Abstract
Homocysteine (Hcy) increases oxidation and inflammation; however, the mechanism of Hcy-induced bone fragility remains unclear. Because selective estrogen modulators (SERMs) have an anti-oxidative effect, SERMs may rescue the Hcy-induced bone fragility. We aimed to examine whether oxidative stress and pro-inflammatory cytokines such as interleukin (IL)-1β and IL-6 are involved in the Hcy-induced apoptosis of osteocytes and whether bazedoxifene (BZA) inhibits the detrimental effects of Hcy. We used mouse osteocyte-like cell lines MLO-Y4-A2 and Ocy454. Apoptosis was examined by DNA fragmentation ELISA and TUNEL staining, and gene expression was evaluated by real-time PCR. Hcy 5 mM significantly increased expressions of NADPH oxidase (Nox)1, Nox2, IL-1β, and IL-6 as well as apoptosis in MLO-Y4-A2 cells. Nox inhibitors, diphenyleneiodonium chloride and apocynin, significantly suppressed Hcy-induced IL-1β and IL-6 expressions. In contrast, an IL-1β receptor antagonist and an IL-6 receptor monoclonal antibody had no effects on Hcy-induced Nox1 and Nox2 expressions, but significantly rescued Hcy-induced apoptosis. BZA (1 nM–1 μM) and 17β estradiol 100 nM significantly rescued Hcy-induced apoptosis, while an estrogen receptor blocker ICI 182,780 reversed the effects of BZA and 17β estradiol. BZA also rescued Hcy-induced apoptosis of Ocy454 cell, and ICI canceled the effect of BZD. Moreover, BZA significantly ameliorated Hcy-induced expressions of Nox1, Nox2, IL-1β, and IL-6, and ICI canceled the effects of BZA on their expressions. Hcy increases apoptosis through stimulating Nox 1 and Nox 2-IL-1β and IL-6 expressions in osteocyte-like cells. BZA inhibits the detrimental effects of Hcy on osteocytes via estrogen receptor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Homocysteine (Hcy) is necessary to form essential amino acids such as methionine and cysteine. In general, plasma concentrations of Hcy are age dependent with higher levels in elderly population, especially in postmenopausal women [1]. Moreover, lifestyle-related diseases and insufficiency of vitamin B12 and folate induce hyperhomocysteinemia [2]. Several studies have shown that elevated plasma Hcy level is a risk factor for osteoporotic fracture [3,4,5]. Van Meurs et al. [4] reported large-scale prospective, population-based studies showing that circulating homocysteine levels were significantly associated with higher osteoporotic fracture risk independently of bone mineral density (BMD) and other potential risk factors for fracture. Therefore, the mechanism by which patients with hyperhomocysteinemia have higher risk of fracture is explained by deterioration of bone quality. Indeed, a previous study showed that plasma homocysteine levels were significantly and independently associated with osteoporotic fracture risk in patients with type 2 diabetes [6], that have an increased risk of fracture despite of normal or high BMD [7,8,9]. However, the mechanism of Hcy-induced bone fragility is not fully understood. Previous studies showed that plasma homocysteine levels were associated with atherosclerotic diseases by increasing oxidative stress [10, 11] and chronic inflammation [12, 13]. Thus, we hypothesized that oxidative stress and pro-inflammatory cytokines might be involved in the Hcy-induced bone fragility. We previously demonstrated that Hcy induces apoptosis of osteoblasts and osteocytes by increasing oxidative stress [14, 15]. However, it is unknown whether or not pro-inflammatory cytokines are involved in the detrimental effects of Hcy.
Selective estrogen receptor modulators (SERMs) are used for the treatment of postmenopausal osteoporosis. Although the increasing effect of SERMs on BMD is lower than that of bisphosphonate, SREMs are as effective as bisphosphonates for preventing vertebral fractures [16], suggesting that SERMs might improve bone quality rather than BMD. A major effect of SERMs on bone metabolism is considered to be inhibition of bone resorption via suppression of osteoclasts activity [17, 18]. However, several studies showed that SERMs might affect osteoblast lineage cells [15, 19, 20]. Osteocytes are the most abundant cells in bone, and they play important roles in coordinating the functions of osteoblasts and osteoclasts [21]. Previous studies have shown that estrogen withdrawal induces osteocyte apoptosis [22], and that treatment with estrogen and SERMs preserve osteocyte viability [19, 23]. We have shown that Hcy induces apoptosis of osteocyte-like MLO-Y4-A2 cells by increasing expressions of NADPH oxidase (Nox) 1 and Nox2, which are dominant oxidative stress-inductive enzymes [14]. However, whether SERMs work protectively against the Hcy-induced apoptosis of osteocytes remains unknown.
Therefore, in this study, we aimed to examine whether pro-inflammatory cytokines such as interleukin (IL)-1β and IL-6 are involved in the Hcy-induced apoptosis of osteocyte-like cells and whether estrogen and bazedoxifene (BZA), one of SERMs, can rescue the Hcy’s effects.
Materials and Methods
Materials
Cell culture medium and supplements were purchased from GIBCO-BRL (Rockville, MD, USA). Hcy was purchased from Wako Pure Chemical Industries (Osaka, Japan). We chose 5 mM Hcy to induce apoptosis based on the previous study [14]. BZA was kindly provided by Pfizer (New York, NY, USA). 17β estradiol was purchased from Sigma-Aldrich (St. Louis, MO, USA). ICI 182,780, an ER inhibitor, was purchased from TOCRIS (Bristol, United Kingdom). IL-1RA, a recombinant murine interleukin-1 receptor antagonist protein, was purchased from Prime Gene (Shanghai, China). MR16-1, an IL-6 receptor monoclonal antibody, was kindly provided by CHUGAI PHARMACEUTICAL (Tokyo, Japan). The Nox inhibitors, diphenyleneiodonium chloride (DPI) and apocynin, were purchased from Enzo Life Sciences (New York, NY, USA) and Santa Cruz Biotech (Santa Cruz, CA, USA), respectively. All other chemicals were of the highest grade available commercially. BZA and 17β estradiol were dissolved in DMSO, and Hcy was in distilled water.
Cell Culture
We used MLO-Y4-A2, a murine long bone-derived osteocytic cell line, which was kindly provided by Dr. Kato (Asahi Kasei Medical Corporation, Tokyo, Japan) and Dr. Bonewald (University of Missouri, USA). As previously described [24], the cells were cultured on type I collagen-coated plates in α-minimum essential medium (α-MEM) supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin without phenol red in 5% CO2 at 37 °C. The culture medium was modified by excluding phenol red because it has estrogenic activity [25]. The culture medium was changed twice weekly.
We used another mouse osteocytic cell line Ocy454 cells. The cells also cultured on collagen-coated plates in α-MEM supplemented with 10% fetal bovine serum at 33 °C. After the cells were confluent, the cells cultured at 37 °C. This cell line is currently available to examine expression of sclerostin [26].
Quantification of Gene Expression by Real-Time PCR
SYBR Green chemistry in conjunction with real-time PCR was used to quantify the amounts of mRNAs for Nox1, Nox2, Il-1β, Il-6, and 36b4, a housekeeping gene, according to an optimized protocol [27, 28]. Total RNA was isolated using Trizol reagent (Invitrogen, San Diego, CA, USA) and further purified by two successive phenol–chloroform extractions. First-strand cDNA was synthesized with an oligo-dT primer and a SuperScript-III cDNA synthesis kit (Invitrogen). Sense and antisense oligonucleotide primers were designed according to published cDNA sequences using Primer Express (version 2.0.0, Applied Biosystems, Carlsbad, CA, USA). The cDNA was amplified with an ABI PRISM 7000 sequence detection system (Applied Biosystems Inc.). The cDNA-specific SYBR Green Mix was incorporated into the PCR buffer provided in the QuantiTect SYBR PCR kit to allow for quantitative detection of the PCR product in a 25-µL reaction volume. The temperature profile of the reaction was 60 °C for 2 min, followed by 95 °C for 15 min and 40 cycles of denaturation at 94 °C for 15 s and annealing and extension at 60 °C for 1 min. Primer sequences were as follows: Nox1, 5ʹ-ATGCCCCTGCTGCTCGAATA-3ʹ and 5ʹ-AAATTGCCCCTCCATTTCCT-3ʹ; Nox2, 5ʹ-ACCGCCATCCACACAATTG-3ʹ and 5ʹ-CCGATGTCAGAGAGAGCTATTGAA-3ʹ; Il-1β, 5ʹ-ACACTCCTTAGTCCTCGGCCA-3ʹ and 5ʹ-TGGTTTCTTGTGACCCTGAGC-3ʹ; Il-6, 5ʹ-GTCACTTTGAGATCTACTCGGCAA-3ʹ and 5ʹ-TGACCACAGTGAGGAATGTCCA-3ʹ; 36b4, 5ʹ-AAGCGCGTCCTGGCATTGTCT-3ʹ and 5ʹ-CCGCAGGGGCAGCAGTGGT-3ʹ.
Assessment of Apoptotic Cell Death
Cell Death Detection ELISA
Apoptosis was assessed by using Cell Death Detection ELISA Plus kit (Roche Molecular Biochemicals, IN, USA) according to the manufacturer’s protocol. MLO-Y4-A2 cells were seeded in 96-well plates at a density of 3000 cells/well and incubated overnight at 37 °C in α-MEM. The next day, the cells were treated with Hcy and/or other agents. On day 2 after treatment, the cells were lysed in 200 μL lysis buffer. After centrifugation, 20 μL of the supernatant was transferred to a streptavidin-coated microplate and exposed to anti-histone antibody (biotin-labeled) and anti-DNA antibody (peroxidase-conjugated) for 2 h at room temperature. Each well was washed 3 times with the incubation buffer, and antibody-nucleosome complexes bound to the microplate were determined spectrophotometrically using 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS). The absorbance measured at 405 nm was proportional to the degree of apoptosis. The results were expressed relative to the control.
TUNEL Staining
Apoptosis of MLO-Y4-A2 cells and Ocy454 cells was also assessed by TUNEL staining using an in situ cell death detection kit (Roche, Germany) according to the manufacturer’s protocol. Briefly, the cells were incubated in chamber slides. After the cells were confluent, agents such as Hcy and BZA were added at their specified concentrations and the cells were incubated for 48 h. Fixation, blocking, and permeabilization were then performed according to the manufacturer’s protocol. Slides were immersed in TUNEL reaction mixture for 60 min at 37 °C in a humidified atmosphere in the dark. Slides were analyzed using optical microscopy. The average value of TUNEL-positive cells in one microscopic field (200 ×) was used to evaluate the degree of apoptosis. TUNEL-positive cells were counted in six or eight randomly selected areas of TUNEL-stained slides, and the average value was calculated.
Statistical Analysis
Results are expressed as mean ± SEM. Differences between groups were evaluated by one-way analysis of variance (ANOVA), followed by Fisher’s protected least significant difference test. For all statistical tests, a value of p < 0.05 was considered to indicate a statistically significant difference.
Results
Effects of BZA and E2 on Hcy-Induced Apoptosis via ER in MLO-Y4-A2 Cells and Ocy454 Cells
To confirm our previous findings [14], we examined the effects of Hcy on cell apoptosis of MLO-Y4-A2 cells. Cell death detection ELISA showed that incubation with Hcy 5 mM significantly increased apoptosis (p < 0.001), and that co-incubation of BZA 100–1000 nM significantly and dose-dependently suppressed Hcy-induced apoptosis (p < 0.01 and p < 0.001, respectively) (Fig. 1a). Moreover, TUNEL staining confirmed the results by ELISA that Hcy significantly induced apoptosis and BZA 10–1000 nM had protective effects against Hcy-induced apoptosis in a dose-dependent manner (p < 0.001) (Fig. 1b, c).
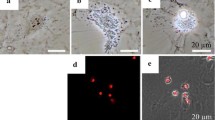
Effects of BZA on Hcy-induced apoptosis of MLO-Y4-A2 cells. After the cells reached confluence, the cells were treated with Hcy in the presence or absence of BZA for 48 h. Apoptosis was analyzed by an ELISA for DNA fragments (a). The result is representative of three different experiments. Quantification of cell count of TUNEL-positive cells (b). A representative picture is shown (c). The result is representative of four different experiments. The results are expressed as the mean ± standard error of mean (SEM). **p<0.01 and ***p<0.001. Cont, control; Hcy, homocysteine (5 mM); BZA, bazedoxifene (1–1000 nM)
We examined the effects of BZA on Hcy-induced apoptosis via ER by using an ER inhibitor ICI. TUNEL staining showed that E2 100 nM significantly inhibited Hcy-induced apoptosis of MLO-Y4-A2 cells (p < 0.001), whereas co-incubation with ICI 100 nM significantly canceled the anti-apoptotic effect of E2 (p < 0.001) (Fig. 2a, b). Moreover, the suppression of Hcy-induced apoptosis by BZA 100 nM was partly but significantly reversed by co-incubation with ICI (p < 0.05) (Fig. 2c, d).
Effects of 17β-estradiol and BZA on apoptosis of MLO-Y4-A2 and OCY454 via ER. After the cells reached confluence, they were treated with indicated agents. a–d: MLO-Y4-A2 cells. e, f: OCY454 cells. After 48 h, quantification of cell count of TUNEL-positive cells (a, c and e). A representative picture is shown (b, d and f). The result is representative of four different experiments. The results are expressed as the mean ± SEM. *p<0.05 and ***p<0.001. Cont, control; Hcy, homocysteine (5 mM); BZA, bazedoxifene (100 nM); E2, 17β-estradiol (100 nM); ICI, ICI 182,780 (100 nM)
Next, we examined the effects of Hcy, BZA, and ICI on cell apoptosis of another osteocytic cell line, OCY454 cells. TUNEL staining confirmed the results as same as MLO-Y4-A2 cells. TUNEL staining showed that incubation with Hcy 5 mM significantly increased apoptosis (p < 0.001), and that co-incubation of BZA 100 nM significantly suppressed Hcy-induced apoptosis (p < 0.001) (Fig. 2e, f). Furthermore, ICI 100 nM significantly canceled the anti-apoptotic effect of BZA (p < 0.001) (Fig. 2e, f).
Effects of BZA on Hcy-Induced Expressions of Nox1, Nox2, Il-1β, and Il-6 via ER in MLO-Y4-A2 Cells
Previously, we have shown that Hcy-induced apoptosis is mediated by Nox 1 and Nox 2 expressions [14]. Therefore, we examined effects of Hcy 5 mM on Il-1β and Il-6 expressions and effects of BZA on Hcy-induced Nox1, Nox2, Il-1β, and Il-6 expressions. MLO-Y4-A2 cells were used for the rest of mechanistic analyses in all results. Real-time PCR showed that Hcy significantly increased expressions of Nox1 and Nox2 (both p < 0.001), and BZA 10–1000 nM significantly suppressed the Hcy-increased Nox1 and Nox2 expressions in a dose-dependent manner (at least p < 0.05) (Fig. 3a, b). Moreover, Hcy significantly increased expressions of Il-1β and Il-6 (both p < 0.001), and BZA 10–1000 nM significantly suppressed the Hcy-increased Il-1β and Il-6 expressions in a dose-dependent manner (at least p < 0.05) (Fig. 3c, d).
Effects of Hcy and BZA on Nox1, Nox2, IL-1β, and Il-6 expressions in MLO-Y4-A2 cells. After the cells reached confluence, they were treated with Hcy and/or BZA for 48 h. Nox1 (a), Nox2 (b), Il-1β (c), and Il-6 (d) mRNA expressions were examined by quantitative real-time PCR. The results are expressed as the mean fold increase over control values ± SEM (n = 8). *p<0.05, **p<0.01, ***p<0.001. Cont, control; Hcy, homocysteine (5 mM); BZA, bazedoxifene (1–1000 nM)
Then, we examined whether BZA decreased the Hcy-increased Nox1, Nox2, IL-1β, and Il-6 expressions via ER. Real-time PCR showed that Hcy 5 mM significantly increased expressions of Nox1 and Nox2 (both p < 0.001), and co-incubation with BZA 100 nM significantly inhibited Hcy-induced upregulation of Nox1 and Nox2 (p < 0.001 and p < 0.01, respectively) (Fig. 4a, b). Moreover, co-incubation with ICI significantly canceled the inhibitory effects of BZA on Hcy-induced upregulation of Nox 1 and Nox 2 expressions (p < 0.01 and p < 0.05, respectively). Furthermore, Hcy 5 mM significantly increased expressions of Il-1β and Il-6 (both p < 0.001), and co-incubation with BZA 100 nM significantly inhibited Hcy-induced upregulation of Il-1β and Il-6 (both p < 0.001) (Fig. 4c, d). Co-incubation with ICI significantly canceled the inhibitory effects of BZA on Hcy-induced upregulation of Il-1β and Il-6 expressions (both p < 0.01).
Effects of BZA on Hcy-induced Nox1, Nox2, IL-1β, and IL-6 expressions via ER in MLO-Y4-A2 cells. After the cells reached confluence, they were treated with indicated agents for 48 h. Nox1 (a), Nox2 (b), Il-1β (c), and Il-6 (d) mRNA expressions were examined by quantitative real-time PCR. The results are expressed as the mean fold increase over control values ± SEM (n = 8). *p<0.05, **p<0.01, ***p<0.001. Cont, control; Hcy, homocysteine (5 mM); BZA, bazedoxifene (100 nM); ICI, ICI 182,780 (100 nM)
Effects of Hcy on Apoptosis via IL-1β ad IL-6 Expressions in MLO-Y4-A2 Cells
To investigate whether IL-1β and IL-6 is involved in Hcy-induced apoptosis of MLO-Y4-A2 cells, we examined whether an IL-1β receptor antagonist IL-1Ra and an IL-6 receptor monoclonal antibody MR16-1 can reverse the effects of Hcy on apoptosis. Cell death detection ELISA showed that IL-1Ra 10–100 ng/mL significantly and dose-dependently inhibited the Hcy-induced apoptosis (at least p < 0.05) (Fig. 5a). TUNEL staining confirmed the results by ELISA that IL-1Ra significantly and dose-dependently inhibited the Hcy-induced apoptosis (all p < 0.001) (Fig. 5b, c). Moreover, ELISA showed that MR16-1 0.1–10 ng/mL significantly and dose-dependently suppressed Hcy-induced apoptosis (at least p < 0.01) (Fig. 6a). TUNEL staining confirmed the results by ELISA that MR16-1 1 and 10 ng/mL significantly and dose-dependently inhibited the Hcy-induced apoptosis (all p < 0.001) (Fig. 6b, c).
Effects of Hcy on apoptosis via IL-1β signal in MLO-Y4-A2 cells. After the cells reached confluence, they were treated with Hcy in the presence or absence of IL-1RA for 48 h. Apoptosis was analyzed by an ELISA for DNA fragments (a). The result is representative of three different experiments. Quantification of cell count of TUNEL-positive cells (b). A representative picture is shown (c). The result is representative of four different experiments. The results are expressed as the mean ± SEM. *p<0.05, **p<0.01, ***p<0.001. Cont, control; Hcy, homocysteine (5 mM); IL-1RA, IL-1β receptor antagonist (10–100 ng/mL)
Effects of Hcy on apoptosis via IL-6 signal in MLO-Y4-A2 cells. After the cells reached confluence, they were treated with Hcy in the presence or absence of MR16-1 for 48 h. Apoptosis was analyzed by an ELISA for DNA fragments (a). The result is representative of three different experiments. Quantification of cell count of TUNEL-positive cells (b). A representative picture is shown (c). The result is representative of four different experiments. The results are expressed as the mean ± SEM. **p<0.01, *** p<0.001. Cont, control; Hcy, homocysteine (5 mM); MR-16-1, an IL-6 monoclonal antibody (0.1–10 ng/mL)
The Association Between Nox and Pro-inflammatory Cytokines in Hcy-Induced Apoptosis
Finally, to investigate the association between Nox and pro-inflammatory cytokines, we examined effects of IL-1Ra and MR16-1 on Hcy-induced Nox1 and Nox2 expressions, and effects of Nox inhibitors on Hcy-induced IL-1β and IL-6 expressions. Real-time PCR analysis showed that neither IL-1Ra nor MR16-1 affected the expressions of Nox1 (Fig. 7a, b) and Nox2 (Fig. 7c, d). In contrast, Nox inhibitors, apocynin 0.1 mM and DPI 1.0 nM, significantly inhibited Hcy-increased Il-1β (both p < 0.01) (Fig. 8a, b) and Il-6 expressions (both p < 0.001) (Fig. 8c, d).
Effects of Hcy on the expressions of Nox1 and Nox2 via IL-1β and IL-6 signals in MLO-Y4-A2 cells. After the cells reached confluence, they were treated with Hcy in the presence or absence of IL-1RA or MR16-1 for 48 h. Total RNA was collected and the mRNA expressions of Nox1 (a, b) and Nox2 (c, d) were examined by quantitative real-time PCR (n = 4). The results are expressed as the mean ± SEM. ***p<0.001, n.s. not significant. Cont, control; Hcy, homocysteine (5 mM); IL-1RA, IL-1β receptor antagonist (100 ng/mL); MR-16-1, an IL-6 monoclonal antibody (10 ng/mL)
Effects of Hcy on the expressions of IL-1β and IL-6 via Nox in MLO-Y4-A2 cells. After the cells reached confluence, they were treated with Hcy in the presence or absence of DPI or Apo for 48 h. Total RNA was collected and the mRNA expressions of Il-1β (a, b) and Il-6 (c, d) were examined by quantitative real-time PCR (n = 5). The results are expressed as the mean ± SEM. ***p<0.001, n.s. not significant. Cont, control; Hcy (5 mM), homocysteine; DPI, diphenyleneiodonium chloride (1.0 nM); Apo, apocynin (0.1 mM)
Discussion
We have previously shown that Hcy increases apoptosis of osteocyte-like MLO-Y4-A2 cells via increasing Nox1 and Nox2 expressions [14]. In this study, Hcy significantly increased IL-1β and IL-6 expressions, and Nox inhibitors suppressed Hcy-induced IL-1β and IL-6 expressions, and blocking IL-1β and IL-6 signals canceled the Hcy-induced apoptosis. These findings suggest that Hcy induced apoptosis of osteocyte-like MLO-Y4-A2 cells through enhancing Nox1 and Nox2-IL-1β and IL-6 pathway. Moreover, BZA significantly inhibits Hcy-induced Nox1, Nox2, IL-1β, and IL-6 expressions as well as apoptosis via ER in MLO-Y4-A2 cells. We also showed BZA rescued Hcy-induced apoptosis via ER in another cell line, Ocy454. These results suggesting that BZA may have a protective effect on Hcy-induced abnormal bone remodeling and bone fragility.
The present study showed that Hcy promoted apoptosis by increasing IL-1β and IL-6 expressions in MLO-Y4-A2 cells. IL-1β and IL-6 are pro-inflammatory cytokines and participate in important signal transmission. Previous studies showed that circulating Hcy levels were positively associated with IL-1β and IL-6 levels [12, 29]. Pro-inflammatory cytokines are known to affect bone metabolism. It has been shown that increased pro-inflammatory cytokines including IL-1β and IL-6 promote osteoclast differentiation and inhibits osteoblast maturation and function, resulting in bone mass reduction [30]. Thaler et al. [31] showed that Hcy dose-dependently increased IL-6 expression in osteoblastic MC3T3-E1 cells, leading to suppression of collagen cross-links. Zhang et al. [32] demonstrated that Hcy increased oxidative stress and pro-inflammatory cytokines including IL-1β expressions in osteoclastic Raw 264.7 cells. However, to our best knowledge, there are no studies on the effects of Hcy on IL-1β and IL-6 expressions in osteocytes thus far. Therefore, this is the first study to show that Hcy increases IL-1β and IL-6 expressions via increasing Nox1 and Nox2 expressions, which result in induction of apoptosis, in osteocyte-like cells.
Oxidative stress is known to be involved in osteoporosis and bone fragility. It has been shown that excessive ROS generation-induced oxidative stress increases apoptosis of osteoblast and osteocyte, resulting in impaired their activity and low bone quality [33]. BZA decreased Hcy-induced apoptosis and expressions of Nox1 and Nox2 in osteocytic cells in this study. We have previously shown that Hcy-induced apoptosis of MLO-Y4-A2 cells was significantly inhibited by anti-oxidant N-acetylcysteine and Nox inhibitors [14], suggesting that oxidative stress is involved in the apoptotic effects of Hcy in osteocytes. Another in vitro study showed that H2O2 induced apoptosis of MLO-Y4 cells, and that 17β-estradiol and raloxifene, one of SERMs, rescued the H2O2-induced apoptosis [34]. Taken together, these results suggest that SERMs potentially rescue Hcy- and oxidative stress-associated osteocyte dysfunction and bone fragility. Indeed, Johnell et al. [35] reported that the efficacy of raloxifene for preventing vertebral fracture is significantly higher in postmenopausal women with diabetes than in those without it. Therefore, further clinical studies are necessary to investigate whether SERMs are useful for preventing osteoporotic fracture especially in subjects with hyperhomocysteinemia and oxidative stress.
The present study showed that BZA significantly inhibited the Hcy-induced pro-inflammatory cytokines such as IL-1β and IL-6 in MLO-Y4-A2 cells. This is the first study to show anti-inflammatory effects of SERMs in osteocytic cells. There are few in vivo and clinical studies showing that SERMs may have anti-inflammatory effects. In animal models of collagen-induced arthritis, BZA reduced serum IL-6 levels and protected against bone loss [36]. Raloxifene significantly decreased circulating IL-6 levels in postmenopausal women [37]. Although further studies are needed, BZA could be useful for protecting against bone fragility caused by Hcy-induced inflammation in patients with osteoporosis.
In this study, Hcy significantly increased apoptosis of MLO-Y4-A2 cells in the absence of 17β-estradiol, whereas co-incubation with 17β-estradiol significantly inhibited the Hcy-induced apoptosis. Furthermore, ER inhibition reversed the effect of 17β-estradiol. These findings suggest that Hcy may affect osteocytes after menopause rather than premenopausal state. On the other hand, as 17β-estradiol, BZA clearly ameliorated Hcy-induced apoptosis; however, co-incubation with ER inhibitor only partly reversed the effect of BZA in two osteocyte-like cell lines. Although the mechanism remains unclear, there are several possibilities. BZA is generated to increase the selectivity to bone compared to 17β-estradiol or raloxifene. BZA binds to not only ERα but also ERβ [38], while 17b-estradiol and raloxifene have higher affinity for ERα than BZA. Because ICI 182,780 is a pure anti-estrogen to ERα [39], ICI may not be suitable to block the effects of BZA. Further, the dose and incubation time of ICI may not be enough for antagonizing BZA. Another possibility is that BZA may rescue Hcy-induced oxidative stress other than ER signal. Mann et al. [19] previously reported that raloxifene and related compound LY117018 inhibited H2O2-induced apoptosis through an estrogen receptor-independent effect. They showed that compounds containing the C3-OH moiety, which is a putative free radical scavenger, had anti-oxidative effects independent of ER signal. Because BZA also has C3-OH moiety same as other SERMs, BZA may protect osteocyte viability against Hcy-induced oxidative stress in the ER independent pathway. Furthermore, Jover-Menqual et al. [40] showed a difference of anti-apoptotic effects between 17β-estradiol and BZA in acute ischemic stroke. While BZA strongly reduced the increased p-ERK1/2 levels under ischemic condition, E2 did not. Therefore, further studies are necessary to clarify the detailed mechanism of anti-oxidative, anti-inflammatory, and anti-apoptotic effects of BZA.
Conclusions
The present study indicated that BZA inhibited Hcy-induced apoptosis of osteocyte-like cells through ER signal. Hcy increased apoptosis through increasing Nox1/2-IL-1β/IL-6 expressions in osteocyte-like cell. Moreover, BZA rescued Hcy-induced apoptosis by inhibiting the Nox1/2-IL-1β/IL-6 pathway in osteocyte-like cells. Therefore, BZA could be a potent therapeutic agent for preventing Hcy-induced apoptosis of osteocytes. To extend the present findings, further in vivo experiments are needed in future.
Abbreviations
- Hcy:
-
Homocysteine
- BZA:
-
Bazedoxifene acetate
- Nox:
-
NADPH oxidase
- DM:
-
Diabetes mellitus
- T2DM:
-
Type 2 diabetes mellitus
- BMD:
-
Bone mineral density
- α-MEM:
-
α-minimum essential medium
- SERM:
-
Selective estrogen receptor modulators
- DPI:
-
Diphenyleneiodonium chloride
- Apo:
-
Apocinin
References
Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Petterson C, De Laet C, Jonsson B (2004) Mortality after osteoporotic fractures. Osteoporos Int 15:38–42
Selhub J, Jacques PF, Wilson PW, Rush D, Rosenberg IH (1993) Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA 270:2693–2698
McLean RR, Jacques PF, Selhub J, Tucker KL, Samelson EJ, Broe KE, Hannan MT, Cupples LA, Kiel DP (2004) Homocysteine as a predictive factor for hip fracture in older persons. N Engl J Med 350:2042–2049
van Meurs JB, Dhonukshe-Rutten RA, Pluijm SM, van der Klift M, de Jonge R, Lindemans J, de Groot LC, Hofman A, Witteman JC, van Leeuwen JP, Breteler MM, Lips P, Pols HA, Uitterlinden AG (2004) Homocysteine levels and the risk of osteoporotic fracture. N Engl J Med 350:2033–2041
Yang J, Hu X, Zhang Q, Cao H, Wang J, Liu B (2012) Homocysteine level and risk of fracture: a meta-analysis and systematic review. Bone 51:376–382
Li J, Zhang H, Yan L, Xie M, Chen J (2014) Fracture is additionally attributed to hyperhomocysteinemia in men and premenopausal women with type 2 diabetes. J Diabetes Investig 5:236–241
Schwartz AV, Sellmeyer DE, Ensrud KE, Cauley JA, Tabor HK, Schreiner PJ, Jamal SA, Black DM, Cummings SR (2001) Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab 86:32–38
Vestergaard P (2007) Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes–a meta-analysis. Osteoporos Int 18:427–444
Yamamoto M, Yamaguchi T, Yamauchi M, Kaji H, Sugimoto T (2009) Diabetic patients have an increased risk of vertebral fractures independent of BMD or diabetic complications. J Bone Mineral Res 24:702–709
Kanani PM, Sinkey CA, Browning RL, Allaman M, Knapp HR, Haynes WG (1999) Role of oxidant stress in endothelial dysfunction produced by experimental hyperhomocyst(e)inemia in humans. Circulation 100:1161–1168
Hoogeveen EK, Kostense PJ, Beks PJ, Mackaay AJ, Jakobs C, Bouter LM, Heine RJ, Stehouwer CD (1998) Hyperhomocysteinemia is associated with an increased risk of cardiovascular disease, especially in non-insulin-dependent diabetes mellitus: a population-based study. Arterioscler Thromb Vasc Biol 18:133–138
Yun J, Kim JY, Kim OY, Jang Y, Chae JS, Kwak JH, Lim HH, Park HY, Lee SH, Lee JH (2011) Associations of plasma homocysteine level with brachial-ankle pulse wave velocity, LDL atherogenicity, and inflammation profile in healthy men. Nutr Metab Cardiovasc Dis NMCD 21:136–143
McCarty MF, O’Keefe JH, DiNicolantonio JJ (2017) Interleukin-1beta may act on hepatocytes to boost plasma homocysteine—the increased cardiovascular risk associated with elevated homocysteine may be mediated by this cytokine. Med Hypotheses 102:78–81
Takeno A, Kanazawa I, Tanaka K, Notsu M, Yokomoto M, Yamaguchi T, Sugimoto T (2015) Activation of AMP-activated protein kinase protects against homocysteine-induced apoptosis of osteocytic MLO-Y4 cells by regulating the expressions of NADPH oxidase 1 (Nox1) and Nox2. Bone 77:135–141
Kanazawa I, Tomita T, Miyazaki S, Ozawa E, Yamamoto LA, Sugimoto T (2017) Bazedoxifene ameliorates homocysteine-induced apoptosis and accumulation of advanced glycation end products by reducing oxidative stress in MC3T3-E1 cells. Calcif Tissue Int 100:286–297
Ellis AG, Reginster JY, Luo X, Bushmakin AG, Williams R, Sutradhar S, Mirkin S, Jansen JP (2014) Indirect comparison of bazedoxifene vs oral bisphosphonates for the prevention of vertebral fractures in postmenopausal osteoporotic women. Curr Med Res Opin 30:1617–1626
Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S (2007) Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 130:811–823
Morita M, Sato Y, Iwasaki R, Kobayashi T, Watanabe R, Oike T, Miyamoto K, Toyama Y, Matsumoto M, Nakamura M, Kawana H, Nakagawa T, Miyamoto T (2016) Selective estrogen receptor modulators suppress hif1alpha protein accumulation in mouse osteoclasts. PLoS ONE 11:e0165922
Mann V, Huber C, Kogianni G, Collins F, Noble B (2007) The antioxidant effect of estrogen and Selective Estrogen Receptor Modulators in the inhibition of osteocyte apoptosis in vitro. Bone 40:674–684
van Essen HW, Holzmann PJ, Blankenstein MA, Lips P, Bravenboer N (2007) Effect of raloxifene treatment on osteocyte apoptosis in postmenopausal women. Calcif Tissue Int 81:183–190
Bellido T (2014) Osteocyte-driven bone remodeling. Calcif Tissue Int 94:25–34
Tomkinson A, Reeve J, Shaw RW, Noble BS (1997) The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J Clin Endocrinol Metab 82:3128–3135
Tomkinson A, Gevers EF, Wit JM, Reeve J, Noble BS (1998) The role of estrogen in the control of rat osteocyte apoptosis. J Bone Miner Res 13:1243–1250
Notsu M, Kanazawa I, Takeno A, Yokomoto-Umakoshi M, Tanaka KI, Yamaguchi T, Sugimoto T (2017) Advanced glycation end product 3 (AGE3) increases apoptosis and the expression of sclerostin by stimulating TGF-beta expression and secretion in osteocyte-like MLO-Y4-A2 cells. Calcif Tissue Int 100:402–411
Welshons WV, Wolf MF, Murphy CS, Jordan VC (1988) Estrogenic activity of phenol red. Mol Cell Endocrinol 57:169–178
Spatz JM, Wein MN, Gooi JH, Qu Y, Garr JL, Liu S, Barry KJ, Uda Y, Lai F, Dedic C, Balcells-Camps M, Kronenberg HM, Babij P, Pajevic PD (2015) The Wnt inhibitor sclerostin is up-regulated by mechanical unloading in osteocytes in vitro. J Biol Chem 290:16744–16758
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Sugimoto T (2010) Fasudil hydrochloride induces osteoblastic differentiation of stromal cell lines, C3H10T1/2 and ST2, via bone morphogenetic protein-2 expression. Endocr J 57:415–421
Gori AM, Corsi AM, Fedi S, Gazzini A, Sofi F, Bartali B, Bandinelli S, Gensini GF, Abbate R, Ferrucci L (2005) A proinflammatory state is associated with hyperhomocysteinemia in the elderly. Am J Clin Nutr 82:335–341
Amarasekara DS, Yu J, Rho J (2015) Bone loss triggered by the cytokine network in inflammatory autoimmune diseases. J Immunol Res 2015:832127
Thaler R, Agsten M, Spitzer S, Paschalis EP, Karlic H, Klaushofer K, Varga F (2011) Homocysteine suppresses the expression of the collagen cross-linker lysyl oxidase involving IL-6, Fli1, and epigenetic DNA methylation. J Biol Chem 286:5578–5588
Zhang Y, He Y, Zong Y, Guo J, Sun L, Ma Y, Dong W, Gui L (2015) 17beta-estradiol attenuates homocysteine-induced oxidative stress and inflammatory response as well as MAPKs cascade via activating PI3-K/Akt signal transduction pathway in Raw 264.7 cells. Acta Biochim Biophys Sin 47:65–72
Arai M, Shibata Y, Pugdee K, Abiko Y, Ogata Y (2007) Effects of reactive oxygen species (ROS) on antioxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life 59:27–33
Saito M, Marumo K, Soshi S, Kida Y, Ushiku C, Shinohara A (2010) Raloxifene ameliorates detrimental enzymatic and nonenzymatic collagen cross-links and bone strength in rabbits with hyperhomocysteinemia. Osteoporos Int 21:655–666
Johnell O, Kanis JA, Black DM, Balogh A, Poor G, Sarkar S, Zhou C, Pavo I (2004) Associations between baseline risk factors and vertebral fracture risk in the Multiple Outcomes of Raloxifene Evaluation (MORE) Study. J Bone Miner Res 19:764–772
Andersson A, Bernardi AI, Stubelius A, Nurkkala-Karlsson M, Ohlsson C, Carlsten H, Islander U (2016) Selective oestrogen receptor modulators lasofoxifene and bazedoxifene inhibit joint inflammation and osteoporosis in ovariectomised mice with collagen-induced arthritis. Rheumatol (Oxf) 55:553–563
Gianni W, Ricci A, Gazzaniga P, Brama M, Pietropaolo M, Votano S, Patane F, Agliano AM, Spera G, Marigliano V, Ammendola S, Agnusdei D, Migliaccio S, Scandurra R (2004) Raloxifene modulates interleukin-6 and tumor necrosis factor-alpha synthesis in vivo: results from a pilot clinical study. J Clin Endocrinol Metab 89:6097–6099
Komm BS, Kharode YP, Bodine PV, Harris HA, Miller CP, Lyttle CR (2005) Bazedoxifene acetate: a selective estrogen receptor modulator with improved selectivity. Endocrinology 146:3999–4008
Jaber BM, Gao T, Huang L, Karmakar S, Smith CL (2006) The pure estrogen receptor antagonist ICI 182,780 promotes a novel interaction of estrogen receptor-alpha with the 3′,5′-cyclic adenosine monophosphate response element-binding protein-binding protein/p300 coactivators. Mol Endocrinol 20:2695–2710
Jover-Mengual T, Castello-Ruiz M, Burguete MC, Jorques M, Lopez-Morales MA, Aliena-Valero A, Jurado-Rodriguez A, Perez S, Centeno JM, Miranda FJ, Alborch E, Torregrosa G, Salom JB (2017) Molecular mechanisms mediating the neuroprotective role of the selective estrogen receptor modulator, bazedoxifene, in acute ischemic stroke: a comparative study with 17beta-estradiol. J Steroid Biochem Mol Biol 171:296–304
Acknowledgements
This study had funding support from Pfizer. Pfizer did not affect the study protocol, interpretation of the results, or discussion. Authors’ roles: MN and IK were responsible for designing and conducting the study. MN performed the experiments and analyzed the data. AT and KT contributed equipment/materials. MN and IK wrote the manuscript. TS reviewed and edited the manuscript. All authors approved the final version. IK takes responsibility for the integrity of the data analysis. The authors thank Keiko Nagira for technical assistance.
Funding
IK and TS have received lecture fees from Pfizer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Masakazu Notsu, Ippei Kanazawa, Ayumu Takeno, Ken-ichiro Tanaka, and Toshitsugu Sugimoto declare that they have no competing interests.
Human and Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Notsu, M., Kanazawa, I., Takeno, A. et al. Bazedoxifene Ameliorates Homocysteine-Induced Apoptosis via NADPH Oxidase-Interleukin 1β and 6 Pathway in Osteocyte-like Cells. Calcif Tissue Int 105, 446–457 (2019). https://doi.org/10.1007/s00223-019-00580-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-019-00580-7