Abstract
Effectiveness of exercise on bone mass is closely related to the mode of exercise training regimen, as well as the study design. This study aimed to determine the effect of different modes of exercise training on lumbar spine and femoral neck bone mineral density (BMD) in older postmenopausal women (PMW). PubMed, CINAHL, Medline, Google Scholar, and Scopus databases and reference lists of included studies were searched up until March 25, 2019 for randomized controlled trials (RCTs) that evaluated the effectiveness of various modes of exercise training in PMW. Sixteen RCTs with 1624 subjects were included. Our study found no significant change in both lumbar spine and femoral neck BMD following exercise training (MD: 0.01 g/cm2; 95% confidence interval (CI) [− 0.01, 0.02] and MD: 0.00 g/cm2; 95% CI [− 0.01, 0.01], respectively). However, subgroup analysis by type of exercise training revealed that lumbar spine BMD (MD: 0.01; 95% CI [0.00, 0.02]) raised significantly when whole-body vibration (WBV) was employed as intervention compared with RCTs that utilized aerobic (MD: − 0.01; 95% CI [− 0.02, − 0.01]), resistance (MD: 0.01; 95% CI [− 0.04, 0.06]), and combined training (MD: 0.03; 95% CI [− 0.01, 0.08]). On the other hand, lumbar spine BMD (MD: − 0.01; 95% CI [− 0.02, − 0.01]) reduced significantly when aerobic exercise training was used as intervention compared with RCTs that utilized resistance training, combined training, and WBV. By contrast, these analyses did not have significant effect on change in femoral neck BMD. WBV is an effective method to improve lumbar spine BMD in older PMW.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a loss of bone mass with a deterioration of bone quality and increased fracture risk. There is a worldwide epidemic associated with increased fracture risk leading to morbidity, mortality, and socioeconomic burden [1, 2]. Some studies have shown that osteoporosis prevalence has increased due to increased life expectancy and sedentary lifestyle, as well as poor dietary habits [3, 4]. Clinically, osteoporosis is a silent disease characterized by increased bone resorption without adequate compensating formation of new bone [5]. After bone mass reaches its peak it remains relatively stable until the onset of menopause in women. Thus, osteoporosis affects postmenopausal women (PMW) because of the suppression or absence of estrogen production [6]. It should be noted that estrogen acts directly on bone by suppression of osteocyte receptors that activate osteoclastic activity.
The rate of change in bone mass and density is greater at sites with predominantly trabecular bone. In older PMW, osteogenic responses with exercise training on bone are more sensitive at the loading sites because PMW over the age of 70 years tend to have lower trabecular and cortical bone mineral density (BMD) and cortical thickness, while younger PMW between 48 and 69 years tend to have higher total cross-sectional area and endosteal circumference [7].
Bones are active dynamic tissues undergoing constant growth via the process of bone modeling and remodeling. Osteocytes are the architect of the bone remodeling process because their interconnected network of cells are capable of detecting mechanical strain and fluid pressure by initiating the process of bone modeling and remodeling. As described by Robling et al. [8], mechanical forces applied to the bone tissue induce interstitial fluid movements along the canaliculi and osteocyte lacunae causing shear stress at the cellular level and deformations of osteocyte plasma membrane. These changes lead to the beginning of the bone remodeling process that stimulates the bone resorption and formation cycle [9]. Removal of these mechanical strains and impact-loading forces, such as physical inactivity or bedrest, lead to low bone mass and BMD. Thus, the application of exercise training with impact loading on bone and whole-body vibration training is to initiate bone formation and prevent bone resorption. Individuals who have BMD value below the osteoporotic level (i.e., femoral or lumbar spine BMD z-score lower than − 2.5 standard deviation of young women), sustained more than half of all hip fractures [10, 11]. Therefore, bone researchers and clinicians believed that biomechanical strength of bone is highly related to BMD, as well as its geometry and microarchitectural parameters.
Another clinical approach to treat or prevent osteoporosis in PMW is by prescribing hormones and anti-resorptive and/or osteogenic medications. This approach has been limited and restricted because of concerns of age-related or poly-drug interaction or side effects. Older women worry regarding the increased risk of hormone therapy linked to breast cancer as well as the unfavorable impacts and expense of the added drugs. On the other hand, it has been reported that various modalities of exercise activity including whole-body vibration (WBV) training plays a significant role in preventing bone loss, and sustaining and enhancing BMD [12, 13] without prescribing anti-resorption drug therapy. Bone mass can be maintained or ameliorated with weight-bearing exercise, resistance training or WBV for enhancing of BMD, and promoting physical health and quality of life in PMW [14,15,16]. For example, two recent meta-analysis studies examining BMD in PMW, reported the beneficial effects of combined resistance training and WBV on BMD, but not isolated resistance training protocols [17, 18]. Mohr and colleagues have also reported an improvement in BMD (leg and hip) in PMW following a 15-week soccer training. Nevertheless, research reported on other exercise modalities in PMW have produced contradictory findings [16, 19,20,21]. In this regard, the effectiveness of exercise on bone mass is closely related to the mode of exercise training regimen, duration and intensity of exercise, as well as the study design.
Eight meta-analyses on physical activity efficacy in PMW were conducted previously [17, 18, 22,23,24,25,26,27], nevertheless, their participants’ range of age differed from the current meta-analysis. Therefore, the purpose of this study was to carry out a systematic review and meta-analysis to clarify the possible effective type of exercise training on BMD in the lumbar spine and femoral neck in older PMW (60 years or more).
Methods
Data Sources and Searches
We performed a detailed search utilizing PubMed, CINAHL, Medline, Google Scholar, and Scopus databases. Search criteria included a mix of both MeSH and free-text terms relating to the keywords of bone mineral density, postmenopausal, exercise training, resistance training, whole-body vibration, aerobic training, walking, physical activity, high-impact exercise, bone loss and exercise, and bone mass. We employed the Boolean search terms (AND, OR, or NOT) to create the search strategy, merging the search terms of the participation in exercise training and the outcomes (lumbar spine and femoral neck BMD). The search strategy including all the items from database inception was developed until March 25, 2019. Then, following the initial screening, systematic reviews, meta-analyses, and all references were also searched to find further studies.
Study Selection
Exercise training randomized controlled trials (RCTs) and controlled trials in PMW were included. In our meta-analysis, exercise training included aerobic (including aerobic training, walking, and weight-bearing training), resistance (including resistance and impact training), combined (aerobic + resistance), and WBV training. Studies included in this meta-analysis compared older PMW in the training and control groups. Two authors independently reviewed the titles, abstracts, and full texts of convenient articles to detect eligible researches.
Inclusion/Exclusion Criteria
For study identification and selection, the following criteria were applied (1) full-text RCTs and controlled trials published in the English language; (2) health PMW aged ≥ 60 years without hormone replacement therapy (HRT) and systematic exercise (less than 2.5 h per week) before study registration; (3) studies in which participants did not receive supplemental calcium and vitamin D other than their daily requirements during the intervention period; (4) study protocols that employed aerobic, resistance, combined aerobic and resistance, and whole-body vibration training, with an intervention period ≥ 6 months (since this is the minimum period used to employed positive impacts on BMD), in a pre-post design with a non-exercise control group. Review articles, literature reviews, conference, abstracts, and study protocols, as well as studies in which the subjects took part in an exercise regimen during the last 6 months have been excluded.
Outcome Measures
The outcome measure was BMD (lumbar spine and/or femoral regions) assessed using dual-energy x-ray absorptiometry (DEXA) or dual-photon absorptiometry (DPA).
Data Extraction
Four authors independently extracted data from each study included in the review. The information extracted included the following:
-
1)
Author, year of publication, and study design;
-
2)
Demographic characteristics of PMW;
-
3)
Exercise interventions feature;
-
4)
Mean and standard deviation (SD) of continuous outcomes;
-
5)
Details of the biomarker evaluation methodology.
Data Synthesis
For all included studies, we summarized the effect size for any outcome by measuring the mean difference between the exercise and control condition from before and following the intervention. If each article published multiple outcomes for the current study, we estimated and reported separately any outcome. Given the similar methods of reporting techniques for outcomes (both femoral neck and lumbar spine BMD), the mean difference (MD) was used. All analyses were performed applying Review Manager 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark). Extracted outcome data were completed using the change in the mean and standard deviation (SD) values. The pre-intervention mean was subtracted from the postintervention mean, and the change SD was calculated applying study group subject numbers in conjunction with group p-values or 95% CI where the change in mean and SD was not reported. In studies that reported standard error of the mean (SEM) data instead of the SD, this value was converted to SD [28]. Where data were not shown in text or tables, and authors could not be contacted, data displayed in figures were extracted or obtained where feasible via GetData Graph Digitizer software. Where an article contained a control group and more than one exercise group, we separately labeled each exercise group and adjusted the sample size of the control group according to the number of exercise groups.
A random-effects inverse variance was utilized. To evaluate the heterogeneity among the studies, the I2 statistic was used, with values > 50% showing substantial heterogeneity [28]. Subgroup analyses were used to recognize potential causes of heterogeneity among the articles. The mode of exercise training (WBV, aerobic, resistance, combined aerobic and resistance) was considered as a predefined source of heterogeneity. We presented meta-analysis applying Forest plots and applied a 5% level of significance to describe the significance of results. The risk of publication bias was measured utilizing funnel plots [29].
Study Quality
Fifteen-point Tool for the Assessment of Study Quality and Reporting in Exercise (TESTEX) scale was utilized for evaluating the study quality and reporting [30]. Two reviewers (GhRMR and AA) independently performed the study quality and reporting assessment, NMR was consulted if discrepancies occurred.
Results
Study and Participant Characteristics
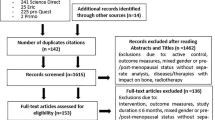
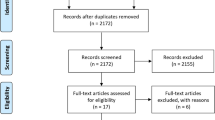
Initially, 1579 articles were found via PubMed, Medline, and Scopus database and hand searching. After duplicate titles, animal studies and exclusion of articles based on abstract and title were removed, 729 full-text articles remained for screening. Full screening resulted in 16 articles meeting the stated inclusion criteria (PRISMA flow diagram; Fig. 1).
The 16 included studies had a total of 1624 participants. There were 903 (55.6%) participants in the exercise group and 721 (44.4%) in the control group. The mean age of participants in the exercise group and the control group was 69.54 ± 4.25 and 70.21 ± 4.28 years, respectively. All included articles were RCTs promulgated since 1992.
Intervention Details
The studies' intervention period ranged from 24 to 120 weeks, with each session's length of range 12–60 min. The reviewed full-text studies that were excluded are supplied in Supplementary Table S1 with reasons.
Of the 16 [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46] included studies, four [32, 33, 35, 37] involved resistance training, four [41, 43, 45, 46] examined aerobic training, three [36, 39, 44] investigated whole-body vibration training, and two [38, 40] investigated combined aerobic + resistance training. Other included studies are isolated aerobic and resistance training [31], isolated whole-body vibration and resistance training [34], isolated combined (aerobic + resistance) and whole-body vibration training [42], each one of the above studies was investigated as one study (Table 1).
BMD Assessment
Seven studies [32, 33, 37, 40, 43, 45, 46] assessed BMD (g/cm2) at the L2-L4 spine, three studies [35, 38, 39] assessed BMD (g/cm2) at the total of lumbar spine, and three studies [34, 42, 44] assessed BMD (g/cm2) at the L1–L4 spine. Moreover, 14 studies [31,32,33,34,35,36,37,38,39,40,41, 43, 45, 46] evaluated BMD (g/cm2) at the femoral neck region. All included studies assessed BMD employing DEXA method.
Outcome Measures
Change in Lumbar Spine BMD
Thirteen studies [32,33,34,35, 37,38,39,40, 42,43,44,45,46] providing a total of 1371 participants (18 intervention groups and 13 control groups) reported changes in lumbar spine BMD as an outcome measure. We combined the results employing the random-effects model and revealed no significant change in lumbar spine BMD after exercise training intervention (MD: 0.01 g/cm2; 95% CI [− 0.01, 0.02]; p = 0.39; Fig. 2).
Change in Femoral Neck BMD
Fourteen studies providing a total of 778 subjects (19 intervention groups and 14 control groups) reported femoral neck BMD as an outcome measure. Pooled results from the random-effects model illustrated that exercise training did not have a significant effect on femoral neck BMD (MD: − 0.00 g/cm2; 95% CI [− 0.01, 0.01]; p = 0.99; Fig. 3).
Subgroup Analysis for Mode of Exercise Training
The results of the subgroup analyses are demonstrated in Table 2. We stratified studies based on the mode of exercise training (WBV, aerobic, resistance, and combined). These analyses revealed that lumbar spine BMD (MD: 0.01; 95% CI [0.00, 0.02]; p = 0.02) raised significantly when WBV training was employed as intervention compared with RCTs that utilized aerobic training (MD: − 0.01; 95% CI [− 0.02, − 0.01]), resistance training (MD: 0.01; 95% CI [− 0.04, 0.06]), and combined training (MD: 0.03; 95% CI [− 0.01, 0.08]). On the other hand, lumbar spine BMD (MD:-0.01; 95% CI [− 0.02, − 0.01]; p < 0.00001) reduced significantly when aerobic exercise training was used as intervention compared with RCTs that utilized resistance training, combined training, and whole-body vibration training. By contrast, the subgroup analyses by type of exercise training (whole-body vibration, aerobic, resistance, and combined training) did not have significant impact on change in femoral neck BMD.
Study Quality
The overall quality of included studies was judged to be moderate to good, with a median TESTEX score of 9.5 (range 8–12) of a maximum score of 15 (Table 3). Each one of the criteria of monitoring of physical activity in the control group, intention to treat analyses, and relative training intensity was met in 6 studies. The criteria of assessor blinding were also met in 5 studies, however, the criteria of allocation concealment were met in only 3 studies. The other TESTEX criteria were each met in at least 50% of trials.
Heterogeneity and Publication Bias
Our analyses in both lumbar spine and femoral neck BMD revealed low heterogeneity (I2 = 42%; p = 0.03 and I2 = 27%; p = 0.14, respectively). The Egger plots suggest risk of publication bias was low (Fig. 4a and b).
Discussion
The primary objective of this study was to undertake a systematic review and meta-analysis of RCTs evaluating the impacts of various types of exercise training on BMD at the lumbar spine and femoral neck in older PMW. The second objective was to assistance provide more evidence on varying modes of exercise training protocols for the aim of determining optimal exercise regimens for older PMW. Our primary analysis shows that various types of exercise training compared to control groups had no significant effects on BMD in either the lumbar spine or the femoral neck. Whereas, the effect of protocols that include WBV appear to be limited to increases in lumbar spine BMD, but not the femoral neck. Yet, aerobic exercise training significantly reduced BMD in the lumbar spine.
Regarding lumbar spine and femoral neck BMD change according to the overall analysis in older participants, our findings differ with the findings of Marques et al. (2012), who found that exercise of mixed loading impact is associated with significant increments in lumbar spine and femoral neck BMD in older adults [47]. In addition, Zhao et al. (2017) reported that combined exercise interventions positively affected the lumbar spine, femoral neck, total hip, and total body BMD compared with the control group [22] that differs from our findings. Our subgroup analysis also failed to indicate a positive effect of combined exercise intervention at the lumbar spine in PMW aged > 60 years. However, both mentioned meta-analyses, the positive change in BMD of the lumbar spine and total femur or femoral neck were studied following only one or two modes of exercise training. According to the findings of Zhao et al. lumbar spine BMD of PMW aged ≥ 60 years was still sensitive to exercise, which designated that other factors other than mechanical stimulus might contribute the beneficial effects, such as exercise-related increase of calcium absorption [48]. Yet, it should be careful to elucidate the findings because subgroup analysis only included a small number of studies.
From clinical research, it is predicted that 60–80 percent of bone mass variation through a lifetime is related to genetics [49]. Under Wolff's law, nevertheless, both mechanical stimuli and quantity of skeletal loading are considered as an active osteogenic promoter [5, 50]. Resistance or impact-loaded exercise training utilizing tensions generated from muscular contraction to stimulate bone cells with strain stress, compression force, and shear stress [51] were used. It should be emphasized that bone formation takes place only when the impact stimulus of physical activity exceeds a certain mechanical strain threshold that is above the accustomed normal daily levels [52].
Land-based running/jogging/walking, as well as step aerobic and cycling exercise involves moderate- to high-impact musculoskeletal loading activity on the lower extremities. Participants developed high muscle strength in lower body, and exhibited gain in BMD in total femur and femoral neck [53]. The land-based resistance training involves high-impact musculoskeletal loading activity on the upper and lower body as well as torso region in a gravitational environment. However, Ryan et al. (1998) reported that 16 weeks of resistance training resulted in no change in BMD in the lumbar spine and femoral neck, and improvement in arm and leg muscular strength in healthy PMW [54]. Liang et al. (2011) reported that 52 weeks of moderate intensity strength training did not induce changes in BMD of the lumbar spine and femoral neck in healthy PMW. But, there was a significant increase in leg muscle strength [55]. Other longitudinal research studies investigating the impact of resistance training on bone mass have shown that PMW's BMD can be enhanced [56,57,58]. It should be noted that both types of land-based training activities might not be suitable for older frail PMW and individuals with osteoporotic fractures. Nevertheless, for the older PMW, enhancing BMD and muscular force expansion also promulgates motor consonance advancement, dynamic balance and postural stabilization, allowing physical autonomy and promoting quality of life [59].
Regarding WBV, the mechanism by which vibration improves BMD is still unclear. WBV exercise has been prescribed for inducing BMD and bone strength [60] and appears to be a safe and effective training modality for maintaining or enhancing bone metabolism in varying populations [61]. Furthermore, WBV training has been used for preventing bone loss in astronauts [34, 42, 60]. Judex and Rubin (2010) proposed a plausible mechanism by which WBV training can induce anabolic or anti-catabolic responses in bone tissue, and that is the direct transmissibility of vibratory signals to bone cells, resulting in osteogenic responses [62]. Rubin (2004) who examined WBV training, observed a significant difference in BMD change between the placebo and the experimental group. At the femoral neck, the placebo group experienced a loss of 2.1% BMD after 1 year, those subjects completed the WBV training with the top compliance (upper quartile) after 1 year showed a 3.3% gain at the same BMD site (p = 0.009), as compared to the mean experimental group gain of 2.7% (p = 0.02). Rubin concluded that WBV training may have a very positive outcome for maintaining and enhancing BMD in PMW [63]. Due to its non-invasive, non-pharmacological nature of intervention, the WBV modality may be an optimal approach of osteoporosis treatment for certain specific populations including PMW [64].
Strengths and Limitations in the Systematic Review and Meta-analysis
To our understanding, this is the first systematic review and meta-analysis to investigate the effectiveness of different modes of exercise training on lumbar spine and femoral neck BMD in healthy older PMW. The strength of the present study is that we pooled all included studies in our analysis and compared the effectiveness of different modes of exercise training on BMD in lumbar spine and femoral neck. Our results show that according to the modes of exercise training, lumbar spine BMD only responded positively to WBV training in older PMW.
Our meta-analysis has some limitations that should be considered. First, the outcome of our meta-analysis is BMD change; however, it has the inherent limitations for bone strength analysis. The bone mineral content and structural adaptation due to exercise training can enhance mechanical load and bone bending strength [65,66,67]. It has been reported that only approximately 60–70 percent of bone strength adaptation can be explained by BMD [68], and other characteristics of the quality of bones, such as microarchitecture, are not included. Hence, in PMW, BMD estimation may not be a perfect indicator of osteogenic response to exercise training. In addition, some trials only included a smaller study population, which tended to weaken the quality of individual study and then posed a threat to risk of bias of our meta-analysis. Finally, concerning the data collection, we computed the mean differences between pre- and postintervention. Notwithstanding, in situations where actual p values within or between groups or 95% CI were unavailable, default p values were applied, and this may have influenced our results. As life expectancy is rising and the number of elderly individuals becoming more sedentary, the development of osteoporotic fracture prevention and treatment regimens is imperative. However, the difficult task of conducting human exercise trials to investigate the impact of exercise training on BMD or osteoporotic fractures as a primary or secondary study endpoint is to deal with an enormous sample size [69] that could provide definite proof that exercise training can positively achieve the ultimate goals of overall fractures prevention in older PMW [70, 71].
Conclusion
The overall conclusion of the present review and meta-analysis was that different modes of exercise training were unable to show improvement or maintenance of BMD in the lumbar spine and femoral neck in older PMW. However, subgroup analysis showed only WBV training that improves the lumbar spine BMD in older PMW, but not other types of exercise training.
References
Christenson E, Jiang X, Kagan R, Schnatz P (2012) Osteoporosis management in post-menopausal women. Minerva Ginecol 64(3):181–194
Lane NE (2006) Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol 194(2):S3–S11
Holroyd C, Cooper C, Dennison E (2008) Epidemiology of osteoporosis. Best Pract Res Clin Endocrinol Metab 22(5):671–685
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17(12):1726–1733
Wolff I, Van Croonenborg J, Kemper H, Kostense P, Twisk J (1999) The effect of exercise training programs on bone mass: a meta-analysis of published controlled trials in pre-and postmenopausal women. Osteoporos Int 9(1):1–12
Gordon JL, Eisenlohr-Moul TA, Rubinow DR, Schrubbe L, Girdler SS (2016) Naturally occurring changes in estradiol concentrations in the menopause transition predict morning cortisol and negative mood in perimenopausal depression. Clin Psychol Sci 4(5):919–935
Stathopoulos KD, Katsimbri P, Atsali E, Metania E, Zoubos AB et al (2011) Age-related differences of bone mass, geometry, and strength in treatment-naive postmenopausal women. A tibia pQCT study. J Clin Densitom 14(1):33–40
Robling AG, Hinant FM, Burr DB, Turner CH (2002) Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J Bone Min Res 17(8):1545–1554
Rochefort G, Pallu S, Benhamou C-L (2010) Osteocyte: the unrecognized side of bone tissue. Osteoporos Int 21(9):1457–1469
Kanis J, Johnell O, Oden A, Dawson A, De Laet C et al (2001) Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int 12(12):989–995
Wainwright SA, Marshall LM, Ensrud KE, Cauley JA, Black DM et al (2005) Hip fracture in women without osteoporosis. J Clin Endocrinol Metab 90(5):2787–2793
Sazy JA, Horstmann HM (1991) Exercise participation after menopause. Clin Sports Med 10(2):359–369
Sinaki M (1989) Exercise and osteoporosis. Arch Phys Med Rehabil 70(3):220–229
Basat H, Esmaeilzadeh S, Eskiyurt N (2013) The effects of strengthening and high-impact exercises on bone metabolism and quality of life in postmenopausal women: a randomized controlled trial. J Back Musculoskelet Rehabil 26(4):427–435
Vélez-Toral M, Godoy-Izquierdo D, de Guevara NML, de Teresa Galván C, Ballesteros AS et al (2017) Improvements in health-related quality of life, cardio-metabolic health, and fitness in postmenopausal women after an exercise plus health promotion intervention: a randomized controlled trial. J Phys Act Health 14(5):336–343
Wen H, Huang T, Li T, Chong P, Ang B (2017) Effects of short-term step aerobics exercise on bone metabolism and functional fitness in postmenopausal women with low bone mass. Osteoporos Int 28(2):539–547
Marín-Cascales E, Alcaraz PE, Ramos-Campo DJ, Martinez-Rodriguez A, Chung LH et al (2018) Whole-body vibration training and bone health in postmenopausal women: a systematic review and meta-analysis. Medicine 97(34):e11918
Zhao R, Zhao M, Xu Z (2015) The effects of differing resistance training modes on the preservation of bone mineral density in postmenopausal women: a meta-analysis. Osteoporos Int 26(5):1605–1618
Moreira LDF, Fronza FCA, dos Santos RN, Zach PL, Kunii IS et al (2014) The benefits of a high-intensity aquatic exercise program (HydrOS) for bone metabolism and bone mass of postmenopausal women. J Bone Min Metab 32(4):411–419
Qin J, Rong X, Zhu G, Jiang Y (2018) The effects of square dancing on bone mineral density and bone turnover markers in patients with postmenopausal osteoporosis. J Mech Med Biol 18(08):1840027
Tantiwiboonchai N, Kritpet T, Yuktanandana P (2017) Effects of Muay Thai aerobic dance on biochemical bone markers and physical fitness in elderly women. J Exerc Physiol Online 20(1):188–199
Zhao R, Zhang M, Zhang Q (2017) The effectiveness of combined exercise interventions for preventing postmenopausal bone loss: a systematic review and meta-analysis. J Orthop Sports Phys Ther 47(4):241–251
Oliveira L, Oliveira R, Pires-Oliveira D (2016) Effects of whole body vibration on bone mineral density in postmenopausal women: a systematic review and meta-analysis. Osteoporos Int 27(10):2913–2933
Martyn-St James M, Carroll S (2006) High-intensity resistance training and postmenopausal bone loss: a meta-analysis. Osteoporos Int 17(8):1225–1240
Kelley GA, Kelley KS, Kohrt WM (2012) Effects of ground and joint reaction force exercise on lumbar spine and femoral neck bone mineral density in postmenopausal women: a meta-analysis of randomized controlled trials. BMC Musculoskelet Disord 13(1):177
Fratini A, Bonci T, Bull AM (2016) Whole body vibration treatments in postmenopausal women can improve bone mineral density: results of a stimulus focussed meta-analysis. PLoS ONE 11(12):e0166774
Martyn-St James M, Carroll S (2010) Effects of different impact exercise modalities on bone mineral density in premenopausal women: a meta-analysis. J Bone Min Metab 28(3):251–267
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Smart NA, Waldron M, Ismail H, Giallauria F, Vigorito C et al (2015) Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int J Evid Based Healthc 13(1):9–18
Marques EA, Wanderley F, Machado L, Sousa F, Viana JL et al (2011) Effects of resistance and aerobic exercise on physical function, bone mineral density, OPG and RANKL in older women. Exp Gerontol 46(7):524–532
Pruitt LA, Taaffe DR, Marcus R (1995) Effects of a one-year high-intensity versus low-intensity resistance training program on bone mineral density in older women. J Bone Min Res 10(11):1788–1795
Rhodes E, Martin A, Taunton J, Donnelly M, Warren J et al (2000) Effects of one year of resistance training on the relation between muscular strength and bone density in elderly women. Brit J Sports Med 34(1):18–22
Verschueren SM, Roelants M, Delecluse C, Swinnen S, Vanderschueren D et al (2004) Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Min Res 19(3):352–359
Chuin A, Labonté M, Tessier D, Khalil A, Bobeuf F et al (2009) Effect of antioxidants combined to resistance training on BMD in elderly women: a pilot study. Osteoporos Int 20(7):1253–1258
Santin-Medeiros F, Santos-Lozano A, Rey-Lopez JP, Vallejo NG (2015) Effects of eight months of whole body vibration training on hip bone mass in older women. Nutr Hosp 31(4):1654–1659
Brentano MA, Cadore EL, Da Silva EM, Ambrosini AB, Coertjens M et al (2008) Physiological adaptations to strength and circuit training in postmenopausal women with bone loss. J Strength Cond Res 22(6):1816–1825
Englund U, Littbrand H, Sondell A, Pettersson U, Bucht G (2005) A 1-year combined weight-bearing training program is beneficial for bone mineral density and neuromuscular function in older women. Osteoporos Int 16(9):1117–1123
Beck BR, Norling TL (2010) The effect of 8 mos of twice-weekly low-or higher intensity whole body vibration on risk factors for postmenopausal hip fracture. Am J Phys Med Rehabil 89(12):997–1009
Park H, Kim KJ, Komatsu T, Park SK, Mutoh Y (2008) Effect of combined exercise training on bone, body balance, and gait ability: a randomized controlled study in community-dwelling elderly women. J Bone Min Metab 26(3):254–259
Korpelainen R, Keinänen-Kiukaanniemi S, Heikkinen J, Väänänen K, Korpelainen J (2006) Effect of impact exercise on bone mineral density in elderly women with low BMD: a population-based randomized controlled 30-month intervention. Osteoporos Int 17(1):109–118
Von Stengel S, Kemmler W, Engelke K, Kalender W (2011) Effects of whole body vibration on bone mineral density and falls: results of the randomized controlled ELVIS study with postmenopausal women. Osteoporos Int 22(1):317–325
Brooke-Wavell K, Jones P, Hardman A, Tsuritani I, Yamada Y (2001) Commencing, continuing and stopping brisk walking: effects on bone mineral density, quantitative ultrasound of bone and markers of bone metabolism in postmenopausal women. Osteoporos Int 12(7):581–587
Leung K, Li C, Tse Y, Choy T, Leung P et al (2014) Effects of 18-month low-magnitude high-frequency vibration on fall rate and fracture risks in 710 community elderly—a cluster-randomized controlled trial. Osteoporos Int 25(6):1785–1795
Lau E, Woo J, Leung P, Swaminathan R, Leung D (1992) The effects of calcium supplementation and exercise on bone density in elderly Chinese women. Osteoporos Int 2(4):168–173
Lord SR, Ward J, Williams P, Zivanovic E (1996) The effects of a community exercise program on fracture risk factors in older women. Osteoporos Int 6(5):361–367
Marques EA, Mota J, Carvalho J (2012) Exercise effects on bone mineral density in older adults: a meta-analysis of randomized controlled trials. Age 34(6):1493–1515
Nelson ME, Fisher EC, Dilmanian FA, Dallal G, Evans W (1991) A 1-y walking program and increased dietary calcium in postmenopausal women: effects on bone. Am J Clin Nutr 53(5):1304–1311
Wagner H, Melhus H, Pedersen NL, Michaëlsson K (2013) Genetic influence on bone phenotypes and body composition: a Swedish twin study. J Bone Min Metab 31(6):681–689
Vainionpää A, Korpelainen R, Sievänen H, Vihriälä E, Leppäluoto J et al (2007) Effect of impact exercise and its intensity on bone geometry at weight-bearing tibia and femur. Bone 40(3):604–611
Yang P, Bruggemann G, Rittweger J (2011) What do we currently know from in vivo bone strain measurements in humans. J Musculoskelet Neuronal Interact 11(1):8–20
Al Nazer R, Lanovaz J, Kawalilak C, Johnston JD, Kontulainen S (2012) Direct in vivo strain measurements in human bone—A systematic literature review. J Biomech 45(1):27–40
Weaver CM, Teegarden D, Lyle RM, McCabe GP, McCabe LD et al (2001) Impact of exercise on bone health and contraindication of oral contraceptive use in young women. Med Sci Sports Exerc 33(6):873–880
Ryan A, Treuth M, Hunter G, Elahi D (1998) Resistive training maintains bone mineral density in postmenopausal women. Calcif Tissue Int 62(4):295–299
Liang M, Braun W, Bassin S, Dutto D, Pontello A et al (2011) Effect of high-impact aerobics and strength training on BMD in young women aged 20–35 years. Int J Sports Med 32(02):100–108
Chien M-Y, Wu Y, Hsu A-T, Yang R, Lai J (2000) Efficacy of a 24-week aerobic exercise program for osteopenic postmenopausal women. Calcif Tissue Int 67(6):443–448
Yamazaki S, Ichimura S, Iwamoto J, Takeda T, Toyama Y (2004) Effect of walking exercise on bone metabolism in postmenopausal women with osteopenia/osteoporosis. J Bone Min Metab 22(5):500–508
Hamaguchi K, Kurihara T, Fujimoto M, Iemitsu M, Sato K et al (2017) The effects of low-repetition and light-load power training on bone mineral density in postmenopausal women with sarcopenia: a pilot study. BMC Geriatr 17(1):102
Wilhelm M, Roskovensky G, Emery K, Manno C, Valek K et al (2012) Effect of resistance exercises on function in older adults with osteoporosis or osteopenia: a systematic review. Physiother Can 64(4):386–394
Torvinen S, Kannus P, SievaÈnen H, JaÈrvinen TA, Pasanen M et al (2002) Effect of a vibration exposure on muscular performance and body balance. Randomized cross-over study. Clin Physiol Funct Imaging 22(2):145–152
Bemben DA, Palmer IJ, Bemben MG, Knehans AW (2010) Effects of combined whole-body vibration and resistance training on muscular strength and bone metabolism in postmenopausal women. Bone 47(3):650–656
Judex S, Rubin CT (2010) Is bone formation induced by high-frequency mechanical signals modulated by muscle activity? J Musculoskelet Neuronal Interact 10(1):3
Rubin C, Recker R, Cullen D, Ryaby J, McCabe J et al (2004) Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Min Res 19(3):343–351
Palombaro KM, Black JD, Buchbinder R, Jette DU (2013) Effectiveness of exercise for managing osteoporosis in women postmenopause. Phys Ther 93(8):1021–1025
Liu-Ambrose TY, Khan KM, Eng JJ, Heinonen A, McKay HA (2004) Both resistance and agility training increase cortical bone density in 75-to 85-year-old women with low bone mass: a 6-month randomized controlled trial. J Clin Densitom 7(4):390–398
Wang Q, Nicholson PH, Suuriniemi M, Lyytikäinen A, Helkala E et al (2004) Relationship of sex hormones to bone geometric properties and mineral density in early pubertal girls. J Clin Endocrinol Metab 89(4):1698–1703
Karinkanta S, Heinonen A, Sievänen H, Uusi-Rasi K, Pasanen M et al (2007) A multi-component exercise regimen to prevent functional decline and bone fragility in home-dwelling elderly women: randomized, controlled trial. Osteoporos Int 18(4):453–462
Ammann PR, Rizzoli R (2003) Bone strength and its determinants. Osteoporos Int 14:13–18
Moayyeri A (2008) The association between physical activity and osteoporotic fractures: a review of the evidence and implications for future research. Ann Epidemiol 18(11):827–835
Kemmler W, Häberle L, Von Stengel S (2013) Effects of exercise on fracture reduction in older adults. Osteoporos Int 24(7):1937–1950
Howe TE, Shea B, Dawson LJ, Downie F, Murray A et al (2011) Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD000333
Funding
This study was not funded by any financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Gholam Rasul Mohammad Rahimi, Neil A. Smart, Michael T.C. Liang, Nahid Bijeh, Alsaeedi L. Albanaqi, Mehrdad Fathi, Arghavan Niyazi, Nasser Mohammad Rahimi declares that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohammad Rahimi, G.R., Smart, N.A., Liang, M.T.C. et al. The Impact of Different Modes of Exercise Training on Bone Mineral Density in Older Postmenopausal Women: A Systematic Review and Meta-analysis Research. Calcif Tissue Int 106, 577–590 (2020). https://doi.org/10.1007/s00223-020-00671-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-020-00671-w