Abstract
In 2001–2002, there were severe declines of eelgrass (Zostera marina) in estuaries along the Nova Scotia coast of the southern Gulf of St. Lawrence. We examined the relationship between the recent invasion of European green crabs (Carcinus maenas) and the eelgrass decline in Benoit Cove in Tracadie Harbour. The abnormally abundant eelgrass wrack consisted mainly of entire shoots, not the usual blades. Three separate methods yielded similar estimates of the rate of shoot removal from the eelgrass beds (direct quadrat counts, “mark–recapture” of tagged shoots in the eelgrass bed, and rate of shoot appearance in the shore wrack). From 14 July to 8 September 2002, the shoot density in the bed fell by about 75 %. Similar rates of decline occurred in crab enclosures (4.4 crabs m−2) placed in the eelgrass bed. Green crab foraging, involving the tearing of shoots and the digging of large pits, was the reason for the drastic decline of the eelgrass bed in Benoit Cove. It is clear that an invasion of green crabs to a region where they had not previously existed can both destroy eelgrass beds and restrict their recovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nova Scotian estuaries bordering the southern Gulf of St. Lawrence have historically contained dense, extensive eelgrass beds (Zostera marina, hereafter referred to as either eelgrass or Zostera beds). Eelgrass beds in these estuaries remain important staging areas for southward migrating Canada Geese in the fall (Seymour 1997; Seymour et al. 2002; Hanson 2004). These eelgrass beds also provided more than enough wrack to allow for its common use as insulation, packed between wooden walls during construction, or piled up around the base of exposed houses in the fall (Wyllie-Echeverria and Cox 1999). Before 2001, pick-up trucks could be quickly filled from the wrack on one beach alone in the Antigonish estuary (AGM, personal observation). However, in 2001, a decline of the eelgrass beds in this estuary occurred that was so drastic that accumulation of eelgrass wrack was negligible. Sampling showed that the biomass of living roots and shoots had declined by about 95 % in the lower part of the Antigonish Harbour (Seymour et al. 2002; Garbary et al. 2004b; Garbary and Miller 2006). Migrating Canada Geese in this area feed on eelgrass rhizomes, and Common Goldeneye ducks (Bucephala clangula) feed on invertebrates associated with eelgrass. Due to the drastic eelgrass decline in 2001, the numbers of Canada Geese and Common Goldeneye using the Antigonish estuary as a staging area declined by about 50 % in the fall of 2001 (Seymour et al. 2002). Declines in eelgrass abundance, not always as drastic as that observed in the Antigonish estuary, were recorded at about the same time in other areas of Nova Scotia (Garbary et al. 2004b; Locke and Hanson 2004; Chapman and Smith 2004). Such a decline had not been observed since the decline of the early 1930s caused by “wasting disease” (Huntsman 1932; Lewis 1932), the infective agent now assumed to be the slime mould Labyrinthula zosterae (Muehlstein et al. 1991).
Declines in eelgrass abundance are increasingly common throughout its entire range (e.g. Orth and Moore 1983; Keser et al. 2003; Frederiksen et al. 2004; Costello and Kenworthy 2011). Many of these declines appear to be related to increased anthropogenic nitrogen input into estuaries, which favours the growth of algae leading to severe shading and death of the eelgrass (Short and Burdick 1996; Hauxwell et al. 2001, 2003). Declines can also be due to pollution, increased turbidity due to sediment suspension (van der Heide et al. 2007), or mechanical damage due to dredging or increased boat traffic. Some declines have been related to infection with L. zosterae (Short et al. 1987, 1988; Muehlstein et al. 1991). In 2001–2002, there were severe declines of eelgrass (Zostera marina) in estuaries along the Nova Scotia coast of the southern Gulf of St. Lawrence.
Davis et al. (1998) reported that the European green crab (Carcinus maenas; hereafter referred to as “green crab”) damaged eelgrass transplants during restoration projects, by ripping shoots in the lower sheath region. The rips included the vascular bundles, and the basal ends of the severed shoots had a “frayed” appearance as a result of the cutting and tearing by the crabs. We noticed that such frayed shoots were abundant in the eelgrass wrack in local estuaries during 2001 and 2002 (Garbary et al. 2004b; Garbary and Miller 2006). In crab enclosure experiments in natural eelgrass beds, Malyshev and Quijón (2011) confirmed that green crabs can sever eelgrass shoots from the rhizome as observed for transplanted eelgrass (Davis et al. 1998).
The green crab was first seen in the estuaries of the southern Gulf of St. Lawrence, including the Antigonish estuary (AG Miller, unpublished), in the mid-1990s. Genetic evidence clearly showed that the invasion of the Gulf was due to a new invasion from Europe (Roman 2006). More detailed descriptions of the northerly migration up the eastern coast of North America, and the recent reinvasion of North America are by Audet et al. (2003), Roman (2006), and Pringle et al. (2011). Mark–recapture experiments in the Antigonish estuary suggested that by the summer of 2001, the population of green crabs was as high as 500,000 within the study area of 1.3 km2 (Campbell 2001).
During a survey of eelgrass populations in local estuaries in June 2002, we entered Benoit Cove, a small cove in Tracadie Harbour, and found it contained a dense population of green crabs. We also observed that the eelgrass wrack on the shore was composed mainly of frayed shoots, characteristic of the cutting and tearing action by green crabs (Davis et al. 1998). In this cove, it was clear the decline of the eelgrass beds was still in progress, in contrast to Antigonish Harbour in which the decline was already largely complete. In the present paper, we describe the observations and experiments we performed to determine whether the drastic decline in Benoit Cove during the summer of 2002 was related to the recent invasion of green crabs.
Materials and methods
Initial survey of Tracadie Harbour
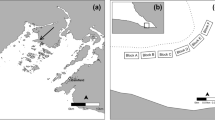
Following the collapse of Zostera beds in Antigonish Harbour in 2001 (Seymour et al. 2002), we initially surveyed Tracadie Harbour on 8 July 2002 to find a study site to evaluate processes associated with the eelgrass decline and to evaluate our hypothesis that this was associated with the invasion of green crab. It was important that the estuary had not been colonized by the invasive seaweed, Codium fragile, that had become common in many estuaries in the southern Gulf of St. Lawrence (Hubbard and Garbary 2002), and which was known to have minor impacts on eelgrass (Garbary et al. 2004a). Tracadie Harbour is an estuary, about 4.2 km × 2.6 km in maximum dimension, that opens into St. Georges Bay at the extreme southern end of the Gulf of St. Lawrence (Fig. 1). A visual inspection of the shorelines and benthos in Tracadie Harbour and the state of eelgrass beds were undertaken. In many areas, the eelgrass had disappeared, although decaying rhizomes were present in the sediment. In some areas, apparently healthy eelgrass beds occurred, e.g. on the harbour side of the gut opening into St. Georges Bay. Based on this initial survey, we selected Benoit Cove as the site for intensive study because of an extensive healthy eelgrass bed, a conspicuous population of green crab, and the isolation of the cove with respect to human disturbance.
a Map of Nova Scotia indicating location of Tracadie Harbour (TH) and Antigonish Harbour (AH), b enlargement showing Tracadie Harbour with location of Benoit Cove (BC) and Condon Cove (CC), which was already devoid of eelgrass by July 2002, c detail of Benoit Cove indicating Sites I and II where eelgrass wrack clearing experiments were conducted
Study site at Benoit Cove
Benoit Cove (45°37.92′N; 61°37.67′W) is a small inlet of 560 m × 220 m in maximum dimensions (Fig. 1) with an area of 68,400 m2 of which about 50,000 m2 consisted of an eelgrass bed in July. It communicates with the larger estuary by an opening of 10–130 m depending upon tide level. Salinity is typically in the mid-20's psu, except during times of freshet, when salinity may be reduced to 10 psu. Water temperature varies from −1.5 °C in winter when the cove has ice cover to about 25 °C in July–August. The tidal regime is semidiurnal with tidal amplitudes of 1.0–1.5 m. At low tide, the average depth is about 1 m. The shorelines consist mostly of sandy gravel with boulders along the eastern shore, and the cove bottom has soft sediment typical of regional estuarine eelgrass meadows. A barrier beach with diverse vegetation on the western shore separates the cove from the main portion of the estuary. Although extensive human development occurs around Tracadie Harbour (farms, scattered residences and cottages, two commercial fishing wharfs), the nearest dwelling to Benoit Cove is about 350 m away. When we began our studies in July 2002, most of the eelgrass bed appeared robust (Fig. 2a, b) and occupied most of the subtidal benthos of Benoit Cove.
Nature of eelgrass wrack in Benoit Cove, Nova Scotia (a) view towards southeast shore of cove showing part of the eelgrass bed and four green crab cages, (b) healthy eelgrass bed in June 2002, (c) north shore showing extensive coverage with eelgrass wrack comprising about 80 % shoots, (d) close-up view of wrack showing comprised mainly of shoots without blades, (e) “frayed shoots” from wrack on the shore, (f) “cut shoots”, with one to three internodes with the terminal internode cut through (arrow), (g) “rhizome shoots” from wrack with long lengths of rhizomes (long arrows) and with roots (short arrows)
Eelgrass wrack
In the absence of the invasive green crab, the Zostera wrack consists primarily of blades (i.e. leaves) that have been naturally abscised from shoots. Regionally, these blades historically accumulated on the shore as loosely packed windrows up to 50 cm deep. Following the invasion of green crabs, the wrack changed dramatically, consisting of detached blades to consisting largely of whole shoots (Fig. 2c, d). Based on the structure of their bases, we termed these as “frayed shoots”, “cut shoots”, or “rhizome shoots” (Fig. 2e–g). Frayed shoots had their bases highly frayed, above the meristem, as if a rasping or ripping action had removed them. The “cut shoots” were cleanly cut one to three internodes below the meristem. The “rhizome shoots” still had an extensive length of rhizome. At each visit to Benoit Cove, we collected a haphazard grab sample containing about 100 shoots from the wrack and determined the frequency of each shoot type.
Determination of shoot density using quadrats
Brass quadrats, 0.25 m × 0.25 m, were attached by 10 m of string to individual snorkellers (up to three snorkellers at any one time). Snorkellers threw the quadrat in a haphazard direction, swam to the quadrat, made a shoot count while underwater, returned to the surface, shouted a value to a recorder on the shore and threw the quadrat in preparation for the next count. This was repeated until 50–103 quadrat counts were obtained.
Shore wrack accumulation
We carried out a shore survey at low tide to measure the total area of wrack using a 30-m tape measure and metre stick (e.g. 25 m along shore at 1 m wide, 13 m along shore at 0.5 m wide, and so on). From these measurements, the total area of wrack was calculated (i.e. 625 m2). The total number of shoots in this wrack was estimated, in four quadrats, 0.5 m × 0.5 m (116 ± 32 shoots m−2, X ± SD). This value was corrected for shoot loss from the cove based on a mark–recapture experiment described below. The values of shoot numbers in wrack could be converted from total number of shoots to total biomass using the value 10 g fresh shoot−1. While we did not collect the detached blades for a similar biomass determination, no more than 15 % of the accumulated wrack consisted of normally abscised blades. It is the release of whole shoots, not unattached blades, from the eelgrass bed that is the unique feature of green crab activity and was the focus of our study.
The determination of the total number of shoots in the wrack and their biomass was used to produce measures of eelgrass bed decline. Furthermore, the difference between this value and the direct measure obtained by the snorkellers provided an estimate of biomass export from Benoit Cove in the form of floating wrack.
Caging experiments
Four square cages with dimensions 1.5 m × 1.5 m (Fig. 2a) were constructed of stiff plastic webbing (mesh size 3 cm × 3 cm) attached to 2.5-m-long stakes, and firmly driven into the soft substratum, with exposed mesh above high tide level. The cages were assigned either as control or experimental based on a random number table. Crabs were removed from all cages with baited traps, and then ten large green crabs (6–8 cm carapace width) were placed in each of the two experimental cages (4.4 crabs m−2). The number of crabs used per cage was based upon estimates of green crab density of 3 crabs m−2 in Antigonish Harbour, Nova Scotia (Campbell 2001) and 5 crabs m−2 in the USA (Young et al. 1999). After 7 days (our regular interval of return to Benoit Cove), the experiment was terminated, and the number of floating shoots was counted in each cage.
Pearson’s chi-squared distribution (Daniel 2005) was used to test the null hypothesis that crabs had no effect on the final frequency distribution of rooted versus floating eelgrass shoots (i.e. test of independence) in the control (no crabs) and test (with crabs) enclosures. The total number of eelgrass shoots in the cages at the time of cage installation was estimated from the results of the intensive quadrat analysis of the eelgrass bed, performed on the day the enclosures were set up (387 ± 68 shoots, X ± SD, N = 100). Two modes of analysis were performed. In the first, we made use of the fact that there were two control enclosures (no crabs) and two test cages (with crabs). Thus, the frequency distribution (at the end of the experiment) of rooted and floating shoots was analysed for the sum of the control pair and the sum of the test pair of enclosures. This summation provided an N value of 1,548 shoots for both the test and the control situation. To more specifically test for spatial effect in the eelgrass bed, the frequency distribution of the four possible pairs of control and test enclosures was also tested for statistical significance. In this analysis, the N value was 774 shoots cage−1.
Bare patch formation
In addition to a generalized thinning of the Zostera bed, areas both near the shore and within the bed were becoming completely devoid of Zostera shoots. In late July, we began to monitor ten of these bare patches by placing wooden dowels or stakes at the margin of each patch, measuring the patches and making scale drawings of the patches on graph paper. On two occasions (August and September), we returned to the patches and added new markers to indicate their expansion. Care was taken not to trample within the bare patch or in the surrounding remnants of the eelgrass. Bare patches that could not be accessed easily without trampling the adjacent Zostera shoots had stakes inserted from a plastic dinghy.
Mark–recapture experiments
Shore experiment
To estimate the total (gross) rate of shoot wrack accumulation on the shore, and therefore, the rate of eelgrass bed decline, it was necessary to determine the extent to which the shoot wrack was relocated to other areas, after it was initially deposited. We selected two different areas of shoreline (Sites I and II in Fig. 1c). In each of these areas, we removed all of the wrack that had accumulated on the shore and deposited it well above the reach of the highest tide. On each of the now bare shores, we placed 100 eelgrass shoots, collected from fresh wrack, that had been tagged with different coloured flagging tape. The ribbons of flagging tape (1 cm × 20 cm) had a small mass (0.5 g) compared to the average 10 g mass of the shoots. We observed no difference in floating behaviour between flagged and non-flagged shoots. Also, the nature of the recovered tagged shoots gave no evidence that water drag on the ribbon had caused their uprooting from the beds, as most had either “frayed” or “cut” not “rhizome” shoots. These tagged shoots were used to estimate the loss rate of already accumulated shoot wrack from the two shore areas due to subsequent ebb tides. After 48 h, we returned and counted the number of tagged shoots remaining in each location. The use of the two colours of flagging tape allowed us not only to estimate the loss of deposited shoots from each area, but also to estimate any shoot exchange between the two shore areas under investigation. We also walked the entire shoreline of Benoit cove to look for tagged shoots that might have been relocated by tidal action.
Open-water experiment
As an addition to the “expanding bare patch” method already described, a “mark–recapture” method similar to that used to estimate the size of animal populations (Bakus 2007) was used to estimate the rate of shoot loss from the eelgrass bed. Investigators in small plastic dinghies paddled (8 August 2002) over the eelgrass bed in the upper part of the cove (area about 13,000 m2), attaching ribbons (2 cm × 20 cm) of flagging tape to the bases of still rooted shoots (N = 200), chosen haphazardly. After 1 week, an intensive search was made for these tagged shoots in the wrack along the shoreline within Benoit Cove, in the floating mats of shoot wrack in the cove, and along the shoreline of Tracadie Harbour itself up to 300 m in each direction from the channel entering Benoit Cove.
Observations on Carcinus maenas
Upon our first entrance into Benoit Cove, numerous green crabs were observed moving along the shore in shallow water parallel to the edge of the eelgrass bed. Crabs were also observed within the eelgrass bed. Numerous crab foraging pits occurred in the shallow mud, unoccupied by eelgrass. In addition to the conspicuous crab pits, the bottom had numerous areas of older pits that appeared as circular discoloured benthos covering up to 50 % of the substratum. The volume of pits was determined by measuring the depth and diameter of recently dug pits (N = 10).
Estimates of green crab abundance were obtained in three ways: (1) setting of baited traps for 30-min periods, (2) counting the number of crabs located in 1-m2 plots, and (3) counting the number of crabs moving past a virtual point along “crab runways” in shallow water parallel to the shore for four 10-min periods.
Results
Eelgrass wrack: detachment forms
Upon our first visit to Benoit Cove (14 July 2002), large areas of intact eelgrass bed with healthy green vegetative and reproductive shoots still existed (Fig. 2b). We noticed that the shoreline was covered with very abundant eelgrass wrack (Fig. 2c), greater than expected for that early in the season, and that most of this wrack (ca. 85 %) was comprised of intact eelgrass shoots (Fig. 2d), and only 15 % of eelgrass blades. The wrack on the shores of Benoit Cove from July 14 onward consisted of entire shoots which we categorize as: (1) “frayed shoots” (Fig. 2e), (2) “cut shoots” (Fig. 2f), and (3) “rhizome shoots” (Fig. 2g) as described in the “Materials and methods”.
The proportions of the three shoot types varied over the summer. Cut shoots showed the least variation, ranging between 15 and 30 % frequency between July and September (Fig. 3a). As the summer progressed, the percentage of frayed shoots dropped from over 80 % to about 10 %, and there was a major increase in the percentage of rhizome shoots in late August and September (Fig. 3a).
a Changing composition of shoot types in eelgrass wrack in Benoit Cove throughout the summer of 2002 (N = ca. 100 for each sampling date); closed circles—frayed shoots; open circles—cut shoots; triangles—rhizome shoots. b Decline in shoot density in eelgrass bed in Benoit Cove in 2002 (plotted points are X ± SEM, N = 50 − 103)
Decline in eelgrass abundance: direct measurement in bed
The heavy accumulation of shoot wrack on the shore (Fig. 2c, d) correlated with a drastic decline in the abundance of shoots (shoot density) in the eelgrass bed (Fig. 3b). As measurements of eelgrass density were not begun until mid-July, when the situation in Benoit Cove was first seen, the overall decline of eelgrass density during 2002 was certainly underestimated. From Fig. 3b, daily rate of loss of 3.6 shoots m−2 day−1 can be calculated for the decline from July 24 to August 8. This rate increased to 4.5 shoots m−2 day−1 in the subsequent 14 days. This provided an overall average of 4.0 shoots m−2 day−1 (or 40 g m−2 day−1) over the total 29-day period. During the subsequent 3 weeks, shoot loss was reduced to 0.6 shoots m−2 day−1. Assuming a similar decline from May 1 to July 24, a period of 85 days, then the eelgrass density on May 1 could have been about 500 shoots m−2.
The reduction in overall shoot density was based on both an overall thinning of eelgrass throughout the bed and formation of bare patches. The data used to construct Fig. 3b also include the actual frequency of quadrats with a given number of shoots. Plotting these frequencies (Fig. 4) shows that although large bare patches were being formed in the bed (see later), there was also an overall thinning of the bed. In Fig. 4, we have plotted the frequency distribution of the shoot densities on each sampling date, as shoots per 20 cm × 20 cm quadrat. By September 14, there were no quadrats with the high shoot densities (i.e. 12–13, 10–11, and 8–9 shoots quadrat−1) observed on July 14.
Decline in eelgrass abundance: indirect measurement from wrack
Forty-eight hours after clearing the two sites of wrack, subsequent accumulation was higher at Site I than at Site II (Table 1). The average values of all variables from both sites appear to be very similar, but the high variance at Site II precludes meaningful statistical comparison of the two sites. However, reasonable errors are obtained when the values for the 10 quadrats at each site are pooled as a common population called Site I + Site II (Table 1 with N = 20). Considering this population, it is evident that the relative proportion of the three shoot types (frayed, cut, rhizome) on July 16 was similar to that obtained on July 14. Overall, frayed shoots were the most abundant, >80 % total shoots, in both cases (cf. Fig. 3a, Table 1). We found that tagged shoots (Fig. 5a) placed on areas cleared of wrack remained in place (i.e. 85.4 % of tagged shoots). This shows that once deposited on the shore, shoots remained largely in place. The combined wrack area of Sites I + II was 29.4 % of the total wrack accumulation area around Benoit Cove.
Green crab impacts in Benoit Cove in 2002 (a) portion of area used for wrack accumulation study with some of the ribbon tagged shoots (arrows), (b) bare patch in eelgrass bed with high turbidity resulting from green crab foraging, (c) change in area of bare patch with initial outline marked by stakes (long arrows) and second outline marked by dowels (short arrows), (d) extensive area that has been cleared by crabs, with abundant remains of foraged clams, (e) single crab foraging pit with exposed rhizomes of eelgrass (arrows), (f) extensive area cleared of eelgrass with numerous, partially eroded crab foraging pits (arrows)
If it is assumed the rate of shoot accumulation at Site I + II is similar to that at the other, unmeasured accumulation sites, then a shoot deposition rate of 81,327 ± 18,513 shoots day−1 can be calculated (Table 1). Given that there are two high tides each day in the region, the deposition rate would be 40,663 ± 9257 shoots tide−1 (Table 1). For all Benoit Cove, this equates to shoot deposition of 407 kg tide−1 or about 814 kg day−1. The total water area of Benoit Cove is about 68,400 m2 and about 50,000 m2 of this was occupied by eelgrass, albeit of already declining density by July 14. The loss rate from the eelgrass bed, based on the shoot deposition in the total shoreline wrack, can be calculated as being 1.6 shoots m−2 day−1, lower than the loss rate (4.1 shoots m−2 day−1) directly observed (Fig. 3b).
Crab cage experiment
The cage experiment showed the ability of green crabs to remove entire shoots from eelgrass beds (Table 2). Mean numbers of floating shoots from the two control and two experimental cages were 7 and 60 shoots, respectively. When the two control and two experimental cages were each considered as single treatments, the chi-squared distribution was significant at p < 0.001 (total N = 1,548). When each cage was considered independently, and all four possible combinations of experimental and control cages were considered, the probability (p) of obtaining these results by chance alone was p < 0.005 (N = 774). Thus, the difference between treatments in the two cage types was highly significant. The presence of some floating shoots in the enclosures without crabs suggests that (a) not all the crabs were removed from the 1.5 m × 1.5 m before the enclosure was complete, (b) crabs were able to burrow into the enclosure during the 7-day experiment, (c) partially damaged shoots only came to the surface after the enclosure was in place, or (d) insertion of the cages resulted in some damage to the bed by uprooting some shoots. The types of shoot (frayed, cut, rhizome) found floating in the enclosures (Table 2) were the same as those outside the cages (Fig. 3a). The loss rates of the intact shoots from the eelgrass growing in the crab enclosures were 4.4 and 2.6 shoots m−2 day−1 (average = 3.5) (Table 2). The shoot density in the eelgrass bed in which the enclosures were placed on July 24 was 172 shoots m−2 (Fig. 3b). Given the loss rate of shoots of 4.1 m2 day−1, a week later the density would have been about 146 shoots m−2.
Bare patch formation
In addition to the overall changes in shoot density throughout the eelgrass bed, we noted the formation of bare patches within the eelgrass bed (Fig. 5b). Changes in the size of the bare patches were highly variable (Table 3). Only one patch (i.e. #5) did not change in size over the observation period. Bare patches tended to double in size between each 3-week monitoring interval (i.e. 0.8, 1.5, and 2.9 m2) and were adjacent to large swatches of eelgrass bed, where the density was conspicuously reduced. Indeed, the thinning (Fig. 5c, d) of the bed adjacent to the bare zones made it increasingly difficult to discern an actual margin. The large apparent change in the size of patch #4 from August to September is an artefact of the fusion of patch #4 with adjacent expanding bare patches, which were not being monitored. The September value for patch #4 was therefore omitted from calculation for mean patch size.
Open-water mark–recapture
Of the 200 tagged shoots, nine were found either as floating wrack or washed up on the shore of Benoit Cove after 7 days. Three tags were found outside the cove washed up on shores within 300 m of the cove entrance. These three tagged shoots give a minimum estimate of 33 % loss of detached shoots from the cove. Assuming we recovered all of the tagged shoots that were detached from the benthos of Benoit Cove gives an estimate of 6 % loss of shoots after 7 days or 0.9 % day−1.
Crab abundance and localization
Measurement of ten pits in Benoit Cove showed them to be essentially circular and to have an average diameter of 23.9 ± 5.0 (X ± SD) cm, an average area of 449 ± 181 cm2, an average depth of 10.8 ± 2.2 cm, and an average volume of 4,845 ± 2,623 cm3. When such large pits are dug amongst eelgrass, green crabs cause severe disruption to the rhizome network of eelgrass (Fig. 5e). It is clear how digging by green crabs will result in “rhizome shoots” in the eelgrass wrack. In calm water, one could observe “frayed shoots” drifting up to the surface. Crabs could be seen tearing off eelgrass shoots as they entered eelgrass patches from the bare intertidal mud. Whether the crabs were trying to clear an area in order to dig a pit, for protection, or to find Mya arenaria (hereafter soft-shell clam), or whether they were just tearing off the eelgrass shoots to facilitate their movement through the eelgrass was unclear. Regardless, these combined activities can clear large continuous areas of eelgrass (Fig. 5b–f).
On July 13, traps were set in various areas of the cove for set times of 30 min. The highest numbers of crabs were captured on the bare intertidal mud and in eelgrass patches adjacent to this mud (Table 4). Crabs were captured in eelgrass patches as far as 10 m from the intertidal mud, but at a much lower rate (Table 4). Direct observation of crabs on the intertidal mud showed an increase in green crab activity during an ebbing tide (Table 4). For various times after the tide turned, the movement in any direction of crabs past a fixed point on the intertidal mud was observed. A peak value of 42 crabs 10 min−1 occurred 1 h after onset of the ebb tide (Table 4).
Discussion
In 2001, there was a drastic decline in the eelgrass beds of the Antigonish Harbour, so drastic that even non-biologists commented upon it. One older observer likened the extent of the eelgrass decline to that of the 1930, caused by the “wasting disease” (AGM, personal communication). The decline was clearly an exceptional event, not at all similar to any natural seasonal changes observed in this region. Our 2002 survey showed that the eelgrass beds in the other local estuaries had already declined or were in the process of declining. Benoit Cove was one of the latter.
Evidence that green crabs caused the drastic decline of eelgrass density in Benoit Cove in 2002 includes the following: (1) Only the crabs could cause the accumulation of detached “frayed” shoots (Fig. 2c–e), rather than the detached blades which normally comprises the eelgrass wrack (Davis et al. 1998); (2) the rate of detached shoot formation was much greater in enclosures to which green crabs had been added than those enclosures which, nominally at least, contained none (Table 2); (3) the rate of shoot detachment (shoots m−2 eelgrass bed day−1) by crabs in the enclosures closely agreed with that determined from a direct measure of density changes in the eelgrass bed (Table 5); (4) much of the decline occurred as the result of expansion of bare patches (Table 3), which was evidently the result of green crab foraging in these bare patches (e.g. Fig. 5b–f). The above symptomatology was consistent in all estuaries in the region, including Antigonish Harbour, especially the abnormal occurrence of shoots rather than blades in the wrack and the co-occurrence of this with high green crab density.
Our observations and experiments in Benoit Cove provided four different estimates of eelgrass decline ranging from 4.1 shoots m−2 day−1 in the cage experiment to 1.1 shoots m−2 day−1 in the mark–recapture experiment (Table 5). Given the constraints of obtaining accurate results from mark–recapture experiments (e.g. Bakus 2007), and our small sample size of 200 marked shoots, it is not surprising that the recapture rate underestimated shoot loss relative to our absolute measure by 75 %. Regardless, to our knowledge, this is the first mark–recapture experiment undertaken with whole plant shoots. The two low values for estimating bed decline based on wrack accumulation and mark–recapture measures are certainly underestimates that reflect the limitations of the methods. However, they provide two estimates of biomass export from the cove of roughly 33 and 60 % of shoots produced due to crab activity, respectively. The value of 60 % was derived as follows from Tables 1 and 5. The direct method gives the most accurate measure of shoot density decline in the eelgrass bed (i.e. 4.0 shoots m−2 day−1). Knowing the area of wrack accumulation (625 m2) and the shoot density in the wrack (116 ± 32 shoots m−2—see “Materials and methods”), then the difference between 4. 0 and 1.6 is an estimate (2.4 shoots m−2 day−1, i.e. 60 %) of export rate from the cove. The 33 % is a clear underestimate of shoot export from Benoit Cove based on the offshore tagging experiment, since it does not take account of the probability that marked shoots were removed more than 300 m away from the cove entrance on ebb tides. Regardless, these values (i.e. 33 and 60 %) are consistent with the large floating rafts of shoots that were observed moving out of the cove during ebb tides during July and August. That the cage experiment and the direct measure of shoot loss gave similar results in terms of rates of shoot loss may be fortuitous given the variation in the two experimental cages, i.e. 5.1 and 2.9 shoots m−2 day−1. Regardless, the congruence of the two approaches is strong evidence that C. maenas was the primary cause of eelgrass loss in the system in 2002.
The rate of shoot deposition on the shore was 81,300 shoots day−1 (Table 1) or 814 kg of shoot biomass per day (based on a value of 10 g shoot−1). From Table 5, we indicate that the total loss was 4 shoots m−2 day−1 of bed area (see Fig. 3b). Using the total bed area of 50,000 m2 results in an estimate of 200,000 shoots (i.e. 2,000 kg) lost per day. This loss includes shoots deposited as wrack in the cove and shoots exported from the cove as floating wrack. The calculation of shoot accumulation on the shore, combined with our estimate of shoot export, gives a similar value to the rate of total rate of shoot loss from the cove.
By observation in this habitat, we estimated a crab density of about 0.6 crabs m−2. Based on mark–recapture experiments, Polk (2008) estimated the crab density in eelgrass beds of Pomquet Harbour (an adjacent estuary) to be 0.78 crabs m−2 in 2006 and 1.04 crabs m−2 in 2007. The rapid development of a high population density of green crabs by 1999, after an introduction about 1994–1995 in Antigonish Harbour, and then the drastic decline of the eelgrass beds in 2001, was noticed by even casual observers. In Antigonish Harbour in 2001, following the drastic decline by 2001, there was very little eelgrass wrack of any kind being deposited on the harbour shores. However, as the eelgrass beds in the harbour began to recover in 2002, the presence of shoot wrack was evident, indicating the continuing effect of green crabs. Frayed shoots in wrack were also a prominent feature in other estuaries in the southern Gulf of St. Lawrence. The first report in the literature of “frayed” and “cut” eelgrass shoots, caused by foraging green crabs, was by Davis et al. (1998) in naturally occurring and transplanted eelgrass beds in Great Bay Estuary, New Hampshire. They provided images of a green crab holding an eelgrass shoots in both chela, then turning while still holding the shoot, bending the shoot towards the sediment, and causing the shoot to be ripped and cut across the sheath, and become separated from the rhizome. No evidence of green crabs consuming (i.e. grazing on) the eelgrass was observed; instead the crabs were probably clearing their way to subsequent digging up of nearby soft-shell clams. The latter are a preferred prey item for green crabs (Ropes 1968; Elner 1981; Grosholz and Ruiz 1996), including those that have recently invaded the southern Gulf of St. Lawrence (Floyd and Williams 2004). The high abundance of the soft-shell clams in Benoit Cove in 2002 was evident from extensive shell debris in the crab foraging patches (Fig. 5d–f).
In Benoit Cove, there was a definite decrease in the proportion of “frayed” shoots, but an increase in the proportion of “rhizome” shoots as summer, and thus the cumulative effect of crab foraging, progressed (Fig. 3a). The extent of bioturbation by the crabs was impressive, resulting in large amounts of sediment being resuspended (Fig. 5b). A local resident, who occasionally canoed in Benoit Cove, likened the suspension cloud near foraging crabs to that caused by a “herd of buffalo”. By early September, the sediment had been so loosened that when one pulled gently on the tip of an eelgrass blade, the shoot with a long length of attached rhizome was pulled up. Pulling on an eelgrass shoot by a foraging crab must have produced same result, and not frayed shoots. The resuspended sediment deposited heavily on the eelgrass blades, especially those bearing epiphytes, and must have severely reduced the rate of photosynthesis (Burke et al. 1996). The bioturbation and decline of eelgrass that we observed associated with foraging crab activity on clams is similar to that observed in Chesapeake Bay eelgrass beds by the Cownose Ray (Orth 1975).
The more usually reported reasons for eelgrass decline also did not apply to the decline in Benoit Cove. As with most eelgrass beds in Nova Scotia, one can find a small percentage of eelgrass blades with blackened areas that may well be due to infection with L. zosterae (Burdick et al. 1993). But most of the shoots, either attached or in the wrack, showed little or no evidence of such infection. Another major cause of eelgrass decline is eutrophication resulting in increased macroalgal growth (e.g. Ulva species) which shades the eelgrass, greatly reducing its ability to photosynthesize (Schmidt et al. 2012). Macroalgae were not abundant in the parts of the eelgrass bed in Benoit Cove, similar to other areas of Tracadie Harbour that we examined (e.g. Condon Cove, Fig. 1b), but large numbers of green crabs were present. As the summer progressed, the eelgrass in Benoit Cove developed a thick coating of resuspended sediment, especially on blades with epiphytes (Fig. 5b). Much of the thick coating on the blades was released upon slight agitation, reflecting the extent of fine sediment accumulation. The covering on the blades would have reduced their rate of photosynthesis, just as increased growth of macroalgae would have. It is also possible that eutrophication of a sort did occur (i.e. via release of nitrate and phosphate from the sediment), due to bioturbation of the sediment by the crabs, and this may have increased the rate of epiphyte growth. If so, this would have been an added stress on the eelgrass caused by the green crabs.
Another mode of shoot detachment by green crabs was recently observed using laboratory-based video recording; this involved the eating or “grazing” of the shoot meristem by juvenile crabs (Malyshev and Quijón 2011). The basal ends of the detached shoots produced by this consumption of the meristem are sharply truncated or “cleanly cut” and not frayed or attached to rhizome internodes. The role of green crab foraging activity, e.g. on clams, versus green crab grazing on eelgrass meristems in the destruction of eelgrass beds, will depend partly on the size structure of the crab population in an eelgrass bed. Malyshev and Quijón (2011) observed that only juvenile green crabs grazed, i.e. ate eelgrass meristems, while adult crabs ripped up shoots.
It is possible that when green crabs initially invade an area, the abundance of soft-shell clams can allow the crabs to feed mainly on this preferred prey (Ropes 1968; Floyd and Williams 2004). This feeding on soft-shell clams requires the crabs to dig up the clams, often located within eelgrass beds, causing the severe disruption we have described in this paper. As the soft-shell clam population declines (Floyd and Williams 2004) as a result of this new form of predation, the crabs are forced to switch to other food sources such as periwinkles, mussels, oysters, small invertebrates, and green algae (Ropes 1968; Elner 1981; Smallegange and van der Meer 2003; Donahue et al. 2009; Pickering and Quijon 2011), which do not require the crabs to dig pits in the mud.
This study was carried out in the context of a phenomenon that had not been previously characterized. The widespread occurrence of this event in our region resulted in a lack of potential control sites. Under these difficulties, we used quantitative data (both experimental and observational) to characterize a rapidly changing habitat and resolve its underlying mechanism. For the first time, we have shown the ability of green crabs to decimate intact beds of eelgrass. In the context of the spread of green crabs in the southern Gulf of St. Lawrence (e.g. Malyshev and Quijón 2011), we have provided a model for the observation and analysis of the impacts of this invasive species as it pertains to Zostera, an ecological engineer of fundamental ecological importance in estuaries.
Postscript
Subsequent to the observations in 2002 in Benoit Cove, we continued to monitor changes in eelgrass beds in the estuaries of St. Georges Bay. Numerous visits each year to Antigonish Harbour have shown a gradual reintroduction of eelgrass beds since the devastation in 2000–2002, and populations of C. maenas appear to be lower (Miller unpublished). However, substantial amounts of “rhizome shoots” still appear on the shoreline wrack due to green crab activity in the eelgrass beds. By 2013, Antigonish Harbour eelgrass beds had recovered to about 60 % of their pre-2000 abundance. Three subsequent trips to Benoit Cove in 2005, 2009, and 2013 showed considerable recovery by 2005 (86 ± 3 shoots m−2, X ± SE). This declined to 28 ± 1 shoots m−2 in early September 2009 suggesting an ongoing impact of green crabs. A complete extirpation of eelgrass was revealed in June 2013, and the substratum was not invaded by macrophytic algae. The shores showed a healthy oyster population; however, the benthos was depauperate, probably due to the presence of very fine sediment throughout the cove. During the 2013 snorkelling survey of approximately 400 m2, only 10 green crabs were observed, i.e. about 0.03 crabs m−2, considerably less than the 0.6 crabs m−2 observed in the same area in 2002.
References
Audet D, Davis DS, Miron G, Moriyasu M, Benhalima K, Campbell R (2003) Geographical expansion of a nonindigenous crab, Carcinus maenas (L.) along the Nova Scotian shore into the southeastern Gulf of St. Lawrence. Can J Shellfish Res 22:255–262
Bakus GJ (2007) Quantitative analysis of marine biological communities. Wiley, Hoboken, NJ
Burdick DM, Short FT, Wolf J (1993) An index to assess and monitor the progression of wasting disease in eelgrass Zostera marina. Mar Ecol Prog Ser 94:83–90
Burke MK, Dennison WC, Moore KA (1996) Non-structural carbohydrate reserves of eelgrass Zostera marina. Mar Ecol Prog Ser 137:195–201
Campbell E (2001) The ecology of the green crab, Carcinus maenas, and its invasion in the southern Gulf of St. Honours Dissertation. St. Francis University, Antigonish, Nova Scotia, Lawrence
Chapman A, Smith J (2004) Quantifying decline of eelgrass beds on the eastern shore of Nova Scotia between 1992 and 2002. In: Hanson AR (ed) Status and conservation of eelgrass (Zostera marina) in Eastern Canada, p 9 (Can Wildlife Service Tech Rep No. 412)
Costello CT, Kenworthy WJ (2011) Twelve-year mapping and change analysis of eelgrass (Zostera marina) areal abundance in Massachusetts (USA) identifies statewide declines. Estuaries Coast 34:232–242
Daniel WW (2005) Biostatistics: a foundation for analysis in the health sciences, 8th edn. Wiley, Hoboken NJ
Davis RC, Short FT, Burdick DM (1998) Quantifying the effects of green crab damage to eelgrass transplants. Rest Ecol 6:297–302
Donahue MJ, Nichols A, Santamaria CA, League-Pike PE, Krediet CJ, Perez KO, Shulman MJ (2009) Predation risk, prey abundance, and the vertical distribution of three brachyuran crabs on Gulf of Maine shores. J Crustac Biol 29:523–531
Elner RW (1981) Diet of green crab Carcinus maenas (L.) from Port Hebert, southwestern Nova Scotia. J Shellfish Res 1:89–94
Floyd T, Williams J (2004) Impact of green crab (Carcinus maenas L.) predation on a population of soft-shell clam (Mya arenaria L.) in the southern Gulf of St. Lawrence. J Shellfish Res 23:457–462
Frederiksen M, Krause-Jensen D, Holmer M, Laursen JS (2004) Long-term changes in area distribution of eelgrass (Zostera marina) in Danish coastal waters. Aquat Bot 78:167–181
Garbary DJ, Miller AG (2006) Green crabs (Carcinus maenas) as the grim reaper: destruction of eelgrass beds in Nova Scotia. J Shellfish Res 25:728
Garbary DJ, Fraser SJ, Hubbard C, Kim KY (2004a) Codium fragile: rhizomatous growth in the Zostera thief of eastern Canada. Helgol Mar Res 58:141–146
Garbary DG, Miller AG, Seymour NS (2004b) Destruction of eelgrass beds in Nova Scotia by the invasive green crab. In: Hanson AR (ed) Status and conservation of eelgrass (Zostera marina) in Eastern Canada, pp 13–14 (Can Wildlife Service Tech Rep No. 412)
Grosholz ED, Ruiz GM (1996) Predicting the impact of introduced marine species: lessons from the multiple invasions of the European green crab. Biol Conserv 78:59–66
Hanson AR (ed) (2004) Status and conservation of eelgrass (Zostera maenas) in Eastern Canada (Can Wildlife Service Tech Rep No. 412)
Hauxwell J, Cebrian J, Valiela I (2001) Macroalgal canopies contribute to eelgrass (Zostera marina) decline in temperate estuarine ecosystems. Ecology 82:1007–1022
Hauxwell J, Cebrian J, Valiela I (2003) Eelgrass Zostera marina loss in temperate estuaries: relationship to land-derived nitrogen loads and effect of light limitation imposed by algae. Mar Ecol Prog Ser 247:59–73
Hubbard CB, Garbary DJ (2002) Morphological variation of Codium fragile (Chlorophyta) in eastern Canada. Bot Mar 45:476–485
Huntsman AG (1932) Disease in eel grass. Prog Rep Atl Biol Stn 5:11–14
Keser M, Swenarton JT, Vozarik JM, Foertch JF (2003) Decline in eelgrass (Zostera marina L.) in Long Island Sound near Millstone Point, Connecticut (USA) unrelated to thermal input. J Sea Res 49:11–26
Lewis H (1932) The eelgrass situation on the Atlantic Coast. In: Trans 19th American Game Conference, New York, NY, pp 409–423 (Held under the auspices of the American Game Association)
Locke A, Hanson JM (2004) Changes in eelgrass in southern Gulf of St. Lawrence estuaries. In: Hanson AR (ed) Status and conservation of eelgrass (Zostera marina) in Eastern Canada, pp 10–12 (Can Wildlife Service Tech Rep No. 412)
Malyshev A, Quijón PA (2011) Disruption of essential habitat by a coastal invader: new evidence of the effects of green crabs on eelgrass beds. ICES J Mar Sci 68:1852–1856
Muehlstein LK, Porter D, Short FT (1991) Labyrinthula zosterae sp. nov., the causative agent of wasting disease of eelgrass Zostera marina. Mycologia 83:180–191
Orth RJ (1975) Destruction of Eelgrass, Zostera marina, by the Cownose Ray, Rhinoptera bonasus, in the Chesapeake Bay. Chesapeake Sci 16:205–208
Orth RJ, Moore KA (1983) Chesapeake Bay: an unprecedented decline in submerged aquatic vegetation. Science 222:51–53
Pickering T, Quijon PA (2011) Potential effects of a non-indigenous predator in its expanded range; assessing green crab, Carcinus maenas, prey preference in a productive coastal area of Atlantic Canada. Mar Biol 158:2065–2078
Polk ZCS (2008) Population estimates and characteristics of the invasive green crab Carcinus maenas. Honours Dissertation. St. Francis Xavier University, Antigonish, Nova Scotia
Pringle JM, Blakeslee AMH, Byers JE, Roman J (2011) Asymmetric control allows an upstream region to control population structure throughout a species’ range. Proc Natl Acad Sci (USA) 108:15288–15293
Roman J (2006) Diluting the founder effect: cryptic invasions expand a marine invader’s range. Proc R Soc B 273:2453–2459
Ropes JW (1968) The feeding habitats of the green crab, Carcinus maenas (L.). Fish Bull 67:183–203
Schmidt AL, Wysmyk JKC, Craig SE, Lotze HK (2012) Regional-scale effects of eutrophication on ecosystem structure and services of seagrass beds. Limnol Oceanogr 57:1389–1402
Seymour NR (1997) Distribution, abundance and activity of migrant Canada geese in Antigonish harbour, Nova Scotia. In: Erskine AJ (ed) Canada goose studies in the Maritime Provinces 1950–1992. Environment Canada, Atlantic Region, Sackville, New Brunswick, pp 70–80
Seymour NR, Miller AG, Garbary DJ (2002) Decline of Canada geese (Branta canadensis) and common goldeneye (Bucephala clangula) associated with a collapse of eelgrass (Zostera marina) in a Nova Scotia estuary. Helgol Mar Res 56:198–202
Short FT, Burdick DM (1996) Quantifying eelgrass habitat loss in relation to housing development and nitrogen loading in Waquoit Bay, Massachussets. Estuaries 19:730–739
Short FT, Muehlstein LK, Porter D (1987) Eelgrass wasting disease: cause and recurrence of a marine epidemic. Biol Bull 173:557–562
Short FT, Ibelings BW, den Hartog C (1988) Comparison of a current eelgrass disease to the wasting disease in the 1930s. Aquat Bot 30:295–304
Smallegange IM, van der Meer J (2003) Why do shore crabs not prefer the most profitable mussels? J Anim Ecol 72:599–607
van der Heide T, van Nes EH, Geerling GW, Smolders AJP, Bouma TJ, van Katwijk MM (2007) Positive feedback in seagrass ecosystems: implications for success in conservation and restoration. Ecosytems 10:1311–1322
Wyllie-Echeverria S, Cox PA (1999) The seagrass (Zostera marina [Zosteraceae]) industry of Nova Scotia (1907–1960). Econ Bot 53:419–426
Young T, Komarow S, Deegan L, Garritt R (1999) Population size and summer home range of the green crabs, Carcinus maenas, in salt marsh tidal creeks. Biol Bull 197:297–299
Acknowledgments
We thank the following for assistance during the course of field work: David Chaisson, Sarah Fraser, Trevor Floyd, Robert Garbary, Ray McCarthy, and Dawn Moxsom. This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada to DJG, AGM and JW.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Kraufvelin.
Rights and permissions
About this article
Cite this article
Garbary, D.J., Miller, A.G., Williams, J. et al. Drastic decline of an extensive eelgrass bed in Nova Scotia due to the activity of the invasive green crab (Carcinus maenas). Mar Biol 161, 3–15 (2014). https://doi.org/10.1007/s00227-013-2323-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-013-2323-4