Abstract
Summary
This meta-analysis synthesized current evidence from 24 clinical trials to evaluate the impact of different resistance training modes on postmenopausal bone loss. Exercise interventions were categorized into two training modes, namely resistance-alone versus combined resistance training protocols. The combined resistance training protocols were defined as the combination of resistance training and high-impact or weight-bearing exercise. The results suggested that the combined resistance training protocols were effective in improving bone mineral density (BMD) at the femoral neck and lumbar spine.
Introduction
The current meta-analysis aimed to examine the effects of combined resistance and resistance-alone training protocols on the preservation of femoral neck and lumbar spine BMD in postmenopausal women.
Methods
An electronic database search was conducted in PubMed, EMBASE, SPORTDiscus, Web of Science, and ProQuest up to March 1, 2014 for the influence of resistance exercise on BMD in postmenopausal women. The study quality was evaluated. The effect sizes were estimated in terms of the standardized mean difference (SMD). A subgroup analysis was conducted by exercise categories.
Results
Twenty-four studies were included in the overall analysis of skeletal response to resistance exercise. The between-study heterogeneity was evident for the hip (I 2 = 46.5 %) and spine (I 2 = 62.3 %). The overall analysis suggested that resistance training significantly increased femoral neck BMD (SMD = 0.303, 95 % confidence interval (95 % CI) = 0.127–0.479, p = 0.001) and lumbar spine BMD (SMD = 0.311, 95 % CI = 0.115–0.507, p = 0.002) in postmenopausal women. However, subgroup analysis indicated that combined resistance training programs significantly affected both the hip BMD (SMD = 0.411, 95 % CI = 0.176–0.645, p = 0.001) and spine BMD (SMD = 0.431, 95 % CI = 0.159–0.702, p = 0.002), whereas resistance-alone protocols only produced nonsignificant positive effects both on the femoral neck and lumbar spine BMD.
Conclusions
Combined resistance exercise protocols appear effective in preserving femoral neck and lumbar spine BMD in postmenopausal women, whereas resistance-alone protocols only produced a nonsignificant positive effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postmenopausal women frequently suffer from persistent bone loss, increased fragility, and a high risk of fracture. Postmenopausal osteoporosis is a major public health problem in both developed and developing countries. It has been estimated that approximately 30 million American women suffered from osteoporosis in 2002, and this population is expected to expand substantially in the future [1]; in China, there were approximately 70 million osteoporotic women along with 30 million women experiencing sustained bone loss in 2006 [2]. The hip and spine are the most common sites at high risk for fracture. It has been reported that in the year 2000 there were an estimated 9.0 million osteoporotic fractures, of which 1.6 million were at the hip and 1.4 million were at the spine [3].Currently, compelling evidence suggests that exercise interventions have great beneficial effects on bone metabolism in older women [4, 5]. Therefore, exercise-associated improvement of bone mineral density (BMD) may be a nonpharmacological strategy for the treatment of age-related osteoporosis.
According to Frost’s mechanostat theory [6], exercises producing high strain can greatly improve the mechanical properties of bone. In support of this notion, progressive high-intensity resistance training has been recognized as an effective strategy for stimulating osteogenic response and preserving BMD in older adults [7]. However, the evidence concerning effects of high-intensity resistance training on postmenopausal women BMD remains controversial [8–21]. Additionally, adaptive bone response to resistance exercise is frequently site-specific [7, 22]. A meta-analysis by Martyn-St James and Carroll [23] synthesizing a body of clinical trials with similar outcomes of interest reported that high-intensity resistance exercise was only effective in preserving postmenopausal bone loss at the lumbar spine.
To explore the optimum resistance training protocols that can best prevent postmenopausal bone loss, a large number of clinical trials with a combined design have been conducted [24–34]. Resistance training is frequently performed integrated with high-impact or weight-bearing exercise to augment the beneficial effects on BMD. These protocols are frequently termed combined resistance training protocols. Current findings have suggested that combined resistance training exercise interventions generated inconsistent results. Several lines of evidence indicated beneficial effects both at the hips [26, 29–31, 33] and spine [26, 29–31, 33, 34], whereas others either did not find positive effects or revealed negative outcomes [25, 27, 32] after combined resistance training interventions. For the inconsistent results, wide variation may exist in the sample size, training frequency, and intensity in the exercise programs. It is necessary to combine the positive and negative outcomes and employ a meta-analysis to reach general conclusions about a body of studies. The current meta-analysis aimed to examine the effects of combined resistance and resistance-alone protocols on preservation of femoral neck and lumbar spine BMD in postmenopausal women.
Methods
This meta-analysis was conducted in accordance with PRISMA recommendations and criteria for reporting of meta-analysis guidelines [35].
Search strategy
An electronic database search was conducted for the influence of resistance exercise on BMD in postmenopausal women. The included criteria are given in Table 1. Briefly, the included studies were controlled trials (CTs) or randomized controlled trials (RCTs). We included CTs in the search strategy because of the limited number of eligible studies and the long-term exercise interventions (several years) frequently available in CTs. The population of interest consisted of healthy postmenopausal women who did not perform regular exercise (less than 2.5 h per week) prior to enrollment in the study. Participants receiving hormone replacement therapy (HRT) or antiresorptive treatment (AT) were excluded. Resistance training intervention lasts for at least 6 months because bone remodeling, bone repair, and the metabolism of ions normally require approximately 6 months. Therefore, detectable skeletal responses to resistance exercise often occur after 6 months.
We searched PubMed, EMBASE, SPORTDiscus, Web of Science, and ProQuest up to March 1, 2014 for studies of the influence of resistance training on BMD in postmenopausal women. Terms used for database searches included “exercise training,” “resistance exercise/training,” “strength exercise/training,” “weight lifting,” “weight training,” “bone density,” and “BMD.” The search was limited to clinical trials and females (see Electronic Supplementary Material, Appendix 1).
Data extraction
All data were extracted and reviewed independently by two authors (MZ and ZX). The details extracted included the following: subject characteristics, sample size, exercise intervention (category, intensity, frequency, and duration), attrition, compliance, HRT and AT use (not shown), regions of interest (ROIs), and BMD values with standard deviations (SDs). Studies published as multiple reports were only included once to prevent duplication in this meta-analysis.
The primary outcomes of the included trials were expressed in terms of areal BMD (BMD g/cm2) assessed by dual X-ray absorptiometry (DXA) or dual photon absorptiometry (DPA). Absolute and relative changes in BMD from baseline to follow-up along with SDs were used for the meta-analysis. If the values of these changes were not available from the original publication or author, these were calculated using baseline and follow-up values. The data extraction followed the methods provided by the Cochrane Reviewers’ Handbook [36].
Risk of bias assessment
Two authors (MZ and ZX) independently assessed the quality of the included studies using a widely utilized instrument [37]. The quality scale is a three-item questionnaire that provides an assessment of bias, specifically focusing on randomization, blinding and withdrawals. All questions are designed to elicit yes (1) or no (0) answers, and the total score ranges from 0 to 5.
Sensitivity analysis
In sensitivity analysis, we excluded five clinical trials [9, 26, 29–31] that were non-RCTs. In the five trials, the randomized assignment and blinding were not properly described. We tested, at the trial level, whether the intervention effects would be affected by excluding the five trials.
Meta-analysis
The primary endpoint was the change in BMD from baseline to follow-up at the lumbar spine or at the femoral neck. The effect sizes associated with exercise interventions were estimated in terms of the standardized mean difference (SMD). The SMD was calculated as follows:
where X e represents the change score from baseline to follow-up in the exercise group, X c represents the change score from baseline to follow-up in the control group, and SDpooled represents the pooled standard deviation for mean difference between the exercise and control groups.
The pooled SD was calculated from the following formula:
where SD pooled is the pooled standard deviation for SMD, n e is the sample size in the exercise group, n c is the sample size in the control group, SD 2e is the square of the standard deviation in the exercise group, and SD 2c is the square of the standard deviation in the control group.
The SMD was chosen over the original metric because of the different ways used to describe data, for example, absolute versus relative changes in BMD. We conducted a subgroup analysis by exercise categories to determine whether different resistance training modes (combined resistance versus resistance-alone training protocols) showed different effects on BMD in elderly women. Furthermore, we used unpaired T-test to determine whether the difference in effect sizes between combined resistance and resistance-alone protocols was significant.
The intention-to-treat (ITT) approach was used in the analysis of the data. If ITT data were not available, the per-protocol approach was used in calculating the pooled effect estimates for the combination of single effects of trials. Heterogeneity of results between studies was determined using a Cochran’s Q test and an alpha value of <0.10 for statistical significance. In addition, I 2 was used to examine inconsistency in the study findings. I 2 values of <25, 25 to <50, 50 to <75, and >75 % were considered to be of low, moderate, high, and very high inconsistency, respectively. The tests for the overall effects (Z-score) were considered as significant at p < 0.05. STATA version 12 (StataCorp LP, TX, USA) was used to perform the meta-analysis and produce the graph.
Results
Study characteristics and quality assessment
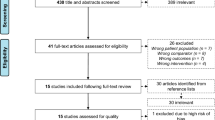
From the searches, 1027 potential abstracts were identified and screened, of which 1003 were excluded (Fig. 1). In all, 24 studies, including a total of 1769 postmenopausal women, met the inclusion criteria, among which 5 trials [9, 26, 29–31] were CTs and 19 trials [8, 10–21, 24, 25, 27, 28, 32–34] were RCTs. Four studies reported the findings of the same program, “EFOPS” [26, 29–31]. Because this was a 12-year follow-up study program and the four trials presented the results of different periods of follow-up, the four studies without duplicate data were also included in the meta-analysis according to the suggestions of the Cochrane Reviewers’ Handbook [36].
All of the studies were conducted with healthy postmenopausal women between the age of 50.5 ± 6.3 and 69.6 ± 4.2 years. The sample size varied from 20 to 320 participants. The studies were conducted in Canada [11, 12, 19, 25], Australia [13, 24], Brazil [10], Belgium [21], UK [15], Germany [26, 29–31, 34], and USA [8, 9, 14, 16–18, 20, 27, 28, 38]. Three studies [15, 25, 34] reported findings on the basis of the ITT approach; most clinical trials only provided per-protocol data. Descriptions of other characteristics of the participants are presented in Table 2.
Studies were awarded methodological quality points for randomization and withdrawals. No studies gained points for blinding (blinding was only used for pharmacological interventions). The quality score of the included studies was relatively low. Seven clinical trials [8, 15, 21, 24, 25, 28, 34] received a quality score of 3, 5 studies [9, 26, 29–31] received a quality score of 1, and 12 trials [10–14, 16–20, 27, 32] received a quality score of 2.
Exercise interventions
Fourteen studies prescribed resistance-alone protocols with a 10- to 20-min warm-up and a 5- to 10-min cooldown [8–21], and 10 clinical trials evaluated combined resistance exercise protocols [24–32, 34] (Table 2). We excluded two study group comparisons [8, 18] because they used low-intensity resistance exercise. Therefore, all of the included trials were based on high-intensity resistance training. The study durations ranged from 6 months to 12 years, with a training frequency of 2–3 times per week in most interventions. With the exception of three studies [25, 33, 34] involving flexibility exercise not expected to have osteogenic effects, most participants in the control groups were asked to continue their normal dietary and physical activity habits. All of the studies measured BMD values immediately after the end of the exercise training intervention. Generally, participant compliance with the exercise interventions was relatively good, ranging from 65 to 90 %.
Meta-analysis
Overall analysis of resistance training impact on postmenopausal bone loss
Our meta-analysis pooled the data from 1769 postmenopausal women in 24 studies. A total of 924 postmenopausal women completed exercise interventions; 845 control participants maintained their original daily dietary and physical activity or received flexibility exercise. Twenty-one and 23 study group comparisons with moderate (I 2 = 46.5 %) and high (I 2 = 62.3 %) levels of heterogeneity, respectively, were included in the overall analysis of the impact of resistance training on femoral neck and lumbar spine BMD in postmenopausal women. Random-effects models, which incorporated an estimate of between-study variation (heterogeneity) in the weighting, were used for calculating overall effect sizes. The findings demonstrated that resistance training interventions significantly increased femoral neck BMD (SMD = 0.303, 95 % confidence interval (95 % CI) = 0.127–0.479, p = 0.001) (Fig. 2) and lumbar spine BMD (SMD = 0.311, 95 % CI = 0.115–0.507, p = 0.002) (Fig. 3) in postmenopausal women.
Different effects of combined resistance versus resistance-alone protocols on BMD
A subgroup analysis was performed to examine the different effects of combined resistance versus resistance-alone protocols on postmenopausal bone loss. Seven (I 2 = 48.3 %) and 10 (I 2 = 71.3 %) heterogeneous study group comparisons were included in the analysis of the impact of combined resistance protocols on femoral neck and lumbar spine BMD, respectively. Random-effects models were used for subgroup analysis. The findings indicated that combined resistance training protocols significantly affected both the hip BMD (SMD = 0.411, 95 % CI = 0.176–0.645, p = 0.001) and spine BMD (SMD = 0.431, 95 % CI = 0.159–0.702, p = 0.002) in postmenopausal women (Table 3). It was estimated that the positive effect sizes contributed to almost 1.8 and 2.4 % increment of BMD at femoral neck and lumbar spine, respectively.
Fourteen (I 2 = 45.6 %) and 13 (I 2 = 48.3 %) moderately heterogeneous study group comparisons were included in the assessment of the influence of resistance-alone protocols on the hip and spine BMD. The results of this assessment suggested that resistance-alone protocols had no significant effects on the preservation of femoral neck BMD (SMD = 0.212, 95 % CI = −0.043–0.468, p = 0.104) or lumbar spine BMD (SMD = 0.180, 95 % CI = −0.09–0.456, p = 0.203) in postmenopausal women (Table 3).
We further determined whether the difference in effect sizes between combined resistance and resistance-alone protocols was significant. Results suggested that the difference was not statistically significant both at the hip (p = 0.149) and spine (p = 0.253) BMD.
Sensitivity analysis
We determined whether the inclusion of RCTs only would affect the effect size estimates. A sensitivity analysis was conducted for 17 (I 2 = 36.2 %) and 18 (I 2 = 36.3 %) study group comparisons at the hip and spine BMD, respectively. The exclusion of five non-RCTs did not significantly alter the effect size estimates for the femoral neck BMD (SMD = 0.261, 95 % CI = 0.071–0.450, p = 0.007) (Table 3). The intervention effects were somewhat weaker for the lumbar spine BMD (SMD = 0.198, 95 % CI = 0.019–0.378, p = 0.030) (Table 3). The sensitivity analysis suggested that our results were relatively robust after inclusion of the five CTs in the final data analysis.
Discussion
This meta-analysis aimed to examine the effects of different modes of resistance training on femoral neck and lumbar spine BMD among postmenopausal women. Systematic searches with assessment based on the inclusion criteria resulted in 24 clinical trials with a population of 1769 postmenopausal women. The overall analysis suggested that resistance exercise interventions were effective in preserving BMD at the femoral neck and lumbar spine in postmenopausal women. However, a subgroup analysis indicated that combined protocols integrating resistance training with high-impact or weight-bearing exercise were effective in improving the hip and spine BMD, whereas resistance-alone protocols only produced nonsignificant positive effects on the prevention of postmenopausal bone loss.
The effects of resistance-alone protocols on BMD
According to Frost’s mechanostat theory, bone can adapt its strength to increased mechanical loading [6]. Therefore, progressive high-intensity resistance training, which usually produces a high level of mechanical strain, is expected to generate beneficial effects on postmenopausal bone health. The overall analysis suggested that the effects of resistance exercise interventions on femoral neck and lumbar spine BMD were significant (Figs. 2 and 3). Because the overall analysis included both combined resistance and resistance-alone exercise protocols, we conducted a subgroup analysis to determine the separate effects of the two different exercise protocols on BMD in older women. Protocols that included only resistance training did not generate significant effects on BMD in older women. These results appeared to be inconsistent with the meta-analysis by Martyn-St James and Carroll in 2006 [23], which reported a significant effect on lumbar spine BMD in postmenopausal women after resistance training interventions. However, this conclusion was based on an analysis including studies that enrolled participants receiving HRT or antiresorptive agents [11, 18, 20, 33, 39]. It is known that the combination of estrogen or antiresorptive agents with exercise may generate additive or synergistic effects on skeletal response [40, 41]. Unlike Martyn-St James and Carroll’s meta-analysis, our study group comparisons did not include subjects receiving pharmacological therapy. Martyn-St James and Carroll also performed a subgroup analysis that differentiated between participants receiving HRT and those not receiving HRT. After the studies with participants receiving HRT were excluded, the results suggested that high-intensity resistance training did not significantly affect either the hip or spine BMD. These results are consistent with our findings. A number of new studies have been conducted since Martyn-St James and Carroll’s meta-analysis. Our meta-analysis shared eight trials with Martyn-St James and Carroll’s review and included six extra trials that were not included in Martyn-St James and Carroll’s study. The findings from another Cochrane analysis [42] suggested a positive response of the hip and spine BMD to high-intensity resistance training. However, it appears that that study was limited because it only included eight clinical trials in the subgroup analysis, and was unable to add substantially to the information provided by Martyn-St James and Carroll’s meta-analysis [23]. Additionally, this previous meta-analysis pooled both absolute and relative change values and used mean difference (MD) methods instead of SMD to estimate the intervention effects. Therefore, the results of this previous meta-analysis might not be definitive.
The effects of combined resistance training protocols on BMD
The subgroup analysis also aimed to identify whether combined resistance training protocols would improve BMD in postmenopausal women. The findings indicated that combined resistance training protocols were effective in significantly improving the hip and spine BMD in postmenopausal women. Our findings are consistent with a meta-analysis by Martyn-St James and Carroll [43] published in 2009. In a subgroup analysis, Martyn-St James and Carroll reported that mixed loading exercise programs integrating impact activity with resistance training were effective in reducing postmenopausal bone loss at the hip and spine. However, the subgroup analysis by Martyn-St James and Carroll was limited because it only included four trials generating five study group comparisons. Additionally, note that the participants in one study group comparison received estrogen treatment [27] and that subjects receiving HRT were also enrolled in two studies [44, 45]. The administration of estrogen may affect the skeletal response to exercise [40]. Because a number of clinical trials were conducted after this previous review, our subgroup analysis included more eligible clinical trials (11 trials) than Martyn-St James and Carroll’s study. In addition, our meta-analysis focused particularly on resistance training, whereas Martyn-St James and Carroll’s meta-analysis primarily concerned impact exercise. Recently, Howe et al. [42] reported in a subgroup analysis that the effects of mixed loading exercise protocols on BMD in postmenopausal women were significant at both the hip and spine. However, the results were also limited by only including six studies.
The positive training effects related to combined resistance exercise interventions have clinical significance. It was estimated that the beneficial effects induced by combined resistance training could contribute to almost 1.8 and 2.4 % BMD gains for the hip and spine in postmenopausal women. The training-related increase in BMD effectively prevented bone loss and greatly benefited postmenopausal women at risk for fracture. Current evidence demonstrates that exercise interventions are effective in fall reduction and fracture prevention [46, 47]. The overall effects could be greater considering the added benefits of exercise-related muscle mass increments, strength gained, joint flexibility and agility, and healthy dynamic movement and good balance, all of which are recognized as independent risk factors for fracture [48].
There is considerable interest in defining the optimum loading type and program to best improve bone strength so that precise exercise prescription guidelines can be developed. Evidence has shown that the skeletal response to loading is modulated by a number of different loading components, including the magnitude, rate, distribution, and number of loading cycles [49–51]. Consistent with these findings, both our meta-analysis and other studies demonstrate that the most effective programs are those involving high-impact, weight-bearing activities (jumping, skipping, dancing, and hopping) in combination with progressive high-intensity resistance training [43, 52]. However, when we directly examined the significance of the difference in the intervention effects between resistance-alone and combined resistance training protocols, the results suggested that the difference was not significant both at the hip and spine BMD. The findings seem not to support the notion that combined resistance training protocols is superior to resistance-alone training protocols for preventing postmenopausal bone loss. However, the nonsignificant results may result from the variation that exists in the sample size, population age, and exercise interventions. Additionally, the most important thing is that combined resistance training not only produces beneficial effects on BMD in postmenopausal women, but the effects are nonsite-specific. Therefore, combined resistance training is an effective strategy for improving postmenopausal bone health. One of the main concerns in bone health practice has been whether combined resistance training is safe for older women. The number of training-related injuries reported in the included studies was very low, indicating that combined resistance training is relatively safe for older women to practice.
Currently, pharmacological treatment remains to be the standard therapy for osteoporosis, with bisphosphonates as a first line of treatment [53]. However, drug treatment only generates a modest BMD increment [54, 55] and has limited effects on the risk factors for fracture, such as weak muscle strength, reduced joint flexibility and agility, and poor dynamic movement and balance. Additionally, the long-term efficacy of pharmacological treatment may be hampered due to low compliance [56, 57], and longtime use of antiresorptive agents is likely to induce adverse effects, such as upper gastrointestinal effects [58–61]. Given that exercise usually generates beneficial effects and hardly induces side effects, combined resistance training has been regarded as a feasible strategy for preventing bone loss in postmenopausal women. However, like pharmacological treatment, low rates of adherence to exercise intervention may present a potential barrier to the improvement of postmenopausal bone health.
For the current meta-analysis, we conducted a systematic review to reduce the potential risk of bias. However, we did not present funnel plots to discuss publication bias because the subjective nature of visual interpretation of funnel plots seems to have limited use in the examination of publication bias in the current meta-analysis.
Study quality assessment and sensitivity analysis
Aspects of methodological quality including randomization, blinding, and statement on withdrawals were assessed by a widely used instrument [37]. Generally, the quality score of the included studies was relatively low. Due to the limited number of studies, we did not restrict subgroup analysis to RCTs. According to the findings of Pildal et al. [62], inadequate concealment of allocation tends to result in overestimates of intervention effects; it appeared that five studies with inadequate allocation concealment included in our meta-analysis failed to avoid this type of bias. However, a more specific meta-analysis by Wood et al. [63] found that intervention effect sizes were overestimated when inadequate sequence generation was present in trials with a subjective outcome but not in those with an objective outcome. Given that the primary outcomes in the included studies were objective measures, namely, absolute values in femoral neck and lumbar spine BMD, inadequate sequence generation may not have posed a real threat. In fact, the sensitivity analysis demonstrated that including the five CTs did not significantly alter the effect size estimates. This result indicates that the inclusion of the five CTs still yielded stable results.
In two studies [11, 25], blinding was used primarily for drug administration but not for exercise training interventions. Therefore, none of the included trials was awarded a point for blinding. It has been reported that a lack of blinding was associated with exaggerated odds ratios [62]. However, this potential bias was less for trials with objective outcomes than for those with subjective outcomes. Given the objective nature of BMD measurement, a lack of blinding in the included studies might not have posed a real threat of bias. This conclusion is important because it is difficult to conduct an exercise intervention study with a double-blinding design. Although it is possible to blind the measurer, few studies performed this type of single blinding.
Only three trials reported ITT data; most of the included studies presented results based on a per-protocol approach. An ITT analysis is preferred because it is unbiased in addressing clinically relevant research questions. The low number of clinical trials that included an ITT analysis might have induced potential bias because attrition was not considered.
Limitations
Our meta-analysis provides definitive evidence that combined resistance training protocols generate nonsite-specific effects on bone loading. The findings are clinically relevant and applicable in older women. However, our meta-analysis has inherent limitations. In all of the included studies, the measurements of BMD were made with DXA or DPA. However, these methods may not be optimal for examining bone strength. Bone can adapt both its mineral content and its structure to increased mechanical loading [39, 64, 65]. It has been reported that BMD only represents approximately 60–70 % of variation in bone strength [66]; it does not incorporate other aspects of bone quality such as microarchitecture. Therefore, BMD measurement may not be a good predictor of skeletal response to resistance training in postmenopausal women. Further detailed studies of postmenopausal women are needed to identify the material changes as well as the structural changes occurring after resistance training intervention. From a clinical perspective, these adaptive processes are important because even small changes in bone geometry and structure can significantly improve bone strength in elderly women. Our meta-analysis was also limited because the data represented highly selected samples of postmenopausal women of varying ages. Additionally, the relatively low quality of the included studies was also a limitation in our meta-analysis. As we included four studies that reported the findings of the same program, “EFOPS,” a 12-year follow-up study, the use of these studies in the meta-analysis might potentially impact the results of pooling intervention effects.
Conclusions
The general conclusions of the present meta-analysis were that progressive high-intensity resistance training tended to be effective for improving or preserving hip and spine BMD in postmenopausal women. However, skeletal adaptation was dependent on training modes, as the combined protocols that integrated resistance training with high-impact or weight-bearing exercises showed significant beneficial effects on postmenopausal bone loss, while resistance-alone protocols did not. Although the number of training-related injuries reported in the included studies was very low, caution is advised when resistance training is performed at home or without supervision because the resistance exercise conducted by the participants in the clinical trials was usually performed with special training equipment and under supervision. The poor quality of several trials reminds us that well-designed studies with large sample sizes are still needed. Further studies are also needed to characterize both material and structural changes to determine exercise-induced gains in bone strength. Nevertheless, combined resistance training protocols furnish a feasible nonpharmacological strategy for preventing postmenopausal bone loss.
References
National Osteoporosis Foundation (2002) America’s bone health: the state of osteoporosis and low bone mass in our nation. National Osteoprosis Foundation, Washington
Zhao D, Wu H, Liu Z (2006) Epidemiology of osteoporosis. In: Liu Z (ed) Bone mineral and clinic practice. Science and Technology Press, Beijing, pp 5–6
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17:1726–1733
Moreira LD, Fronza FC, Dos Santos RN, Zach PL, Kunii IS, Hayashi LF, Teixeira LR, Kruel LF, Castro ML (2013) The benefits of a high-intensity aquatic exercise program (HydrOS) for bone metabolism and bone mass of postmenopausal women. J Bone Miner Metab. doi:10.1007/s00774-00013-00509-y
Basat H, Esmaeilzadeh S, Eskiyurt N (2013) The effects of strengthening and high-impact exercises on bone metabolism and quality of life in postmenopausal women: a randomized controlled trial. J back Musculoskelet Rehabil 26:427–435
Frost HM (1987) Bone “mass” and the “mechanostat”: a proposal. Anat Rec 219:1–9
Ryan AS, Ivey FM, Hurlbut DE, Martel GF, Lemmer JT, Sorkin JD, Metter EJ, Fleg JL, Hurley BF (2004) Regional bone mineral density after resistive training in young and older men and women. Scand J Med Sci Sports 14:16–23
Bemben DA, Fetters NL, Bemben MG, Nabavi N, Koh ET (2000) Musculoskeletal responses to high- and low-intensity resistance training in early postmenopausal women. Med Sci Sports Exerc 32:1949–1957
Bemben DA, Palmer IJ, Bemben MG, Knehans AW (2010) Effects of combined whole-body vibration and resistance training on muscular strength and bone metabolism in postmenopausal women. Bone 47:650–656
Bocalini DS, Serra AJ, dos Santos L, Murad N, Levy RF (2009) Strength training preserves the bone mineral density of postmenopausal women without hormone replacement therapy. J Aging Health 21:519–527
Chilibeck PD, Davison KS, Whiting SJ, Suzuki Y, Janzen CL, Peloso P (2002) The effect of strength training combined with bisphosphonate (etidronate) therapy on bone mineral, lean tissue, and fat mass in postmenopausal women. Can J Physiol Pharmacol 80:941–950
Chuin A, Labonte M, Tessier D, Khalil A, Bobeuf F, Doyon CY, Rieth N, Dionne IJ (2009) Effect of antioxidants combined to resistance training on BMD in elderly women: a pilot study. Osteoporos Int J Established Result Coop Eur Found Osteoporos Natl Osteoporos Found U S A 20:1253–1258
Kerr D, Ackland T, Maslen B, Morton A, Prince R (2001) Resistance training over 2 years increases bone mass in calcium-replete postmenopausal women. J Bone Miner Res Off J Am Soc Bone Miner Res 16:175–181
Maddalozzo GF, Widrick JJ, Cardinal BJ, Winters-Stone KM, Hoffman MA, Snow CM (2007) The effects of hormone replacement therapy and resistance training on spine bone mineral density in early postmenopausal women. Bone 40:1244–1251
Marques EA, Wanderley F, Machado L, Sousa F, Viana JL, Moreira-Goncalves D, Moreira P, Mota J, Carvalho J (2011) Effects of resistance and aerobic exercise on physical function, bone mineral density, OPG and RANKL in older women. Exp Gerontol 46:524–532
Nelson ME, Fiatarone MA, Morganti CM, Trice I, Greenberg RA, Evans WJ (1994) Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures. A randomized controlled trial. JAMA J Am Med Assoc 272:1909–1914
Pruitt LA, Jackson RD, Bartels RL, Lehnhard HJ (1992) Weight-training effects on bone mineral density in early postmenopausal women. J Bone Miner Res Off J Am Soc Bone Miner Res 7:179–185
Pruitt LA, Taaffe DR, Marcus R (1995) Effects of a one-year high-intensity versus low-intensity resistance training program on bone mineral density in older women. J Bone Miner Res Off J Am Soc Bone Miner Res 10:1788–1795
Rhodes EC, Martin AD, Taunton JE, Donnelly M, Warren J, Elliot J (2000) Effects of one year of resistance training on the relation between muscular strength and bone density in elderly women. Br J Sports Med 34:18–22
Smidt GL, Lin SY, O’Dwyer KD, Blanpied PR (1992) The effect of high-intensity trunk exercise on bone mineral density of postmenopausal women. Spine 17:280–285
Verschueren SM, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S (2004) Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Miner Res Off J Am Soc Bone Miner Res 19:352–359
Notomi T, Okazaki Y, Okimoto N, Saitoh S, Nakamura T, Suzuki M (2000) A comparison of resistance and aerobic training for mass, strength and turnover of bone in growing rats. Eur J Appl Physiol 83:469–474
Martyn-St James M, Carroll S (2006) High-intensity resistance training and postmenopausal bone loss: a meta-analysis. Osteoporos Int J Established Result Coop Eur Found Osteoporos Natl Osteoporos Found U S A 17:1225–1240
Bolton KL, Egerton T, Wark J, Wee E, Matthews B, Kelly A, Craven R, Kantor S, Bennell KL (2012) Effects of exercise on bone density and falls risk factors in post-menopausal women with osteopenia: a randomised controlled trial. J Sci Med Sport 15:102–109
Chilibeck PD, Vatanparast H, Pierson R, Case A, Olatunbosun O, Whiting SJ, Beck TJ, Pahwa P, Biem HJ (2013) Effect of exercise training combined with isoflavone supplementation on bone and lipids in postmenopausal women: a randomized clinical trial. J Bone Miner Res Off J Am Soc Bone Miner Res 28:780–793
Engelke K, Kemmler W, Lauber D, Beeskow C, Pintag R, Kalender WA (2006) Exercise maintains bone density at spine and hip EFOPS: a 3-year longitudinal study in early postmenopausal women. Osteoporos Int J Established Result Coop Eur Found Osteoporos Natl Osteoporos Found U S A 17:133–142
Going S, Lohman T, Houtkooper L et al (2003) Effects of exercise on bone mineral density in calcium-replete postmenopausal women with and without hormone replacement therapy. Osteoporos Int J Established Result Coop Eur Found Osteoporos Natl Osteoporos Found U S A 14:637–643
Jessup JV, Horne C, Vishen RK, Wheeler D (2003) Effects of exercise on bone density, balance, and self-efficacy in older women. Biol Res Nurs 4:171–180
Kemmler W, Engelke K, Weineck J, Hensen J, Kalender WA (2003) The Erlangen Fitness Osteoporosis Prevention Study: a controlled exercise trial in early postmenopausal women with low bone density-first-year results. Arch Phys Med Rehabil 84:673–682
Kemmler W, Lauber D, Weineck J, Hensen J, Kalender W, Engelke K (2004) Benefits of 2 years of intense exercise on bone density, physical fitness, and blood lipids in early postmenopausal osteopenic women: results of the Erlangen Fitness Osteoporosis Prevention Study (EFOPS). Arch Intern Med 164:1084–1091
Kemmler W, von Stengel S, Bebenek M, Engelke K, Hentschke C, Kalender WA (2012) Exercise and fractures in postmenopausal women: 12-year results of the Erlangen Fitness and Osteoporosis Prevention Study (EFOPS). Osteoporos Int J Established Result Coop Eur Found Osteoporos Natl Osteoporos Found U S A 23:1267–1276
Milliken LA, Going SB, Houtkooper LB, Flint-Wagner HG, Figueroa A, Metcalfe LL, Blew RM, Sharp SC, Lohman TG (2003) Effects of exercise training on bone remodeling, insulin-like growth factors, and bone mineral density in postmenopausal women with and without hormone replacement therapy. Calcif Tissue Int 72:478–484
Villareal DT, Binder EF, Yarasheski KE, Williams DB, Brown M, Sinacore DR, Kohrt WM (2003) Effects of exercise training added to ongoing hormone replacement therapy on bone mineral density in frail elderly women. J Am Geriatr Soc 51:985–990
von Stengel S, Kemmler W, Engelke K, Kalender WA (2011) Effects of whole body vibration on bone mineral density and falls: results of the randomized controlled ELVIS study with postmenopausal women. Osteoporos Int J Established Result Coop Eur Found Osteoporos Natl Osteoporos Found U S A 22:317–325
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(264–269):W264
Higgins J, Green S (2008) Cochrane reviewers’ handbook 5.0.1 (updated September 2008). The Cochrane Library. Wiley, Chichester
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
Milliken LA, Wilhelmy J, Martin CJ, Finkenthal N, Cussler E, Metcalfe L, Guido TA, Going SB, Lohman TG (2006) Depressive symptoms and changes in body weight exert independent and site-specific effects on bone in postmenopausal women exercising for 1 year. J Gerontol A: Biol Med Sci 61:488–494
Liu-Ambrose TY, Khan KM, Eng JJ, Heinonen A, McKay HA (2004) Both resistance and agility training increase cortical bone density in 75- to 85-year-old women with low bone mass: a 6-month randomized controlled trial. J Clin Densitom Off J Int Soc Clin Densitom 7:390–398
Tobias JH (2003) At the crossroads of skeletal responses to estrogen and exercise. Trends Endocrinol Metab TEM 14:441–443
Zhang J, Gao R, Cao P, Yuan W (2014) Additive effects of antiresorptive agents and exercise on lumbar spine bone mineral density in adults with low bone mass: a meta-analysis. Osteoporos Int J Established Result Coop Eur Found Osteoporos Natl Osteoporos Found U S A 25:1585–1594
Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, Harbour RT, Caldwell LM, Creed G (2011) Exercise for preventing and treating osteoporosis in postmenopausal women. The Cochrane database of systematic reviews CD000333
Martyn-St James M, Carroll S (2009) A meta-analysis of impact exercise on postmenopausal bone loss: the case for mixed loading exercise programmes. Br J Sports Med 43:898–908
Shaw JM, Snow CM (1998) Weighted vest exercise improves indices of fall risk in older women. J Gerontol A: Biol Med Sci 53:M53–M58
Snow CM, Shaw JM, Winters KM, Witzke KA (2000) Long-term exercise using weighted vests prevents hip bone loss in postmenopausal women. J Gerontol A: Biol Med Sci 55:M489–M491
El-Khoury F, Cassou B, Charles MA, Dargent-Molina P (2013) The effect of fall prevention exercise programmes on fall induced injuries in community dwelling older adults: systematic review and meta-analysis of randomised controlled trials. BMJ 347:f6234
Karinkanta S, Piirtola M, Sievanen H, Uusi-Rasi K, Kannus P (2010) Physical therapy approaches to reduce fall and fracture risk among older adults. Nat Rev Endocrinol 6:396–407
Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, Genant HK, Palermo L, Scott J, Vogt TM (1993) Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet 341:72–75
Lanyon LE (1987) Functional strain in bone tissue as an objective, and controlling stimulus for adaptive bone remodelling. J Biomech 20:1083–1093
Borer KT (2005) Physical activity in the prevention and amelioration of osteoporosis in women: interaction of mechanical, hormonal and dietary factors. Sports Med 35:779–830
Rubin CT, McLeod KJ (1994) Promotion of bony ingrowth by frequency-specific, low-amplitude mechanical strain. Clin Orthop Relat Res 298:165–174
Martyn-St James M, Carroll S (2010) Effects of different impact exercise modalities on bone mineral density in premenopausal women: a meta-analysis. J Bone Miner Metab 28:251–267
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377:1276–1287
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62:e1–e34
Harris ST, Watts NB, Genant HK et al (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA J Am Med Assoc 282:1344–1352
Rabenda V, Mertens R, Fabri V, Vanoverloop J, Sumkay F, Vannecke C, Deswaef A, Verpooten GA, Reginster JY (2008) Adherence to bisphosphonates therapy and hip fracture risk in osteoporotic women. Osteoporos Int J Established Result Coop Eur Found Osteoporos Natl Osteoporos Found U S A 19:811–818
Cramer JA, Gold DT, Silverman SL, Lewiecki EM (2007) A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int J Established Result Coop Eur Found Osteoporos Natl Osteoporos Found U S A 18:1023–1031
Reid IR (2013) Osteoporosis treatment: focus on safety. Eur J Intern Med 24:691–697
Miller PD, Derman RJ (2010) What is the best balance of benefits and risks among anti-resorptive therapies for postmenopausal osteoporosis? Osteoporos Int J Established Result Coop Eur Found Osteoporos Natl Osteoporos Found U S A 21:1793–1802
Rossouw JE, Anderson GL, Prentice RL et al (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA J Am Med Assoc 288:321–333
Kennel KA, Drake MT (2009) Adverse effects of bisphosphonates: implications for osteoporosis management. Mayo Clin Proc 84:632–637, quiz 638
Pildal J, Hrobjartsson A, Jorgensen KJ, Hilden J, Altman DG, Gotzsche PC (2007) Impact of allocation concealment on conclusions drawn from meta-analyses of randomized trials. Int J Epidemiol 36:847–857
Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, Gluud C, Martin RM, Wood AJ, Sterne JA (2008) Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 336:601–605
Wang Q, Nicholson PH, Suuriniemi M, Lyytikainen A, Helkala E, Alen M, Suominen H, Cheng S (2004) Relationship of sex hormones to bone geometric properties and mineral density in early pubertal girls. J Clin Endocrinol Metab 89:1698–1703
Karinkanta S, Heinonen A, Sievanen H, Uusi-Rasi K, Pasanen M, Ojala K, Fogelholm M, Kannus P (2007) A multi-component exercise regimen to prevent functional decline and bone fragility in home-dwelling elderly women: randomized, controlled trial. Osteoporos Int J Established Result Coop Eur Found Osteoporos Natl Osteoporos Found U S A 18:453–462
Ammann P, Rizzoli R (2003) Bone strength and its determinants. Osteoporos Int 14:S13–S18
Acknowledgments
This material was based upon the work funded by Zhejiang Provincial Natural Science Foundation of China under Grant No. LY14H070001.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Zhao, R., Zhao, M. & Xu, Z. The effects of differing resistance training modes on the preservation of bone mineral density in postmenopausal women: a meta-analysis. Osteoporos Int 26, 1605–1618 (2015). https://doi.org/10.1007/s00198-015-3034-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3034-0