusters identified by DAPC using a priori sampling location information for datasets excluding clones at a all five populations and b BARC populations only; and density plots for c the first discriminant axis for all five populations showing the differentiation of GON, d the first discriminant axis for BARC populations, and e second discriminant axis for BARC populations
Concordant patterns of population structure in BARC were revealed by model-based clustering in Geneland. Geneland identified the most likely number of genetic clusters as K = 3 and assigned individuals from LCR to one cluster, MLP and CLY to a second cluster, and CNG individuals to a third, based on a tessellation map of estimated cluster membership and posterior probability isoclines demarcating inferred genetic landscapes (Fig. 5). Fine-scale spatial structure within BARC is supported by pairwise FST values for the clone-corrected dataset, where all comparisons between BARC populations were significant (CI95% lower bound > 0), except for MLP and CLY (FST = 0.0127; Table 2). For the all-ramet dataset, however, all pairwise FST values among BARC populations were significant, indicating the influence of clonality on genetic differentiation. AMOVA reveals significant partitioning of genetic variance among the 3 BARC groups, for both all-ramet (FCT = 0.0532, P < 0.001) and clone-corrected datasets (FCT = 0.0173, P < 0.001).
Genetic connectivity in BARC
The relative migration network constructed in DivMigrate shows weak albeit symmetric levels of gene flow (m) between the outgroup population GON and BARC populations (mean m = 0.28, Fig. 6). Within BARC, populations of P. acuta were found to be highly connected but with varying levels of migration and bidirectional symmetry. The centrally located CLY was found to be strongly connected to all three sites (mean m = 0.69). The strongest level of relative migration was detected within the CNG–CLY edges which also exhibited asymmetric connectivity (1.0/0.54). Almost symmetric but strong connectivity was found in the CLY–MLP edge with relative migration values of 0.61/0.81 despite the 9-km distance between the two sites. Interestingly, CLY was also found to be highly connected to LCR (0.42/0.72) which is in the opposite side of the reef system (Fig. 1c), in contrast with the LCR–MLP link which exhibited weak relative migration values (0.33/0.33) despite the shorter, 5-km distance between them. It is important to note, however, that relative migration values estimated by DivMigrate are not congruent with effective migration rates or the actual number of migrants per generation. DivMigrate assumes idealized conditions (e.g., drift-mutation equilibrium across populations) to expedite calculations but effectively highlight directional components of genetic divergence between pairs of populations (Sundqvist et al. 2016).
Discussion
This study estimated the levels of genetic differentiation and the relative contribution of sexual and asexual modes of reproduction in the maintenance of four populations of Pocillopora acuta in a non-contiguous reef system in northwestern Philippines. Through near-exhaustive sampling and genotyping of adult colonies, we were able to take a snapshot of clonal richness and quantify the contribution of clonally and sexually produced ramets to each of the four BARC populations. Moreover, genetic data provided insight into the spatial range of realized dispersal, specifically of clonal propagules, covering a maximum distance of 22 km within the reef complex.
Clonal structure and the potential role of disturbance
Signatures of asexual reproduction in P. acuta were detected as ramets having identical genotypes across 16 microsatellite loci, highly unlikely to result from independent events of sexual reproduction. This complements previous reports of clonal reproduction for the species (Gélin et al. 2017b; Nakajima et al. 2018; Smith et al. 2019). Across the 5 sampled sites, adult colonies were predominantly derived from asexual reproduction (GO/GE < 0.5 at all locations; R < 0.5 in 3 of 5 sites), indicating the important role of asexually produced propagules in recruitment and maintenance of P. acuta populations in the Bolinao–Anda reefs. Genotyping exhaustively collected colonies in the BARC also reveals the occurrence of 24–60 unique MLGs per site and numerous singleton MLGs which could potentially indicate sexually produced recruits (Fig. 2). These singleton MLGs significantly contributed to the genotypic richness in each study site. However, whether these recruits were produced via external fertilization of spawned gametes or internal rearing of sexual planulae could only be resolved with rigorous in situ observations or histological analyses of local samples (e.g., Permata et al. 2000). While strong support for a brooding strategy of P. damicornis s.l. is provided by ex situ monitoring experiments of planulation in Bolinao (Villanueva et al. 2008), external fertilization cannot be discounted considering observations of simultaneous planulation and gamete release by the same coral colony for P. damicornis and P. acuta in other geographic regions (Schmidt-Roach et al. 2012, 2014a; Smith et al. 2019).
Geographic variability in clonal structure of anthozoans has been attributed to the influence of physical disturbance, habitat availability, population density, and size structure (Hunter 1993; Coffroth and Lasker 1998; Baums et al. 2006; Pinzón et al. 2012; Foster et al. 2013). The effects of disturbance vary across coral species, depending on how disturbance affects propagule production and colony survival (Coffroth and Lasker 1998). For P. acuta populations in the BARC, the influence of local-scale disturbance at the level of the reef is evident in the pronounced differences in clonality between areas experiencing different levels of wave exposure (REI; Villanoy et al. 2013). Populations in low-disturbance sites CLY and CNG, in the inner Lingayen Gulf sheltered from strong wave energies, exhibit two-fold greater proportions of asexual reproduction relative to populations in medium-disturbance sites MLP and LCR. This is consistent with the models of Williams (1975) and Sebens and Thorne (1985) where asexual reproduction in stable environments acts to maintain locally adapted clones, and sexual reproduction in moderately disturbed environments is a means to generate diversity for natural selection.
Genotypic diversity increasing with disturbance, i.e., clonality decreasing with disturbance, has also been observed in Porites compressa (Hunter 1993) where disturbed areas are characterized by high genotypic diversity, low density, and small colony size. Similar trends have been reported for P. damicornis s.l. populations in the Gulf of California (Pinzón et al. 2012) and P. acuta in the Réunion Island (Gélin et al. 2017b), with higher clonality in physically protected sites, and greater proportions of sexual reproduction at sites exposed to greater wave energies or storm frequency. In contrast, patterns of genotypic diversity decreasing with disturbance, i.e., clonality increasing with physical disturbance, have also been reported for P. damicornis s.l. in southern Japan (Adjeroud and Tsuchiya 1999) and for other coral species such as the gorgonian Plexaura kuna (Coffroth and Lasker 1998) and Montastrea annularis (Foster et al. 2013), attributed to the accelerated production of clonal fragments. For P. acuta in the BARC, reduced clonality in sites with heightened disturbance levels suggests the limited contribution of colony fragmentation to population maintenance. While colonies derived from asexual reproduction are genetically indistinguishable, observations on colony morphology, clonal aggregation, and fragment abundance can provide insight into larval versus fragmentation origins (Highsmith 1982; Stoddart 1984a; Richmond 1985, 1987a; Adjeroud and Tsuchiya 1999). Within sites, our observations of many genets comprising clonemates that belong to same size groups (i.e., standard deviation of colony sizes among clonemates ranging from 0–5 cm; Online Resource 5, 6) imply a likelihood of colonies originating from the same larval clutch. The occurrence of many clonal ramets in positions generally unsuitable for fragment reattachment, i.e., extending from the side of coral heads, as well as the lack of fine-scale spatial aggregation of clones further support this inference.
For species and populations that rely more on larval recruitment over fragmentation, the influence of disturbance could also occur through effects on the growth and survival of ramets and the consequences of limited space (Fig. 2; Coffroth and Lasker 1998). Ramet survival is postulated to be higher in more stable environments (i.e., more sheltered sites) which allows for the growth and expansion of well-suited clones. Competitive exclusion by locally adapted clones, whether through greater reproductive output in larger colonies (Hughes et al. 1992; Harrison 2011), or as transcriptomic response favoring growth (Bay and Palumbi 2017), increases competition for space as well as mortality for smaller and newer recruits, yielding a lower overall genetic diversity. At the same time, reproduction of prevalent clonal genotypes also skews diversity and decreases genotypic evenness. This is exemplified by CNG where lower levels of wave action allowed for the growth of colonies to a mean size of 19.48 cm (vs. 10.57 cm in MLP) and for three clonal MLGs (out of 50) to represent almost half of the population (Fig. 3), resulting in the lowest recorded genotypic diversity (R = 0.333, D* = 0.887; Table 1) and equitability (V′H″ = 0.770) in the BARC. On the other hand, higher levels of disturbance increase ramet mortality, yielding smaller colonies and a more even distribution of genotypes. As ramet survival decreases, more spaces are opened for recruiting new genets, reducing competition, and ultimately increasing genetic diversity. Accordingly, in sites more exposed to higher wave energies, smaller and presumably younger colonies of P. acuta were observed, which could reflect the higher turn-over rates in these populations. Higher genotypic diversity and evenness estimated in the less populated MLP (R = 0.605, D* = 0.964, V'H" = 0.946) and the lower-REI site CLY (R = 0.416, D* = 0.950, V'H" = 0.852) suggest the indirect effects of a gradient of disturbance to genetic diversity.
An ontogenetic shift towards greater proportions of asexually produced larvae in larger and presumably more mature colonies is known for P. damicornis s.l. (Combosch and Vollmer 2013). If P. acuta also exhibits the same intrinsic shift in reproductive mode, then the influence of disturbance to the genetic diversity of BARC populations could also be indirectly associated with the relative proportions of sexual and asexual modes of reproduction as controlled by each population’s size structure. Stronger wave action means fewer and smaller colonies, contributing higher proportions of sexual larvae, while more stable environments may lead to higher colony density and larger colonies, which contribute more asexual than sexual larvae, yielding highly clonal clutches. Indeed, this scenario of population size structure influencing clonality may be a more likely explanation for the observed levels of clonal diversity in the BARC, in contrast to a hypothesis proposed by Smith et al. (2019) for P. acuta which suggests that the likelihood of sexual reproduction may be density-driven, i.e., higher population density may result in greater sperm availability and a higher proportion of sexual reproduction. Further investigations into the reproductive behavior of P. acuta, particularly the investments into asexual and sexual reproduction in various contexts of ontogeny and environment, coupled with the use of higher resolution genomic markers, will be essential towards uncovering the mechanisms of reproduction that this species employs, which will provide insight into its persistence and putative resilience.
Genetic differentiation and restricted dispersal
Asexual reproduction contributes to genetic differentiation of P. acuta in the Bolinao–Anda reefs. Genetic differentiation is more pronounced for the all-ramet dataset than the clone-corrected dataset, and accounting for clonal structure and the frequency distribution of clonemates results in greater population genetic variance. The occurrence of shared MLGs between sites indicates that realized dispersal of clonal propagules of P. acuta within the BARC may reach up to 22 km (between LCR and CNG). Comparable spatial scales of dispersal have been reported for P. damicornis s.l. and P. acuta, with clones shared among populations up to 40 km apart (Souter et al. 2009; Gélin et al. 2017b), although broader scale dispersal of clonal propagules has also been reported from 120 km (Thomas et al. 2014) up to 200 km (Adjeroud et al. 2014), attributed to the influence of oceanic circulation. However, in this study, the few shared MLGs (9 out of 196 MLGs) and the private MLGs accounting for 46% of the total number of colonies sampled suggest that the asexually produced propagules of P. acuta in BARC are largely retained (over a sampling area of 400 m2) and that the realized dispersal of asexual propagules across broader scales between reefs (> 5 km) is not as common.
Clonemates distributed over broader distances between reefs are likely the result of dispersal of ameiotic larvae. Long-distance dispersal of bailed-out polyps is unlikely considering their slightly negative buoyancy and very low resettlement rates under experimental conditions (Sammarco 1982; Fordyce et al. 2017). Meanwhile, limited observations of unattached colony fragments in BARC, together with the reported low survival rates of P. damicornis s.l. in field conditions (Liddle and Kay 1987) suggest that colony fragmentation might not substantially account for connectivity between reefs. Other factors such as substratum type, tissue regeneration rates, and local environmental conditions of salinity, predation, and sedimentation will determine the successful recruitment of colony fragments (reviewed in Lizcano-Sandoval et al. 2018). In situ studies on larval recruitment and genetic parentage analysis, coupled with observations on fragmentation and establishment success, are needed to examine the relative contributions of fragmentation versus larval origins in the Bolinao–Anda reefs. Likewise, incorporating integral oceanographic processes can greatly enrich this exposition and is recommended for future investigations.
Sexually derived propagules also exhibit limited dispersal within the BARC. Excluding clonal replicates results in lower values of genetic differentiation, suggesting broader spatial scales of dispersal and connectivity for sexually derived versus asexually derived propagules. However, selection may have also influenced the abundance and distribution of adult clonemates. While this study cannot assess the effect of selection on adult clonal distribution, selection is expected to result in an underestimation of the relative contribution of sexual reproduction and estimates of effective propagule dispersal distances. Moreover, this study cannot quantify dispersal distances of sexually derived propagules, which can be better estimated by genetic parentage analyses necessitating exhaustive sampling not only of adult colonies but also of newly settled recruits or early juveniles.
Despite analyzing a relatively small domain such as the BARC, significant genetic differentiation is detected among P. acuta populations suggesting the generally limited larval dispersal of this species, at least between the three genetic clusters inferred: LCR, MLP–CLY, and CNG. While demographic connectivity is evident based on the occurrence of shared MLGs, this was apparently insufficient to homogenize the distribution of alleles within the reef complex. These results are consistent with previous studies reporting genetic structure at scales of 10s of kilometers for P. damicornis s.l. (Stoddart 1984a; Benzie et al. 1995; Miller and Ayre 2004; Sherman et al. 2006; Whitaker 2006; Combosch and Vollmer 2011; Adjeroud et al. 2014) and P. acuta (Gélin et al. 2017b). The observed fine-scale spatial structure suggests that even though the species is capable of delaying metamorphosis and settlement for extended periods up to 200 days (Harrigan 1973; Richmond 1987b), a large proportion of its planulae may actually settle within a shorter time period, between 2 and 7 days based on other observations (Ayre and Hughes 2000; Harii et al. 2002; Villanueva et al. 2008). In contrast, a few pre-taxonomic revision studies report a counter-intuitive combination of genetic structure at small spatial scales over 10s of kilometers, against a backdrop of broad-scale panmixia over 100–1000s of kilometers (Ayre et al. 1997; Ayre and Hughes 2000; Torda et al. 2013b). This taxon-muddled, geography-linked conundrum consequently necessitates a comprehensive reevaluation of many aspects of this species’ biology and ecology, ideally viewed at different spatial scales (Gorospe and Karl 2013, 2015; Gorospe et al. 2015).
Implications for management
Resilience-based management dictates that in any conservation effort, be it in designating areas for protection, constructing reserve networks, or restoring damaged reefs, the maintenance of genetic diversity and pathways of connectivity among reef populations should be ensured (McLeod et al. 2019). In the context of reef restoration, the observed structuring of diversity in Bolinao–Anda populations requires that any coral gardening or transplantation activity should be contained within the inferred genetic boundaries. The limited dispersal revealed in P. acuta means that each of these genetic groups are somehow distinct and if reef resilience is an objective of such intervention, then the maintenance of this genetic diversity should be prioritized.
Management schemes like setting up marine protected areas (MPAs) have been shown to be more effective when implemented in source populations rather than sink populations (Crowder et al. 2000; White et al. 2009). Therefore, information on the directionality of connectivity and source-sink characteristics of populations are important for effective MPA planning. The relative migration network we presented depicts an overall interconnectedness within BARC (Fig. 6). The asymmetric rates of migration indicate sites CNG and LCR as potential source populations of larvae for the medially situated MLP–CLY cluster. This by itself should already compel for stronger protective measures in the distal areas of the reef system. The spatial boundaries of this study, however, could have limited the actual range of realized dispersal and may be expanded in the future to give insights for wider scale management. Broader scale population structuring and connectivity (e.g., basin scale, archipelagic scale), with an integration of oceanographic assessment or modeling, could be helpful and should also be considered in planning for networks of marine reserves to deliver a more holistic investigation.
This study underlines the utility and importance of contextualizing site conditions and species biology in designing or improving management. The gene flow and variability in wave energy and genetic diversity found among the study sites can be speculated to contribute to the resilience of this naturally and anthropogenically disturbed region: a relatively stable reef site offers strong demographic supply, sites disturbed by strong wave energies provide genetic novelty, while all sites are linked through interconnectivity. These patterns of diversity with potential functionality found in Bolinao–Anda, if maintained with protection, could serve as a model resilient metapopulation and provide substantiation for implementing more integrative and comprehensive management approaches. There are 1620 reported MPAs in the Philippines but many of these are less than 1 km2 (Cabral et al. 2014) due to budgetary constraints and impediments brought by recurring shifts in each local government unit’s priorities (Horigue et al. 2012). Furthermore, most of these were not fully designed to be part of functionally connected, resilience-oriented ecological networks of MPAs which would have helped preserve ecosystem function (Horigue et al. 2012, 2014). Given limited resources, it is important to augment current management interventions with spatial planning supported by genetic information to maximize the ecological returns of these conservation efforts. Exploring the benefits of functional genetic diversity among populations should also be considered by assessing fine-scale genetic and demographic characteristics exhibited by populations within prospective protected areas. Since pocilloporid corals are often pioneer reef-building species, such information will provide valuable insights into the recovery of degraded reefs, the resilience of existing populations, and the pace of local adaptation to changing conditions.
Data availability
Multilocus genotypes and metadata for all samples used in this study have been deposited in Dryad (https://doi.org/10.5061/dryad.t1g1jwt0x).
References
Adjeroud M, Tsuchiya M (1999) Genetic variation and clonal structure in the scleractinian coral Pocillopora damicornis in the Ryukyu Archipelago, southern Japan. Mar Biol 134:753–760. https://doi.org/10.1007/s002270050592
Adjeroud M, Guérécheau A, Vidal-Dupiol J, Flot J-F, Arnaud-Haond S, Bonhomme F (2014) Genetic diversity, clonality and connectivity in the scleractinian coral Pocillopora damicornis: a multi-scale analysis in an insular, fragmented reef system. Mar Biol 161:531–541. https://doi.org/10.1007/s00227-013-2355-9
Alberto F, Gouveia L, Arnaud-Haond S, Pérez-Lloréns JL, Duarte CM, Serrão EA (2005) Within-population spatial genetic structure, neighbourhood size and clonal subrange in the seagrass Cymodocea nodosa. Mol Ecol 14:2669–2681. https://doi.org/10.1111/j.1365-294X.2005.02640.x
Arnaud-Haond S, Belkhir K (2007) GENCLONE: a computer program to analyse genotypic data, test for clonality and describe spatial clonal organization. Mol Ecol Notes 7:15–17. https://doi.org/10.1111/j.1471-8286.2006.01522.x
Arnaud-Haond S, Duarte CM, Alberto F, Serrão EA (2007) Standardizing methods to address clonality in population studies. Mol Ecol 16:5115–5139. https://doi.org/10.1111/j.1365-294X.2007.03535.x
Ayre DJ, Hughes TP (2000) Genotypic diversity and gene flow in brooding and spawning corals along the Great Barrier Reef, Australia. Evolution (NY) 54:1590–1605. https://doi.org/10.1111/j.0014-3820.2000.tb00704.x
Ayre DJ, Resing JM (1986) Sexual and asexual production of planulae in reef corals. Mar Biol 90:187–190. https://doi.org/10.1007/BF00569126
Ayre DJ, Hughes TP, Standish RJ (1997) Genetic differentiation, reproductive mode, and gene flow in the brooding coral Pocillopora damicornis along the Great Barrier Reef, Australia. Mar Ecol Prog Ser 159:175–187. https://doi.org/10.3354/meps159175
Bailleul D, Stoeckel S, Arnaud-Haond S (2016) RClone: a package to identify MultiLocus Clonal Lineages and handle clonal data sets in R. Methods Ecol Evol 7:966–970. https://doi.org/10.1111/2041-210X.12550
Baird AH, Guest JR, Willis BL (2009) Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu Rev Ecol Evol Syst 40:551–571. https://doi.org/10.1146/annurev.ecolsys.110308.120220
Baums IB, Miller MW, Hellberg ME (2006) Geographic variation in clonal structure in a reef-building Caribbean coral, Acropora palmata. Ecol Monogr 76:503–519. https://doi.org/10.1890/0012-9615(2006)076[0503:GVICSI]2.0.CO;2
Bay RA, Palumbi SR (2017) Transcriptome predictors of coral survival and growth in a highly variable environment. Ecol Evol 7:4794–4803. https://doi.org/10.1002/ece3.2685
Benzie JAH, Haskell A, Lehman H (1995) Variation in the genetic composition of coral (Pocillopora damicornis and Acropora palifera) populations from different reef habitats. Mar Biol 121:731–739. https://doi.org/10.1007/BF00349309
Cabral RB, Aliño PM, Balingit ACM, Alis CM, Arceo HO, Nañola CL, Geronimo RC (2014) The Philippine marine protected area (MPA) database. Philipp Sci Lett 7:300–308
Carlon DB (1999) The evolution of mating systems in tropical reef corals. Trends Ecol Evol 14:491–495. https://doi.org/10.1016/S0169-5347(99)01709-7
Chapuis MP, Estoup A (2007) Microsatellite null alleles and estimation of population differentiation. Mol Biol Evol 24:621–631. https://doi.org/10.1093/molbev/msl191
Chiazzari B, Magalon H, Gélin P, Macdonald A (2019) Living on the edge: assessing the diversity of South African Pocillopora on the margins of the Southwestern Indian Ocean. PLoS ONE 14:e0220477. https://doi.org/10.1371/journal.pone.0220477
Coffroth MA, Lasker HR (1998) Population structure of a clonal gorgonian coral: the interplay between clonal reproduction and disturbance. Evolution (NY) 52:379. https://doi.org/10.2307/2411075
Combosch DJ, Vollmer SV (2011) Population genetics of an ecosystem-defining reef coral Pocillopora damicornis in the Tropical Eastern Pacific. PLoS ONE 6:e21200. https://doi.org/10.1371/journal.pone.0021200
Combosch DJ, Vollmer SV (2013) Mixed asexual and sexual reproduction in the Indo-Pacific reef coral Pocillopora damicornis. Ecol Evol 3:3379–3387. https://doi.org/10.1002/ece3.721
Connell JH (1978) Diversity in tropical rain forests and coral reefs. Science 199:1302–1310. https://doi.org/10.1126/science.199.4335.1302
Crabbe MJC (2012) Environmental effects on coral growth and recruitment in the Caribbean. J Mar Biol Assoc UK 92:747–752. https://doi.org/10.1017/S0025315411001913
Crabbe MJC, Mendes JM, Warner GF (2002) Lack of recruitment of non-branching corals in Discovery Bay is linked to severe storms. Bull Mar Sci 70:939–945
Crowder LB, Lyman SJ, Figueira WF, Priddy J (2000) Source-sink population dynamics and the problem of siting marine reserves. Bull Mar Sci 66:799–820
De Palmas S, Soto D, Denis V, Ho MJ, Chen CA (2018) Molecular assessment of Pocillopora verrucosa (Scleractinia; Pocilloporidae) distribution along a depth gradient in Ludao, Taiwan. PeerJ. https://doi.org/10.7717/peerj.5797
Dorken ME, Eckert CG (2001) Severely reduced sexual reproduction in northern populations of a clonal plant, Decodon verticillatus (Lythraceae). J Ecol 89:339–350
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491
Flot J-F, Magalon H, Cruaud C, Couloux A, Tillier S (2008) Patterns of genetic structure among Hawaiian corals of the genus Pocillopora yield clusters of individuals that are compatible with morphology. C R Biol 331:239–247. https://doi.org/10.1016/j.crvi.2007.12.003
Fordyce AJ, Camp EF, Ainsworth TD (2017) Polyp bailout in Pocillopora damicornis following thermal stress. F1000Res 6:687. https://doi.org/10.12688/f1000research.11522.1
Foster NL, Baums IB, Sanchez JA, Paris CB, Chollett I, Agudelo CL, Vermeij MJA, Mumby PJ (2013) Hurricane-driven patterns of clonality in an ecosystem engineer: the Caribbean coral Montastraea annularis. PLoS ONE 8:e53283. https://doi.org/10.1371/journal.pone.0053283
Gaither MR, Szabó Z, Crepeau MW, Bird CE, Toonen RJ (2011) Preservation of corals in salt-saturated DMSO buffer is superior to ethanol for PCR experiments. Coral Reefs 30:329–333. https://doi.org/10.1007/s00338-010-0687-1
Gélin P, Postaire B, Fauvelot C, Magalon H (2017a) Reevaluating species number, distribution and endemism of the coral genus Pocillopora Lamarck, 1816 using species delimitation methods and microsatellites. Mol Phylogenet Evol 109:430–446. https://doi.org/10.1016/j.ympev.2017.01.018
Gélin P, Fauvelot C, Mehn V, Bureau S, Rouzé H, Magalon H (2017b) Superclone expansion, long-distance clonal dispersal and local genetic structuring in the coral Pocillopora damicornis type β in Reunion Island, South Western Indian Ocean. PLoS ONE 12:e0169692. https://doi.org/10.1371/journal.pone.0169692
Gélin P, Pirog A, Fauvelot C, Magalon H (2018) High genetic differentiation and low connectivity in the coral Pocillopora damicornis type β at different spatial scales in the Southwestern Indian Ocean and the Tropical Southwestern Pacific. Mar Biol 165:167. https://doi.org/10.1007/s00227-018-3428-6
Gorospe KD, Karl SA (2013) Genetic relatedness does not retain spatial pattern across multiple spatial scales: dispersal and colonization in the coral, Pocillopora damicornis. Mol Ecol 22:3721–3736. https://doi.org/10.1111/mec.12335
Gorospe KD, Karl SA (2015) Depth as an organizing force in Pocillopora damicornis: intra-reef genetic architecture. PLoS ONE 10:1–17. https://doi.org/10.1371/journal.pone.0122127
Gorospe KD, Donahue MJ, Karl SA (2015) The importance of sampling design: spatial patterns and clonality in estimating the genetic diversity of coral reefs. Mar Biol 162:917–928. https://doi.org/10.1007/s00227-015-2634-8
Guillot G, Mortier F, Estoup A (2005) GENELAND: a computer package for landscape genetics. Mol Ecol Notes 5:712–715. https://doi.org/10.1111/j.1471-8286.2005.01031.x
Harii S, Kayanne H, Takigawa H, Hayashibara T, Yamamoto M (2002) Larval survivorship, competency periods and settlement of two brooding corals, Heliopora coerulea and Pocillopora damicornis. Mar Biol 141:39–46. https://doi.org/10.1007/s00227-002-0812-y
Harper JL (1977) Population biology of plants. Academic Press, London
Harrigan JF (1973) The planula larva of Pocillopora damicornis: lunar periodicity of swarming and substratum selection behavior (Parts I and II). University of Hawai‘i, Manoa
Harrison PL (2011) Sexual reproduction of scleractinian corals. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Dordrecht, pp 59–85
Harrison PL, Wallace CC (1990) Reproduction, dispersal and recruitment of scleractinian corals. In: Dubinsky Z (ed) Ecosystems of the world 25: coral reefs. Elsevier, Amsterdam, pp 133–207
Hedrick PW (2005) A standardized genetic differentiation measure. Evolution 59:1633–1638. https://doi.org/10.1111/j.0014-3820.2005.tb01814.x
Hellberg ME (2007) Footprints on water: the genetic wake of dispersal among reefs. Coral Reefs 26:463–473. https://doi.org/10.1007/s00338-007-0205-2
Highsmith RC (1982) Reproduction by fragmentation in corals. Mar Ecol Prog Ser 7:207–226. https://doi.org/10.3354/meps007207
Horigue V, Aliño PM, White AT, Pressey RL (2012) Marine protected area networks in the Philippines: trends and challenges for establishment and governance. Ocean Coast Manag 64:15–26. https://doi.org/10.1016/j.ocecoaman.2012.04.012
Horigue V, Aliño PM, Pressey RL (2014) Evaluating management performance of marine protected area networks in the Philippines. Ocean Coast Manag 95:11–25. https://doi.org/10.1016/j.ocecoaman.2014.03.023
Hughes TP, Ayre DJ, Connell JH (1992) The evolutionary ecology of corals. Trends Ecol Evol 7:292–295. https://doi.org/10.1016/0169-5347(92)90225-Z
Hunter CL (1993) Genotypic variation and clonal structure in coral populations with different disturbance histories. Evolution (NY) 47:1213–1228. https://doi.org/10.1111/j.1558-5646.1993.tb02148.x
Jackson JBC (1986) Modes of dispersal of clonal benthic invertebrates: consequences for species’ distributions and genetic structure of local populations. Bull Mar Sci 39:588–606
Johnston EC, Forsman ZH, Flot J-F, Schmidt-Roach S, Pinzón JH, Knapp ISS, Toonen RJ (2017) A genomic glance through the fog of plasticity and diversification in Pocillopora. Sci Rep 7:1–11. https://doi.org/10.1038/s41598-017-06085-3
Jombart T, Devillard S, Balloux F (2010) Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genet 11:94. https://doi.org/10.1186/1471-2156-11-94
Jones GP, Almany GR, Russ GR, Sale PF, Steneck RS, van Oppen MJH, Willis BL (2009) Larval retention and connectivity among populations of corals and reef fishes: history, advances and challenges. Coral Reefs 28:307–325. https://doi.org/10.1007/s00338-009-0469-9
Karlson RH, Hughes TP, Karlson SR (1996) Density-dependent dynamics of soft coral aggregations: the significance of clonal growth and form. Ecology 77:1592–1599. https://doi.org/10.2307/2265554
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. https://doi.org/10.1093/bioinformatics/bts199
Keenan K, Mcginnity P, Cross TF, Crozier WW, Prodöhl PA (2013) DiveRsity: an R package for the estimation and exploration of population genetics parameters and their associated errors. Methods Ecol Evol 4:782–788. https://doi.org/10.1111/2041-210X.12067
Kitano Y, Nagai S, Ueno M, Yasuda N (2015) Most Pocillopora damicornis around Yaeyama Islands are Pocillopora acuta according to mitochondrial ORF sequences. Galaxea J Coral Reef Stud 17:21–22. https://doi.org/10.3755/galaxea.17.21
Liddle MJ, Kay AM (1987) Resistance, survival and recovery of trampled corals on the Great Barrier Reef. Biol Conserv 42:1–18. https://doi.org/10.1016/0006-3207(87)90049-8
Lizcano-Sandoval LD, Londoño-Cruz E, Zapata FA (2018) Growth and survival of Pocillopora damicornis (Scleractinia: Pocilloporidae) coral fragments and their potential for coral reef restoration in the Tropical Eastern Pacific. Mar Biol Res 14:887–897. https://doi.org/10.1080/17451000.2018.1528011
Loiselle BA, Sork VL, Nason J, Graham C (1995) Spatial genetic structure of a tropical understory shrub, Psychotria officinalis (Rubiaceae). Am J Bot 82:1420–1425. https://doi.org/10.1002/j.1537-2197.1995.tb12679.x
Magalon H, Samadi S, Richard M, Adjeroud M, Veuille M (2004) Development of coral and zooxanthella-specific microsatellites in three species of Pocillopora (Cnidaria, Scleractinia) from French Polynesia. Mol Ecol Notes 4:206–208. https://doi.org/10.1111/j.1471-8286.2004.00618.x
Malhotra A, Fonseca MS (2007) WEMo (Wave Exposure Model): Formulation. Procedures and Validation, NOAA Tech Memo NOS NCCOS, p 65
Mayfield AB, Bruckner AW, Chen C, Chen C-S (2015) A survey of pocilloporid corals and their endosymbiotic dinoflagellate communities in the Austral and Cook Islands of the South Pacific. Platax 12:1–17
Mayfield AB, Chen C-S, Dempsey AC (2017) Biomarker profiling in reef corals of Tonga’s Ha‘apai and Vava‘u archipelagos. PLoS ONE 12:e0185857. https://doi.org/10.1371/journal.pone.0185857
McLeod E, Anthony KRN, Mumby PJ, Maynard JA, Beeden R, Graham NAJ, Heron SF, Hoegh-Guldberg O, Jupiter S, MacGowan P, Mangubhai S, Marshall N, Marshall PA, McClanahan TR, McLeod K, Nyström M, Obura DO, Parker B, Possingham HP, Salm RV, Tamelander J (2019) The future of resilience-based management in coral reef ecosystems. J Environ Manag 233:291–301. https://doi.org/10.1016/j.jenvman.2018.11.034
Miller KJ, Ayre DJ (2004) The role of sexual and asexual reproduction in structuring high latitude populations of the reef coral Pocillopora damicornis. Heredity (Edinb) 92:557–568. https://doi.org/10.1038/sj.hdy.6800459
Nakajima Y, Chuang PS, Ueda N, Mitarai S (2018) First evidence of asexual recruitment of Pocillopora acuta in Okinawa Island using genotypic identification. PeerJ. https://doi.org/10.7717/peerj.5915
Oury N, Gélin P, Massé LM, Magalon H (2019) First study of asexual planulae in the coral Pocillopora damicornis type β SSH05c from the southwestern Indian Ocean. Coral Reefs 38:499–503. https://doi.org/10.1007/s00338-019-01800-0
Palumbi SR (2003) Population genetics, demographic connectivity, and the design of marine reserves. Ecol Appl 13:146–158. https://doi.org/10.1890/1051-0761(2003)013[0146:PGDCAT]2.0.CO;2
Permata WD, Kinzie RA, Hidaka M (2000) Histological studies on the origin of planulae of the coral Pocillopora damicornis. Mar Ecol Prog Ser 200:191–200. https://doi.org/10.3354/meps200191
Pielou EC (1969) An introduction to mathematical ecology. Wiley, New York
Pielou EC (1975) Ecological diversity. Wiley, New York
Pinzón JH, Reyes-Bonilla H, Baums IB, LaJeunesse TC (2012) Contrasting clonal structure among Pocillopora (Scleractinia) communities at two environmentally distinct sites in the Gulf of California. Coral Reefs 31:765–777. https://doi.org/10.1007/s00338-012-0887-y
Pinzón JH, Sampayo E, Cox E, Chauka LJ, Chen CA, Voolstra CR, LaJeunesse TC (2013) Blind to morphology: genetics identifies several widespread ecologically common species and few endemics among Indo-Pacific cauliflower corals (Pocillopora, Scleractinia). J Biogeogr 40:1595–1608. https://doi.org/10.1111/jbi.12110
Poquita-Du RC, Ng CSL, Bin LJ, Afiq-Rosli L, Tay YC, Todd PA, Chou LM, Huang D (2017) New evidence shows that Pocillopora ‘damicornis-like’ corals in Singapore are actually Pocillopora acuta (Scleractinia: Pocilloporidae). Biodivers Data J 5:e11407. https://doi.org/10.3897/BDJ.5.e11407
Raymond M, Rousset F (1995) GENEPOP version 1.2: population genetics software for exact tests and ecumenicism. J Hered 86:248–249. https://doi.org/10.1093/oxfordjournals.jhered.a111573
Rice WR (1989) Analyzing tables of statistical tests. Evolution (NY) 43:223–225. https://doi.org/10.2307/2409177
Richmond RH (1985) Reversible metamorphosis in coral planula larvae. Mar Ecol Prog Ser 22:181–185. https://doi.org/10.3354/meps022181
Richmond RH (1987a) Energetic relationships and biogeographical differences among fecundity, growth and reproduction in the reef coral Pocillopora damicornis. Bull Mar Sci 41:594–604
Richmond RH (1987b) Energetics, competency, and long-distance dispersal of planula larvae of the coral Pocillopora damicornis. Mar Biol 93:527–533. https://doi.org/10.1007/BF00392790
Richmond RH, Hunter CL (1990) Reproduction and recruitment of corals: comparisons among the Caribbean, the Tropical Pacific, and the Red Sea. Mar Ecol Prog Ser 60:185–203. https://doi.org/10.3354/meps060185
Rousset F (2008) GENEPOP’007: a complete re-implementation of the GENEPOP software for Windows and Linux. Mol Ecol Resour 8:103–106. https://doi.org/10.1111/j.1471-8286.2007.01931.x
Sammarco PW (1982) Polyp bail-out: an escape response to environmental stress and a new means of reproduction in corals. Mar Ecol Prog Ser 10:57–65
Schmidt-Roach S, Miller KJ, Woolsey E, Gerlach G, Baird AH (2012) Broadcast spawning by Pocillopora species on the Great Barrier Reef. PLoS ONE 7:e50847. https://doi.org/10.1371/journal.pone.0050847
Schmidt-Roach S, Lundgren P, Miller KJ, Gerlach G, Noreen AME, Andreakis N (2013) Assessing hidden species diversity in the coral Pocillopora damicornis from Eastern Australia. Coral Reefs 32:161–172. https://doi.org/10.1007/s00338-012-0959-z
Schmidt-Roach S, Johnston EC, Fontana S, Jury CP, Forsman ZH (2014a) Daytime spawning of Pocillopora species in Kaneohe Bay, Hawai‘i. Galaxea 16:11–12. https://doi.org/10.3755/galaxea.16.11
Schmidt-Roach S, Miller KJ, Lundgren P, Andreakis N (2014b) With eyes wide open: A revision of species within and closely related to the Pocillopora damicornis species complex (Scleractinia; Pocilloporidae) using morphology and genetics. Zool J Linn Soc 170:1–33. https://doi.org/10.1111/zoj.12092
Sebens KP, Thorne BL (1985) Coexistence of clones, clonal diversity, and the effects of disturbance. In: Jackson JBC, Buss LW, Cook RE (eds) Population biology and evolution of clonal organisms. Yale University Press, New Haven, pp 357–396
Sherman CDH, Ayre DJ, Miller KJ (2006) Asexual reproduction does not produce clonal populations of the brooding coral Pocillopora damicornis on the Great Barrier Reef, Australia. Coral Reefs 25:7–18. https://doi.org/10.1007/s00338-005-0053-x
Smith HA, Moya A, Cantin NE, van Oppen MJ, Torda G (2019) Observations of simultaneous sperm release and larval planulation suggest reproductive assurance in the coral Pocillopora acuta. Front Mar Sci 6:362. https://doi.org/10.3389/fmars.2019.00362
Souter P (2010) Hidden genetic diversity in a key model species of coral. Mar Biol 157:875–885. https://doi.org/10.1007/s00227-009-1370-3
Souter P, Henriksson O, Olsson N, Grahn M (2009) Patterns of genetic structuring in the coral Pocillopora damicornis on reefs in East Africa. BMC Ecol 9:19. https://doi.org/10.1186/1472-6785-9-19
Stambler N (2011) Zooxanthellae: the yellow symbionts inside animals. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Dordrecht, pp 87–106
Starger CJ, Yeoh SSR, Dai C-F, Baker AC, Desalle R (2008) Ten polymorphic STR loci in the cosmopolitan reef coral, Pocillopora damicornis. Mol Ecol Resour 8:619–621. https://doi.org/10.1111/j.1471-8286.2007.02017.x
Stella JS, Pratchett MS, Hutchings PA, Jones GP (2011) Coral-associated invertebrates: diversity, ecological importance and vulnerability to disturbance. Oceanogr Mar Biol Annu Rev 49:43–104
Stoddart JA (1984a) Genetic differentiation amongst populations of the coral Pocillopora damicornis off southwestern Australia. Coral Reefs 3:149–156. https://doi.org/10.1007/BF00301959
Stoddart JA (1984b) Genetical structure within populations of the coral Pocillopora damicornis. Mar Biol 81:19–30. https://doi.org/10.1007/BF00397621
Stoddart JA, Taylor JF (1988) Genotypic diversity: estimation and prediction in samples. Genetics 118:705–711
Sundqvist L, Keenan K, Zackrisson M, Prodöhl PA, Kleinhans D (2016) Directional genetic differentiation and relative migration. Ecol Evol 6:3461–3475. https://doi.org/10.1002/ece3.2096
Thomas L, Kendrick GA, Stat M, Travaille KL, Shedrawi G, Kennington WJ (2014) Population genetic structure of the Pocillopora damicornis morphospecies along Ningaloo Reef, Western Australia. Mar Ecol Prog Ser 513:111–119. https://doi.org/10.3354/meps10893
Toonen RJ, Andrews KR, Baums IB, Bird CE, Concepcion GT, Daly-Engel TS, Eble JA, Faucci A, Gaither MR, Iacchei M, Puritz JB, Schultz JK, Skillings DJ, Timmers MA, Bowen BW (2011) Defining boundaries for ecosystem-based management: a multispecies case study of marine connectivity across the Hawaiian Archipelago. J Mar Biol. https://doi.org/10.1155/2011/460173
Torda G, Schmidt-Roach S, Peplow LM, Lundgren P, van Oppen MJH (2013a) A rapid genetic assay for the identification of the most common Pocillopora damicornis genetic lineages on the Great Barrier Reef. PLoS ONE 8:e58447. https://doi.org/10.1371/journal.pone.0058447
Torda G, Lundgren P, Willis BL, van Oppen MJH (2013b) Genetic assignment of recruits reveals short- and long-distance larval dispersal in Pocillopora damicornis on the Great Barrier Reef. Mol Ecol 22:5821–5834. https://doi.org/10.1111/mec.12539
Torda G, Lundgren P, Willis BL, van Oppen MJH (2013c) Revisiting the connectivity puzzle of the common coral Pocillopora damicornis. Mol Ecol 22:5805–5820. https://doi.org/10.1111/mec.12540
Torres AF, Ravago-Gotanco R (2018) Rarity of the “common” coral Pocillopora damicornis in the western Philippine archipelago. Coral Reefs 37:1209–1216. https://doi.org/10.1007/s00338-018-1729-3
van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538. https://doi.org/10.1111/j.1471-8286.2004.00684.x
van Oppen MJH, Gates RD (2006) Conservation genetics and the resilience of reef-building corals. Mol Ecol 15:3863–3883. https://doi.org/10.1111/j.1365-294X.2006.03026.x
Vekemans X, Hardy OJ (2004) New insights from fine-scale spatial genetic structure analyses in plant populations. Mol Ecol 13:921–935. https://doi.org/10.1046/j.1365-294X.2004.02076.x
Veron JE, Pichon M (1976) Scleractinia of Eastern Australia, Part I. Families Thamnasteridae, Astrocoeniidae, Pocilloporidae. Aust Inst Mar Sci Monogr Ser 1:1–86. https://doi.org/10.5962/bhl.title.60617
Villanoy CL, Salamante E, Cabrera OC (2013) Chapter 3. Exposure: waves and storm surges. Vulnerability assessment tools for coastal ecosystems: a guidebook. MERF, Quezon City, pp 44–54
Villanueva RD, Yap HT, Montaño MNE (2008) Timing of planulation by pocilloporid corals in the northwestern Philippines. Mar Ecol Prog Ser 370:111–119. https://doi.org/10.3354/meps07659
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution (NY) 38:1358–1370. https://doi.org/10.1111/j.1558-5646.1984.tb05657.x
Whitaker K (2006) Genetic evidence for mixed modes of reproduction in the coral Pocillopora damicornis and its effect on population structure. Mar Ecol Prog Ser 306:115–124. https://doi.org/10.3354/meps306115
White JW, Botsford LW, Hastings A, Largier JL (2009) Population persistence in marine reserve networks: incorporating spatial heterogeneities in larval dispersal. Mar Ecol Prog Ser 398:49–67. https://doi.org/10.3354/meps08327
Williams GC (1975) Sex and evolution. Princeton University Press, Princeton
Williams DE, Miller MW, Kramer KL (2008) Recruitment failure in Florida Keys Acropora palmata, a threatened Caribbean coral. Coral Reefs 27:697–705. https://doi.org/10.1007/s00338-008-0386-3
Williams DE, Miller MW, Baums IB (2014) Cryptic changes in the genetic structure of a highly clonal coral population and the relationship with ecological performance. Coral Reefs 33:595–606. https://doi.org/10.1007/s00338-014-1157-y
Acknowledgements
This study was supported by the Philippine Department of Science and Technology (DOST)—Philippine Council for Agriculture, Aquatic and Natural Resources Research and Development, the Department of Environment and Natural Resources—Biodiversity Management Bureau, and the University of the Philippines Marine Science Institute. The authors thank Darryl Valino, Mikhael Tañedo, Romer Albino, David Siquioco, Lovely Heyres, and Emmeline Jamodiong for assisting with field collections. Relative wave exposure data were produced by Cesar Villanoy and Erlinda Salamante with the support of DOST. This is MSI contribution number 475, HIMB publication number 1829, and SOEST number 11155.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable national and institutional guidelines for sampling of corals have been followed. Collections for this study were done with permission (Gratuitous Permit No. 0102-15) issued by the Philippine Department of Agriculture—Bureau of Fisheries and Aquatic Resources.
Additional information
Responsible Editor: S. Uthicke.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by S. Schmidt-Roach and N. Yasuda.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Torres, A.F., Forsman, Z.H. & Ravago-Gotanco, R. Shifts in coral clonality along a gradient of disturbance: insights on reproduction and dispersal of Pocillopora acuta. Mar Biol 167, 161 (2020). https://doi.org/10.1007/s00227-020-03777-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-020-03777-9