Abstract
Elasmobranch fishes (sharks, skates, and rays) are hypothesized to use environmental cues, such as the geomagnetic field (GMF), to navigate across the ocean. However, testing the sensory and navigation abilities of large highly migratory fishes in the field is challenging. This laboratory study tested whether the yellow stingray, Urobatis jamaicensis, could detect and distinguish between the GMF cues used by other magnetically sensitive species to actively determine their location. Stingrays were divided into two cohorts for initial behavioral conditioning: one was trained to associate a change in GMF intensity with an aversive stimulus, whereas the other was trained using a change in GMF inclination angle. Individuals from each cohort remained naïve to the GMF conditioning stimulus used to condition the other cohort. The combined group learned the initial association within a mean (± SE) of 184.0 ± 34.8 trials. Next, stingrays from each cohort were randomly exposed to their original GMF conditioning stimulus and the novel GMF stimulus. The original magnetic stimulus continued to be reinforced, whereas the novel stimulus was not. The group demonstrated a significantly different response to the original (reinforced) and novel (non-reinforced) stimuli, which indicates that stingrays could distinguish between the intensity and inclination angle of a magnetic field. This experiment is the first to show that a batoid (skate or ray) can detect and distinguish between changes in GMF intensity and inclination angle, and supports the idea that elasmobranchs might use GMF cues to form a magnetically based cognitive map and derive a sense of location.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Navigation, in the truest sense, is demonstrated when an animal can migrate through unfamiliar territory, actively determine its current location relative to that of a known goal, then use this positional information to calculate and maintain the correct heading toward the final goal (Bingman and Cheng 2005; Gould 1998, 2011; Griffin 1952; Kramer 1953, 1957). The location determining component of navigation is a cognitive ability called the map step, whereas the direction-finding component is known as the compass step (Kramer 1953, 1957). Animals can use multiple types of cognitive maps and compasses in a hierarchical manner depending upon the scale of the navigational task, the availability of environmental cues, and the sensory modalities employed (Able 1991; Gould 1998, 2004). The map step involves a series of interrelated mechanisms for detecting, coding, classifying, and recalling salient environmental cues, which are then arranged into a coherent fashion to form the cognitive map (reviewed in Able 2001). One of the simplest types of “map” is seen in animals that, at a certain time, migrate with a particular vector (i.e., direction and distance), yet have no preference for precise locations within their habitats (reviewed in Able 1991, 2001). Other maps require an animal to learn a series of outgoing vectors between specific locations and then recall these vectors in reverse order, with the opposite orientation, for the return trip home (reviewed in Able 2001). Additional complexity is seen in animals that remember a series of outgoing vectors and then calculate a novel return vector back to the original location (reviewed in Able 1991, 2001; Collet and Graham 2004). Animals that stay within a limited area can devote additional cognitive resources to memorizing the locations of specific landmarks, and the vectors between them, to form a cognitive map of their home range (reviewed in Able 2001; Collet and Graham 2004; O’Keefe and Nadel 1978). If environmental cues, such as odor or sound gradients, co-occur with the spatial position of known landmarks, then an animal could learn to associate the relative values of these cues with the locations of various landmarks (reviewed in Able 2001; Collet and Graham 2004). The advantage of this system is that if the animal encounters these cues in an unknown territory, it might be able to actively compare the current values of the cues to those stored in its memory as a way to determine its present location and maintain the correct orientation toward its goal (reviewed in Able 2001; Collet and Graham 2004; Gould 1998).
Elasmobranchs (sharks, skates, and rays) undertake regular migrations, exhibit philopatry, seasonal residency, and maintain home ranges (reviewed in Hueter et al. 2005; Speed et al. 2010; Chapman et al. 2015; Flowers et al. 2016), but the sensory modalities and cues that these fishes use to navigate between specific habitats are not well known. Temporary sensory deprivation and physical displacement (< 10 km) has shown that leopard (Triakis semifasciata (Nosal et al. 2016)) and blacktip (Carcharhinus limbatus (Gardiner et al. 2015)) sharks use olfactory cues to navigate from an unfamiliar location back to the site of their original capture. However, the dispersal of chemical cues is limited by the lateral movement of currents within a vertically stratified water column and temporal fluctuations in source odorant concentration. Consequently, spatiotemporal changes in the availability of a cue can reduce its utility for migrating over longer distances and render the cue effective only for localized navigation. Conversely, deposits of magnetic compounds in the seafloor result in minute localized gradients (± 0.6%) within the geomagnetic field (GMF) that might serve as temporally stable cues for magnetically sensitive species as they navigate over relatively short distances (Klimley 1993). The molten outer core of the Earth creates the GMF, which can serve as a global cue for long-distance migrations because it is ubiquitous over large spatial scales (~ 1000 km) and fluctuates little over the lifetime of many species. The GMF at any geographic location can be quantified by a vector with an overall intensity of ~ 20–70 µT and an inclination angle (measured with respect to the surface of the Earth) that ranges from + 90° to − 90° (Fig. 1). These quantities change predictably with latitude such that the intensity is weakest at the equator and greatest at the magnetic poles, and the inclination angle is parallel to the surface of the Earth at the equator and orthogonal at the poles (Fig. 1a, b). If an animal is sensitive to GMF intensity or inclination angle, it could potentially use either cue to derive a general sense of latitude (Lohmann and Lohmann 1994, 1996; Lohmann et al. 2007; Gould 1998). Interestingly, the isolines of GMF intensity and inclination angle intersect at skewed angles (≤ 20°, Fig. 1c, d) to create a bicoordinate system of unique geomagnetic signatures across the globe that is analogous to latitude and longitude. Therefore, an animal that is sensitive to both GMF cues could potentially use them to determine a more precise sense of its location (Lohmann and Lohmann 1994, 1996, Lohmann et al. 2007; Gould 1998). Because the angular difference between the GMF intensity and inclination angle isolines is greater near the poles (Fig. 1d) compared to the equator (Fig. 1c), sensitivity to both cues might better enable location determination at higher latitudes (Boström et al. 2012; Gould 1998). Magnetoreceptive species, such as the loggerhead sea turtle (Caretta caretta (Lohmann and Lohmann 1994, 1996; Putman et al. 2011)), chinook salmon (Oncorhynchus tshawytscha (Putman et al. 2013, 2014)), pink salmon (O. gorbuscha (Putman et al. 2020)), Atlantic salmon (Salmo salar (Scanlan et al. 2018)), and European eel (Anguilla anguilla (Naisbett-Jones et al. 2017)), can use GMF intensity and inclination angle to form a bicoordinate magnetic map, use it to determine their current location, and make navigational course corrections toward their goal. Interestingly, juvenile sea turtles, salmon, and eels are known to imprint on the GMF signatures of their natal habitats (Lohmann 1991; Naisbett-Jones et al. 2017; Putman et al. 2011, 2014) and, as adults, use these magnetic data to successfully migrate back to these specific locations for reproduction.
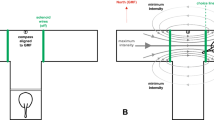
a–d Schematic diagram of the Earth and geomagnetic field (redrawn from Lohmann et al. 2007). The overall vectors (a) of the GMF (black arrows), intensity (red lines) and inclination angle (blue lines) isolines. Total GMF intensity (b) is the vector sum of the horizontal and vertical components, whereas GMF inclination angle is defined with respect to the surface of the Earth. The angular difference between intensity (red) and inclination angle (blue) isolines is generally greatest at higher (d) compared to lower (c) latitudes
The goal of this study was to determine if a stingray can detect and distinguish between GMF intensity and inclination angle, two cues that aid in magnetic-based navigation and are necessary to form a bicoordinate geomagnetic map. Elasmobranchs have been hypothesized to use the GMF to orient and navigate between habitats (Kalmijn 1974; Klimley 1993; Paulin 1995). Behavioral conditioning studies have confirmed a general sensitivity to magnetic fields in the scalloped hammerhead (Sphyrna lewini (Meyer et al. 2005)) and sandbar sharks (Carcharhinus plumbeus, (Meyer et al. 2005; Anderson et al. 2017)), and the short-tailed (Dasyatis brevicaudata (Kirschvink et al. 2001)) and yellow (U. jamaicensis (Newton and Kajiura 2017)) stingrays. The round (Urobatis halleri (Kalmijn 1978)) and yellow (Newton and Kajiura 2020) stingrays can discriminate GMF polarity, and bonnethead sharks (Sphyrna tiburo (Keller 2020) can use GMF intensity and inclination angle to derive a sense of location. Small point sources of magnetic stimuli, such as the permanent magnets used by Newton and Kajiura (2017) and the electromagnets used by other researchers (Anderson et al. 2017; Meyer et al. 2005; Kalmijn 1978), create GMF gradients that vary in both intensity and inclination angle. Therefore, we created a set of magnetic coils that generated a uniform field and could manipulate GMF intensity and inclination angle independently (Merritt et al. 1983). Behavioral studies on magnetoreception require test subjects to move several body lengths through space in order to adequately sample and respond to magnetic stimuli (Wiltschko and Wiltschko 2007). Based on the calculations of Merritt et al. (1983), creating uniform GMF stimuli across an experimental arena requires an electromagnet with linear dimensions that are more than twice those of the arena. These spatial limitations made the yellow stingray an ideal test species because it is small, readily learns operant conditioning tasks, and is magnetically sensitive (Newton and Kajiura 2017). Long-distance migration is not required for GMF intensity and inclination angle sensitivity (e.g., Scanlan et al. 2018) and the seasonal migratory patterns of the yellow stingray are unknown. Nevertheless, the yellow stingray might exhibit relatively large (~ 30 km) seasonal movements similar to those of the congeneric round stingray (U. halleri (Vaudo and Lowe 2006)). In either case, the yellow stingray might use GMF cues to actively determine its location and maintain the correct heading during short- or long-distance navigational tasks. Because finding migrating benthic stingrays in the field is prohibitively challenging, we performed a laboratory study that tested if the yellow stingray could be conditioned to associate an aversive stimulus with changes in GMF intensity and inclination angle, and if it could discriminate between these two magnetic cues.
Methods
Experimental apparatus
The experimental apparatus was located in a magnetically uniform portion of the laboratory (26°21′56.3″N, 80°04′17.1″W) and consisted of an acrylic tank (122 × 61 × 30 cm) on a wooden stand (127 × 66 × 85 cm) surrounded by a magnetic coil system (Fig. 2). The coils were constructed of 38 mm diameter PVC pipe (2 × 2 × 2 m overall); then a set of four horizontal and four vertical coils were spaced 0.74, 0.52, and 0.74 m apart (Merritt et al. 1983). A continuous loop of 14-gauge wire was wrapped onto each set of coils with 26, 11, 11, and 26 windings on the individual coils (Merritt et al. 1983). The apparatus was aligned with the N–S axis of the GMF (Fig. 2) and positioned such that the resultant magnetic fields produced by each set of coils were aligned with the vertical and horizontal components of the local GMF. The coil system was powered by a two-channel, 30 W DC power supply (BK Precision, Yorba Linda, CA), while the direction and magnitude of the electric current, and the resultant magnetic field, were controlled by a remote control system of electrical switches and circuits. Visual confirmation of magnetic field application and direction was confirmed by four red LEDs placed outside the tank but within the field of view of an overhead video camera (Sony HDR CX360, Tokyo, Japan). A tri-axial magnetometer (Model #3AMG, Alphalab Inc., Salt Lake City, UT) and xSensor software (Crossbow Inertial Systems, Milpitas, CA) for the iPhone (Apple, Cupertino, CA) confirmed the presence, intensity, inclination angle, and uniformity of the applied magnetic fields within the acrylic tank. Confounding visual cues were removed by covering the outside of the acrylic tank and the coil system with opaque white plastic sheeting. Four overhead compact fluorescent lamps (55 W, 3860 lm, 2700 K) placed at the upper corners of the coil system provided even illumination.
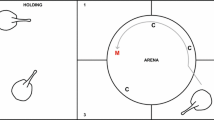
a–d Schematic diagram of the top down (a) and lateral views (b–d) of the magnetic coil system and shuttlebox aquarium. Thick dark gray lines represent the PVC frame and copper wire windings that form the four horizontal and four vertical coils. Water flow in and out of the aquarium is indicated by the small light blue arrows (a). Red dots (a) indicate four light emitting diodes (LEDs) located outside the arena, but within the field of view of the camera. The LEDs were paired with the horizontal and vertical coils and signified the presence and direction of the magnetic stimuli during each trial. Solid light gray lines and arrows indicate the ambient geomagnetic field (a–d). Solid red arrows (c) indicate stimulus conditions that only changed magnetic field intensity, whereas the solid blue arrows (d) indicate stimuli that changed only the inclination angle of the magnetic field. Arrow direction indicates magnetic north and arrow size indicates the relative intensity of the field. Dashed green arrows (c, d) indicate stingray swimming across the aquarium midline as a conditioned response to magnetic stimuli
Ambient GMF, magnetic, and aversive stimuli
The ambient GMF in the laboratory had an intensity (F) of 45 µT (26.8 µT horizontal, 36.9 µT vertical) with an inclination angle (I) of + 55° with respect to the surface of the Earth (Fig. 2a, b). Note that by convention, the Z-axis is positive in the downward direction and a positive GMF inclination angle means that the field lines are pointed toward the Earth’s surface. The magnetic conditioning stimuli (Fig. 2c, d) consisted of either doubling the GMF intensity to 90 µT (∆F = + 45 µT) while keeping the ambient inclination angle constant, or increasing the GMF inclination angle to − 5° (∆I = − 60°) while keeping the ambient intensity constant. Stimuli were cycled at a rate of 1 Hz. These magnetic stimuli are not ecologically relevant because a stingray would not encounter such large cyclical changes in GMF intensity or inclination angle in nature. However, we chose these parameters to make the magnetic cues more obvious and to facilitate learning the association between the positive magnetic and aversive stimuli. Electrophysiological data (Adrianov et al. 1974; Akoev et al. 1976; Brown and Ilyinsky 1978) support the hypothesis that changes in magnetic fields are detected by the electroreceptive cells in the ampullae of Lorenzini (Kalmijn 1978, 1982). This scenario postulates that elasmobranchs use their electroreceptors to detect electrical artifacts induced as they swim through the ambient GMF within electrically conductive seawater. If so, then a stingray resting on the substrate is unlikely to detect a single change in the GMF because physical movement is required for this induction-based mechanism to work and for animals to adequately sample and respond to magnetic stimuli (Wiltschko and Wiltschko 2007). Swimming has an inherent time component as an animal moves a given distance over time. Therefore, we suggest that for stationary animals, varying the GMF with time can mimic a rapid change in physical position that is necessary for GMF stimulus detection. Because elasmobranch electroreceptors are best tuned to detect AC stimuli that oscillate at low frequencies (0.1–15 Hz; Adrianov et al. 1974; Peters and Evers 1985; Montgomery 1984; Tricas and New 1998), we chose to cycle the magnetic stimuli at a rate of 1 Hz. Prior sensory discrimination studies have occasionally used electric shocks as aversive stimuli to behaviorally condition elasmobranchs (Kelly and Nelson 1975; Nelson 1967; Schwarze et al. 2013), but if the hypothesis of Kalmijn (1978, 1982) is true, then electric shocks would likely impede the potential detection of GMF stimuli by the electroreceptors. Therefore, we used a mechanotactile aversive stimulus that involved prodding the stingray with a rubber-coated plastic rod to elicit an escape response.
Subjects
Yellow stingrays (n = 7; 5 males, 2 females; DW = 10–25 cm) were housed in a flow-through aquarium (244 × 122 cm) supplied with mechanically filtered and UV-treated seawater. Stingrays were kept on a 14:10 h light:dark cycle and fed a diet of squid, fish, and shrimp ad libitum every other day. Once the subjects were successfully eating for at least 2 weeks, they were acclimatized to the experimental apparatus. Approximately 10–15 min after transport to the experimental tank, the stingrays would rest along the bottom of the tank in a manner consistent with their normal behavior in the husbandry tanks. At this point the subjects began the conditioning procedure.
Detection of GMF intensity or inclination angle stimuli by naïve stingrays
Subjects (n = 5) were assigned to two treatment groups where the positive stimulus was either a change in magnetic field intensity or inclination angle. Trials commenced after an individual was resting on the bottom of the experimental tank for 10–15 min. Naïve subjects were given magnetic stimuli without aversive stimulus pairing for a session of 10 trials. We began behavioral conditioning trials, which consisted of turning the positive magnetic stimulus on; then after 10 s had elapsed, the aversive stimulus was administered concurrent with the positive stimulus until the stingray shuttled across the midline of the tank. At this point both the positive and aversive stimuli ceased, and the trial concluded. The behavioral response to the GMF stimulus before the application of the aversive stimulus was then recorded. The total positive stimulus presentation lasted between 11 and 15 s and the aversive stimulus presentation was 1–5 s. The stingray was allowed to rest for an interval of 20–40 s and the next trial began once the stingray was resting on the bottom. Training sessions consisted of a block of 10 trials, and two training sessions occurred each day, with a 10–15 min rest period between each session. Once a subject reached the learning criterion (see below) training stopped and magnetic stimulus discrimination trials began the following day.
Discrimination between GMF intensity and inclination angle by experienced stingrays
Stingrays (n = 5) that underwent magnetic stimulus detection training were then trained to discriminate between GMF intensity and inclination angle stimuli. One magnetic stimulus was termed positive and the other negative, because they were either paired with an aversive stimulus or not, respectively. Due to the nature of the magnetic coil setup, the positive and negative stimuli could not be produced simultaneously, so they were presented sequentially to the stingrays in a pseudorandom order. Pilot studies showed that two different reinforcement strategies helped the stingrays to demonstrate magnetic stimulus discrimination; however, the small size of the acrylic tank rendered the combination of aversive and appetitive reinforcement ineffective. Therefore, we continued reinforcing the original GMF conditioning stimulus to distinguish it from the novel unreinforced stimulus and minimize confusing the subjects. In other words, individuals that were initially trained to use magnetic field intensity as the positive stimulus continued this procedure in addition to being exposed to magnetic field inclination angle as the negative stimulus, and vice versa. Each discrimination session was a balanced design of 20 total trials where the positive or negative stimuli were presented no more than three times in a row. The behavioral responses were recorded on video and scored as in the initial conditioning procedure.
Discrimination between GMF intensity and inclination angle by naïve stingrays
We hypothesized that overtraining might lead to a decline in the overall magnetic stimulus discrimination responses among stingrays across sessions. Therefore, we trained and tested naïve stingrays (n = 2) according to the aforementioned GMF stimulus discrimination protocol, but they did not undergo the initial conditioning procedure of pairing GMF stimuli with aversive stimuli.
Data collection and analysis
Conditioned behavioral responses for each trial were counted only if they were robust demonstrations of learning and visible on the video record. Observer bias was minimized during data collection by blinding the primary observer to the onset and type of magnetic stimulus used. The primary observer reliability (95%) was confirmed by a blind second observer and disagreements were resolved by using the most conservative interpretation of the observed behavior. In all experiments, the only conditioned response data used for the calculations were those trials where a stingray swam across the midline of the tank. Additional responses to GMF stimuli were more subtle and difficult to score consistently, so they were excluded from our analyses. For stationary stingrays, these behaviors involved flinching the pectoral fins upward (≥ 5 mm), or abruptly swimming more than one body length (BL) from their original position. Likewise, swimming stingrays would occasionally stop swimming and freeze in place, or exhibit a sharp turn (> 90°–180°) from an established vector without crossing the midline of the tank.
During magnetic stimulus detection trials, individual stingrays had to consistently give an above average conditioned response (i.e., shuttling across the midline) to demonstrate that they had learned to associate the positive magnetic and aversive stimuli. Based on previous work (Newton and Kajiura 2020), we set the learning criterion to a ≥ 60% conditioned response rate per session for a minimum of three consecutive sessions, until the overall performance was significantly different from chance (Chi square test, X20.05 (1) = 3.84). The mean conditioned response of individuals for the first three sessions was compared to those of the final three sessions (Wilcoxon matched-pairs signed-rank test, p ≤ 0.05). The small sample size precluded comparing the mean number of trials to criterion between magnetic treatments (GMF intensity and inclination angle) or sex. For the group of stingrays in the magnetic stimulus discrimination experiments (n = 7), we compared the mean conditioned response (across 14 sessions) to positive GMF stimuli (reinforced) to that of negative stimuli (not reinforced) for each stingray (paired t test, p ≤ 0.05).
Results
During the initial pre-training sessions, naïve yellow stingrays showed no discernible response to the GMF intensity or inclination angle stimuli that were not paired with aversive stimuli. In the initial magnetic stimulus detection experiments, stingrays (n = 5) reached the learning criterion within a mean (± SE) of 184.0 ± 34.8 trials (range 90–300). Individuals varied in their ability to learn the association between the positive and aversive stimuli (Fig. 3a–e) and demonstrated learning within (a) 300 trials (X2 = 9.6, p ≤ 0.005), (b) 150 trials (X2 = 7.6, p ≤ 0.01), (c) 170 trials (X2 = 13.2, p ≤ 0.001), (d) 90 trials (X2 = 4.4, p ≤ 0.05), and (e) 210 trials (X2 = 20.0, p ≤ 0.001). The small sample size and high variance precluded statistical testing for differences in response among treatments or sex. Nevertheless, qualitative observations suggest that stingrays trained to detect changes in GMF intensity reached the criterion in a similar mean (± SE) number of trials (n = 2; 225.0 ± 75.0; range 150–300) compared to those trained with changes in inclination angle (n = 3; 156.7 ± 35.3; range 90–210). Likewise, male stingrays (n = 3; 226.7 ± 38.4; range 170–300) did not take more trials to learn the association than females (n = 2; 120.0 ± 30.0; range 90–150). Additionally, there was a significant increase (W = 0, p = 0.03) in the overall mean (± SE) conditioned response (Fig. 4) between the initial three sessions (1.4 responses per session ± 0.3) and the final three sessions (7.7 responses per session ± 0.4).
a–e The yellow stingray can detect changes in GMF intensity and inclination angle. Learning acquisition curves for individual yellow stingrays (n = 5) conditioned to associate a change in GMF intensity (red lines and symbols) or inclination angle (blue lines and symbols) with an aversive stimulus until they reached the learning criterion (dotted gray line). Square and circle symbols signify male and female stingrays, respectively. Sessions were composed of ten trials and two sessions occurred each day. Individual stingrays (a–e) reached the learning criterion by: a 300 trials**, b 150 trials**, c 170 trials****, d 90 trials*, and e 210 trials**** (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.005; ****p ≤ 0.001)
The yellow stingray can learn to associate GMF stimuli with an aversive stimulus. The mean (± SE) conditioned response per session of stingrays (n = 5) trained to associate either a change in GMF field intensity (red lines and symbols) or inclination angle (blue lines and symbols) with an aversive stimulus for the first three sessions (empty symbols) compared to final three sessions (solid symbols). Squares indicate male stingrays, whereas circles indicate females. All subjects showed a significant increase in the mean number of conditioned responses to magnetic stimuli (W = 0, p = 0.03)
The experienced stingrays, along with two naïve individuals, underwent GMF intensity and inclination angle discrimination trials. Data from individual stingrays, both naïve and experienced, showed no consistent trends across consecutive sessions, so the data were pooled. Overall, the group (n = 7) demonstrated a significant (t6 = 6.727, p = 0.0005) and consistent ability to discriminate between positive and negative GMF stimuli for 14 sessions (Fig. 5). Interestingly, the overall response to the positive GMF stimuli was lower than that of the negative stimuli, despite pairing the positive and aversive stimuli together.
The yellow stingray can discriminate between GMF intensity and inclination angle stimuli. The mean number of responses per session, across 14 sessions, for each stingray to GMF+ (reinforced) and GMF− (not reinforced) stimuli. Stingrays showed a significantly higher overall response to negative compared to positive magnetic stimuli (t6 = 6.727, p = 0.0005). Unique symbols connected by gray lines indicate individual stingrays (n = 7): stingrays A–E underwent initial GMF detection training and stingrays N1–2 were naïve to detection training. Red symbols indicate the mean response of an individual to a change in GMF intensity, whereas blue symbols indicate the mean response for the same individual to a change in GMF inclination angle. Stingrays were separated into two groups: in one cohort (n = 3) the positive stimulus was a change in GMF intensity (red GMF+), and the negative stimulus was a change in GMF inclination angle (blue GMF−), and vice versa for the second cohort (n = 4; blue GMF+ and red GMF−)
Discussion
Naive yellow stingrays gave no observable response to a rapid and large change in the ambient GMF intensity or inclination angle, but our data show that this species can learn to associate both of these cues with an aversive stimulus and elicit an escape response. The small sample size prevented us from testing if there was a quantifiable difference between the responses of males and females, or among individuals trained with changes in intensity or inclination angle. Overall, stingrays responded differently to the two GMF cues when one was reinforced and the other was not, which suggests that changes in GMF intensity and inclination angle were perceived differently. If so, then these data support the idea that stingrays, and perhaps other elasmobranchs, might use the GMF to actively determine their location during migrations.
Detection of GMF intensity and inclination angle
Our results are similar to previous conditioning experiments on electromagnetic cue perception in urolophid stingrays and sensory discrimination studies in sharks that used aversive reinforcement (Table 1). Under the same learning criteria as this study, it took the yellow stingray a comparable number of trials to learn that GMF polarity cues indicated the location of food rewards (159 ± 28 trails; Newton and Kajiura 2020). Under more stringent criteria, the yellow stingray learned to distinguish between the positive and negative poles of a prey-simulating electric field (180 ± 8.2 trials; Siciliano et al. 2013), but it took 3.7X fewer trials to locate hidden magnets for food rewards (50.5 ± 2.6 trials; Newton and Kajiura 2017). Interestingly, Kalmijn (1978) paired aversive and appetitive stimuli with the two opposite poles of a magnetic field to train the congeneric round stingray to swim to specific locations within an aquarium. However, this dual reinforcement strategy yielded results (163 ± 89.5 trials) akin to those of other studies, save the one that used very strong magnets (Newton and Kajiura 2017). Despite differences in methodology, the general trend in urolophid stingrays could be that stronger conditioning stimuli facilitate learning more than different reinforcement strategies. Results similar to ours were found when researchers paired aversive electric stimuli with the onset of green lights to determine the neural substrates of fear conditioning in the gray (Chiloscyllium griseum) and brown banded (C. punctatum) bamboo sharks (150 ± 12 and 123 ± 29 trials, respectively; Schwarze et al. 2013). Electric shocks were paired with pure-tone audio signals to investigate the auditory capabilities of the lemon (Negaprion brevirostris (Nelson 1967)) and horn sharks (Heterodontus francisci (Kelly and Nelson 1975)). Nelson (1967) found that lemon sharks took 100 trials to discriminate between two acoustic tones when each was paired with electric shocks, but they took 3X fewer (33) trials to discriminate between two tones when one was paired with an appetitive and the other an aversive stimulus. It is interesting that a dual reinforcement strategy might have facilitated learning acquisition in the lemon shark but not in the round stingray. It is noteworthy to mention that Clark (1959) successfully trained the lemon shark to strike a visually distinct target for food within 30 trials, which is on par with the results of Newton and Kajiura (2017). The faster learning shown in these two studies compared to ours might be because they used “obvious” localized conditioning stimuli (a high contrast visual target and a strong magnet) instead of relatively weak global conditioning stimuli (changes in GMF). Of course, the different methods and sensory modalities prevent direct comparison across these studies, but the effects of reinforcement strategy and stimulus strength on learning acquisition in elasmobranchs appear to be an area worthy of further investigation.
Logistical constraints prevented us from fully testing for sexual differences in reaching the learning criteria during the initial GMF detection task, or in discriminating between GMF stimuli. If such a difference exists, we propose that it might be due to factors known to increase system sensitivity, such as sexual dimorphisms in sensory receptor surface area (Crooks and Waring 2013), receptor innervation (Kempster et al. 2013), or seasonal plasticity in receptor function (Sisneros and Tricas 2000). The congeneric round stingray has shown differences in the electrosensory-mediated behavior of males and females during the reproductive season (Tricas et al. 1995), which is a result of seasonal differences in the levels of sex hormones circulating in the blood (Sisneros and Tricas 2000). Throughout the year, the electroreceptors of Atlantic stingrays (Hypanus sabina) are primarily used for predation and have a broad tuning curve that is capable of detecting a wide variety of bioelectric fields produced by prey. However, as the reproductive season approaches and androgen levels increase, the electroreceptors of male stingrays discharge more frequently and become better tuned to detect low frequency stimuli (0.1–4 Hz) compared to those of females (Sisneros and Tricas 2000). This shift in the sensory tuning of males corresponds to the bioelectric signatures produced by conspecific stingrays and allows the males to better detect cryptic females buried in the sand. Because our experiments were conducted during the spring and late summer reproductive seasons of the yellow stingray (Fahy et al. 2007), our males might have experienced a similar shift in electrosensory tuning. If the mechanism of elasmobranch magnetic stimulus detection is mediated by the electrosensory system (Kalmijn 1978, 1982), then a shift in electrosensory tuning and increased sensitivity to low frequency stimuli might result in male yellow stingrays learning a task faster or responding to GMF stimuli more robustly than females.
Discrimination between GMF intensity and inclination angle, and ecological implications
The overall response rate for the group of stingrays to negative stimuli was statistically greater than to positive stimuli, which indicates that changes in GMF intensity and inclination angle were perceived differently. However, it is not clear why subjects would flee magnetic stimuli that were not aversively reinforced more often than those that were reinforced. It is also unclear why the difference between average responses, although consistently and statistically different across several consecutive sessions, was not much larger in magnitude. Perhaps, the lack of reinforcement to negative GMF stimuli confused the subjects and they generalized their conditioned response to flee during any change in the GMF. If so, then it might be worth determining if the yellow stingray can group magnetic stimuli into categories similar to how the gray bamboo shark (Fuss et al. 2014, 2017; Schluessel et al. 2014) and cichlid (Pseudotropheus zebra (Schluessel et al. 2012, 2014, 2015, 2018)) use visual cues and motion to categorize objects into different groups.
However, it is equally plausible that the somewhat similar response rates to positive and negative stimuli were because the distinction between changes in GMF intensity and inclination angle are not ecologically relevant cues for this species. The yellow stingray is distributed from the northern to the southern subtropical waters of the western Atlantic Ocean (Piercy et al. 2006) where the angular difference between the isolines of GMF intensity and inclination angle range between 0° and 5° (Boström et al. 2012). This minute difference makes a bicoordinate magnetic map less useful because both GMF cues provide nearly the same latitudinal information with very little distinction in longitude (Fig. 1c; Boström et al. 2012). A magnetically sensitive species that evolved under these conditions might experience less selective pressure to discriminate between these cues compared to species that evolved at higher latitudes where this angular difference between GMF intensity and inclination angle isolines is greater (Fig. 1d). If so, then animals at lower latitudes might navigate east–west by following the magnetic isolines until they reach a continental land mass and then travel north–south along the coastline toward their final goal (Lohmann and Lohmann 2006; Lohmann et al. 2007; Berdahl et al. 2016; Endres et al. 2016).
Alternatively, the small angular difference between the intensity and inclination angle isolines could select for magnetoreceptive animals with high sensory acuity. The bobolink (Dolichonyx oryzivorus) can detect magnetic intensities as low as 0.05–0.2 µT (Beason and Semm 1987; Semm and Beason 1990), the sandbar shark can detect intensities of 0.03 µT (Anderson et al. 2017), and the thorny skate (Amblyraja radiata) can detect intensity changes as low as 1 nT (Brown et al. 1979). The American alligator (Alligator mississippiensis) can detect changes in magnetic inclination angle as small as 0.01°–0.2° (Rodda 1984) and the red-spotted newt (Notophthalmus viridiscens) can detect inclination angle changes from 0.5° to 2.0° (Fischer et al. 2001; Phillips et al. 2002). We did not test the sensory acuity of the yellow stingray, but it would be interesting to conduct a comparative experiment across species to see if acuity to GMF stimuli correlates with a species’ latitudinal distribution or the spatiotemporal scale of migrations deduced from behavioral tracking data.
This study provides the first behavioral evidence that a stingray can detect and distinguish between the GMF cues used by other species to derive a sense of location. Magnetic pseudo-displacement experiments have shown that juvenile loggerhead sea turtles (Lohmann and Lohmann 1994, 1996; Putman et al. 2011), European eels (Naisbett-Jones et al. 2017), Chinook (Putman et al. 2014), Atlantic (Scanlan et al. 2018), and pink salmon (Putman et al. 2020) use these cues to form a bicoordinate magnetic map and gain a sense of location. Recent evidence indicates that bonnethead sharks can use the GMF to derive a sense of location and orient toward a home range (Keller 2020); however, this ability has yet to be shown in any batoid (skate or ray). The ocellate river stingray (Potamotrygon motoro) is believed to use visual cues to form a cognitive map and solve a four-arm maze navigational task (Schluessel and Bleckmann 2005) and it is likely that cognitive maps exist in other batoids. Additional GMF pseudo-displacement work on a migratory batoid is necessary to unequivocally establish that skates and rays use a geomagnetic map to determine their location during navigational tasks.
Mechanistic considerations
Unfortunately, the mechanism of elasmobranch magnetoreception remains unresolved and we do not know if the yellow stingray can detect GMF stimuli directly through an undiscovered magnetoreceptor, or indirectly via the ampullae of Lorenzini, as proposed by Kalmijn (1978, 1982). Behavioral evidence that supports direct magnetoreception comes from the short-tailed stingray (Walker et al. 2003) and the sandbar shark (Anderson et al. 2017), whereas electrophysiological data from the ampullae of Lorenzini in the thornback ray (Raja clavata (Akoev et al. 1976; Brown and Ilyinsky 1978)) and common stingray (Dasyatis pastinaca (Adrianov et al. 1974; Akoev et al. 1976; Brown and Ilyinsky 1978)) support the mechanism of electroreceptor-mediated magnetic field detection. Anderson et al. (2017) behaviorally conditioned sandbar sharks to respond to weak magnetic stimuli (> 0.03 µT) that generated electrical artifacts of 73 nV cm−1. The stimuli that we used (~ 45 µT), and those of the electrophysiological studies (~ 80–200 µT; Adrianov et al. 1974; Akoev et al. 1976; Brown and Ilyinsky 1978), undoubtedly induced electrical artifacts that were above the median behavioral response thresholds of the yellow stingray (22 nV cm−1; Bedore et al. 2014), and the physiological thresholds of the thornback ray and common stingray. Therefore, until this mechanistic issue is resolved, the most conservative and plausible interpretation of our data is that the yellow stingray was detecting the electric fields induced by changes to the ambient GMF intensity and inclination angle. If chondrichthyans do use their electroreceptors to detect, encode, and perceive magnetic stimuli, the next step is to uncover how these fishes might distinguish between natural geomagnetic and bioelectric cues.
Data availability
The datasets created during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Able KP (1991) Common themes and variations in animal orientation systems. Am Zool 31:157–167
Able K (2001) The concepts and terminology of bird navigation. J Avian Biol 32:174–183
Adrianov GN, Brown HR, Ilyinsky OB (1974) Responses of central neurons to electrical and magnetic stimuli of the ampullae of Lorenzini in the Black Sea skate. J Comp Physiol 93:287–299
Akoev GN, Ilyinsky OB, Zadan PM (1976) Responses of electroreceptors (ampullae of Lorenzini) of skates to electric and magnetic fields. J Comp Physiol 106:127–136
Anderson JM, Clegg TM, Véras LVMV, Holland KH (2017) Insight into shark magnetic field perception from empirical observations. Sci Rep 7:1042. https://doi.org/10.1038/s41598-017-11459-8
Beason RC, Semm P (1987) Magnetic responses of the trigeminal nerve system of the bobolink (Dolichonyx oryzivorus). Neurosci Lett 80:229–234
Bedore CB, Harris LL, Kajiura SM (2014) Behavioral responses of batoid elasmobranchs to prey-simulating electric fields are correlated to peripheral sensory morphology and ecology. Zoology 117:95–103
Berdahl A, Westley PAH, Levin SA, Couzin ID, Quinn TP (2016) A collective navigation hypothesis for homeward migration in anadromous salmonids. Fish Fish 17:525–542
Bingman VP, Cheng K (2005) Mechanisms of animal global navigation: comparative perspectives and enduring challenges. Ethol Ecol Evol 17:295–318
Boström JE, Åkesson S, Alerstam T (2012) Where on earth can animals use a geomagnetic bi-coordinate map for navigation? Ecography 35:1039–1047
Brown HR, Ilyinsky OB (1978) The ampullae of Lorenzini in the magnetic field. J Comp Physiol 126:333–341
Brown HR, Ilyinsky OB, Muraveiko VM, Corshkov ES, Fonarev GA (1979) Evidence that geomagnetic variations can be detected by lorenzinian ampullae. Nature 277:649–650
Chapman DD, Feldheim KA, Papastamatiou YP, Hueter RE (2015) There and back again: a review of residency and return migrations in sharks, with implications for population structure and management. Annu Rev Mar Sci 7:547–570
Clark E (1959) Instrumental conditioning of lemon sharks. Science 130:217–218
Collet TS, Graham P (2004) Animal navigation: path integration, visual landmarks and cognitive maps. Curr Biol 14:R475–R477
Crooks N, Waring CP (2013) A study into the sexual dimorphisms of the Ampullae of Lorenzini in the lesser-spotted catshark, Scyliorhinus canicula (Linnaeus, 1758). Environ Biol Fish 96:585–590
Endres CS, Putman NF, Ernst DA, Kurth JA, Lohmann CF, Lohmann KJ (2016) Mulit-modal homing in sea turtles: modeling dual use of geomagnetic and chemical cues in island-finding. Front Behav Neurosci 10:19. https://doi.org/10.3389/fnbeh.2016.00019
Fahy DP, Spieler RE, Hamlett WC (2007) Preliminary observations on the reproductive cycle and uterine fecundity of the yellow stingray, Urobatis jamaicensis (Elasmobranchii: Myliobatiformes: Urolophidae) in Southeast Florida, USA. Raffles Bull Zool Suppl 14:131–139
Fischer JH, Freake MJ, Phillips JB (2001) Evidence for the use of magnetic map information by an amphibian. Anim Behav 62:1–10
Flowers KI, Ajemian MJ, Bassos-Hull K, Feldheim KA, Hueter RE, Papastamatiou YP, Chapman DD (2016) A review of batoid philopatry, with implications for future research and population management. Mar Ecol Prog Ser 562:251–261
Fuss T, Bleckmann H, Schluessel V (2014) Visual discrimination abilities in the grey bamboo shark, Chiloscyllium griseum. Zoology 117:104–111
Fuss T, Russnak V, Stehr K, Schluessel V (2017) World in motion: perception and discrimination of movement in juvenile grey bamboo sharks (Chiloscyllium griseum). Anim Behav Cogn 4(3):223–241. https://doi.org/10.26451/abc.04.03.03.2017
Gardiner JM, Whitney NM, Hueter RE (2015) Smells like home: the role of olfactory cues in the homing behavior of blacktip sharks, Carcharhinus limbatus. Integr Comp Biol 55(3):495–506
Gould JL (1998) Sensory bases of navigation. Curr Biol 8(20):R731–R738
Gould JL (2004) Animal navigation. Curr Biol 14(6):R221–R224
Gould JL (2011) Animal navigation: longitude at last. Curr Biol 21(6):R225–R227
Griffin DR (1952) Bird navigation. Biol Rev Camb Philos Soc 27:359–400
Hueter RE, Heupel MR, Heist EJ, Keeney DB (2005) Evidence of philopatry in sharks and implications for the management of shark fisheries. J Northwest Atl Fish Sci 35:239–247
Kalmijn AJ (1974) The detection of electric fields from inanimate and animate sources other than electric organs. In: Fessard A (ed) Handbook of sensory physiology III/3: electroreceptors and other specialized receptors in lower vertebrates. Springer, Berlin, pp 147–200
Kalmijn AJ (1978) Experimental evidence of geomagnetic orientation in elasmobranch fishes. In: Schmidt-Koening K, Keaton WT (eds) Animal migration, navigation, and homing. Springer, Berlin, pp 347–353
Kalmijn AJ (1982) Electric and magnetic field detection in elasmobranch fishes. Science 218:916–918
Keller BA (2020) The spatiotemporal ecology of the bonnethead shark, Sphyrna tiburo: migration, parturition and magnetic-based navigation. PhD Dissertation, Florida State University, College of Arts and Sciences, Tallahassee, FL, USA
Kelly JC, Nelson DR (1975) Hearing thresholds of the horn shark, Heterodontus francisci. J Acoust Soc Am 58:905
Kempster RM, Garza-Gisholt E, Egeberg CA, Hart NS, O’Shea OR, Collin SP (2013) Sexual dimorphism of the electrosensory system: a quantitative analysis of nerve axons in the dorsal anterior lateral line nerve of the blue-spotted fantail ray (Taeniura lymma). Brain Behav Evol 8:226–235
Kirschvink JL, Walker MW, Diebel CE (2001) Magnetite-based magnetoreception. Curr Opin Neurobiol 11:462–467
Klimley AP (1993) Highly directional swimming by scalloped hammerhead sharks, Sphyrna lewini, and subsurface irradiance, temperature, bathymetry, and geomagnetic-field. Mar Biol 117:1–22
Kramer G (1953) Wird die Sonnenhöhe bei der Heimfinde orientierung ververtet? J Ornithol 94:201–219
Kramer G (1957) Experiments in bird orientation and their interpretation. Ibis 99:196–227
Lohmann KJ (1991) Magnetic orientation by hatchling loggerhead sea turtles (Caretta caretta). J Exp Biol 155:37–49
Lohmann KJ, Lohmann CMF (1994) Detection of magnetic inclination angle by sea turtles: a possible mechanism for determining latitude. J Exp Biol 194:23–32
Lohmann KJ, Lohmann CMF (1996) Detection of magnetic field intensity by sea turtles. Nature 380:59–61
Lohmann KJ, Lohmann CMF (2006) Sea turtles, lobsters, and oceanic maps. Mar Freshw Behav Physiol 39:49–64
Lohmann KJ, Lohmann CMF, Putman NF (2007) Magnetic maps in animals. J Exp Biol 210:3697–3705
Merritt R, Purcell C, Stroink G (1983) Uniform magnetic field produced by three, four and five square coils. Rev Sci Instrum 54:879–882
Meyer CG, Holland KN, Papastamatiou YP (2005) Sharks can detect changes in the geomagnetic field. J R Soc Interface 2:129–130
Montgomery JC (1984) Frequency response characteristics of primary and secondary neurons in the electrosensory neurons in the electrosensory system of the thornback ray. Comp Biochem Physiol 79A:189–195
Naisbett-Jones LC, Putman NF, Stephenson JF, Ladak S, Young KA (2017) A magnetic map leads juvenile European eels to the Gulf Stream. Curr Biol. https://doi.org/10.1016/j.cub.2017.03.015
Nelson DR (1967) Hearing thresholds, frequency discrimination and acoustic orientation in the lemon shark, Negaprion brevirostris (Poey). Bull Mar Sci 17(3):741–767
Newton KC, Kajiura SM (2017) Magnetic field discrimination, learning and memory in the yellow stingray, Urobatis jamaicensis. Anim Cogn 20(4):603–614
Newton KC, Kajiura SM (2020) The yellow stingray, Urobatis jamaicensis, can use magnetic field polarity to orient in space and solve a maze. Mar Biol 167:36. https://doi.org/10.1007/s00227-019-3643-9
Nosal AP, Chao Y, Farrara JD, Chai F, Hastings PA (2016) Olfaction contributes to pelagic navigation in a coastal shark. PLoS ONE 11(1):e0143758. https://doi.org/10.1371/journal.pone.0143758
O’Keefe J, Nadel L (1978) The hippocampus as a cognitive map. Oxford University Press/Clarendon Press, Oxford
Paulin MG (1995) Electrorecpetion and the compass sense of sharks. J Theor Biol 174:325–339
Peters RC, Evers HP (1985) Frequency selectivity in the ampullary system of an elasmobranch fish (Scyliorhinus canicula). J Exp Biol 118:99–109
Phillips JB, Freake MJ, Fischer JH, Borland SC (2002) Behavioral titration of a magnetic map coordinate. J Comp Physiol A 188:157–160
Piercy AN, Snelson FF, Grubbs RD (2006) Urobatis jamaicensis. IUCN Red List of Threatened Species. Version 2016-1. http://www.iucnredlist.org/details/60109/0. Accessed 30 Mar 2017
Putman NF, Endres CS, Lohmann CMF, Lohmann KJ (2011) Longitude perception and bicoordinate magnetic maps in sea turtles. Curr Biol 21:463–466
Putman NF, Lohman KJ, Putman EJ, Quinn TP, Klimley AP, Noakes DLG (2013) Evidence for geomagnetic imprinting as a homing mechanism in pacific salmon. Curr Biol 23(4):312–316
Putman NF, Scanlon MM, Billman EJ, O’Neil JP, Couture RB, Quinn TP, Lohmann KJ, Noakes DLG (2014) An inherited magnetic map guides ocean navigation in juvenile pacific salmon. Curr Biol 24(4):446–450
Putman NF, Williams CR, Gallagher EP, Dittman AH (2020) A sense of place: pink salmon use a magnetic map for orientation. J Exp Biol 223:jeb218735
Rodda GH (1984) The orientation and navigation of juvenile alligators: evidence of magnetic sensitivity. J Comp Physiol 154:649–658
Scanlan MM, Putman NF, Pollock AM, Noakes DL (2018) Magnetic map in nonanadromous Atlantic salmon. PNAS 115:10995–10999
Schluessel V, Bleckmann H (2005) Spatial memory and orientation strategies in the elasmobranch Potamotrygon motoro. J Comp Physiol A 191:695–706
Schluessel V, Fricke G, Bleckmann H (2012) Visual discrimination and object categorization in the cichlid Pseudotropheus sp. Anim Cogn 15:525–537
Schluessel V, Beil O, Weber T, Bleckmann H (2014) Symmetry perception in bamboo sharks (Chiloscyllium griseum) and Malawi cichlids (Pseudotropheus sp.). Anim Cogn 17(5):1187–1205
Schluessel V, Kortekamp N, Cortes JA, Klein A, Bleckmann H (2015) Perception and discrimination of movement and biological motion patterns in fish. Anim Cogn 18(5):1077–1091. https://doi.org/10.1007/s10071-015-0876-y
Schluessel V, Hiller J, Krueger M (2018) Discrimination of movement and visual transfer abilities in cichlids (Pseudotropheus zebra). Behav Ecol Sociobiol 72:61. https://doi.org/10.1007/s00265-018-2476-8
Schwarze S, Bleckmann H, Schluessel V (2013) Avoidance conditioning in bamboo sharks (Chiloscyllium griseum and C. punctatum): behavioral and neuroanatomical aspects. J Comp Physiol A 199(10):843–856
Semm P, Beason RC (1990) Responses to small magnetic variations by the trigeminal system of the bobolink. Brain Res Bull 25:735–740
Siciliano AM, Kajiura SM, Long JH, Porter ME (2013) Are you positive? Electric dipole polarity discrimination in the yellow stingray (Urobatis jamaicensis). Biol Bull 225:85–91
Sisneros JA, Tricas TC (2000) Androgen-induced changes in response dynamics of primary ampullary electrosensory primary afferent neurons. J Neurosci 20(22):8586–8595
Speed CW, Field IC, Meekan MG, Bradshaw CJA (2010) Complexities of coastal shark movements and their implications for management. Mar Ecol Prog Ser 408:275–293
Tricas T, New JG (1998) Sensitivity and response dynamics of elasmobranch electrosensory primary afferent neurons to near threshold fields. J Comp Physiol A 182:89–101
Tricas TC, Michael SW, Sisneros JA (1995) Electrosensory optimization to conspecific phasic signals for mating. Neurosci Lett 202:129–132
Vaudo JJ, Lowe CG (2006) Movement patterns of the round stingray Urobatis halleri (Cooper) near a thermal outfall. J Fish Biol 68:1756–1766
Walker MM, Diebel CE, Kirschvink JL (2003) Detection and use of the Earth’s magnetic field by aquatic vertebrates. In: Collin SP, Marshall NJ (eds) Sensory processing in aquatic environments. Springer, New York, pp 53–74
Wiltschko W, Wiltschko R (2007) Conditioning to magnetic directions. NeuroReport 18:949–950
Acknowledgements
This research was supported by grants to KCN from the Florida Atlantic University (FAU) Graduate Grant, the Save Our Seas Foundation Small Grant, the Henry F. Mollet Research Award from the American Elasmobranch Society, the Gordon Gilbert Graduate Scholarship from the Friends of Gumbo Limbo Nature Center, and the PADI Foundation Grant. The authors thank S. Creager, A. Murakami, E. Cave, L. Celano, J. Noble, G. Gil, B. Bowers, K. Kramer, and S. Ramirez for help with stingray collection and husbandry, and R. Stackman and M. Salmon for assistance with experimental design and animal training protocols.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of animal welfare and ethics
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in these studies involving animals were in accordance with the ethical standards of the Florida Atlantic University Institutional Animal Care and Use Committee under protocols A23-13 and A16-33. Animals were collected pursuant to Florida Fish and Wildlife Conservation Commission Special Activities License SAL 15-1413A-SR.
Significance
Previous work has shown that elasmobranchs (sharks, skates, and rays) can detect magnetic stimuli and might use the Earth’s magnetic field as a navigational cue. However, the specific nature of the geomagnetic cues that elasmobranchs can detect are largely unknown. This study used behavioral conditioning to demonstrate that the yellow stingray, Urobatis jamaicensis, can detect changes in the intensity and inclination angle to the geomagnetic field. These cues change predictably with latitude and are used by other magnetically sensitive species to determine their location during long distance navigation.
Additional information
Responsible Editor: J. Carlson.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by P. Klimley and an undisclosed expert.
Rights and permissions
About this article
Cite this article
Newton, K.C., Kajiura, S.M. The yellow stingray (Urobatis jamaicensis) can discriminate the geomagnetic cues necessary for a bicoordinate magnetic map. Mar Biol 167, 151 (2020). https://doi.org/10.1007/s00227-020-03763-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-020-03763-1