Abstract
Elasmobranch fishes (sharks, skates, and rays) have been hypothesized to use the geomagnetic field (GMF) to maintain a sense of direction as they navigate throughout their environment. However, it is difficult to test the sensory ecology and spatial orientation ability of large highly migratory fishes in the field. Therefore, we performed behavioral conditioning experiments on a small magnetically sensitive species, the yellow stingray (Urobatis jamaicensis), in the laboratory. We trained individuals to use the polarity, or the north–south direction, of the GMF as a cue to orient in space and navigate a T-maze for a food reward. Subjects were split into two groups that learned to associate the direction of magnetic north or south as the indicator of the reward location. Stingrays reached the learning criterion within a mean (± SE) of 158.6 ± 28.4 trials. Subjects were then reverse trained to use the previously unrewarded magnetic stimulus of the opposite polarity as the new cue for the reward location. Overall, the stingrays reached the reversal criterion in significantly fewer trials (120 ± 13.8) compared to the initial procedure. These data show that the yellow stingray can learn to associate changes in GMF polarity with a reward, relearn a behavioral task when the reward contingency is modified, and learn a reversal procedure faster than the initial association. These data support the idea that the yellow stingray, and perhaps other elasmobranchs, might use GMF polarity as a cue to orient and maintain a heading during navigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Orientation is an integral part of animal navigation where an organism aligns itself with respect to an external cue (Berthold 2001; Gould 1998) to maintain a desired heading. However, calculating a heading requires that the animal know its current position relative to that of its goal so that it can determine the correct direction in which to travel (Gould 1998; 2004). The animal can then use an appropriate environmental cue, such as visual landmarks, localized sounds or odor gradients, the position of the sun, or the direction of the geomagnetic field (GMF) as an external point of reference to maintain the correct orientation. Animals can use different types of cues to form distinct cognitive compasses and employ them as needed when environmental stimuli cease to propagate and become unreliable (Able 1991; Gould 1998). The physical nature of a cue and how it behaves in a medium, such as seawater, will determine how effective that cue is for navigating over a given spatiotemporal scale. Cues that originate from localized sources tend to diminish rapidly with space and time, which makes them effective beacons or landmarks (Shettleworth and Sutton 2005; Cheng 2012) for relatively short distance navigation. Conversely, global cues such as celestial rotation or GMF polarity fluctuate very little over small spatiotemporal scales and are well suited for navigational tasks that can last several months and span thousands of kilometers.
The GMF has polarity, or a north–south directional component, because on the surface of the Earth it is emitted from the magnetic north pole located in the southern hemisphere and terminates at the magnetic south pole in the northern hemisphere. The GMF at any geographic location can be described by a vector with an overall intensity of 20–70 µT and an inclination angle (measured relative to the surface of the Earth) that ranges from + 90° to − 90°. These quantities change predictably with latitude in that the largest values define the magnetic poles and the smallest values define the magnetic equator. Therefore, magnetically sensitive species can potentially use the intensity and inclination angle of the GMF as global cues to actively determine their location. Experimental manipulation of the ambient GMF can simulate a large physical displacement and will induce migrating loggerhead sea turtle hatchlings (Caretta caretta (Lohmann 1991)), sockeye salmon fry (Oncorhynchus nerka (Putman et al. 2011, 2014)), and juvenile European eels (Anguilla anguilla (Naisbett et al. 2017)) to alter their swimming vectors and reorient towards their original goal. This behavior in juveniles implies that GMF-based location and wayfinding are innate to these species and are essential for naïve individuals embarking upon their first migration (Lohmann 1991; Naisbett et al. 2017; Putman et al. 2011, 2014).
Experimental evidence indicates that magnetically sensitive species can use either the inclination angle or the polarity of the GMF as a cue to orient and maintain a desired heading. The loggerhead sea turtle (Light et al. 1993) and European robin (Erithacus rubecula (Wiltschko and Wiltschko 1972)) use a magnetic inclination compass to discriminate how the vertical component of the GMF gradually shifts from + 90º at the north magnetic pole to − 90º at the south magnetic pole. Conversely, sockeye salmon fry (Quinn 1980; Quinn et al. 1981) and smolts (Quinn and Brannon 1982), chum salmon fry (O. keta (Quinn and Groot 1983)), the American eel (Anguilla rostrata (Souza et al. 1988), European eel (Tesch et al. 1992)), zebrafish (Danio rerio (Krylov et al. 2016, Myklatun et al. 2018; Osipova et al. 2016, Takebe et al. 2012)), medaka (Oryzias latipes (Myklatun et al. 2018)), and mole rat (Cryptomys hottentotus (Marhold et al. 1997)) will reorient themselves to maintain a desired heading after GMF is experimentally shifted in the horizontal plane. The direction of the horizontal GMF component can be used as a polarity-based compass that functions similarly to the way that ferromagnetic particles align with magnetic field lines and point toward the poles of a magnet. In elasmobranch fishes (sharks, skates, and rays), magnetic sensitivity has been demonstrated in the round stingray (Urobatis halleri (Kalmijn 1978)), sandbar shark (Carcharhinus plumbeus (Meyer et al. 2005; Anderson et al. 2017)), scalloped hammerhead shark (Sphyrna lewini (Meyer et al. 2005)), short-tailed stingray (Bathytoshia brevicaudata (Walker et al. 2003)), common stingray (Dasyatis pastinaca (Adrianov et al. 1974; Akoev et al. 1976; Brown and Ilyinsky 1978)), thornback ray (Raja clavata (Akoev et al. 1976; Brown and Ilyinsky 1978)), and yellow stingray (Urobatis jamaicensis (Newton and Kajiura 2017)). However, the only example of spontaneous orientation by an elasmobranch to a magnetic field was reported in captive leopard sharks (Triakis semifasciata (Kalmijn 1974)) that aligned to the north–south axis of the GMF.
Elasmobranchs are K-selected and iteroparous fishes, and many species exhibit a wide range of migratory behaviors and philopatric preferences to specific locations (reviewed in Hueter et al. 2005; Speed et al. 2010; Chapman et al. 2015; Flowers et al. 2016). Sharks and rays are amenable to various behavioral conditioning procedures including those that require spatial orientation, rapid learning, and accurate memory recall (reviewed in Schluessel 2015). As an elasmobranch gains experience performing spatial orientation tasks, it is likely that subsequent tasks with a similar structure will be learned faster, with improved memory recall and consistent expression of relevant behaviors. The spatial orientation and cognitive abilities of the grey bamboo shark, (Chiloscyllium griseum (Fuss et al. 2014a, b, c)) and freshwater ocellate stingray (Potamotrygon motoro (Schluessel and Bleckmann 2005; Schluessel et al. 2015; Schluessel and Ober 2018; Daniel and Schluessel 2019)) have been well characterized (Table 1); however, learning performance across subsequent tasks has not been extensively studied in elasmobranch fishes. Reversal learning is a type of operant conditioning where, once the initial learning criterion is reached, the reward contingency is reversed and the animal is retrained to respond to the previously unrewarded stimulus (Pavlov 1927). This technique was originally used to quantify intelligence or the ability of an animal to learn (Bitterman 1965), but now it is used to interpret how species make decisions (Shettleworth 2010), to compare the learning flexibility of species across different situations (Day et al. 1999), and to compare the cognitive abilities among species (Bond et al. 2007). In regard to spatial tasks, reversal learning can indicate how quickly a species recognizes and categorizes landmarks (e.g., Fuss et al. 2014c) or its behavioral flexibility in response to changing environmental cues.
The purpose of this study was to determine if the yellow stingray can detect and use changes in GMF polarity to solve a spatial orientation task. Elasmobranchs have been hypothesized to use the GMF as a navigational cue (Kalmijn 1974; Klimley 1993; Paulin 1995) and if so, they might be able to detect and use the polarity of the GMF to orient towards a goal. The yellow stingray is a magnetically sensitive elasmobranch that readily learns operant conditioning tasks and is small enough to use in spatial orientation experiments (Newton and Kajiura 2017). This benthic species is found in seagrass beds and nearshore reefs (Fahy 2004) and is distributed from North Carolina to Venezuela (Piercy et al. 2006). If the yellow stingray migrates over spatiotemporal scales similar to that of its congener, the round stingray (Vaudo and Lowe 2006), then the ability to orient to GMF stimuli could be beneficial during migrations. This study aims to test if the yellow stingray can use the polarity of a magnetic field as a cue to solve a T-maze task for a food reward, successfully complete a reversal learning procedure and learn subsequent spatial tasks in significantly fewer trials.
Methods
Yellow stingrays (n = 7; DW = 12–26 cm) were captured via hand nets from a local population (26°47′01.1"N, 80°02′47.8"W) and housed in husbandry tanks (244 × 122 cm) with flow through seawater and a 14:10 light:dark cycle. Stingrays were separated into two cohorts of three or four individuals and fed a mixture of shrimp, fish, and squid ad libitum (approximately 5% body weight, BW) every day until training commenced. The stingrays went on a reduced food intake of 3% BW per day to ensure a proper motivational state for behavioral conditioning.
Experimental apparatus
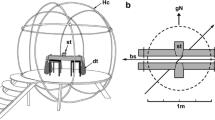
A T-maze was constructed of PVC pipe (2 cm diameter) and covered in thick (1 mm) plastic sheeting (Fig. 1a). The walls of the maze were 46 cm high and spaced 40 cm apart, the start arm was 72 cm long and the right and left arms were 88 cm long. Removable gates (46 × 40 cm) made of PVC pipe and plastic sheeting were used to block off compartments and isolate test subjects within an arm of the maze. The experimental tank (244 × 122 cm) was positioned with the short side aligned with the north–south axis of the GMF. The T-maze was placed into the tank and positioned such that the starting compartment was located in the magnetic south, the intersection in the magnetic north, and the right and left arms to the magnetic east and west (i.e., parallel to the magnetic equator), respectively (Fig. 1a). Fresh seawater flowed from the right and left arms towards the intersection and out of the maze at the start arm. A layer of fine sand covered the bottom of the tank and maze to a depth of 2 cm. A continuous loop of insulated 14-gauge single core copper wire was wrapped 12 times around the cross-section of the maze to the immediate left and right of the intersection (24 wraps total) then connected to a 30 W DC power supply (Model #TP3005DM, TEK Power, Montclair, CA). This formed a horizontal solenoid that allowed the north–south axis of the ambient GMF to be shifted towards the magnetic east (right) or west (left) at the intersection of the T-maze (Fig. 1b). A series of inline switches controlled the onset and polarity of electric current (5 V, 5 A) to the solenoid and in turn shifted the polarity of the magnetic field stimulus. A tri-axial magnetometer (Model #3AMG, Alphalab Inc., Salt Lake City, UT) and a waterproof compass located at the intersection confirmed the direction and consistency of the magnetic stimulus during trials. Two fluorescent lights at a height of 2.5 m evenly illuminated the setup from either side of the tank and a high-definition video camera (Sony HDR CX360) was suspended 3.5 m above the center of the tank to record all trials for subsequent analysis.
a–b Schematic diagram of the T-maze setup from an overheard view. The geomagnetic field is indicated by the red arrow, whereas gray lines and arrows indicate the isolines of the magnetic field applied by the green solenoid coils located at the maze intersection. Prior to the start of a trial (a) the stingray was behind a gate in the start arm and the magnetic stimulus was switched off. The trial began (b) as the magnetic stimulus was switched on, the gate was lifted, and the stingray swam into the intersection and choose to turn right. In this example, the stingray was trained to use magnetic north (N) as the indicator for the correct arm and it received a food reward after passing the magnetic coils (thick green lines)
Ambient GMF and magnetic polarity stimuli
The ambient GMF in Boca Raton, FL produced a uniform field with an overall intensity of 45 µT (26.8 µT horizontal, 36.9 µT vertical) and an inclination angle of − 55° across the entire setup. Because the short arm of the maze (start—> intersection) was aligned with the north–south axis of the GMF, and the GMF was uniform across the lateral axis of the maze, there was no directional information from left to right. The solenoid consistently generated a net maximum magnetic field intensity (38 µT) along its central axis with intensity isolines that radiated outward (30 cm) until they faded to ambient values just before the start arm gate (Fig. 1b). The field formed horizontal intensity isolines along the left–right axis of the choice arms and an intensity gradient from the solenoid central axis down the start arm (Fig. 1b). Therefore, a stingray experienced a magnetic intensity gradient as it swam from the start position to the intersection of the maze but not as it moved laterally towards the left or right arms. Consequently, the only magnetic cue available along the lateral (left–right) axis of the T-maze was the direction, or the N—> S polarity, of the resultant magnetic field applied by the solenoid (Fig. 1b).
Pretraining
Stingrays were allowed to acclimatize to the experimental tank for 24 h in groups of four. Then, individuals took turns learning to associate food with the right and left ends of the T-maze for 6 days. Shrimp and squid were cut into ten pieces for each individual (~ 3% BW per day) and awarded equally between the right and left arms in a pseudo randomized order. Food morsels were delivered at the end of a plastic rod to ensure accurate and timely delivery once a stingray swam into the right or left arm. When a stingray learned to associate both the right and left arms with food, it was placed behind the gate in the starting arm and training commenced. Once the stingray was resting on the sand, food odorant (1 g squid homogenized with 15 mL H2O) was given to stimulate search behavior. When the subject touched the center of the gate, the gate was raised, and the stingray swam throughout the maze until it went into an arm to receive food. Food was equally distributed between the right and left arms and data from pretraining confirmed the absence of a turn bias.
Initial behavioral conditioning
Individual stingrays were separated into two groups, in which either the north (N) or the south (S) pole of the GMF were used as a positive stimulus (i.e., conditioning stimulus) to indicate the correct arm to receive the food reward. During conditioning, the N or S pole of the GMF at the maze intersection was shifted to the right or left (Fig. 1b). During a session, the positive stimulus was presented in a balanced yet randomized order with no more than two successive presentations in a given direction. Naïve stingrays showed no observable innate response to the magnetic conditioning stimuli so the change in GMF polarity was deemed to be neutral. Training sessions began with a 15-minute acclimation period where the stingray could explore the maze at will. Ten trials were conducted during a session and sessions occurred daily for a maximum of five sessions per week. Trials consisted of an individual stingray being coaxed into the start arm, the gate lowered into place, and 15 mL of food odorant injected into the starting area. Once the stingray began search behavior, the positive stimulus was switched on, the gate was raised, and the subject swam towards the intersection. A choice was defined as the first time the entire body of the stingray fully crossed the solenoid wires located outside the intersection (Fig. 1). If the animal made a correct choice, the positive stimulus remained on during food administration to ensure spatiotemporal overlap of the positive stimulus, food reward, and correct behavioral response (i.e. correct arm choice). When the stingray finished eating, the positive stimulus was turned off and the subject swam about the maze (~ 30–45 s) until it returned to the starting area. If the stingray made an incorrect choice, the positive stimulus was turned off and a gate was lowered to isolate the animal in the incorrect arm (~ 30–45 s) thereby reinforcing the negative stimulus with the incorrect choice and lack of a food reward. After the trial was over, the stingray was gently ushered back into the starting area and the gate was lowered into place. Water flushed the maze for 120–240 s and the sand was raked to eliminate any residual visual or olfactory cues from the previous trial.
Reversal training
Due to a parasitic infection, two stingrays died after the initial training and the remaining stingrays (n = 5) were given a 3-week interval of antibiotics on a full feeding schedule. Reversal training proceeded as previously described except that the previously unrewarded negative stimulus became the new positive stimulus for conditioning. In other words, stingrays that were initially trained to use N to indicate the correct arm for food rewards were retrained to use S to indicate the correct choice, and vice versa.
Data collection and analysis
If the right and left arms of the T-maze and the difference between N and S stimuli are equal, then the likelihood of making a correct or incorrect choice should be equal. Therefore, small or random fluctuations in the number of correct responses for a session should approach chance (~ 50%) across multiple sessions. Previous conditioning procedures on elasmobranchs (Daniel and Schluessel 2019; Fuss et al 2014a, b, c; 2018) used learning criteria that individuals must give the correct response in ≥ 70% of the ten trials within a session for three consecutive sessions (Chi-square test, \(\chi^{2}\)0.05 (1) = 3.84). The critical value for a statistically significant difference can be reached by a ≥ 90% correct response rate for one session (\(\chi^{2}\) = 6.4), ≥ 80% for two consecutive sessions (\(\chi^{2}\)= 6.2), ≥ 70% for three consecutive sessions (\(\chi^{2}\) = 4.8), ≥ 60% for ten consecutive sessions (\(\chi^{2}\) = 4.0), or through any combination of these rates. We found that some yellow stingrays gave robust correct responses (≥ 80%) for one or two sessions, whereas others gave weaker responses (≥ 60%) across several consecutive sessions. Therefore, we set our learning criterion to a ≥ 60% correct response rate for a minimum of three to a maximum of seven consecutive sessions. This criterion allowed us to include the small but statistically significant effect of learning observed in one individual, accommodate the variation in correct response rates among multiple individuals, and observe established procedures that require subjects to consistently demonstrate learning across multiple sessions. For the group of stingrays, the overall mean correct response of the first two sessions was compared to those of the final two sessions (Wilcoxon matched pairs signed rank test, p ≤ 0.05). The mean number of sessions to criterion was compared for stingrays that underwent initial and reversal training (paired t test, p ≤ 0.05).
Results
Naïve yellow stingrays did not display any innate reaction to magnetic N or S positive stimuli or indicate turn biases during the initial pretraining sessions. Stingrays (n = 7) reached the learning criterion during the initial stimulus training within a mean (± SE) of 158.6 ± 28.4 trials (range = 50–250). The sample size prevented statistical comparisons among stingrays grouped by treatment and sex; however, qualitative comparisons suggest that there was no difference in the mean number of trials to criterion between males (n = 3; 126.7 ± 17.6; range = 10–160) and females (n = 4; 182.5 ± 47.2; range = 50–250), or between stingrays trained with N (162.5 ± 33.8; range = 100–250) and those with S as the positive stimulus (153.3 ± 57.8; range = 50–250). The overall mean number of correct responses per session (Fig. 2) of the initial conditioning experiments significantly increased (W = 28.0; p = 0.0078) from the initial two sessions (4.2 ± 0.4, range = 2–7) compared to the final two sessions (6.9 ± 0.3; range = 6–9).
The yellow stingray can be trained to use magnetic polarity as a positive stimulus to solve a T-maze task, then it can relearn the task when the reward contingency is reversed. Stingrays trained during the initial conditioning paradigm (n = 7) showed a significant increase in the mean (± SE) number of correct responses from the first two sessions to the final two sessions (p ≤ 0.01). Likewise, stingrays that underwent the serial reversal paradigm (n = 5) showed a significant increase in the mean (± SE) number of responses from the first two sessions to the final two sessions (p ≤ 0.05). Symbols indicate individual stingrays
Individuals (n = 5) that underwent reversal training (Fig. 3) took significantly fewer trials (t = 2.513, df = 4; p = 0.0329) to reach the reversal criterion (120 ± 13.8; range = 80–150) compared to their initial training criterion (180 ± 37; range = 50–250). The reverse trained stingrays demonstrated a significant increase (W = 15.0; p = 0.0312) in the overall mean number of correct responses per session (Fig. 2) from the initial two sessions (4.8 ± 0.5; range = 3–8) compared to the final two sessions (7.1 ± 0.3; range = 6–9). Qualitative comparisons suggest that there was no difference in the overall mean number of trials to criterion between those reverse trained with N (110 ± 20.8; range = 80–150) and those reverse trained with S (135 ± 15; range = 120–150) as the positive stimulus.
The yellow stingray can solve a serial reversal task in fewer trials compared to the initial conditioning paradigm. The mean (± SE) number of sessions to criterion for stingrays (n = 5) that learned to use magnetic field polarity to solve a T-maze task during reversal training was significantly fewer compared to the initial conditioning experiments (p ≤ 0.05). Symbols indicate individual stingrays
Individual learning curves (Fig. 4a–g) show the number of trials (range = 50–250), the number of consecutive sessions (range = 3–7) to criterion among individuals, the positive stimulus used for conditioning (N or S), stingray sex, and conditioning procedure (initial, reversal). Individuals stingrays reached the learning criterion for initial training (Fig. 4a–g) within: (a) 120 trials (\(\chi^{2}\) = 7.2, p ≤ 0.01), (b) 100 trials (\(\chi^{2}\) = 4.4, p ≤ 0.05), (c) 250 trials (\(\chi^{2}\) = 5.6, p ≤ 0.05), (d) 180 trials (\(\chi^{2}\) = 4.0, p ≤ 0.05), (e) 170 trials (\(\chi^{2}\) = 6.4, p ≤ 0.05), (f) 250 trials (\(\chi^{2}\) = 7.2, p ≤ 0.01), and (g) 50 trials (\(\chi^{2}\) = 5.2, p ≤ 0.05). After a 3-week interval, stingrays were reverse trained to associate the opposite magnetic field polarity with a food reward by: (c) 150 trials (\(\chi^{2}\) = 4.0, p ≤ 0.05), (d) 120 trials (\(\chi^{2}\) = 4.8, p ≤ 0.05), (e) 100 trials (\(\chi^{2}\) = 10.4, p ≤ 0.005), (f) 150 trials (\(\chi^{2}\) = 7.6, p ≤ 0.01), and (g) 80 trials (\(\chi^{2}\) = 7.2, p ≤ 0.01). Interestingly, one female stingray had the fastest initial learning curve (Fig. 4g) and was the only individual that took longer to learn the reversal task, compared to the other reverse trained individuals (n = 4) that averaged 33% fewer sessions to reach the second criterion.
a–g Learning acquisition curves for yellow stingrays conditioned to use magnetic field polarity (positive stimulus) as a cue to solve a T-maze task for a food reward. Red lines indicate female stingrays and blue lines are males. Squares indicate that the positive stimulus is magnetic north and circles are magnetic south. Solid lines and symbols indicate the first series of training sessions for a positive stimulus, whereas empty symbols and dotted lines indicate reversal training to the opposite positive stimulus. Sessions of ten trials occurred daily and the number of correct choices for a session is plotted. Individual stingrays (n = 7) reached the learning criterion for initial training by: a 120 trials**, b 100 trials*, c 250 trials*, d 180 trials*, e 170 trials* f 250 trials**, and g 50 trials*. Stingrays were reverse trained (n = 5) and reached the learning criterion by: c 150 trials*, d 120 trials*, e 100 trials***, f 150 trials**, and g 80 trials**. (* = p ≤ 0.05; ** = p ≤ 0.01; *** = p ≤ 0.005)
Discussion
This study demonstrated that the yellow stingray can be behaviorally conditioned to use the polarity of an altered GMF to solve a T-maze task. During the initial training, stingrays learned to associate the direction of either magnetic N or S with the location of a food reward. After a break in training, stingrays were then reverse trained to use the previously unrewarded stimulus of the opposite polarity as the new cue of the reward location. Learning performance increased as the initial and reversal training sessions progressed, but the group learned the reversal task in significantly fewer trials compared to the initial procedure. Qualitative observations suggest that stingrays learned to use N or S as the positive stimulus equally well in the initial and reversal experiments. Furthermore, the lack of innate response by naïve subjects to N or S stimuli confirms that a change in GMF polarity was an appropriate neutral stimulus for associative learning (Molet and Miller 2014).
The yellow stingray and the congeneric round stingray took a comparable number of trials to learn tasks that used GMF polarity as cues to indicate the location of food rewards (Table 1; Kalmijn 1978). Kirschvink (1989) argued that the presence of inadvertent chemical, visual, and tactile cues and magnetic gradients were potentially confounding variables that might better explain the results of Kalmijn (1978). We accounted for these non-magnetic variables and ensured that our solenoid generated a horizontal magnetic field with no lateral intensity gradients that might give directional information to a stingray (Fig. 1b). The only magnetic cue available along the left–right horizontal axis of the T-maze was the polarity of the resultant magnetic field applied by the solenoid (Fig. 1b). Thus, we are confident that the presentations of the N and S stimuli only differed in polarity and were the only environmental cues indicating the correct choice for the location of food rewards.
The yellow stingray appeared to learn this spatial task at a rate commensurate to the freshwater ocellate stingray (Table 1) that was trained to solve a variety of mazes using visual cues and different orientation strategies (Schluessel and Bleckmann 2005), visual landmarks and habitat geometry (Schluessel et al. 2015), and visual landmarks and directional cues (Schluessel and Ober 2018). However, the learning criteria for these studies were based on a minimum time to complete the tasks (Schluessel et al. 2015; Schluessel and Ober 2018) or a higher number of correct responses (≥ 80% for three consecutive sessions; Schluessel and Bleckmann 2005) which makes direct comparison difficult. A prior experiment on the yellow stingray (Newton and Kajiura 2017) used permanent magnets to train subjects (≥ 75% correct for three consecutive sessions) to associate magnetic stimuli with food rewards in threefold fewer trials compared to this study (Table 1). The greater number of trials it took yellow stingrays to learn the tasks of this study compared to those of previous experiments might reflect the relative difficulty in associating subtle GMF stimuli with spatial orientation behavior. The cognitive flexibility of the freshwater stingray was determined using visual stimuli, food rewards, a serial reversal learning paradigm and a stricter learning criterion (≥ 70% correct for three consecutive sessions; Daniel and Schluessel 2019). Interestingly, these individuals (n = 7) learned the first reversal task in 3X the mean number of trials (678 ± 52 SE) compared to their initial training (217 ± 41 SE), but each subsequent reversal showed improved learning. Logistical constraints prevented a similar long-term study on the yellow stingray and a stricter learning criterion would have taken longer to achieve. Future studies should investigate how learning criterion rigor affects reversal learning performance in sharks and rays, because species with higher behavioral flexibility might respond more favorably to rapid changes in environmental conditions.
Spatial learning acquisition could have been influenced by the yellow stingray using an orientation strategy that focused on environmental stimuli other than the positive magnetic stimulus. Egocentric orientation strategies require a subject to remember the location of objects (or stimuli) with respect to the self, whereas allocentric strategies rely on associations made between the location of objects (or stimuli) relative to one another or the overall environment (Burgess 2006). These two systems can operate independently or in unison (Burgess 2006), as seen in the freshwater stingray which can employ egocentric and allocentric strategies to orient inside a four-arm maze (Schluessel and Bleckmann 2005). However, when given a choice, it prefers to use an allocentric strategy based on environmental geometry to find hidden feeding stations (Schluessel et al. 2015) and turn-based directional information over landmarks to navigate a labyrinth (Schluessel and Ober 2018). If the yellow and freshwater stingrays have the same learning preferences, then our stingrays might have initially navigated the T-maze using turn-based directional information or habitat geometry instead of the GMF polarity cues localized to the maze intersection.
Studies using a variety of sensory modalities and methods on mammals (Chow et al. 2015), avians (Bond et al. 2007), teleosts (Parker et al. 2012), and insects (Strang and Sherry 2014) have shown that serial reversal conditioning can result in faster rates of learning and fewer mistakes with each subsequent switch in the reinforcement contingency. Similar improvements in reversal learning were shown in the freshwater stingray that used visual stimuli to solve a four-arm maze and then learned a reversal procedure in 20% fewer trials compared to the initial task (Schluessel and Bleckmann 2005). Despite the increased number of trials to criterion for the freshwater stingray during the first reversal, learning rates improved slightly across three additional reversals (Daniel and Schluessel 2019). Interestingly, the learning performance of the grey bamboo shark under visual discrimination training declined during a single reversal task compared to that of the initial procedure (Fuss et al. 2014a). In our experiments, the yellow stingray learned a single reversal procedure in 33% fewer trials compared to the initial conditioning regimen, which supports the idea of an overall positive transfer of learning between the two procedures (Warburton and Hughes 2011). Due to differences in methodology and sensory modality tested, it is unclear why our results differ from those of other elasmobranch reversal training studies. If the yellow stingray can use what it learns in one spatial orientation context to facilitate learning in another, then the positive transfer of learning could allow migrating individuals to navigate more efficiently though habitats and reduce the metabolic costs associated with searching behaviors. This could be especially useful if an individual must switch between sensory modalities (Gardiner et al. 2014) due to changes in cue availability or reliability as it navigates across different spatiotemporal scales (Nosal et al. 2016).
It was beyond the scope of this study to determine the mechanism of magnetic stimulus detection, so it remains unclear how the yellow stingray detected the changes in GMF polarity. Kalmijn (1978) hypothesized that elasmobranchs use their ampullae of Lorenzini to detect the electrical artifacts induced as they swim through the GMF within the conductive medium of seawater. In this scenario, an elasmobranch could use the GMF to determine a compass heading because the direction and magnitude of the induced electrical current is a function of the swimming vector relative to that of the GMF (Kalmijn 1978; Paulin 1995; Molteno and Kennedy 2009). Because the yellow stingray can discriminate between the positive and negative poles of an electrical field (Siciliano et al. 2013), it can likely distinguish a reversal in the direction of an induced electric current. Electrophysiological experiments have shown that the electroreceptors of the thornback ray and common stingray respond to changes in GMF intensity and that electroreceptor excitation or inhibition depends upon the direction (i.e., polarity) of the applied magnetic field (Akoev et al. 1976; Brown and Ilyinsky 1978). Recent evidence supports the idea that the sandbar shark might detect magnetic stimuli directly and indirectly using magnetite- and electroreceptor-based mechanisms, respectively (Anderson et al. 2017). Because a definitive magnetoreceptive cell has not been found in any elasmobranch, it is likely that our yellow stingrays used their ampullae of Lorenzini to discern the direction of the electrical currents induced as they moved through magnetic fields with opposite polarities.
Although we do not understand how elasmobranchs detect magnetic cues, our results demonstrate that the yellow stingray can detect the polarity of a magnetic field and use it to spatially orient and solve navigational tasks. This supports the idea that Urolophid stingrays, and perhaps other elasmobranchs, might use the GMF as a cue to maintain a compass heading as they migrate towards a desired location. Future studies could simulate large physical displacements by manipulating the ambient GMF on migrating elasmobranchs and quantifying their orientation response. The inherent challenges of these studies include finding species that naturally orient in a preferred direction, do not require large husbandry tanks for ram ventilation, and creating magnetic coils of sufficient size to deliver accurate stimuli. If elasmobranchs do use the GMF to actively determine their current location and maintain a course heading towards a goal, it might explain how sharks and rays can navigate across featureless marine habitats and repeatedly arrive at specific locations for feeding, mating, and parturition.
Data availability
The datasets created during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Able KP (1991) Common themes and variations in animal orientation systems. Amer Zool 31:157–167
Adrianov GN, Brown HR, Ilyinsky OB (1974) Responses of central neurons to electrical and magnetic stimuli of the ampullae of Lorenzini in the Black Sea skate. J Comp Physiol 93:287–299
Akoev GN, Ilyinsky OB, Zadan PM (1976) Responses of electroreceptors (ampullae of Lorenzini) of skates to electric and magnetic fields. J Comp Physiol 106:127–136
Anderson JM, Clegg TM, Véras LVMV, Holland KH (2017) Insight into shark magnetic field perception from empirical observations. Sci Rep 7:1042. https://doi.org/10.1038/s41598-017-11459-8
Berthold P (2001) Bird Migration: a general survey. Oxford University Press, Oxford, UK, p 272
Bitterman ME (1965) Phyletic differences in learning. Am Psychol 20:395–410
Bond AB, Kamil A, Balda RP (2007) Serial reversal learning and the evolution of behavioral flexibility in three species of North American corvids Gymnorhinus cyanocephalus, Nucifraga Columbiana, Aphelocoma californica. J Comp Psychol 121(4):372–379
Brown HR, Ilyinsky OB (1978) The ampullae of Lorenzini in the magnetic field. J Comp Physiol 126:333–341
Burgess N (2006) Spatial memory how egocentric and allocentric combine. Trends Cogn Sci 10:551–557
Chapman DD, Feldheim KA, Papastamatiou YP, Hueter RE (2015) There and back again: a review of residency and return migrations in sharks, with implications for population structure and management. Ann Rev Mar Sci 7:547–570
Cheng K (2012) How to navigate without maps: the power of taxon-like navigation in ants. Comp Cogn Behav Rev 7:1–22. https://doi.org/10.3819/ccbr.2012.70001
Chow PKY, Leaver LA, Wang M, Lea SEG (2015) Serial reversal learning in gray squirrels: learning efficiency as a function of learning and change of tactics. J Exp Psych 41:343–353
Daniel MMM, Schluessel V (2019) Serial reversal learning in freshwater stingrays (Potamotrygon motoro). Anim Cogn. https://doi.org/10.1007/s10071-019-01321
Day LB, Crews D, Wilczynski W (1999) Spatial and reversal learning in congeneric lizards with different foraging strategies. Anim Behav 57:393–407
Fahy DP (2004) Diel activity patterns, space utilization, seasonal distribution and population structure of the yellow stingray (Urobatis jamaicensis) in South Florida with comments on reproduction. (MS Thesis) Nova Southeastern University Oceanographic Center, Dania Beach, Florida, USA
Flowers KI, Ajemian MJ, Bassos-Hull K, Feldheim KA, Hueter RE, Papastamatiou YP, Chapman DD (2016) A review of batoid philopatry, with implications for future research and population management. Mar Ecol Prog Ser 562:251–261
Fuss T, Schluessel V (2018) Immediate early gene expression related to learning and retention of a visual discrimination task in bamboo sharks (Chiloscyllium griseum). Brain Struct Func 223:3975–4003. https://doi.org/10.1007/s00429-018-1728-8
Fuss T, Bleckmann H, Schluessel V (2014a) The shark (Chiloscyllium griseum) can orient using turn responses before and after partial telencephalon ablation. J Comp Physiol A 200:19–35
Fuss T, Bleckmann H, Schluessel V (2014b) Place learning prior to and after telencephalon ablation in bamboo and coral cat sharks (Chiloscyllium griseum and Atelomycterus marmoratus). J Comp Physiol A 200:37–52
Fuss T, Schluessel V, Bleckmann H (2014c) Visual discrimination abilities in the gray bamboo shark (Chiloscyllium griseum). Zool 117:104–111
Gardiner JM, Atema J, Hueter RE, Motta PJ (2014) Multisensory integration and behavioral plasticity in sharks from different ecological niches. PLoS ONE 9(4):e93036
Gould JL (1998) Sensory bases of navigation. Curr Biol 8:R731–R738
Gould JL (2004) Animal navigation. Curr Biol 14:R221–R224
Hueter RE, Heupel MR, Heist EJ, Keeney DB (2005) Evidence of philopatry in sharks and implications for the management of shark fisheries. J Northw Atl Fish Sci 35:239–247
Kalmijn AJ (1974) The detection of electric fields from inanimate and animate sources other than electric organs. In: Fessard A (ed) Handbook of sensory physiology III/3: electroreceptors and other specialized receptors in lower vertebrates. Heidelberg, Berlin, pp 147–200
Kalmijn AJ (1978) Experimental evidence of geomagnetic orientation in elasmobranch fishes. In: Schmidt-Koening K, Keaton WT (eds) Animal Migration, Navigation, and Homing. Springer-Verlag, Berlin, pp 347–353
Kirschvink JL (1989) Magnetite biomineralization and geomagnetic sensitivity in animals: an update and recommendations for future study. Bioelectromagnetics 10:239–259
Klimley AP (1993) Highly directional swimming by scalloped hammerhead sharks, Sphyrna lewini, and subsurface irradiance, temperature, bathymetry, and geomagnetic field. Mar Biol 117:1–22
Krylov VV, Osipova EA, Pavlova VV, Batrakova AA (2016) Influence of magnetic field on spatial orientation in zebrafish (Danio rerio) and roach (Rutilus rutilus) (Cyprinidae). J Ichthyol 56(3):456–461
Light P, Salmon M, Lohmann KJ (1993) Geomagnetic orientation of loggerhead sea turtles: evidence for an inclination compass. J Exp Biol 182:1–10
Lohmann KJ (1991) Magnetic orientation by hatchling loggerhead sea turtles (Caretta caretta). J Exp Biol 155:37–49
Marhold S, Burda H, Wiltschko W (1997) A magnetic polarity compass for direction finding in a subterranean mammal. Naturwissenschaften 84:421–423
Meyer CG, Holland KN, Papastamatiou YP (2005) Sharks can detect changes in the geomagnetic field. J R Soc Interface 2:129–130
Molet M, Miller RM (2014) Timing an attribute of associative learning. Behav Processes 0:4–14
Molteno TCA, Kennedy WL (2009) Navigation by induction-based magnetoreception in elasmobranch fishes. J Biophys 4575:380976
Myklatun A, Lauri A, Eder SHK, Cappetta M, Shcherbakov D, Wurst W, Winklhofer M, Westmeyer GG (2018) Zebrafish and medaka offer insights into the neurobehavioral correlates of vertebrate magnetoreception. Nature Comm 9:802. https://doi.org/10.1038/s41467-018-03090-6
Naisbett-Jones LC, Putman NF, Stephenson JF, Ladak S, Young KA (2017) A magnetic map leads juvenile European eels to the Gulf Stream. Curr Biol 27:1236–1240
Newton KC, Kajiura SM (2017) Magnetic field discrimination, learning and memory in the yellow stingray (Urobatis jamaicensis). Anim Cogn 20:603–614
Nosal AP, Chao Y, Farrara JD, Chai F, Hastings PA (2016) Olfaction contributes to pelagic navigation in a coastal shark. PLoS ONE 11(1):e0143758. https://doi.org/10.1371/journal.pone.0143758
Osipova EA, Pavlova VV, Nepomnyaschchikh VA, Kylov VV (2016) Influence of magnetic field on zebrafish activity and orientation in a plus maze. Behav Proc 122:80–86
Parker MO, Gavira J, Haigh A, Millington ME, Brown VJ, Combe FJ, Brennan CH (2012) Discrimination reversal and attentional sets in zebrafish (Danio rerio). Behav Brain Res 232:264–268
Paulin MG (1995) Electroreception and the compass sense of sharks. J Theor Biol 174:325–339
Pavlov IP (1927) Lectures on conditioned reflexes. International Publishers, New York, NY
Piercy AN, Snelson FF, Grubbs RD (2006) Urobatis jamaicensis. IUCN Red List of Threatened Species. Version 2016–1. https://www.iucnredlist.org/details/60109/0 Accessed 30 March 2019
Putman NF, Endres CS, Lohmann CMF, Lohmann KJ (2011) Longitude perception and bicoordinate magnetic maps in sea turtles. Curr Biol 21:463–466
Putman NF, Scanlon MM, Billman EJ, O’Neil JP, Couture RB, Quinn TP, Lohmann KJ, Noakes DLG (2014) An inherited magnetic map guides ocean navigation in juvenile pacific salmon. Curr Biol 24:446–450
Quinn TP (1980) Evidence of celestial and magnetic compass orientation in lake migrating sockeye salmon fry. J Comp Physiol 137:243–248. https://doi.org/10.1007/BF00657119
Quinn TP, Brannon EL (1982) The use of celestial and magnetic cues by orienting sockeye salmon smolts. J Comp Physiol 147:547–552. https://doi.org/10.1007/BF00612020
Quinn TP, Groot C (1983) Orientation of chum salmon (Oncorhynchus keta) after internal and external magnetic field alteration. Can J Fish Aquat Sci 40:1598–1606. https://doi.org/10.1139/f83-185
Quinn TP, Merrill RT, Brannon EL (1981) Magnetic field detection in Sockeye Salmon. J Exp Zool 217:137–142. https://doi.org/10.1002/jez.1402170114
Schluessel V (2015) Who would have thought that “Jaws” also has brains? Cognitive functions in elasmobranchs. Anim Cogn 18(1):19–37
Schluessel V, Bleckmann H (2005) Spatial memory and orientation strategies in the elasmobranch (Potamotrygon motoro). J Comp Physiol A 191:695–706
Schluessel V, Ober C (2018) How to get out of a maze? Stingrays (Potamotrygon motoro) use directional over landmark information when provided with both in a spatial task. Evol Ecol Res 19:619–637
Schluessel V, Herzog H, Scherpenstein M (2015) Seeing the forest before the trees-spatial orientation in freshwater stingrays (Potamotrygon motoro) in a hole-board task. Behav Proc 119:105–115
Shettleworth SJ (2010) Cognition, evolution and behavior, 2nd edn. Oxford University Press, New York
Shettleworth SJ, Sutton JE (2005) Multiple systems of spatial learning: dead reckoning and beacon-homing in rats. J Exp Psychol Anim Behav Process 31:125–141
Siciliano AM, Kajiura SM, Long JH, Porter MP (2013) Are you positive? Electric dipole polarity discrimination in the yellow stingray (Urobatis jamaicensis). Biol Bull 225:85–91
Souza JJ, Poluhowich JJ, Guerra RJ (1988) Orientation responses of American eels, (Anguilla rostrata) to varying magnetic fields. Comp Biochem Physiol A 90:57–61. https://doi.org/10.1016/0300-9629(88)91005-5
Speed CW, Field IC, Meekan MG, Bradshaw CJA (2010) Complexities of coastal shark movements and their implications for management. Mar Ecol Prog Ser 408:275–293
Strang CG, Sherry DF (2014) Serial reversal learning in bumblebees (Bombus impatiens). Anim Cogn 17:723–734
Takebe A, Furutani T, Wada T, Koinuma M, Kubo Y, Okano K, Okano T (2012) Zebrafish respond to the geomagnetic field by bimodal and group-dependent orientation. Sci Rep 2:727. https://doi.org/10.1038/srep00727
Tesch FW, Wendt T, Karlsson L (1992) Influence of geomagnetism and salinity on orientation of the eel (Anguilla anguilla), as evident from laboratory experiments. Ecol Freshwater Fish 1:52–60. https://doi.org/10.1111/j.1600-0633.1992
Vaudo JJ, Lowe CG (2006) Movement patterns of the round stingray (Urobatis halleri) (Cooper) near a thermal outfall. J Fish Biol 68:1756–1766
Walker MM, Diebel CE, Kirschvink JL (2003) Detection and use of the Earth’s magnetic field by aquatic vertebrates. In: Collin SP, Marshall NJ (eds) Sensory processing in aquatic environments. Springer-Verlag, New York, pp 53–74
Warburton K, Hughes R (2011) Learning of foraging skills by fish. In: Brown C, Laland K, Krause J (eds) Fish cognition and behavior, 2nd edn. Wiley-Blackwell, Chichester, UK, pp 10–35
Wiltschko W, Wiltschko R (1972) Magnetic compass of European robins. Science 176:62–64
Acknowledgements
This research was supported by grants to KCN from the Florida Atlantic University Graduate Research and Inquiry Program Award, the Save Our Seas Foundation Small Grant, the Student Research Award from the American Elasmobranch Society, The Grant in Aid of Research from the Society for Integrative and Comparative Biology, the Gordon Gilbert Graduate Scholarship from the Friends of Gumbo Limbo Nature Center, and the PADI Foundation Grant. We thank S. Creager, A. Murakami, E. Cave, L. Celano, J. Noble, G. Gil, B. Bowers, K. Kramer, and S. Ramirez for help with stingray collection and husbandry, and R. Stackman and M. Salmon for assistance with experimental design and animal training protocols. Animals were collected pursuant to Florida Fish and Wildlife Conservation Commission Special Activities License SAL 15-1413A-SR
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of animal welfare and ethics
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the Florida Atlantic University Institutional Animal Care and Use Committee.
Additional information
Responsible Editor: J. Carlson.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by undisclosed experts.
Rights and permissions
About this article
Cite this article
Newton, K.C., Kajiura, S.M. The yellow stingray (Urobatis jamaicensis) can use magnetic field polarity to orient in space and solve a maze. Mar Biol 167, 36 (2020). https://doi.org/10.1007/s00227-019-3643-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-019-3643-9