Abstract
We have investigated the relationship between genotypic diversity, the mode of production of brooded larvae and disturbance in a range of reef habitats, in order to resolve the disparity between the reproductive mode and population structure reported for the brooding coral Pocillopora damicornis. Within 14 sites across six habitats, the ratio of the observed (G o) to the expected (G e) genotypic diversity ranged from 69 to 100% of that expected for random mating. At three other sites in two habitats the G o /G e ranged from 35 to 53%. Two of these sites were recently bleached, suggesting that asexual recruitment may be favoured after disturbance. Nevertheless, our data suggest that brooded larvae, from each of five habitats surveyed, were asexually produced. While clonal recruitment may be important in disturbed habitats, the lack of clonality detected, both in this and earlier surveys of 40 other sites, implies that a disturbance is normally insufficient to explain this species’ continued investment in clonal reproduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Corals display a diverse range of reproductive patterns, with many showing the capacity for both sexual reproduction (via internal fertilisation or broadcast spawning) and asexual reproduction (via fragmentation and the asexual production of brooded larvae) (Harrison and Wallace 1990). Theoretical predictions of the relative roles of sexual and asexual reproduction suggest that organisms with mixed life history strategies use sexual reproduction to produce genotypically diverse and widely dispersed propagules, thus enabling the colonisation of distant or unstable habitats. In contrast, asexual reproduction will be used to restock or maintain populations within the parental habitat patch (Williams 1975; Maynard Smith 1978; Bell 1982).
The effect of habitat stability and levels of disturbance on sexual and asexual reproduction may, however, be complex. For example, many sessile marine invertebrates, such as sponges and branching corals, fragment and disperse under the action of storms and strong waves (Tunnicliffe 1981; Highsmith 1982; Wulff 1985, 1991; Lasker 1990); while, in contrast, many freshwater zooplankton species reproduce sexually in response to physically stressful or changing environmental conditions (Hebert and Ward 1976; Hebert 1978; Hebert et al. 1988; Hughes 1989). Simulations of genotypic diversity and population structure, in response to disturbances, predict that maximum genotypic diversity will occur in populations subjected to intermediate levels of disturbance (Sebens and Thorne 1985); however, the relationship between habitat stability and the levels of genotypic diversity may be more complex.
For the brooding coral Pocillopora damicornis, in Western Australia and Hawaii, the reproductive biology and population structure appears to support the predicted roles of sexual and asexual reproduction within a single life history. The colonies are asexually viviparous and local populations are highly clonal and genetically distinct (Stoddart 1983, 1984); although population genetic and histological evidence implies that broadcast spawning may be used for longer distance colonisation (Stoddart and Black 1985; Stoddart 1988; Ward 1992). In contrast, on Australia’s Great Barrier Reef (GBR), the populations of P. damicornis typically show high levels of genotypic diversity and low levels of genetic differentiation that are consistent with predominantly sexual reproduction and recruitment (Benzie et al. 1995; Ayre et al. 1997; Ayre and Miller 2004; Miller and Ayre 2004). A recent study, however, has shown that, for at least one location on the southern GBR, the brooded larvae of P. damicornis are produced asexually (Ayre and Miller 2004); hence the reproductive effort and the genotypic diversity of local populations appears to be mismatched.
A number of hypotheses can be proposed to explain the observed disparity between population genetic structure of P. damicornis and the prevalence of asexual reproduction in this species. First, habitats with differing physical and biological characteristics may give rise to differences in the genetic composition of populations, due to varying contributions of sexual and asexual reproduction, and the selection of locally adapted genotypes within habitats (Williams 1975; Potts 1984; Jackson 1986). The resolution of this paradox may, therefore, lie in the expansion of studies to determine whether reproductive modes and the relative contribution of sexual and asexual reproduction vary among different reef habitats. Second, asexual reproduction may be an adaptation that allows the exploitation of newly available substrata after a disturbance event. The availability of suitable space after a disturbance event may allow for the rapid re-colonisation of these areas by the localised recruitment of asexually generated larvae from surviving colonies. Hence, populations that have recently been impacted or which have suffered population decline, may show high levels of clonality among new recruits. Third, the presence of cryptic or sibling species within P. damicornis may have masked our ability to detect asexual recruitment in previous genetic studies. P. damicornis shows high levels of morphological variability and several authors have reported local and regional variations in morphological, life history and physiological characters (Richmond and Jokiel 1984; Knowlton 1993; Takabayashi and Hoegh-Guldberg 1995; Ayre and Hughes 2000). Populations of P. damicornis on the GBR may actually comprise two or more taxonomic groups that have different reproductive strategies, with only the asexually brooding taxa releasing larvae during the experimental collections of Stoddart (1983) and Ayre and Miller (2004). The earlier examinations of the genotypes of broods by Stoddart (1983) and Ayre and Miller (2004) inevitably focused only on those colonies that released planulae, and ignored other sympatric adults. Genotypic surveys are therefore needed to determine whether brooding P. damicornis colonies are genetically distinct from non-brooding colonies and, hence, represent different taxonomic groups.
To test these hypotheses we used a combination of genetic and histological data to assess the population structure and mode of reproduction of P. damicornis from the southern GBR. Our aims were to: (1) determine the genetic structure and, hence, the contribution of sexual and asexual reproduction to recruitment within populations of P. damicornis from six different reef habitats at One Tree Island, on the southern GBR, (2) determine the genotypic diversity of recruits after a major disturbance event (3) determine whether the mode of production of brooded larvae varies among different reef habitats, and (4) test for the presence of cryptic species that might have different reproductive modes.
Materials and methods
Study site and sample collection
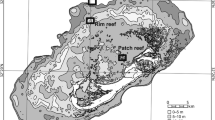
We sampled populations of P. damicornis at One Tree Island Reef (23° 30′ S; 152° 06′ E) on the southern GBR, Australia (Fig. 1). We collected samples from two to three sites, within each of six reef habitats, during November/December 2001–2003. Four habitats were within the lagoon (reef flat, lagoon wall at 5 m depth, patch reefs at 2 m depth, and micro-atolls at 2 m depth) and the other two habitats included samples from the upper and lower reef slope (upper reef slope at 2–6 m depth and the lower reef slope at 7–11 m depth) (Fig. 1). Separation between habitats varied from 200 to 1,000 m, except for reef slope habitats where equivalently numbered upper and lower reef slope sites were separated by only 10–15 m (Fig. 1). We selected these habitats a priori because they represented a range of habitats in which P. damicornis is found and differed in their physical and biological characteristics to that of the reef crest (Done 1982). We purposely avoided the reef crest habitat as it has been the focus of most other studies of P. damicornis on the GBR. The two micro-atoll sites that we sampled showed evidence of a disturbance, due to a major coral bleaching event that affected this area in 1998 (Baird and Marshall 1998; Booth and Beretta 2002). The effects of this disturbance were clearly patchy, with Booth and Beretta (2002) reporting a significant decrease in the coral cover (primarily of the Pocilloporid species which dominated the lagoon) in two of four lagoon sites, but negligible change in live coral cover in two other lagoon sites.
We made collections of 2–5 cm branch fragments, from 39 to 50 coral colonies from each site (25–50 m2), within the six reef habitats (except the micro-atoll sites, where we collected from 94 to 100 specimens) (Table 1). The collections consisted of a haphazard selection of available colonies within each site (sites separated by 50–100 m), which included both small colonies (<7 cm colony diameter) and larger colonies (>8 cm colony diameter). To avoid the collection of clonal fragments, we sampled only those colonies firmly attached to the reef matrix and which displayed a symmetrical growth form consistent with the growth from a settled larva. We placed each fragment in an individual zip-lock bag, two-thirds filled with seawater, for transport back to the laboratory where they were immediately frozen in liquid nitrogen. The samples were subsequently stored at −80°C, prior to genotyping, using allozyme electrophoresis.
Electrophoresis
Allozyme electrophoresis was carried out on horizontal starch gels (12% w/v) using a tris citrate (TC8), tris-EDTA-borate (TEB) or tris-maleate (TM) buffer (buffers 5, 6, and 9 respectively of Selander et al. 1971). We determined the genotypes of P. damicornis samples, collected from each of the six reef habitats, for eight allozyme loci that had previously been found to be variable at sites within the GBR (Ayre et al. 1997). We assayed glucosephosphate isomerase (Gpi1, EC 5.3.1.9), malate dehydrogenase (Mdh1&2, EC 1.1.1.37), and mannose phosphate isomerase (Mpi, EC 5.3.1.8) on buffer TC8; hexokinase (Hk1&2, EC 2.7.1.1) and phosphoglucomutase (Pgm2, EC 5.4.2.2) on buffer TM; leucyl proline peptidase (Lpp, EC 3.4.11), and leucyl glycylglycyl peptidase (Lggp1&2, EC 3.4.11) on buffer TEB. We detected between two and six alleles at each locus, and described the alleles numerically, in order of decreasing electrophoretic mobility.
Larval collections
To assess whether brooded larvae were produced sexually or asexually across different reef habitats, we collected larvae from adult colonies of P. damicornis during the main summer planulation period (Tanner 1996) in November/December 2002 and 2003. Four to 15 colonies were collected from each of the six reef habitats and held in individual aquaria on wet tables for 6–8 days, using a flow-through seawater system. We collected larvae using overflow traps lined with 200 μm plankton mesh. We successfully collected larvae from a total of 12 colonies taken from five of the six reef habitats (no larvae were released from micro-atoll colonies during the experimental collections). We used allozyme electrophoresis to compare the genotypes of adult colonies and their broods to determine whether the larvae were produced sexually or asexually. The methods used were as described above, although larvae were scored for only a subset of the loci previously described, and an additional locus, leucyl tryosine peptidase (Ltp, EC 3.4.11), was scored for some larvae. The larvae were genotyped within 24 h of release, as per Ayre and Miller (2004).
Testing for cryptic species
Colonies that were held in aquaria, during the main planulation period, and which did not release larvae were examined histologically for the presence of larvae. For each colony we decalcified a middle branch fragment, for 24 h in 10% hydrochloric acid, and then viewed these under a stereomicroscope, examining the body cavity and mesenteries for the presence of larvae. To determine whether non-brooding colonies represented a different or more complex taxonomic group to those colonies that did release larvae, tissue samples of all colonies used for larvae collections were taken and the 8-locus allozyme genotypes determined as described above. We then calculated the genetic similarity among all brooding and non-brooding colonies as Nei’s (1978) genetic distance, and used a principle coordinate analysis to test for the presence of genetic groups that relate to the presence of cryptic species. However, the absence of brooded larvae in some colonies does not exclude the possibility that these colonies may have already released brooded larvae, or may not have begun their brooding cycle.
Statistical analyses
Fine-scale population structure and levels of genetic subdivision
We expressed levels of genetic and genotypic variation within sites as the mean numbers of alleles per locus (n a), effective number of alleles per locus (n e), observed (H o), and expected heterozygosity (H e), calculated using GENEPOP Version 3.4 (Raymond and Rousset 1995). We tested for differences in the level of genetic variation among habitats, using a single-factor ANOVA for each of the three measures. To ensure that our larger sample sizes from the two micro-atoll sites did not influence diversity measures, we randomly selected 50 individuals from each of these two sites when testing for the heterogeneity of genetic diversity among habitats.
In order to determine whether each locus assorted independently, we tested each pair-wise combination of loci for linkage disequilibrium (Weir 1979) for each site, using GENEPOP Version 3.4. From a total of 450 pair-wise tests, only 22 significant inter-locus associations were detected (p<0.05); however, only eight of these associations (all for micro-atoll site 2) remained significant after the application of a sequential Bonferroni correction (Rice 1989).
We quantified levels of population subdivision, using a hierarchical analysis of standardised genetic variance (F) statistics (Wright 1969), to partition genetic variation within and among habitats. Subscripts were used to denote the source of variation: F SH, variation among sites within each habitat; F HT, variation among habitats within the total; and F ST, total variation among all sites. We calculated these parameters using the formulations of Weir and Cockerham (1984), using the program TFPGA (Miller 1997) which executes numerical re-sampling (jackknifing) to provide estimates of variances across loci. The values of F were judged to be statistically significant when zero lay outside the 95% confidence interval of the mean. Nei’s (1978) unbiased genetic distance (D) was used to examine the genetic relationship among sites and habitats, and UPGMA analysis and trees were drawn using the program TFPGA. The robustness of each node was evaluated by bootstrapping allele frequencies 100 times.
Since the level of allelic differentiation of habitats may be partially confounded by the effects of varying degrees of geographic separation, we tested for correlation between matrices of pairwise F ST/(1-F ST) values and the geographical distances (m) between sites, using a Mantel test (10,000 permutation, GENEPOP Version 3.4).
Assessing relative contributions of sexual and asexual reproduction
In order to assess the relative effect of sexual and asexual reproduction on the genetic composition of populations, we first calculated the magnitude and direction of departures from Hardy–Weinberg equilibria for each locus within each site. The departures were expressed as Wright’s (1978) fixation index, f, where positive and negative values represent deficits or excess of heterozygotes, respectively. For those loci that were sufficiently variable (i.e. frequency of the most common allele <95%, Hedrick 2000), we employed chi-square tests to determine whether the observed numbers of heterozygotes were significantly different from those expected under Hardy–Weinberg equilibria, using the genetics program GENEPOP Version 3.4. To reduce the chance of type I errors we applied a sequential Bonferroni correction. Second, for each site we compared the number of colonies sampled (N) to the number of unique multi-locus genotypes (N g) detected. We then compared the ratio of observed multi-locus genotypic diversity (G o) to that expected under conditions of sexual reproduction with free recombination (G e), as described by Stoddart and Taylor (1988). We tested for significance departures from unity by determining if G o lay outside the 95% confidence interval of G e (Stoddart and Taylor 1988). To reduce the chance of type I errors we applied a sequential Bonferroni correction.
Assessing mode of reproduction
To assess whether brooded larvae are produced sexually or asexually, we compared the genotypes of larvae to that of their brood parent. Outcrossing is likely to result in a diverse set of genotypes and the presence of non-maternal alleles, while selfing would result in an increase in homozygous offspring in comparison to a heterozygous parent. If an adult colony were heterozygous at a particular locus, we would then expect, on average, a maximum of 50% of the brood to be identically heterozygous to the parent, if the larvae were generated via sexual reproduction (the value expected for self-fertilization or exclusive mating, with either another identically heterozygous individual(s) or an individual homozygous, for one of the two parental alleles). We, therefore, calculated the maximum probability that sets of ‘n’ brooded larvae could be identically heterozygous at one or more loci (as a result of sexual reproduction) as the product of the single locus probabilities, where for any given locus: p=0.5n. This assumes independent assortment and free recombination (Black and Johnson 1979).
Results
Genetic variation among sites and habitats
We detected consistently high levels of allelic diversity in our collections of P. damicornis from all sites and habitats (Table 1). Overall, the average number of alleles per locus, across all sites, was 3.75±1.49 (SE), with an average of 1.71±0.57 effective alleles per locus and a mean expected heterozygosity of 0.33±0.21. However, although differences among habitats were relatively slight, coral populations from the reef slope had significantly higher numbers of effective alleles (F 4,12=10.81, p=0.0006) and greater levels of expected heterozygosity (F 4,12=9.74, p=0.001) than coral populations in the lagoon (Table 1).
We used levels of single locus heterozygosity to test for departures from random mating at each site. Of 99 single locus tests across eight loci, we found 30 cases of departures from expected values, with all except one representing heterozygous deficits. However, only five of these remained significant after a sequential Bonferroni correction of significance levels and all were heterozygote deficits (Table 2). The large and consistent heterozygous deficits detected cannot be explained by simple asexual reproduction, as this should generate similar levels of both heterozygous excesses and deficits, and are more likely to reflect breeding between closely related individuals or Wahlund effects.
Population subdivision
The hierarchical analysis of F-statistics revealed significant levels of population subdivision among all sites (p<0.05, Table 3). The mean F ST (±SE) across eight loci was 0.06±0.01, with most of this variation (83%) due to the variation among habitats (F HT=0.05±0.02). We detected little genetic differentiation among sites within each habitat (F SH ranging from 0.001 to 0.02, Table 3).
The UPGMA cluster analysis, based on Nei’s (1978) unbiased genetic distance (D), clearly indicated that the majority of genetic differentiation observed between habitats was due to differences among the lagoon and reef slope populations (Fig. 2). Sites sampled from a habitat showed a high similarity and generally clustered together (i.e. sites in the same habitat were more similar than sites from other habitats). However, our analysis using a Mantel test did reveal some evidence of significant isolation by distance among all sites (p<0.001), although this relationship explains only 35% of the total variation.
Dendrogram showing the genetic relationship between 17 collections of P. damicornis made within the lagoon and outer reef habitats at One Tree Island on the southern GBR, Australia. Nei’s genetic distance (1978) was calculated based on data for eight enzyme encoding loci and clustering was determined using UPGMA. Bootstrapped values over 35% (based on 100 randomisations) are shown next to corresponding nodes
The relative importance of sexual and asexual reproduction among habitats
The genotypic composition of sites revealed that the relative importance of sexual and asexual recruitment varied across habitats. We detected high levels of genotypic diversity within collections of P. damicornis from the lagoon wall, reef flat, lower reef slope, upper reef slope, and for two sites within the patch reef habitat, consistent with sexually derived recruitment (Table 4). For these sites the number of unique genotypes (N g) detected, compared to the number of colonies sampled (N) was consistently high with N g /N ranging from 0.68 to 0.92 (Table 4). Some replicate genotypes were found within these sites, but most were represented by only two or three individuals and no single clone was numerically dominant. Within these sites we detected 69–100% (G o/G e=0.69 to 1.00) of the genotypic diversity expected for sexual reproduction (Table 4). The separate analysis of large and small (and potentially younger) colonies, from each site, revealed no increased levels of clonality within either group, with G o/G e ranging from 0.74 to 1.12 for larger colonies and 0.83–1.09 for smaller colonies (data not shown).
In contrast, within the two micro-atoll sites and patch reef site 2, we detected low levels of genotypic richness (N g /N=0.54 to 0.65), with one genotype represented by 18 individuals in micro-atoll 2. Collections from both micro-atoll sites and patch reef site 2 showed only 35–53% of the genotypic diversity expected for sexual reproduction (G o/G e=0.35 to 0.53, Table 4). This suggests that, within these three sites, asexual recruitment may be more important for maintaining populations. The separate analysis of larger and smaller colonies showed higher levels of genotypic diversity, with 39–68% of the diversity expected for sexual reproduction detected among larger colonies and 57–72% of the diversity expected for sexual reproduction detected for collections of smaller colonies (data not shown).
Variation in mode of production of brooded planulae among habitats
In total, 23 colonies released larvae, but only 12 released enough larvae (>4) to produce a powerful test for evidence of asexual reproduction. Despite the high levels of allelic diversity that we detected within samples of P. damicornis at the One Tree Island Reef (Table 1), we found that all larval genotypes from colonies collected from five reef habitats were electrophoretically identical to their brood parent (262 larvae from 12 adult colonies, Table 5). Six of the 12 broods produced larvae (110 larvae in total) that displayed genotypes that were identically heterozygous to the brood parent for at least one locus, the probability of this occurring due to sexual reproduction is extremely small (p=7.7×10−34). Moreover we detected 28 larvae that were identically heterozygous to the brood parent for three loci, again an extremely unlikely occurrence due to sexual reproduction (p=5.17×10−26). None of the larvae that we genotyped displayed non-maternal alleles.
Tests for the presence of cryptic species
The microscopic examination of colonies, used for the collection of brooded larvae, revealed that of the 42 colonies that did not release larvae, four of these were brooding larvae within their polyps, but had not released their broods during the experimental period. Based on these results, 27 colonies were identified as brooding and 38 colonies as non-brooding. A principal coordinates analysis, based on a genetic distance matrix, revealed no obvious structuring within our One Tree Island collection (Fig. 3) and, although the analysis was relatively weak (the first two principle components accounted for only 41% of the variation), it was clear that the set of non-brooding colonies did not contain any distinct genetic groupings that could potentially represent a cryptic species.
Principal coordinates analysis, based on a genetic distance matrix (Nei 1978), for individual brooding and non-brooding colonies of P. damicornis collected from the One Tree Island Reef on the southern GBR, Australia
Discussion
Our data for populations of P. damicornis from a wide variety of reef habitats, confirm and extend the scale of the apparent mismatch between the reproductive mode and localised recruitment, reported by Ayre and Miller (2004) for GBR populations of P. damicornis; but also indicate that clonal recruitment may be favoured within recently disturbed sites. In contrast to our expectations, we found that populations of P. damicornis, from 14 of 17 sites, displayed high levels of genotypic diversity, consistent with recruitment from sexual reproduction. However, our electrophoretic analysis of the genotypes of brooded larvae, from a range of habitats, suggested that all broods surveyed in this study were produced asexually. Interestingly, we detected significantly lower levels of genotypic diversity at three sites within the reef lagoon, two of which were known to have undergone severe disturbances in the last 6 years. The apparent mismatch between the reproductive mode and recruitment could not be explained by the presence of cryptic species at One Tree Island (Ayre et al. 1991; Knowlton et al. 1992; Miller and Benzie 1997). Rather, we found that a principal coordinates analysis plot, based on a genetic distance matrix, showed that all colonies tested (including brooding and non-brooding colonies) formed a single genetic cluster.
Variation in genotypic diversity across habitats
We had anticipated that the apparent paradox implied by Ayre and Miller’s (2004) finding, that reef flat and reef crest colonies of P. damicornis produce clonal broods while reef crest populations are typically highly diverse (Benzie et al. 1995; Ayre et al. 1997; Ayre and Miller 2004), might be resolved if local populations in other habitats displayed high levels of clonality. We reasoned that the persistence of asexual viviparity on reef crests could, therefore, be maintained despite apparently high levels of sexual recruitment if asexual reproduction were favoured elsewhere, and that the reef crests were effectively a genetic sink (Ayre and Miller 2004). However, our data provides no such simple explanation, since we found that asexual recruitment contributes little to maintaining local populations of P. damicornis within each of six other reef habitats. Indeed, sites from most of the sheltered lagoon habitats (lagoon wall, patch reefs, and reef flat) showed similar levels of genotypic diversity to sites on the highly speciose and heterogeneous reef slopes. The high levels of genotypic diversity within these sites were comparable not only to those from earlier studies of P. damicornis on the GBR, but were also similar to levels reported for exclusively sexually brooding corals (e.g. Ayre and Dufty 1994; Ayre and Hughes 2000), including two other Pocilloporids.
The hypothesis that asexual larvae disperse over greater distances than the spatial scales, sampled in this and earlier studies, may provide an explanation for the apparent lack of clonal structure in these populations. Competency and energetic studies of brooded larvae of P. damicornis from Hawaii and Japan suggest that brooded larvae may, indeed, be capable of remaining in the plankton for extended periods (up to 100 days), and therefore have the potential to be widely dispersed (Richmond 1987; Isomura and Nishihira 2001; Harii et al. 2002). It is clear from this study that the asexually produced larvae of P. damicornis, in populations on the GBR, are rarely successful in recruiting close to their brood parent. This means that either asexual propagules are used for widespread dispersal or remain unused and do not recruit in the majority of reef habitats (though disturbed habitats may provide an exception to this). While it is not possible to reject the hypothesis that the genotypic diversity of local populations of P. damicornis is maintained by the widespread dispersal of asexually generated larvae, it is difficult to understand the selective process that would favour the evolution and maintenance of a life history that used both sexual and asexual modes of reproduction for the widespread dispersal of propagules. For organisms with both sexually and asexually generated propagules, theory predicts that asexual reproduction will be used to restock the parental habitat and sexual reproduction will be used for widespread dispersal or the colonisation of more heterogeneous habitats (Williams 1975). This dichotomy of roles is well supported from studies in a number of taxa (Ayre 1984; Hoffmann 1986; Hebert et al. 1988; Darling et al. 2004). Direct comparisons of the relative dispersal capabilities and the relative fitness of sexually versus asexually generated larvae, are clearly needed to understand the conditions under which either mode of reproduction is likely to be important. Interestingly, despite P. damicornis being one of the most (if not the most) intensively studied coral, there have been no direct observations of broadcast spawning or the capture of sexually generated larvae for direct comparisons with asexually produced larvae. Additionally, more intensive and extensive sampling of populations is needed to identify the potential scale of dispersal of asexually produced larvae. P. damicornis can occur at very high densities and may require the sampling of a large proportion of a population (i.e. thousands of individuals), including both adults and recent recruits, to determine the scale of dispersal and recruitment of asexually generated larvae.
Low levels of genotypic diversity within disturbed habitats
While asexual reproduction may be more commonly associated with the absence of disturbances or the predictability of the parental habitat (Williams 1975), we expect that disturbances, such as bleaching or cyclonic storms, would have a simplifying effect on normally speciose coral habitats that might favour localised asexual recruitment for a period of years. During such periods, coral densities and species richness are reduced (Hughes et al. 1992; Connell 1997; Hughes and Connell 1999) and, hence, the potential for both interspecific and interclonal competition is also reduced (Connell et al. 1997). This could facilitate the re-colonisation of this space through the recruitment of clonal larvae produced by a few surviving adults. Our results support this hypothesis, since we detected high levels of asexual recruitment in three lagoon sites, two of which are known to have experienced recent (i.e., in the previous 6 years) disturbance events. We detected only 35–53% of the genotypic diversity expected for sexual reproduction, with free recombination, within these sites. A major bleaching event in 1998 had resulted in large mortalities of adult corals (including P. damicornis) within a number of lagoonal sites at the One Tree Island Reef (Booth and Beretta 2002). A study by Benzie et al. (1995), prior to the bleaching event, had found high levels of genotypic diversity and little evidence of asexual recruitment within the micro-atoll habitats, including one of the sites sampled in this study. Benzie et al. (1995) found that micro-atoll two (i.e., micro-atoll one in this study) showed high levels of genotypic diversity (G o /G e=0.93) and few replicated genotypes (N g/N=0.87), while we detected considerably lower levels of genotypic diversity (G o /G e=0.53) and unique multi-locus genotypes (N g/N=0.55). This decrease in genotypic diversity, within the micro-atoll site, may be explained by the re-colonisation of this space by asexually produced larvae produced by the surviving adults after the bleaching event. All the genotypes of the smaller colonies sampled within these sites were represented by at least one of the adult colonies, thus supporting the hypothesis that the larvae originated from adult colonies within these sites. Localised recruitment, by even a small proportion of asexually brooded larvae, could lead to reduced diversity in an expanding population. However, the continued recruitment of larvae from other areas may, subsequently, lead to an increase in diversity over time. Additionally, micro-atoll sites typically form enclosed ponds at low tide, which is likely to facilitate the retention of asexual generated larvae. The structure and local hydronamic regimes of habitats may, therefore, also play an important role in determining the level of clonality within a habitat and may also explain the higher levels of clonality observed in Western Australian populations, which typically consist of small embayments that may facilitate the local retention of larvae (Stoddart 1984). Therefore, the lack of localised recruitment of asexually generated larvae in other habitats may, in large part, be due to a combination of longer periods of time since the disturbance event and differences in local hydrodynamic regimes that do not favour the retention of asexually produced larvae within the parental habitat.
Genetic variation and subdivision
Our analysis of genetic variation found significant but modest levels of genetic differentiation among all sites (F ST=0.06). However, closer inspection implies that there is very little differentiation among sets of neighbouring sites within habitats. Our hierarchical analysis of F ST suggested that the majority of the variation present was due to differences between habitats, rather than between sites within habitats. However, we recognise that the relatively larger level of variation, which we report among habitats rather than among sites (0.045 vs. 0.028-0.001), does include both the effects of variation among habitats and some effect of isolation by distance. The occurrence of isolation by distance implies that individuals within neighbouring sites are more closely related than sets from distant sites; nevertheless, this should not reduce the value of comparisons of genotypic diversity or reproductive modes across habitats, as these characteristics can vary over finer spatial scales (e.g. variation among patch reefs, Table 4).
Taken together, our data indicate that asexually brooded larvae of P. damicornis do not contribute significantly to maintaining local populations in the majority of reef habitats; although our data do suggest that asexual recruitment may be favoured after a disturbance event, presumably when competition is reduced. The extent of clonal recruitment in a wider variety of disturbed habitats warrants further investigation to determine if this type of disturbance is frequent enough to drive the persistence of this asexual mode of reproduction. Comparisons of genotypes of brooded larvae to that of their brood parent is also needed to clarify the mode of production of brooded larvae, in locations such as Japan, where a sexual origin of larvae has been inferred form histological studies (Permata et al. 2000). Finally, until comparative studies of dispersal and the relative fitness of sexually versus asexually produced larvae, under different environmental conditions, have been carried out, it will be difficult to understand under which conditions either mode of reproduction would be favoured.
References
Ayre DJ (1984) The effects of sexual and asexual reproduction on geographic variation in the sea anemone Actinia tenebrosa. Oecologia 62:222–229
Ayre DJ, Dufty S (1994) Evidence for restricted gene flow in the viviparous coral Seriatopora hystrix on Australia Great Barrier Reef. Evolution 48:1183–1201
Ayre DJ, Hughes TP (2000) Genotypic diversity and gene flow in brooding and spawning corals along the Great Barrier Reef, Australia. Evolution 54:1590–1605
Ayre DJ, Hughes TP, Standish RS (1997) Genetic differentiation, reproductive mode, and gene flow in the brooding coral Pocillopora damicornis along the Great Barrier Reef, Australia. Mar Ecol Prog Ser 159:175–187
Ayre DJ, Miller KJ (2004) Where do clonal larvae go? Adult genotypic diversity conflicts with reproductive effort in the brooding coral Pocillopora damicornis. Mar Ecol Prog Ser 277:95–105
Ayre DJ, Veron JEN, Dufty SL (1991) The corals Acropora palifera and Acropora cuneata are genetically and ecologically distinct. Coral Reefs 10:13–18
Baird AH, Marshall PA (1998) Mass bleaching of corals on the Great Barrier Reef. Coral Reefs 17:376
Bell G (1982) The masterpiece of nature: the evolution and genetics of sexuality. Croom Helm, London
Benzie JAH, Haskell A, Lehman H (1995) Variation in the genetic composition of coral (Pocillopora damicornis and Acropora palifera) populations from different reef habitats. Mar Biol 121:731–739
Black R, Johnson MS (1979) Asexual viviparity and population genetics of Actinia tenebrosa. Mar Biol 53:27–31
Booth DJ, Beretta GA (2002) Changes in a fish assemblage after a coral bleaching event. Mar Ecol Prog Ser 245:205–212
Connell JH (1997) Disturbance and recovery of coral assemblages. Coral Reefs 16(Suppl):S101–S113
Connell JH, Hughes TP, Wallace CC (1997) A 30-year study of coral abundance, recruitment, and disturbance at several scales in space and time. Ecol Monogr 67:461–488
Darling JA, Reitzel AM, Finnerty JR (2004) Regional population structure of a widely introduced estuarine invertebrate: Nematostella vectensis Stephenson in New England. Mol Ecol 13:2969–2981
Done TJ (1982) Patterns in the distribution of coral communities across the central Great Barrier Reef. Coral Reefs 1:95–107
Harii S, Kayanne H, Takigawa H, Hayashibara T, Yamamoto M (2002) Larval survivorship, competency periods and settlement of two brooding corals, Heliopora coerulea and Pocillopora damicornis. Mar Biol 141:39–46
Harrison PL, Wallace CC (1990) Coral Reefs. Ecosystems of the world. Elsevier, Amsterdam
Hebert PDN, Ward RD (1976) Enzyme variability in natural populations of Daphnia magna.IV. Ecological differentiation and frequency changes of genotypes at Audley End. Heredity 36:331–341
Hebert PDN, Ward RD, Weider LJ (1988) Clonal diversity patterns and breeding-system variation in Daphnia pulex, an asexual–sexual complex. Evolution 42:147–159
Hebert PN (1978) Adaptive significance of cyclomorphosis in Daphnia: more possibilities. Freshw Biol 8:313–320
Hedrick PW (2000) Genetics of populations. Jones and Bartlett, Boston, MA
Highsmith RC (1982) Reproduction by fragmentation in corals. Mar Ecol Prog Ser 7:207–226
Hoffmann RJ (1986) Variation in contributions of asexual reproduction to the genetic structure of populations of the sea anemone Metridium senile. Evolution 40:357–365
Hughes RN (1989) A functional biology of clonal animals. Chapman and Hall, NY
Hughes TP, Ayre D, Connell JH (1992) The evolutionary ecology of corals. Trends Ecol Evol 7:292–295
Hughes TP, Connell JH (1999) Multiple stressors on coral reefs: a long-term perspective. Limnol Oceanogr 44:932–940
Isomura N, Nishihira M (2001) Size variation of planulae and its effect on the lifetime of planulae in three pocilloporid corals. Coral Reefs 20:309–315
Jackson JBC (1986) Modes of dispersal of clonal benthic invertebrates: consequences for species distributions and genetic structure of local populations. Bull Mar Sci 39(2):588–606
Knowlton N (1993) Sibling species in the sea. Annu Rev Ecol Syst 24:189–216
Knowlton N, Weil E, Weigt LA, Guzman HM (1992) Sibling species in Montastraea annularis, coral bleaching, and the coral climate record. Science 255:330–333
Lasker HR (1990) Clonal propagation and population dynamics of a Gorgonian coral. Ecology 71:1578–1589
Maynard Smith J (1978) The evolution of sex. Cambridge University Press, Cambridge
Miller KJ, Ayre DJ (2004) The role of sexual and asexual reproduction in structuring high latitude populations of the reef coral Pocillopora damicornis. Heredity 92:557–568
Miller KJ, Benzie JAH (1997) No clear genetic distinction between morphological species within the coral genus Platygyra. Bul Mar Sci 61:907–917
Miller MP (1997) Tools for population genetyic analysis (TFPGA) 1.3: A Windows program for the analysis of allozyme and molecular population genetic data. http://www.marksgeneticsoftware.net/tfpga.htm
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Permata WD, Kinzie RA III, Hidaka M (2000) Histological studies on the origin of planulae of the coral Pocillopora damicornis. Mar Ecol Prog Ser 200:191–200
Potts DC (1984) Natural selection in experimental populations of reef-building corals scleractinia. Evolution 38:1059–1078
Raymond M, Rousset F (1995) Genepop (Version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Richmond RH (1987) Energetics, competence, and long-distance dispersal of planula larvae of the coral Pocillopora damicornis. Mar Biol 93:527–533
Richmond RH, Jokiel PL (1984) Lunar periodicity in larva release in the reef coral Pocillopora damicornis at Enewetak, Marshall slands and Hawaii, USA. Bul Mar Sci 34:280–287
Sebens KP, Thorne BL (1985) Coexistence of clones, clonal diversity, and the effects of disturbance. In: Jackson JBC (ed) Population biology and evolution of clonal organisms. Yale University Press, New Haven, pp 357–398
Selander RK, Smith MH, Yank SY, Johnston WE, Gentry JB (1971) Biochemical polymorphism and systematics in the genus Peromyscus. I. Variation in the old-field mouse (Peromyscus polionotus). Stud Genet 6:49–90
Stoddart JA (1983) Asexual production of planulae in the coral Pocillopora damicornis. Mar Biol 76:279–284
Stoddart JA (1984) Genetic differentiation among populations of the coral Pocillopora damicornis off southwestern Australia. Coral Reefs 3:149–156
Stoddart JA (1988) Coral populations fringing islands larval connections. Aust J Mar Freshw Res 39:109–116
Stoddart JA, Black R (1985) Cycles of gametogenesis and planulation in the coral Pocillopora damicornis. Mar Ecol Prog Ser 23:153–164
Stoddart JA, Taylor JF (1988) Genotypic diversity—estimation and prediction in samples. Genetics 118:705–711
Takabayashi M, Hoegh-Guldberg O (1995) Ecological and physiological differences between two colour morphs of the coral Pocillopora damicornis. Mar Biol 123:705–714
Tanner JE (1996) Seasonality and lunar periodicity in the reproduction of Pocilloporid corals. Coral Reefs 15:59–66
Tunnicliffe V (1981) Breakage and propagation of the stony coral Acropora cervicornis. Proc Natl Acad Sci-Biol 78:2427–2431
Ward S (1992) Evidence for broadcast spawning as well as brooding in the scleractinian coral Pocillopora damicornis. Mar Biol 112:641–646
Weir BS (1979) Inferences about linkage disequilibrium. Biometrics 35:235–254
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38(6):1358–1370
Williams GC (1975) Sex and evolution. Princeton University Press, Princeton
Wright S (1969) The evolution and genetics of populations, vol 2. The theory of gene frequencies. University of Chicago Press, Chicago, IL
Wright S (1978) Evolution and the genetics of populations. 4. Variability within and among natural populations. University of Chicago Press, Chicago, IL
Wulff JL (1985) Dispersal and survival of fragments of coral reef sponges. Proc 5th Int Coral Reef Congr, Tahiti 5:119–124
Wulff JL (1991) Asexual fragmentation, genotype success, and population dynamics of erect branching sponges. J Exp Mar Biol Ecol 149:227–247
Acknowledgements
We thank Mike Johnson and two anonymous reviewers for comments on the manuscript. M. Martin, J. Dunn and C. Mundy helped with field and laboratory work. K. and P. Beinssen assisted at the University of Sydney’s One Tree Island research station. This work was supported, in part, by two student grants to C.D.H. Sherman by the Great Barrier Reef Marines Park Authority and by an ARC Discovery Grant to D.J. Ayre and K.J. Miller. This is publication #258 from the Ecology and Genetics Group at the University of Wollongong.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Biological Editor H.R. Lasker
Rights and permissions
About this article
Cite this article
Sherman, C., Ayre, D. & Miller, K. Asexual reproduction does not produce clonal populations of the brooding coral Pocillopora damicornis on the Great Barrier Reef, Australia. Coral Reefs 25, 7–18 (2006). https://doi.org/10.1007/s00338-005-0053-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-005-0053-x