Abstract
Over the past decades, several studies have revealed that the traditional view of the Antarctic Circumpolar Current (ACC) as an agent for species dispersal in the Southern Ocean is not applicable to all taxa. Some species are actually circum-Antarctically or circum-sub-Antarctically distributed, but some other species actually comprise species’ complexes, with cryptic taxa occurring at different areas. However, to date, few of the invertebrate species formerly reported as widespread in the Southern Ocean have been re-analyzed using genetic techniques. This study examined whether two geographically distant areas of the sub-Antarctic region under the influence of the ACC, the Southern tip of South America (SSA) and the Prince Edward Islands (PEI), share some marine invertebrate species. For that, members of two genera of bivalves, Gaimardia and Hiatella, were selected. As part of this study, we found extremely low genetic differentiation between specimens from SSA and PEI. In addition, shared haplotypes were found between these two areas. Our results confirm that Gaimardia trapesina and one same species of Hiatella (“Hiatella O”) are present in both areas. Given that these two species are found on macroalgae, natural rafts appear as the most plausible means of dispersal of juveniles and adults, although in the case of Hiatella O, additional larval dispersion cannot be discarded. In any of these cases, dispersion should be facilitated (or even determined) by the ACC. Thus, this study provides new evidence in favour of considering the ACC as an effective dispersive agent in the Southern Ocean.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Antarctic Circumpolar Current (ACC) is considered a primary force promoting events of dispersion, isolation and speciation in Southern Ocean (Patarnello et al. 1996; Beu et al. 1997). This current, originated about 25–23 million years ago (in the late Oligocene) (Lyle et al. 2007), flows around the Antarctic continent in a west–east direction. It is delimited by the sub-Antarctic Front in the north and the Southern ACC Front in the south, encompassing in between the Antarctic Polar Front (Orsi et al. 1995). These frontal zones have been frequently regarded as natural barriers to genetic exchanges between species located within and outside the ACC, as well as those occurring at both sides of the ACC (Barker et al. 2007; Hunter and Halanych 2008).
Historically, based on the overall morphological similarities, several invertebrate species have been reported as widely distributed in the Southern Ocean, showing circum-Antarctic or circum-sub-Antarctic patterns (e.g., Dell 1972). In this regard, several authors have suggested that the ACC could be responsible for this distributional patterns, either through the dispersion of larval stages (as members of plankton community) or of juvenile/adult specimens by rafting (e.g., Fell 1962; Dell 1972). Additionally, some authors (e.g., Castilla and Guiñez 2000) regarded the anthropogenic dispersal as an alternative way for explaining this distributional pattern in some taxa. However, these hypotheses were seldom supported by factual evidence. In the last decades, the usage of genetic techniques applied to the taxa occurring in the Southern Ocean revealed contrasting distributional patterns: although some species appear to be actually widely distributed (e.g., Nikula et al. 2010; Moon et al. 2017), several other species previously regarded as widely distributed actually proved to correspond to species’ complexes, with “cryptic” species occurring in different localities (e.g., Wilson et al. 2007; Allcock et al. 2011). This fact led some authors to conclude that few taxa actually show circum-Antarctic or circum-sub-Antarctic distributions (e.g., Clarke et al. 2007: 620). However, to date, it is difficult to determine what “few taxa” exactly means, as a reduced number of the taxa previously considered as widespread have been carefully evaluated using genetic techniques. Among bivalves, for instance, only a few families were thus far genetically investigated, including Ostreidae (Ó Foighil et al. 1999), Limidae (Page and Linse 2002) and Mytilidae (Zbawicka et al. 2019), all of them belonging to the subclass Autobranchia. The results obtained by these studies are not conclusive: the two first confirm the occurrence of widespread species, while Zbawicka et al. (2019) revealed the presence of different (cryptic) species, with allopatric distributions, along the sub-Antarctic area. Considering the great diversification of bivalves in this area, and the broad geographic distributions exhibited by several other bivalve genera, this group of invertebrates appears particularly attractive for providing new insights on the biogeography of the Southern Ocean.
Gaimardia (Gaimardiidae) and Hiatella (Hiatellidae) are two genera members of the subclass Heterodonta which include species currently regarded as circum-sub-Antarctic. According to MolluscaBase (2020), Gaimardia comprises 11 valid species, all of them occurring in the southern hemisphere at high latitudes, including Southern South America, the Scotia Arc islands, Prince Edward Islands, Kerguelen, Tasmania, Macquarie, and New Zealand (Dell 1964; Zelaya 2005). Based on morphological characters, two of these species (Gaimardia trapesina and G. adamsiorum) are considered as widespread in sub-Antarctic waters, although the conspecificity of specimens from distant areas was never confirmed with genetic techniques; the remaining nine species of Gaimardia are considered as having much smaller distributional ranges (i.e., restricted to one or a few archipelagos, or to some particular areas). To date, the distinction of Gaimardia species is not easy. Most of these species remain only known from their original description (usually lacking relevant information for species distinction) and are imperfectly figured or not figured at all. A systematic revision encompassing these species has been never performed. Members of this genus lack a free-living larva and exclusively live on macroalgae (Helmuth et al. 1994; Ituarte 2009; Chaparro et al. 2011; Zelaya et al. 2019). Gaimardia trapesina, for instance, lives on the giant kelp Macrocystis pyrifera, where in fact it is the most abundant epibiotic species on the fronds (Dayton 1985a; Adami and Gordillo 1999; Puccinelli et al. 2018).
The knowledge on Hiatella is not much better than that of Gaimardia. Hiatella is a worldwide distributed genus, but all species have been described based on their shell morphology, a character that has proved to be greatly variable, being dependent on the local / particular conditions, where individuals grow (Lezin and Flyachinskaya 2015). Molecular studies in members of Hiatella are still scarce. Laakkonen et al. (2015) recognized two lineages (molecular species) of Hiatella in the southern hemisphere (which they referred as Hiatella A and Hiatella B), and 11 lineages in the northern hemisphere (Hiatella C-M). Layton et al. (2016) found an additional molecular species in the northern hemisphere (which he named Hiatella N). Out of these molecularly investigated species, only Hiatella A appears in the area under de incidence of the CCA. Members of Hiatella may be found over a great variety of substrates, including the giant kelp (Gordillo 2001; Laakkonen et al. 2015). Species of Hiatella for which some developmental information is available reveal the presence of a planktonic larval stage (e.g., Schejter et al. 2010; Díaz and Campos 2014).
The fact that some species of both Gaimardia and Hiatella live on macroalgae suggests that rafting may be a responsible driver for their current circum-sub-Antarctic pattern of distribution. Several biological and environmental factors have been reported to cause breakage of stipes and detachment of the holdfasts of the giant kelp (Barrales and Lobban 1975; Santelices and Ojeda 1984; Dayton 1985b; Duggins et al. 2001). These factors lead to the generation of kelp rafts, which are dispersed in the Southern Ocean, with the aid of the ACC, consequently dispersing the organisms living on them (Fraser et al. 2010; Nikula et al. 2010; Gillespie et al. 2011; Waters et al. 2018; Avila et al. 2020). In this regard, Gaimardia trapesina appears as an emblematic species in sub-Antarctic waters, because it was the first species in the area in which the dispersion by rafting was documented: Helmuth et al. (1994) provided evidence of specimens of G. trapesina dispersing on Macrocystis rafts from South America to South Georgia (about 1500 km away). Considering that kelp rafts are commonly found in the Southern Ocean (Schiel and Foster 2015) this transport could potentially work for other (more distant) areas too, as in fact it was suggested by some authors (e.g., Dell 1964; Castilla and Guiñez 2000), although this hypothesis was never formally proved.
The aim of this study is to examine if two geographically distant areas of the sub-Antarctic region, the Southern tip of South America (SSA) and the Prince Edwards Islands (PEI), actually share some faunistic elements. For that, members of two genera of bivalves (Gaimardia and Hiatella) with different modes of life and reproduction are selected as particular case studies.
Materials and methods
Studied areas
The SSA encompasses the Atlantic and Pacific coasts of the American continent. The term is here restricted to refer to the waters south of 47°S, including Isla de los Estados and Burdwood Bank. SSA corresponds to an old geological area, originated from the Gondwana break out (Faure and Mensing 2010). The Pacific coast of SSA is under the influence of the West Wind Drift, a subantarctic water flow that contacts the Chilean coast at about 48°S (Camus 2001; Thiel et al. 2007). This gives origin to the Cape Horn Current, which flows towards the south along the Pacific coast, surrounding the southern tip of South America, heading eastwards towards the Atlantic Ocean. The Cape Horn Current gives origin to the Patagonian shelf waters and the Malvinas/Falkland Current, which flow northwards parallel to the Argentine coast. The Malvinas/Falkland Current deflects eastwards off the continental slope at about 40–45°S (Stramma and England 1999).
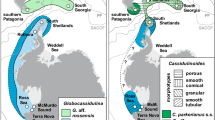
The PEI is a relatively young volcanic archipelago, comprising Marion Island (originated about 0.45 million years ago) and Prince Edward Island (originated about 0.2 million years ago) (McDougall et al. 2001; Chown et al. 2008). This archipelago is located in the Indian sector of the Southern Ocean (Fig. 1).
a–c Study area (a) and sampling sites of Gaimardia (circles) and Hiatella (triangles), at (b) the Southern tip of South America (SSA) and (c) the Prince Edward Islands (PEI: light blue). ACC Antarctic Circumpolar Current; BB Burdwood Bank (purple); BC Beagle Channel (orange); CF Chilean Fjords (red); IE Isla de los Estados (yellow); PD Puerto Deseado (green); SAF sub-Antarctic Front; SACC Southern ACC Front; TF Atlantic coast of Tierra del Fuego (light orange)
Sample collection
Studied specimens of Gaimardia were collected by hand from fronds of the giant kelp Macrocystis pyrifera attached to the substrate in the shallow subtidal. In SSA, samples were taken in 2011 and 2012, in the Chilean Fjords (CF) (Pacific coast), in the Beagle Channel (BC), in Isla de los Estados (IE) and Puerto Deseado (PD) (Atlantic coast) and in PEI, in 2016 and 2017 (Fig. 1).
Studied specimens of Hiatella were sorted from sediment dredged from 82 to 415 m depth, in SSA at Tierra del Fuego (TF) and Burdwood Bank (BB) and in PEI, during expeditions of BO Puerto Deseado, GC-189 Prefecto García and RV SA Agulhas II in 2016 and 2017 (Fig. 1). On BO Puerto Deseado and GC-189 Prefecto García a dredge of 4 mm2 mesh-size net of 20 × 60×60 cm, was used, while on RV SA Agulhas II a dredge of 30 × 100×100 cm and a mesh size of 1 cm2 was used.
All samples were preserved in 100% ethanol until further processing. Voucher specimens were deposited at the Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Buenos Aires, Argentina.
Due to the impossibility to confirm a-priori the conspecificity of the studied specimens of Gaimardia and Hiatella from SSA and PEI, we refer to these materials as “Gaimardia” and “Hiatella”, in the Results section.
Genetic studies
Total DNA was extracted from adductor muscles using a CTAB/Proteinase K method (Dayrat et al. 2011). For specimens of Gaimardia, fractions of the mitochondrial Cytochrome Oxidase subunit I (COI), mitochondrial ribosomal gene 16S, and the nuclear Internal Transcribed Spacer region (ITS1-5.8S-ITS2, from now on “ITS”) were amplified, using the universal primers LCO/HCO (Folmer et al. 1994), 16Sar/16Sbr (Palumbi 1996) and ITS5/ITS4 (White et al. 1990), respectively. For specimens of Hiatella, a COI fraction was amplified using the HiaHd/HiaHr primers (Laakkonen et al. 2015). Amplifications were performed by routine polymerase chain reaction (PCR).
PCR products were visualized in 2% agarose gels, cleaned-up with enzymes (EXO I-FastAP, Thermo Scientific®) and sent out to Macrogen, Inc. (Korea) for sequencing on both ways. Sequences were trimmed and refined with chromatograms guidance prior to assemblage, and checked manually. MEGA version 10.0.5 (Kumar et al. 2018) was used for aligning sequences using Clustal W with default settings, and to calculate uncorrected p-distances (from now on “p-dist”). DnaSP version 5 (Librado and Rozas 2009) was used to calculate haplotype diversity (H) and nucleotide diversity (π).
Information on voucher specimens, sampled localities and GenBank accession numbers, are provided in (Table 1).
Phylogenetic reconstructions of sequences of Gaimardia were performed for each marker individually, and combining the three in a concatenated analysis using a subset of 11 specimens (Table 1). A 16S sequence of Gaimardia trapesina from Tierra del Fuego, Argentina, available from GenBank (KX713220.1) was included in the analysis, as well as COI and 16S sequences of Cyamiomactra laminifera (KC429131, KC429293.1), another Cyamioidea, here used as outgroup. ITS fragments are not currently available in GenBank for any other Cyamioidea, and therefore, the phylogenetic reconstruction performed with this marker could not be rooted.
For the phylogenetic reconstruction of specimens of Hiatella, we included the sequences obtained by Laakkonen et al. (2015); (Hiatella A-M: GeneBank accession numbers KP767805–KP761044). Sequences of Panopea generosa (KJ125418.1) and Panopea globosa (KJ125413.1) were used as outgroups.
The best evolutionary models were selected with AIC criterion in jModeltest version 2.1.10 (Darriba et al. 2012). Maximum Likelihood (ML) reconstructions were performed in MEGA, with node support evaluated through 1000 bootstrap replicates. Bayesian inference (BI) analyses were performed in MrBayes version 3.2 (Ronquist et al. 2012), with four simultaneous runs of 100 generations each and a sample frequency of 100, until average standard deviation of split frequencies reached ≤ 0.001; phylogenetic trees were summarized with a 10% burn-in value. Haplotype networks were built using a median-joining method (Bandelt et al. 1999) in Network version 5.0.1.0 (available at https://www.fluxus-engineering.com/sharenet.htm).
Results
The case Gaimardia
In total, 23 specimens of Gaimardia provided genetic information for analyses: 12 for 16S, 21 for COI, and nine for ITS. Of these, eight specimens successfully amplified for all gene loci, enabling the concatenated analysis.
The alignment of the twelve 16S sequences (Table 1) had a length of 481 bp and resulted in only two variable sites, corresponding to singletons. The phylogenetic reconstructions confirmed the monophyly of specimens of Gaimardia from SSA and PEI altogether, with high support both in the ML and BI analyses. Within the Gaimardia clade, the 16S did not resolve any grouping.
The alignment of the nine sequences of ITS (Table 1) was 1361 bp long, with nine variable sites and six singletons. The phylogenetic reconstructions based on ITS did not resolve any grouping within the Gaimardia clade.
The alignment of the 21 COI sequences (Table 1) had a length of 529 bp with 21 variable sites (4%), with a total of 15 singletons. Low levels of genetic divergence were registered for COI among the considered sequences (p-dist = 0–1.7%) (Table 2). These sequences of Gaimardia showed high levels of differentiation with Cyamiomactra laminifera (p-dist = 16.8%). The phylogenetic reconstructions confirmed the monophyly of specimens of Gaimardia from SSA and PEI altogether, with high support both in the ML and BI analyses. Within the Gaimardia clade, the COI marker consistently recovered a subclade, which encompasses all the specimens from BC, IE and CF, with high Bayesian posterior probability (0.98) but moderate bootstrap support in ML (71%). COI showed relatively low genetic differentiation among the five studied localities (average p-dist = 0.6%).These differences were low among different localities of SSA, and somewhat greater between PEI and each locality at SSA or SSA as a whole (Table 2). The p-dist between PEI and CF, BC, or IE were higher than the respective within-site distances. However, the distances between PEI and PD were lower than the average distance among specimens from PEI (Table 2).
The concatenated alignment of 16S, COI and ITS revealed a distinctive grouping within the Gaimardia clade. In this case the BC + IE + CF specimens also formed a subclade, with 80% bootstrap support and 0.97 posterior probability (Fig. 2a). In addition, in the concatenated analysis by BI, a second subclade, comprising the specimens from PD, was recovered with moderately good posterior probability (0.86).
Case Gaimardia. a Tree obtained by Bayesian Inference for COI, ITS and 16S concatenated markers (GTR + G model). Posterior probabilities > 95% are indicated with “*”; maximum likelihood bootstrap support > 80 are indicated with “°”. b, c Median-joining haplotype networks of: (b) COI and (c) ITS. Numbers on branches correspond to number of mutated sites; size of circles is proportional to the number of specimens per haplotype, according to the scale provided. Specimen codes (“G1”– “G23”) correspond to those listed in (Table 1). For colour codes see (Fig. 1)
Among the 21 COI sequences, 15 different haplotypes were recognized, separated by a maximum of nine mutations. Standard diversity indices revealed high haplotype diversity and moderate nucleotide diversity (H = 0.95; π = 0.007), a fact also evidenced among sequences from BC (H = 0.90; π = 0.003) and PEI (H = 0.97; π = 0.008). Contrastingly, relatively low diversity was found among sequences from PD (H = 0.40; π = 0.0008). The reconstruction of the median-joining network showed a relatively short topology, with low-frequency haplotypes (Fig. 2b). All studied localities showed exclusive haplotypes, with the exception of IE, which shared the same haplotype of BC. The network revealed certain association of the haplotypes with the geographic origin of the specimens. BC, IE and CF were grouped by very similar haplotypes, and connected to the most frequent haplotype of PD by only two substitutional steps. PEI specimens showed the greatest number of haplotypes (eight), and were separated by the greatest number of steps among them (up to eight). These specimens from PEI resulted in two haplotype groups, connected independently to the PD main haplotype. The first group (“PEI I”) showed a star-like topology with its central haplotype separated from the main haplotype in PD by only two steps. The second group (“PEI II”) comprised two additional haplotypes (Fig. 2b). The haplotypes grouped in “PEI I” were similarly differentiated from BC + IE + CF than from “PEI II” (p-dist = 1.1% and 0.9%, respectively).
Among the nine ITS sequences, seven distinct haplotypes were found, most of them represented by a single sequence, resulting in high haplotype diversity and low nucleotide diversity for this marker (H = 0.92; π = 0.002). The ITS haplotype network revealed no geographic structure. In fact, one shared haplotype was found in BC, IE and PEI (Fig. 2c).
When comparing the COI and ITS haplotype networks, no concordant results were found in the position of the specimens. The PD specimens that shared one central haplotype in the COI network were distinct in ITS. Likewise, the PEI specimen that shared a common haplotype with BC and IE in the ITS network, was distant in COI.
The case Hiatella
In total, 17 specimens of Hiatella provided genetic information for analyses. The COI alignment (187 sequences, 796 bp) was highly-conserved among the new sequenced specimens from SSA and PEI, with only three variable sites. However, the inclusion of the sequences of the lineages of Hiatella reported by Laakkonen et al. (2015) resulted in a total of 279 variable sites (35%). High levels of genetic divergence were found between the newly obtained sequences and any other previously known molecular species (p-dist > 19.1%; Table 3), in contrast to the minimum divergence among the material studied herein (p-dist = 0.06%; Table 3), and the divergence between the specimens from SSA and PEI (p-dist = 0.06%). The material here studied was closer to Laakkonen et al.’s (2015) molecular species from the southern hemisphere (i.e., Hiatella A: from the Magellan Strait, and Hiatella B: from New Zealand) than any other lineage from the northern hemisphere (Table 3).
The phylogenetic reconstructions revealed the monophyly of the studied material, with high bootstrap support and bayesian probability (98% and 1.00, respectively) (Fig. 3a). No subclades were recovered among these sequences, which showed a basal position to the previously known Hiatella species, although lacking adequate support (Fig. 3a).
Case Hiatella. Tree obtained by Bayesian Inference for COI (TIM3 + I + G model). Posterior probabilities > 95% are indicated with “*”; maximum likelihood bootstrap support > 80 are indicated with “°”. Terminals “A” to “M” correspond to Laakkonen et al.’s (2015) molecular species (GenBank accession numbers KP767805 to KP761044); terminals grouped in “O” correspond to newly sequenced specimens. b Median-joining haplotype network for COI. Numbers on branches correspond to the number of mutated sites; size of circles is proportional to the number of specimens per haplotype, according to the scale provided. Specimen codes (“H1”–“H17”) correspond to those listed in (Table 1). For colour codes see (Fig. 1)
Only four haplotypes were recognized in the sequences obtained as part of this study (Fig. 3b): one, the dominant, being present in all sampled localities (TF + IE + BB + PEI), two haplotypes exclusive from BB, and the remaining exclusive from PEI. The last three haplotypes appear separated by only one or two mutational steps from the main haplotype.
Discussion
In recent years, several studies have analyzed the connectivity and origin of the marine fauna from different sub-Antarctic archipelagos (e.g., Waters 2008; Cumming et al. 2014; Moon et al. 2017; González-Wevar et al. 2018; and references therein), although few of these studies have considered the fauna of PEI. González-Wevar et al. (2014) provided genetic evidence of the occurrence at PEI of a gastropod species (Nacella delesserti) that is not present in SSA or any other area in the Southern Ocean (where other species of Nacella are present). This fact would suggest certain degree of faunistic isolation of PEI. On the contrary, the bivalves considered in the present study show extremely low genetic differentiation, together with the presence of shared haplotypes between PEI and SSA, revealing that the same species of Gaimardia and Hiatella are present in these two areas. These results are similar to those reported for the bivalve Limatula pygmaea (Page and Linse 2002), and the isopods Septemserolis septemcarinata and Limnoria stephenseni (Leese et al. 2010; Nikula et al. 2010); suggesting that this wide distributional pattern is not unusual, and that it occurs in species with different modes of life and reproduction. The low genetic differentiation here found between specimens from SSA and PEI does not seem to be originated in the used molecular markers. In fact, two of the fragments here used proved to be successful in distinguishing other cyamioidean and hiatelloidean species: the 16S sequences obtained by Zelaya et al. (2019), resulted in p-dist from 15 to 18.8% among species of Cyamiocardium; similarly, the COI marker shows high divergence of lineages in Hiatella (11.2–20.2%), according to Laakkonen et al. (2015). A taxonomical revision of the species of Gaimardia occurring in the Southern Ocean (Güller and Zelaya, in prep) allows us to confirm that the taxon involved in this study is G. trapesina. The species of Hiatella cannot be determined with confidence, as none of the nominal species of this genus described or reported from the Southern Ocean (and even worldwide) is properly defined. Despite that, the species involved in the present study does not correspond to any of the molecular species, previously recognized by Laakkonen et al. (2015) or Layton et al. (2016), either from the southern or the northern hemisphere, revealing that the molecular diversity of Hiatella is even greater than previously known. For the purpose of the subsequent discussion, the taxon involved in this study is referred as Hiatella O.
The little genetic differentiation found in this study for ITS sequences (fast-evolving nuclear loci), supports the hypothesis of relatively recent events of colonization of PEI by Gaimardia trapesina. In addition, the presence of two different groups of haplotypes of G. trapesina at PEI, which diverge from SSA’s main haplotype (when considering COI marker) is compatible with the occurrence of more than one event of colonization of this archipelago. This is in agreement with the findings by Page and Linse (2002) for Limatula pygmaea (in that case, based on ITS-1 sequences).
How can species occur in geographically distant areas?
The occurrence of a same species in two geographically distant areas is usually explained as a consequence of a common history of these areas (i.e., geological or tectonic connections), anthropogenic transport or natural dispersive events (Castilla and Guiñez 2000). The different geologic histories of SSA and PEI argue against the first of those alternatives to explain the co-occurrence of these taxa in these two areas. Anthropogenic transport does not seem to have major significance for explaining the occurrence of these two taxa at PEI either, as both Gaimardia trapesina and Hiatella O are relatively small-sized species, which do not represent human food-source or are involved in aquaculture activities. On the other hand, the PEI historically have had only occasional ships traffic, due to the distant location of the islands from usual sea routes (Cooper 2008; Leese et al. 2010); and studies on the sea-chests fauna of the few ships visiting this archipelago (Lee and Chown 2007) did not report these species.
On the contrary, some biological and ecological aspects of the two species considered in this study support the hypothesis that natural dispersive events have been the main responsible drivers for their current pattern of distribution. Gaimardia trapesina exclusively live on the giant kelp Macrocystis pyrifera (Dayton 1985a; Adami and Gordillo 1999; Puccinelli et al. 2018), on which all the specimens here studied, either from SSA and PEI, were collected. Specimens of Hiatella O can also be occasionally found on M. pyrifera, where early juveniles are usually present on the fronds and larger specimens on the holdfasts (Zelaya, pers obs). The present study takes Helmuth et al.’s (1994) findings even further, revealing that specimens of G. trapesina may be dispersed in this way, over much greater distances, thus occurring in SSA and PEI (6500 km away). Furthermore, members of the genus Hiatella have been also found living on the bull kelp Durvillaea antarctica (López et al. 2018), which according to Smith (2002) is likely to be more important for dispersal over larger spatial scales than M. pyrifera in the Southern Ocean. Therefore, rafting on macroalgae represents the most probable way in which G. trapesina (and perhaps also Hiatella O) has reached the PEI.
The dispersion of Hiatella O does not merely restrict to the rafting on macroalgae. Members of this genus have been also reported associated to pumice (Velasquez et al. 2018). Considering that pumice is frequently found in several sub-Antarctic localities (Risso et al. 2002; Bryan et al. 2012), and that pumice from South Sandwich Islands have been recovered in quite distant localities, such as New Zealand (Coombs and Landis 1966), this alternative of dispersion cannot be discarded. In fact, rafting on pumice was regarded as the most probable way of long-distance dispersal in Ostrea chilensis (Ó Foighil et al. 1999). In addition to rafting, another way of dispersion for Hiatella O arises from the free-living larval stage present in members of this genus (Gordillo 2001; Greene and Grizzle 2007). In conclusion, the natural dispersion of larvae, juveniles or adults appears as the most probable alternative to explain the presence of Gaimardia trapesina and Hiatella O in PEI, a phenomenon which, in any way, must have been facilitated by the ACC. This current is regarded as the strongest marine current of the world (Olbers et al. 2004), with an average speed of about 40 cm.s−1 and jet surface speeds reaching 0.6 m.s−1 (Whitworth et al. 1982; Meredith et al. 2011). The simulations of particle movements in the Southern Ocean performed by Fraser et al. (2018) provide evidence in favour of regarding the ACC as a potential way allowing the dispersion from SSA to PEI. But even if assuming that the ACC keeps its maximum speed, covering the distance between SSA and PEI (6500 km) would take at least 125 days, which represent more than the estimated floating periods for rafts of Macrocystis pyrifera (100 days, according to Helmuth et al. 1994; Hu and Fraser 2016). Additionally, species of Hiatella have a shorter larval phase (of about 30–60 days, fide López et al. (2018)). However, rare meteorological and extreme oceanographic conditions can reduce the time required for such large-scale dispersive events (Gillespie et al. 2011). An alternative explanation to such successful dispersion arises in the fact that both G. trapesina and Hiatella have been reported in intermediate localities between SSA and PEI, such as the Malvinas/Falkland Islands and South Georgia; and Hiatella also at Gough Island (Dell 1964). In all these archipelagos, M. pyrifera and D. antarctica are also present (Fraser et al. 2009; Macaya and Zuccarello 2010a, b; Caselle et al. 2018). In addition, small-sized specimens of Hiatella have been found in some intermediate localities between SSA and PEI. Thus, the intermediate archipelagos between SSA and PEI could have acted/be acting as stepping-stones for the colonisations of PEI by specimens of Gaimardia trapesina and Hiatella O, which it is compatible with the route of colonization of the different sub-Antarctic islands proposed by Fraser et al. (2012).
Future perspectives
Several issues, to be addressed in the future, arise from this study:
-
1.
Are recurrent events of colonization of PEI coming from a single source or multiple sources? Some of the results obtained for Gaimardia trapesina in this study, as well as previous findings from Page and Linse (2002) on Limatula pygmaea, suggest the presence of different lineages of these species at PEI. Such pattern may be interpreted as a consequence of different events of colonization from a single source or, alternatively, as a consequence of specimens from different localities of the Southern Ocean arriving to PEI. Incorporating specimens from intermediate areas (i.e., Malvinas/Falkland Islands, South Georgia, South Sandwich Islands, Gough Island) could help to evaluate the connectivity of PEI with each of these areas, and the potential multiple origins of the marine invertebrate fauna present today at PEI.
-
2.
How frequent is the circum-sub-Antarctic pattern here reported? The contrasting results between species shared between PEI and SSA (e.g., Gaimardia trapesina, Limatula pygmaea and Hiatella O) vs. endemic species at PEI (Nacella delesserti), reveal the necessity of carefully revising each species previously considered as circum-sub-Antarctic based exclusively on their morphological similarity.
-
3.
What is the proper name for Hiatella O? To solve this issue, a systematic revision of the numerous nominal species of Hiatella reported for the southern hemisphere is crucial. Such revision should encompass topotypic specimens and consider genetic information, particularly taking into account the plasticity exhibited by members of the genus Hiatella in shell morphology (Beu 1971; Lezin and Flyachinskaya 2015).
References
Adami M, Gordillo S (1999) Structure and dynamics of the biota associated with Macrocystis pyrifera (Phaeophyta) from the Beagle Channel Tierra del Fuego. Sci Mar. 63:183–191
Allcock AL, Barratt I, Eléaume M, Linse K, Norman MD, Smith PJ, Steinke D, Stevens DW, Strugnell JM (2011) Cryptic speciation and the circumpolarity debate: a case study on endemic Southern Ocean octopuses using the COI barcode of life. Deep-Sea Res II 58:242–249. https://doi.org/10.1016/j.dsr2.2010.05.016
Avila C, Angulo-Preckler C, Martín-Martín RP, Figuerola B, Griffiths HJ, Waller CL (2020) Invasive marine species discovered on non–native kelp rafts in the warmest Antarctic island. Sci Rep 10:1639. https://doi.org/10.1038/s41598-020-58561-y
Barker PF, Filippelli GM, Florindo F, Martin EE, Scher HD (2007) Onset and role of the Antarctic Circumpolar Current. Deep Sea Res II 54:2388–2398. https://doi.org/10.1016/j.dsr2.2007.07.028
Bandelt HJ, Forster P, Röhl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48. https://doi.org/10.1093/oxfordjournals.molbev.a026036
Barrales H, Lobban CS (1975) The comparative ecology of Macrocystis pyrifera, with emphasis in the forest of Chubut, Argentina. J Ecol 63:657–677. https://doi.org/10.2307/2258743
Beu AG (1971) New light on the variation and taxonomy of the bivalve Hiatella. New Zeal J Geol Geop 14:64–66. https://doi.org/10.1080/00288306.1971.10422459
Beu AG, Griffin M, Maxwell PA (1997) Opening of the drake passage gateway and late miocene to pleistocene cooling reflected in southern ocean molluscan dispersal: evidence from New Zealand and Argentina. Tectonophysics 281:83–97. https://doi.org/10.1016/S0040-1951(97)00160-1
Bryan SE, Cook AG, Evans JP, Hebden K, Hurrey L, Colls P, Jell JS, Weatherley D, Firn J (2012) Rapid, long-distance dispersal by pumice rafting. PLoS ONE 7:e40583. https://doi.org/10.1371/journal.pone.0040583
Camus PA (2001) Biogeografía marina de Chile continental. Rev Chil Hist Nat 74:587–617. https://doi.org/10.4067/S0716-078X2001000300008
Caselle JE, Hamilton SL, Davis K, Thompson CDH, Turchik A, Jenkinson R, Simpson D, Sala E (2018) First quantification of subtidal community structure at Tristan da Cunha Islands in the remote South Atlantic: from kelp forests to the deep sea. PLoS ONE 13:e019516. https://doi.org/10.1371/journal.pone.0195167
Castilla JC, Guiñez R (2000) Disjoint geographical distribution of intertidal and nearshore benthic invertebrates in the Southern Hemisphere. Rev Chil Hist Nat 73:585–603. https://doi.org/10.4067/S0716-078X2000000400004
Chaparro OR, Schmidt AJ, Pardo LM, Andrade PV, Wagner CE, Cubillos VM (2011) Reproductive strategy of the semelparous clam Gaimardia bahamondei (Bivalvia, Gaimardiidae). Invertebr Biol 130:49–59. https://doi.org/10.1111/j.1744-7410.2010.00218.x
Chown SL, Lee JE, Shaw JD (2008) Conservation of Southern Ocean islands: invertebrates as exemplars. J Insect Conserv 12:277–291. https://doi.org/10.1007/s10841-008-9151-8
Clarke A, Griffiths HJ, Linse K, Barnes DKA, Crame JA (2007) How well do we know the Antarctic marine fauna? A preliminary study of macroecological and biogeographical patterns in Southern Ocean gastropod and bivalve molluscs. Divers Distrib 13:620–632. https://doi.org/10.1111/j.1472-4642.2007.00380.x
Coombs DS, Landis CA (1966) Pumice from South Sandwich eruption of March 1962 reaches New Zealand. Nature 209:289–290
Cooper J (2008) Human history. In: Chown SL, Froneman PW (eds) The Prince Edward Islands, Land-sea interactions in a changing ecosystem. Sun Press, Stellenbosch, pp 331–350
Cumming RA, Nikula R, Spencer HG, Waters JM (2014) Transoceanic genetic similarities of kelp-associated sea slug populations: long-distance dispersal via rafting? J Biogeogr 41:2357–2370. https://doi.org/10.1111/jbi.12376
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772. https://doi.org/10.1038/nmeth.2109
Dayrat B, Conrad M, Balayan S, White TR, Albrecht C, Golding R, Gomes SR, Harasewych MG, de Frias Martins AM (2011) Phylogenetic relationships and evolution of pulmonate gastropods (Mollusca): New insights from increased taxon sampling. Mol Phylogenet Evol 59:425–437. https://doi.org/10.1016/j.ympev.2011.02.014
Dayton PK (1985a) The structure and regulation of some South American kelp communities. Ecol Monogr 55:447–468. https://doi.org/10.2307/2937131
Dayton PK (1985b) Ecology of kelp communities. Annu Rev Ecol Systemat 16:215–245. https://doi.org/10.1146/annurev.es.16.110185.001243
Dell RK (1964) Antarctic and subantarctic Mollusca: Amphineura, Scaphopoda and Bivalvia. Discover Rep 33:93–250
Dell RK (1972) Antarctic benthos. Adv Mar Biol 10:1–216
Díaz P, Campos B (2014) Ontogenia de la concha larval y postlarval de cuatro especies de bivalvos de la costa del Pacífico sureste. Rev Biol Mar Oceanog 49:175–191. https://doi.org/10.4067/S0718-19572014000200002
Duggins D, Eckman JE, Siddon CE, Klinger T (2001) Interactive roles of mesograzers and current flow in survival of kelps. Mar Ecol Prog Ser 223:143–155. https://doi.org/10.3354/meps223143
Faure G, Mensing TM (2010) Break-up of Gondwana and assembly of Antarctica. In: Faure G, Mensing TM (eds) The transantarctic mountains: Rocks, ice, meteorites and water. Springer, Dordrecht, pp 491–515
Fell HB (1962) West-Wind-Drift dispersal of echinoderms in the Southern hemisphere. Nature 193:759–761. https://doi.org/10.1038/193759a0
Foighil ÓD, Marshall BA, Hilbish TJ, Pino MA (1999) Trans-Pacific range extension by rafting is inferred for the flat oyster Ostrea chilensis. Biol Bull 196:122–126
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial Cytochrome C Oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Fraser CI, Nikula R, Spencer HG, Waters JM (2009) Kelp genes reveal effects of subantarctic sea ice during the Last Glacial Maximum. P Natl Acad Sci USA 106:3249–3253. https://doi.org/10.1073/pnas.0810635106
Fraser CI, Nikula R, Waters JM (2010) Oceanic rafting by a coastal community. Proc R Soc B 278:649–655. https://doi.org/10.1098/rspb.2010.1117
Fraser CI, Nikula R, Ruzzante DE, Waters JM (2012) Poleward bound: biological impacts of Southern Hemisphere glaciation. Trends Ecol Evol 27:462–471. https://doi.org/10.1016/j.tree.2012.04.011
Fraser CI, Morrison AK, McC Hogg A, Macaya EC, van Sebille E, Ryan PG, Padovan A, Jack C, Valdivia N, Waters JM (2018) Antarctica’s ecological isolation will be broken by storm-driven dispersal and warming. Nat Clim Change 8:704–708. https://doi.org/10.1038/s41558-018-0209-7
Gillespie RG, Waters JM, Fraser CI, Nikula R, Baldwin BG, Roderick GK (2011) Long-distance dispersal - a framework for hypothesis testing. Trends Ecol Evol 27:47–55. https://doi.org/10.1016/j.tree.2011.08.009
González-Wevar CA, Chown SL, Morley S, Coria N, Saucéde T, Poulin E (2014) Out of Antarctica: quaternary colonization of sub-Antarctic Marion Island by the limpet genus Nacella Patellogastropoda: Nacellidae). Polar Biol 39:77–89. https://doi.org/10.1007/s00300-014-1620-9
González-Wevar CA, Segovia NI, Rosenfeld S, Ojeda J, Hüne M, Naretto J, Saucède T, Brickle P, Morley S, Féral J-P, Spencer HG, Poulin E (2018) Unexpected absence of island endemics: long-distance dispersal in higher latitude sub-Antarctic Siphonaria (Gastropoda: Euthyneura) species. J Biogeogr 45:874–884. https://doi.org/10.1111/jbi.13174
Gordillo S (2001) Puzzling distribution of the fossil and living genus Hiatella (Bivalvia). Palaeogeogr, Palaeoclimatol, Palaeoecol 165:231–249. https://doi.org/10.1016/S0031-0182(00)00162-0
Greene JK, Grizzle RE (2007) Successional development of fouling communities on open ocean aquaculture fish cages in the western Gulf of Maine, USA. Aquaculture 262:289–301. https://doi.org/10.1016/j.aquaculture.2006.11.003
Helmuth B, Veit RR, Holberton R (1994) Long-distance dispersal of a subantarctic brooding bivalve (Gaimardia trapesina) by kelp-rafting. Mar Biol 120:421–426. https://doi.org/10.1007/BF00680216
Hu ZM, Fraser C (2016) Seaweed phylogeography. Adaptation and evolution of seaweeds under environmental change, Springer, Dordrecht, Netherlands
Hunter RL, Halanych KM (2008) Evaluating connectivity in the brooding brittle star Astrotoma agassizii across the Drake Passage in the Southern Ocean. J Hered 99:137–148. https://doi.org/10.1093/jhered/esm119
Ituarte C (2009) Unusual modes of oogenesis and brooding in bivalves: the case of Gaimardia trapesina (Mollusca: Gaimardiidae). Invertebr Biol 128:243–251. https://doi.org/10.1111/j.1744-7410.2009.00171.x
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Laakkonen HM, Strelkov P, Väinölä R (2015) Molecular lineage diversity and inter-oceanic biogeographical history in Hiatella (Mollusca, Bivalvia). Zool Scr 44:383–402. https://doi.org/10.1111/zsc.12105
Layton KKS, Martel AL, Hebert DN (2016) Geographic patterns of genetic diversity in two species complexes of Canadian marine bivalves. J Moll Stud 82:282–291. https://doi.org/10.1093/mollus/eyv056
Lee JE, Chown SL (2007) Mytilus on the move: transport of an invasive bivalve to the Antarctic. Mar Ecol Progr Ser 339:307–310. https://doi.org/10.3354/meps339307
Leese F, Agrawal S, Held C (2010) Long-distance island hopping without dispersal stages: transportation across major zoogeographic barriers in a Southern Ocean isopod. Sci Nat 97:583–594. https://doi.org/10.1007/s00114-010-0674-y
Lezin P, Flyachinskaya L (2015) Shell sculpture formation in bivalves of the genus Hiatella under different temperature conditions. J Mar Biol Assoc UK 95:1621–1627. https://doi.org/10.1017/S0025315415000259
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452. https://doi.org/10.1093/bioinformatics/btp187
López BA, Macaya EC, Rivadeneira MM, Tala F, Tellier F, Thiel M (2018) Epibiont communities on stranded kelp rafts of Durvillaea antarctica (Fucales, Phaeophyceae) – Do positive interactions facilitate range extensions? J Biogeogr 45:1833–1845. https://doi.org/10.1111/jbi.13375
Lyle M, Gibbs S, Moore TC, Rea DK (2007) Late Oligocene initiation of the Antarctic circumpolar current: evidence from the South Pacific. Geology 35:691–694. https://doi.org/10.1130/G23806A.1
Macaya EC, Zuccarello GC (2010a) DNA barcoding and genetic divergence in the giant kelp Macrocystis (Laminariales). J Phycol 46:736–742. https://doi.org/10.1111/j.1529-8817.2010.00845.x
Macaya EC, Zuccarello GC (2010b) Genetic structure of the giant kelp Macrocystis pyrifera along the southeastern Pacific. Mar Ecol Prog Ser 420:103–112. https://doi.org/10.3354/meps08893
McDougall I, Verwoerd W, Chevallier L (2001) K-Ar geochronology of Marion Island, Southern Ocean. Geol Mag 1:1–17. https://doi.org/10.1017/S0016756801005039
Meredith MP, Woodworth PL, Chereskin TK, Marshall DP, Allison LC, Bigg GR, Donohue K, Heywood KJ, Hughes CW, Hibbert A, Mc Hogg A, Johnson HL, Jullion L, King BA, Leach H, Lenn Y-D, Morales Maqueda MA, Munday DR, Naveira Garabato AC, Provost C, Sallée J-B, Sprintall J (2011) Sustained monitoring of the Southern Ocean at Drake Passage past achievements and future priorities. Rev Geophys 49:4005. https://doi.org/10.1029/2010RG000348
MolluscaBase eds (2020) World register of Marine Species. https://www.marinespecies.org/ (Access 27 Jan 2020)
Moon KL, Chown SL, Fraser CI (2017) Reconsidering connectivity in the sub-Antarctic. Biol Rev 92:2164–2181. https://doi.org/10.1111/brv.12327
Nikula R, Fraser C, Spencer H, Waters J (2010) Circumpolar dispersal by rafting in two subantarctic kelp-dwelling crustaceans. Mar Ecol Prog Ser 405:221–230. https://doi.org/10.3354/meps08523
Olbers D, Borowski D, Völker C, Wölff JO (2004) The dynamical balance, transport and circulation of the Antarctic Circumpolar Current. Antarct Sci 16:439–470. https://doi.org/10.1017/S0954102004002251
Orsi AH, Whitworth TIII, Nowlin WD Jr (1995) On the meridional extent and fronts of the Antarctic Circumpolar Current. Deep-Sea Res I 42:641–673. https://doi.org/10.1016/0967-0637(95)00021-W
Palumbi SR (1996) Nucleic acids II: the polymerase chain reaction. In: Hillis DM, Moritz C, Mable BK (eds) Molecular systematics. Sinauer Associates, Sunderland, pp 205–247
Page TJ, Linse K (2002) More evidence of speciation and dispersal across the Antarctic Polar Front through molecular systematics of Southern Ocean Limatula (Bivalvia: Limidae). Polar Biol 25:818–826. https://doi.org/10.1007/s00300-002-0414-7
Patarnello T, Bargelloni L, Varotto V, Battaglia B (1996) Krill evolution and the Antarctic ocean currents: evidence of vicariant speciation as inferred by molecular data. Mar Biol 126:603–608. https://doi.org/10.1007/BF00351327
Puccinelli E, von der Meden CEO, McQuaid CD, Ansorge IJ (2018) Biological characteristics of the rafting bivalve Gaimardia trapesina in the Southern Ocean. Mar Biol 165:172. https://doi.org/10.1007/s00227-018-3430-z
Risso C, Scasso RA, Aparicio A (2002) Presence of large pumice blocks on Tierra del Fuego and South Shetland Islands shorelines, from 1962 South Sandwich Islands eruption. Mar Geol 186:413–422. https://doi.org/10.1016/S0025-3227(02)00190-1
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. https://doi.org/10.1093/sysbio/sys029
Santelices B, Ojeda FP (1984) Population dynamics of coastal forests of Macrocystis pyrifera in Puerto Toro, Isla Navarino, Southern Chile. Mar Ecol Prog Ser 14:175–183
Schejter L, Bremec C, Waloszek D, Escolar M (2010) Recently settled stages and larval developmental mode of the bivalves Zygochlamys patagonica and Hiatella meridionalis in the Argentine Sea. J Shellfish Res 29:63–67. https://doi.org/10.2983/035.029.0127
Schiel DR, Foster MS (2015) The biology and ecology of giant kelp forests. University of California Press, Oakland
Smith SDA (2002) Kelp rafts in the Southern Ocean. Global Ecol Biogeogr 11:67–69. https://doi.org/10.1046/j.1466-822X.2001.00259.x
Stramma L, England M (1999) On the water masses and mean circulation of the south Atlantic ocean. J Geophys Res 104:863–883. https://doi.org/10.1029/1999JC900139
Thiel M, Macaya EC, Acuña E, Arntz W, Bastias H, Brokordt K, Camus PA, Castilla JC, Castro LR, Cortes M, Dumont CP, Scribano R, Fernandez M, Gajardo JA, Gaimer CF, Gomez I, Gonzalez AE, Gonzalez HE, Haye PA, Illanes JE, Iriarte JL, Lancellotti DA, Luna Jorquera G, Luxoro C, Manriquez PH, Marin V, Muñoz P, Navarrete SA, Perez E, Poulin E, Sellanes J, Hito Sepulveda H, Stotz W, Tala F, Thomas A, Vargas CA, Vasquez JA, Alonso Vega JM (2007) The Humboldt current system of northern and central Chile. Oceanogr Mar Biol Ann Rev 45:195–344. https://doi.org/10.1201/9781420050943.ch6
Velasquez E, Bryan S, Ekins M, Cook AG, Hurrey L, Firn J (2018) Age and area predict patterns of species richness in pumice rafts contingent on climatic zone encountered. Ecol Evol 8:5034–5046. https://doi.org/10.1002/ece3.3980
Waters JM (2008) Driven by the West Wind Drift? A synthesis of southern temperate marine biogeography, with new directions for dispersalism. J Biogeogr 35:417–427. https://doi.org/10.1111/j.1365-2699.2007.01724.x
Waters JM, King TM, Fraser CI, Garden C (2018) Rafting dispersal in a brooding southern sea star (Asteroidea: Anasterias). Invertebr Syst 32:253–258. https://doi.org/10.1071/IS17037
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Michael DHG, Innis A, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–322
Whitworth T, Nowlin WD, Worley SJ (1982) The net transport of the Antarctic Circumpolar Current through Drake Passage. J Physiol Oceanogr 12:960–971. https://doi.org/10.1175/1520-0485(1982)012<0960:TNTOTA>2.0.CO;2
Wilson NG, Hunter RL, Lockhart SJ, Halanych KM (2007) Multiple lineages and absence of panmixia in the “circumpolar” crinoid Promachocrinus kerguelensis from the Atlantic sector of Antarctica. Mar Biol 152:895–904. https://doi.org/10.1007/s00227-007-0742-9
Zbawicka M, Gardner JPA, Wenne R (2019) Cryptic diversity in smooth-shelled mussels on Southern Ocean islands: Connectivity, hybridisation and a marine invasion. Front Zool 6:16–32. https://doi.org/10.1186/s12983-019-0332-y
Zelaya DG (2005) The bivalves from the Scotia Arc islands species richness and faunistic affinities. Sci Mar. 69:113–122. https://doi.org/10.3989/scimar.2005.69s2113
Zelaya DG, Güller M, Ituarte CI (2019) Filling a blank in bivalve taxonomy: an integrative analysis of Cyamioidea (Mollusca: Bivalvia). Zool J Linn Soc. https://doi.org/10.1093/zoolinnean/zlz144
Acknowledgements
This study was funded by the South African National Antarctic Program (SANAP) and the South African Department of Environmental Affairs and Tourism (grants 105539 and 110735 to Dr. Sarah Fawcett; grant 110719 to Prof. Isabelle Ansorge); and by Agencia Nacional de Promoción Científica y Tecnológica (PICT 2016-2983) and Universidad de Buenos Aires (UBACYT 20020150100195BA) to DZ. Part of the samples were obtained through funds of the Argentine National Law Nº 26875. Special thanks go to Captain Bengu and his officers and crew of the SA Agulhas II for their assistance at sea; and to the crews of the BO Puerto Deseado and GC-189 Prefecto García. Cristián Ituarte, Matías Urcola, María del Mar Eivers and the staff of Huinay Scientific Field Station kindly provided some of the specimens for this study. The authors are grateful to Alex Gottlieb, who provided advice on molecular studies. We thank B. M. and an anonymous reviewer who greatly improved the final version of the manuscript. MG and DZ are members of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). This work is the contribution Nº 39 of the MPA Namuncurá/Burdwood Bank.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the study conception, data collection, analysis and manuscript writing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for sampling, care and experimental use of organisms for this study have been followed and all necessary approvals have been obtained.
Availability of data and material
The datasets generated and analyzed during the current study are available in the GenBank repository. GenBank accession numbers are indicated in Table 1 accepted.
Additional information
Responsible Editor: A. Atkinson.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by undisclosed experts.
Rights and permissions
About this article
Cite this article
Güller, M., Puccinelli, E. & Zelaya, D.G. The Antarctic Circumpolar Current as a dispersive agent in the Southern Ocean: evidence from bivalves. Mar Biol 167, 143 (2020). https://doi.org/10.1007/s00227-020-03746-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-020-03746-2