Abstract
Summary
We demonstrate a reduction in enzymatic divalent immature and trivalent pyridinium cross-links and an increase in the nonenzymatic cross-link, pentosidine (Pen), in rabbits with methionine (Met)-induced hyperhomocysteinemia. Such detrimental cross-link formation in bone was ameliorated by raloxifene (RLX) treatment.
Introduction
Collagen cross-links are determinants of bone quality. Homocysteine (Hcys) interferes with collagen cross-linking. Because RLX is thought to ameliorate bone quality, we investigated whether RLX ameliorated hyperhomocysteinemia-induced cross-link abnormalities using a Met-rich diet rabbit model.
Methods
We divided New Zealand white rabbits into six groups (n = 6 per group): baseline control, sham operation, sham + 1% Met diet, ovariectomy (OVX), 1% Met diet + OVX, OVX + RLX (10 mg/kg/day), and 1% Met diet + OVX + RLX. RLX was administered for 16 weeks. We measured the amount of enzymatic immature and mature pyridinium cross-links and the nonenzymatic cross-link, Pen, and correlated the cross-link content to bone strength.
Results
Hcys levels were significantly higher in the Met diet groups than in the normal diet groups. Met-fed rabbits with or without OVX showed a significant reduction of enzymatic cross-links, whereas an increase in Pen was observed in Met-fed rabbits with OVX. The cross-link content of the RLX-treated Met-fed rabbits with OVX was restored to similar levels as the sham group, accompanied by an improvement of bone strength.
Conclusion
These results demonstrate that hyperhomocysteinemia reduced bone strength via a reduction of enzymatic cross-links and an increase of nonenzymatic cross-links. RLX may ameliorate hyperhomocysteinemia-induced detrimental cross-linking in rabbits with OVX and may improve bone strength via the amelioration of collagen cross-links.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Impaired bone quality and bone loss have been proposed as a cause of increased bone fragility in osteoporosis [1–3]. Collagen cross-link formation in bone plays an important role in bone strength [3–8] and the proper biological function of bone [9–11]. Thus, collagen cross-links are thought to be a determinant of bone quality at a material level. A moderately increased plasma content of homocysteine (Hcys) in the general population may indicate a risk of fracture that is independent of bone mineral density (BMD) [12, 13]. McKusick first proposed that hyperhomocysteinemia might interfere with normal enzymatic collagen cross-link formation [14], and this proposed mechanism was confirmed indirectly in homocystinuric patients. Lubec et al. [15] demonstrated that abnormalities of serum collagen metabolic markers, such as the carboxyterminal propeptide of type I collagen and the carboxyterminal telopeptide of collagen type I, in ten patients with homocystinuria may reflect abnormalities of bone collagen cross-linking. The McKusick hypothesis, however, still remains controversial because they did not analyze the actual content of collagen cross-links in bone.
Collagen cross-links can be divided into lysyl oxidase-mediated enzymatic cross-links and glycation- or oxidation-induced nonenzymatic cross-links (advanced glycation end products [AGEs] such as pentosidine [Pen] and vesperlysine). These two types differ in the mechanism of formation and in function. Enzymatic cross-links form by a two-stage process. Initially, divalent immature cross-links, ketoimines, are formed via the action of lysyl oxidase and lysyl hydroxylases [11, 16]. A portion of divalent immature cross-links then undergoes a spontaneous reaction to form trivalent mature pyridinium or pyrrole cross-links. Enzymatic cross-link formation increases bone strength within a beneficial range without brittleness [5, 6, 8]. Several investigations concerning enzymatic cross-links have focused only on mature pyridinium cross-links (pyridinoline [Pyr], deoxypyridinoline [Dpyr]) without accounting for the presence of immature cross-links (dehydro-dihydroxylysinonorleucine [deH-DHLNL], dehydro-hydroxylysinonorleucine [deH-HLNL], dehydro-lysinonorleucine [deH-LNL]). However, immature cross-links may also affect the mechanical function of bone [6, 7]. These observations have led to the proposal that a simultaneous estimation of both immature and mature cross-links is necessary to elucidate the actual role of enzymatic cross-link formation in bone.
The AGEs-type of cross-links, such as Pen and vesperlysine, are formed spontaneously by nonenzymatic glycation [4, 5] or oxidation reactions [17]. In contrast to the positive effects bestowed by enzymatic cross-links, AGEs cross-links deteriorate the mechanical and biological functions of bone [4–6]. An excessive formation of Pen in bone is thought to make collagen fibers brittle, thereby leading to a deterioration of the mechanical properties of bone, particularly postyield properties and toughness [4, 18].
Blouin et al. [19] recently demonstrated using Fourier transform infrared imaging (FTIRI) that the collagen cross-link ratio (Pyr/DHLNL) was significantly higher in hip fracture cases with hyperhomocysteinemia (mean plasma level of Hcys = 28.12 ± 7.99 nmol/mL) than cases with normal Hcys plasma levels (mean plasma level of Hcys = 7.02 ± 0.73 nmol/mL). These changes were independent of bone mineral characteristics. In addition, we reported, in a case-controlled study, a significant reduction in the actual amount of enzymatic cross-links and a marked increased in AGEs cross-link, Pen, in bone from patients with postmenopausal osteoporotic hip fractures and hyperhomocysteinemia (n = 25, mean plasma level of Hcys = 12.94 ± 3.38 nmol/mL) [20]. However, these two studies were cross-sectional studies so it could not be determined whether Hcys caused collagen cross-link abnormalities.
Raloxifene (RLX), a selective estrogen receptor modulator, decreases fracture risk in osteoporosis to a similar extent as bisphosphonate despite a smaller increase in BMD [21, 22]. Therefore, it is postulated that RLX may show favorable effects on collagen quantitative and qualitative changes [23]. Interestingly, RLX treatment of postmenopausal women reduced circulating Hcys levels in two randomized controlled studies [24, 25]. However, to date, there are no available data regarding the effects of RLX on bone collagen cross-link formation in hyperhomocysteinemia.
Due to the lack of data regarding the relationship between Hcys levels in sera and enzymatic and AGEs cross-link formation in human bone, in this study, we investigated whether hyperhomocysteinemia affects enzymatic immature and mature cross-links and nonenzymatic cross-link contents in bone. We used the conventional methionine (Met)-rich diet rabbit model, which induces hyperhomocysteinemia. Rabbits with induced hyperhomocysteinemia are a well-established model that has been successfully used as an experimental cardiovascular disease model [26, 27]. Moreover, we used rabbits since, unlike rats or mice, they exhibit Haversian bone remodeling similar to humans and bone remodeling may affect collagen nonenzymatic cross-linking [23]. In a preliminary study, we confirmed that nonenzymatic cross-link content in aged rabbit (3 to 4 years of age) is significantly higher than that of skeletally immature rabbits. Because similar hyperhomocysteinemic dog or monkey models, animals which also exhibit bone remodeling, have not yet been established, we used the rabbits. Since RLX is used to treat patients in an estrogen-deficient state (postmenopausal women), we also investigated whether RLX ameliorated hyperhomocysteinemia-induced cross-link abnormalities using the conventional Met-rich diet rabbit model where estrogen deficiency was achieved by ovariectomy (OVX).
Materials and methods
Animals
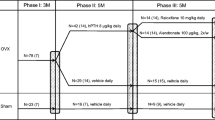
Forty-two 5-month-old (3.4 ± 0.19 kg body weight), skeletally mature, female New Zealand white rabbits were purchased from Kitayama Labes (Nagano, Japan). Animals were acclimatized for 2 weeks and maintained with a 12-h light–dark cycle at room temperature with ad libitum access to water.
Experimental animal model
The rabbits were divided randomly into six groups based on their body weight (n = 6 per group). Group 1: the baseline control, which were euthanized at day 0. Group 2 (sham group): sham operation. Group 3 (Met group): sham operation with a 1% Met diet. Group 4 (OVX): ovariectomized (OVX) with vehicle (1.5% carboxymethyl cellulose, 2 mL/kg/day). Group 5 (Met/OVX group): 1% Met diet with OVX and vehicle. Group 6 (OVX/RLX group): OVX and RLX (RLX; LY139481, Eli Lilly, Indianapolis, IN, USA; 10 mg/kg/day). Group 7 (Met/OVX/RLX group): OVX with a 1% Met diet and RLX. RLX or vehicle was administered by oral gavage every day for 16 weeks after an initial 16 weeks of 1% Met diet with OVX. Sham operation and OVX was performed under ketamine hydrochloride (0.6 mL/kg administered intraperitoneally [i.p.]) anesthesia. Met was added as described above to a regular diet (RC4, Oriental Yeast, Tokyo, Japan) containing 1.4% calcium and 0.6% phosphorus. These rabbits were pair-fed with 100 g/day throughout the study. RLX dosage was chosen according to previous studies using rabbit models for RLX [27]. Previous pharmacokinetic data in rabbits have shown that the doses chosen produce plasma RLX concentrations in a similar range to those found in postmenopausal women treated with RLX [27]. After treatment for 16 weeks, the rabbits were anesthetized with pentobarbital, 60 mg/kg i.p., and euthanized by exsanguination. Fasting blood was withdrawn from the left ventricle and centrifuged. Plasma Hcys levels were measured as a fluorescence derivative by high-performance liquid chromatography (HPLC) [28]. One day before killing, the rabbits were placed in metabolic cages for a fasting 24-h urine collection. Total urinary Dpyr was determined after acid hydrolysis using our established HPLC method [29]. The femur was collected for analyses of BMD, bone mechanical tests, and collagen cross-links. All experiments were approved by the Experimental Animal Ethics Committee at our institution and conducted in accordance with guidelines concerning the management and handling of experimental animals.
Bone mineral density
Mid-diaphyses of the femurs were scanned using a pQCT device (XCT Research SA+, Stratec Medizintechnik, Pforzheim, Germany) with pixel dimensions of 0.12 × 0.12 mm and a slice thickness of 0.77 mm. The volumetric cortical BMD (Ct.BMD; mg/cm3), periosteal circumference, and endocortical circumference were analyzed using the pQCT software, Rev. 6.00B [30]. The cortical region was determined with a threshold value of 690 mg/cm3 [30].
Collagen content in bone
Collagen content in the central one third of the femoral bone was calculated from hydroxyproline (Hyp) content, which was expressed as a percentage of tissue dry weight.
An aliquot of bone powder was weighed and hydrolyzed in 6 M hydrochloric acid at 110°C for 24 h. The amount of collagen in bone was determined from the Hyp content measured by HPLC. It was assumed that collagen weighed 7.5 times the measured Hyp weight, with a molecular weight of 300,000 [29]. The resulting data were used to calculate the cross-link values as moles per mole of collagen [29].
Measurement of collagen cross-links
Reduction of collagen in bone was performed using sodium borohydride (NaBH4; Sigma-Aldrich, St Louis, MO, USA). Subsequent quantification of reducible immature, nonreducible mature, and nonenzymatic cross-links, Pen, in the central one third of the femoral bone was carried out by HPLC as previously described [29]. Briefly, each sample of bone powder was demineralized twice with 0.5 M ethylenediaminetetraacetic acid in 50 mM Tris buffer, pH 7.4, for 96 h at 4°C. Demineralized bone residues were then sequentially suspended in potassium phosphate buffer, pH 7.6 (ionic strength = 0.15), and reduced at 37°C with nonradioactive NaBH4. Reduced specimens were hydrolyzed in 6 M hydrochloric acid at 110°C for 24 h. Hydrolysates were then analyzed for cross-links using a Shimadzu LC9 HPLC fitted with a cation exchange column (0.9 × 10 cm; Aa pack-Na, Jasco, Tokyo, Japan) linked to an inline fluorescence flow monitor (RF10AXL, Shimadzu, Shizuoka, Japan). We determined the content of enzymatic cross-links, such as immature reducible and mature nonreducible, and nonenzymatic cross-links (AGEs). Lysyl oxidase-mediated reducible immature cross-links (deH-DHLNL, deH-HLNL, and deH-LNL) were identified and quantified according to their reduced forms (DHLNL, HLNL, and LNL, respectively). Reducible immature cross-links and common amino acids, such as Hyp, were detected by o-phthalaldehyde derivatization using a postcolumn method, whereas enzymatic nonreducible mature cross-links, such as Pyr and Dpyr, and nonenzymatic glycation-induced cross-links, Pen, were detected by natural fluorescence. Our established HPLC system enables us to determine enzymatic and nonenzymatic cross-link contents within a linear range from 0.2 to 600 pmol in bone specimens. Contents of each cross-link were expressed as moles per mole of collagen. We confirmed that the hydrolysates of demineralized and reduced bone specimens consisted of glycine in the range of 322 to 310 residues per 1,000 amino acids and greater than 80 residues Hyp per 1,000 amino acids, which is in accordance with the known amino acid composition of type I collagen [29].
Bone mechanical tests
Immediately after collection, the femurs were mechanically tested by positioning them on the lower supports of a three-point bending fixture and load was applied at the midpoint using a material testing machine (MZ-500D, Maruto, Tokyo, Japan) [31]. The specimen was placed on the two lower support bars (40 mm apart) with the convex side facing toward the loading bar and the loading bar was positioned at the midpoint of the specimen. A load was applied at a strain rate of 2.5 mm/min until the breakpoint. Structural mechanical properties such as ultimate load, stiffness, and work to ultimate load were automatically determined from the load–displacement curve by a connected computer. Load and displacement data were converted to stress and strain and used to compute the bending intrinsic material properties according to the method of Turner et al. [32]. The intrinsic material properties such as ultimate stress, elastic modulus, and toughness were calculated by normalizing the structural parameters as follows: ultimate stress = (ultimate load × L × anterior–posterior diameter)/(8 × cross-sectional moment of inertia), elastic modulus = stiffness + L 3/(48 × cross-sectional moment of inertia), toughness = 0.75 × energy absorption × anterior–posterior diameter2/(L × cross-sectional moment of inertia). L represents the bottom of support span and an anterior–posterior diameter and a cross-sectional moment of inertia were from pQCT scans.
Statistical analysis
All data are expressed as the mean ± standard deviation (SD). Data were compared using one-way analysis of variance with a Games–Howell post hoc test using SPSS statistical software. Values of p < 0.05 were considered statistically significant. Pearson correlations and linear regression analyses were performed between collagen cross-link parameters and bone mechanical properties or levels of serum Hcys.
Results
In general, the animals tolerated the surgery and experimental treatments without complications. There was no significant difference in body weight between the baseline control group and all animals at the end of the experiment, which is consistent with a previously reported rabbit study (Table 1) [27]. No significant differences were observed in plasma Hcys levels, urinary excretion of Dpyr, body weight, bone size, and femoral BMD between the baseline control and sham group (Table 1).
Estrogen deficiency after OVX was confirmed at necropsy by significantly decreased uterine weights and by a significant reduction in serum estradiol levels in all OVX and Met/OVX groups (9.1 ± 3.6 pg/mL) whatever the time point and regardless of treatment compared with the sham group, which exhibited mean estradiol levels of 24.2 ± 5.4 pg/mL at euthanasia. This is consistent with previous reports [27, 33].
Plasma Hcys levels
Plasma Hcys levels were significantly higher in Met-fed rabbits (Met group = 158.9 ± 48.5 nmol/mL, p < 0.0001 and Met/OVX group = 163.2 ± 34.5 nmol/mL, p < 0.0001) than in the sham group (11.2 ± 1.2 nmol/mL). A higher content of plasma Hcys levels was also evident in the OVX group (13.9 ± 1.9 nmol/mL) than in the sham group, but this was not significantly higher (p = 0.08; Table 1). Plasma Hcys levels in Met-fed rabbits were consistent with those previously reported in a similar rabbit model [34, 35]. When comparing plasma Hcys levels between RLX treatment groups (OVX/RLX and Met/OVX/RLX groups) and nontreated groups (OVX and Met/OVX groups), a trend (p = 0.08) toward lower content was evident in the OVX/RLX group than the OVX group, while a marked decreased of 52% was observed in the Met/OVX/RLX group compared with the Met/OVX group (p < 0.0001; Table 1).
Bone resorption marker, bone size, BMD, and collagen content
There was no difference in either urinary excretion of the bone resorption marker, Dpyr, bone size, or cortical BMD in midfemoral diaphysis between the baseline control group and the sham group (Table 1). This was also evident when comparing all groups at the end of the experiment (Table 1). These results of BMD after OVX in rabbits are consistent with those previously reported in a similar OVX rabbit model [33]. No significant difference was observed in bone collagen content between the baseline control and sham groups. The Met and OVX groups showed no significant difference in bone collagen content compared with the sham group, whereas a significant decrease of 18% (p = 0.0003) was observed in the Met/OVX group compared with the sham group. This reduction in collagen content in the Met/OVX group was restored to the same level as the sham group by treatment with RLX (Table 2).
Amount of enzymatic cross-links and nonenzymatic cross-links
Comparing the baseline control and sham groups, there was no significant difference in the cross-link parameters or bone mechanical properties in the femur (Fig. 1, Tables 2 and 3).
Total enzymatic cross-links (a) and nonenzymatic cross-links, Pen (b) in rabbit femoral bones, subjected to 1% methionine diet (Met), sham operation (Sham), ovariectomy (OVX), 1% Met diet with OVX (Met + OVX), and raloxifene (RLX) treatment (10 mg/kg/day for 16 weeks). The enzymatic cross-link content was estimated by the sum of immature divalent (DHLNL, HLNL, and LNL) and mature trivalent pyridinium (Pyr and Dpyr) cross-links. Mean values ± SD
Met-fed rabbits, such as the Met and Met/OVX groups, exhibited a significant reduction in content of both immature and mature enzymatic cross-links. Consequently, the total enzymatic cross-links (the sum of DHLNL, HLNL, LNL, Pyr, and Dpyr) were markedly decreased by 21% (p = 0.0003) for the Met group, 25% (p < 0.0001) for the Met/OVX group, and 24% (p < 0.0001) for the OVX group compared with the sham group (Fig. 1a and Table 2). Immature and mature enzymatic cross-links in the RLX-treated OVX and Met/OVX groups were restored to the same levels as that of the sham group. There was a significantly higher content of total enzymatic cross-links in the RLX-treated OVX (p < 0.0001) and Met/OVX (p = 0.0008) groups than in the nontreated OVX and Met/OVX groups (Fig. 1a and Table 2). While the differences in total enzymatic cross-links among the groups may correlate with plasma Hcys levels, we analyzed the relationship between collagen cross-link parameters and plasma levels of Hcys by Pearson correlation analyses (Table 4). The sums of immature and mature enzymatic cross-links were significantly and negatively correlated with plasma Hcys levels (r = −0.475, p = 0.0034). Similarly, total immature (r = −0.470, p = 0.0038) and mature (r = −0.238, p = 0.022) enzymatic cross-links associated negatively with plasma Hcys levels (Table 4).
A marked increase in Pen content was evident in the Met group (1.8-fold higher, p = 0.002) and the Met/OVX group (2.0-fold higher, p = 0.0002) compared with the sham group (Fig. 1b and Table 2). A significant reduction in Pen content (−69%, p < 0.0001) was evident in the RLX-treated Met/OVX group compared with the nontreated Met/OVX group, whereas there was no significant difference between OVX and OVX/RLX groups (Fig. 1b and Table 2). As shown in Table 4, there was a significant positive correlation between Pen content and plasma Hcys levels (r = 0.578, p = 0.0002).
Bone mechanical properties
There was no difference in bone strength between the baseline control and the sham group (Table 3). No significant association was observed between BMD in the femur mid-diaphysis and bone mechanical properties (Table 4). Although there was no significant difference in BMD and bone size among the groups, the bone mechanical properties were significantly different (Tables 3 and 4).
Met-fed groups, such as the Met and Met/OVX groups, showed a marked reduction in ultimate load (−12%, p = 0.066 for the Met group and −16%, p = 0.029 for the Met/OVX group) compared with the sham group. Toughness was similarly reduced in Met-fed groups (−31%, p = 0.0017 for the Met group and −30%, p = 0.007 for the Met/OVX group) compared with the sham group. However, toughness was significantly higher in the RLX-treated OVX (energy absorption = 59%, p = 0.023; toughness = 31%, p = 0.011) and Met/OVX group (toughness = 149%, p = 0.003) than the nontreated OVX and Met/OVX group, respectively (Table 3). Consequently, these bone mechanical parameters in the RLX-treated groups were restored to the same levels as those of the sham group.
While changes in mechanical properties may correlate with collagen cross-link formation, we analyzed the relationship between collagen cross-link parameters and bone mechanical properties. As shown in Table 4, the content of Pen had a significant negative association with toughness.
Discussion
Rabbits with hyperhomocysteinemia induced by a Met diet and OVX rabbits showing estrogen deficiencies have been successfully used as experimental cardiovascular disease models [26, 27]. Rabbits are the smallest species known to have Haversian bone remodeling processes. Bone remodeling also affects collagen nonenzymatic cross-linking [23, 36], so studying rabbits may be useful for assessing the role of nonenzymatic cross-links. However, there are a limited number of bone studies in OVX rabbits [33, 37]. The pathognomonic issue in rabbits is that OVX alone might not be enough to develop low BMD despite estrogen deficiency [33].
Recently, data have accumulated that enzymatic cross-links and nonenzymatic cross-links affect material properties of bone [4–6, 18]. In human bone, there is a well-known age-related marked increase in AGEs-type cross-link, Pen [4, 29]. Such age-related accumulation of AGEs cross-linking in bone collagen is an independent determinant of bone mechanical properties [4]. Silva et al. [38] reported that there was no significant age-related increase in the nonenzymatic cross-link, Pen, in a senescence-accelerated mouse strain P6 (SAMP6), which is a murine model of senile osteoporosis. Also, Wistar rats did not exhibit a significant age-related increase in the nonenzymatic cross-link, Pen [6]. However, postmenopausal hip fracture patients with hyperhomocysteinemia show an excessive formation of the nonenzymatic cross-link, Pen, in bone [20]. To examine the effect of RLX on cross-links in bone, we used a rabbit model of hyperhomocysteinemia, which, unlike mice and rats, showed similar abnormalities of enzymatic and nonenzymatic cross-links to human bone induced by hyperhomocysteinemia. We also investigated estrogen deficiency, which was induced by OVX, in rabbits with hyperhomocysteinemia in order to study cross-link formation and estrogen status since this is closer to the conditions humans experience when using RLX.
Hyperhomocysteinemia and enzymatic and nonenzymatic collagen cross-links
In the present study, we show the adverse effects of hyperhomocysteinemia on enzymatic immature divalent and mature trivalent pyridinium cross-link formation and on formation of the nonenzymatic cross-link, Pen, in bone. However, RLX ameliorated detrimental cross-link formation and restored bone strength in rabbits with hyperhomocysteinemia. Recently, Blouin et al. [19] demonstrated that hip fracture cases with mildly elevated hyperhomocysteinemia have an abnormal cross-link ratio (Pyr/DHLNL). In addition, we previously reported that a reduction in the total amount of enzymatic cross-links and an excessive accumulation of the nonenzymatic cross-link, Pen, were evident in patients with hip fractures and hyperhomocysteinemia [20, 39]. These data support the McKusick [14] and Lubec [15] hypotheses, cohort studies [12, 13], and our previously reported clinical studies on a 5-year prospective study of 502 postmenopausal women with moderate hyperhomocysteinemia. In the latter study, moderate hyperhomocysteinemia induced by a TT genotype of the MTHFR polymorphism (C677T) was independent of the risk of fractures [40]. This indicated that hyperhomocysteinemia in the general population may increase bone fragility via a reduction in bone quality, that is, collagen cross-link deterioration, independent of changes in BMD.
The present study shows a significant reduction in enzymatic cross-links in the OVX group (Fig. 1 and Table 3). Oxlund et al. [7] demonstrated in an animal study that impaired enzymatic cross-link formation in bone induced by beta-aminopropionitrile, which irreversibly inhibits the enzyme lysyl oxidase, resulted in a significant decrease in bone strength down to approximately 30% of control animals. We previously reported in preclinical diabetic rats that a significant reduction in enzymatic cross-link formation was accompanied by a 25% decrease in bone strength at a material level, independent of BMD [6]. At preclinical diabetic stages, this model did not show any change in nonenzymatic AGEs cross-links [6]. In terms of osteoblastic activity, altered enzymatic cross-linking by beta-aminopropionitrile treatment deteriorated osteoblastic differentiation and lathyrogen cross-link formation [41]. These results indicate that proper enzymatic cross-link formation in bone matrix is indispensable for both bone mechanical strength and osteoblastic differentiation.
Interestingly, Ozasa et al. [42] showed in a mouse OVX model that lysyl oxidase activity decreased down to 25% 3 days after OVX, but activity was completely rescued by estradiol injection. This indicates that estrogen may be a regulatory factor of enzymatic cross-link formation. Hak et al. [43] in a population-based study (n = 12,675) and Russo et al. [44] in a case-controlled study showed that menopause itself is an independent risk factor for elevated plasma Hcys levels. In this study, plasma Hcys levels in the OVX group were somewhat higher than in the sham group. These results indicate that a reduction of the amount of immature and mature enzymatic cross-links in the OVX group might be attributed to mildly elevated circulating Hcys levels as well as estrogen deficiency, although further investigations regarding the actual enzymatic activity of lysyl oxidase in bone are needed. In contrast, AGEs cross-link formation did not show a significant increase in the OVX group. Therefore, estrogen deficiency may impair formation of enzymatic cross-links rather than quantitatively change nonenzymatic AGEs cross-link formation.
In this study, we demonstrate that hyperhomocysteinemia itself induced collagen cross-link deterioration in bone in the Met and Met/OVX groups, which coincided with a marked decrease in bone toughness, but without a change in BMD (Tables 2, 3, and 4). It is worthy to note that there was a significant reduction in enzymatic cross-link formation and a 1.8-fold or 2.0-fold increase in the nonenzymatic AGEs cross-link, Pen, in the Met or Met/OVX groups, respectively, compared with the sham group (Fig. 1 and Table 2). Since a reduction in enzymatic cross-links and excessive formation of the nonenzymatic AGEs cross-link, Pen, is thought to reduce the mechanical properties of bone, particularly toughness, and consequently make collagen fibers brittle [5, 18], hyperhomocysteinemia may deteriorate toughness of bone via alterations of cross-link formation (Fig. 1 and Tables 2, 3, and 4). This result may support our previous human bone biopsy study where the content of enzymatic cross-links was significantly decreased and nonenzymatic cross-links, Pen, were markedly increased in bone from postmenopausal osteoporotic hip fracture cases with moderately elevated plasma Hcys levels [20].
In contrast to our results, Herrmann et al. [45] demonstrated that chronic hyperhomocysteinemia (circulating Hcys level; 27 to 120 nmol/mL) induced by Met feeding reduced bone strength in a rat model. This may be due to a decrease in BMD via increased osteoclastic activity, although they did not show any information on collagen cross-link status. We confirmed that there was no change in BMD or urinary excretion of the bone resorption marker, Dpyr, in Met-fed groups, such as the Met and Met/OVX groups, in this rabbit study (Table 1). The reason for the differences may relate to species variation and the method by which hyperhomocysteinemia was induced. Hypomethylation induced by Met loading also has a crucial role in the pathogenesis of hyperhomocysteinemia. Herrmann et al. [45] demonstrated a distinct increase in hypomethylation of Met in bone tissue and Hcys-loaded animals that significantly reduced cellular methylation capacity, which may cause a profound disturbance of cellular activity. Therefore, hypomethylation induced by a Met-rich diet may be another factor involved in the adverse effects of hyperhomocysteinemia on bone.
The conventional Met-fed animal model is considered to be an artificial hyperhomocysteinemia because of extremely high levels of Hcys in sera. Hermann et al. used rat models with hyperhomocysteinemia induced by a Met-rich diet or Hcys-rich diet [45, 46]. In their reports, the experimental period was 10 to 20 weeks and their Hcys plasma levels were 27 to 120 nmol/mL. In this rabbit study, a Met-rich diet resulted in a significant increase of plasma Hcys levels (158.9 ± 48.5 nmol/mL), which was consistent with those previously reported in a similar rabbit model [34, 35]. Adverse effects of moderately elevated plasma Hcys levels on bone quality in the general population may gradually accumulate in bone over several decades. Thus, the adverse effects of moderately elevated Hcys may only surface in the elderly. In contrast, the Met-fed rabbit model shows a significantly higher content of plasma Hcys levels than the general population. Consequently, bone collagen cross-link abnormalities may occur to a similar extent as our previously reported human study after only 8 months of exposure of hyperhomocysteinemia in rabbits. Tovorek et al. [26] also showed that 6 to 9 months of loading of Met-rich diet resulted in atherogenic changes in a rabbit model. Further investigations are required to establish whether long-term exposure (years) induces a similar deterioration in cross-links in a longer lifespan animal.
Combining the previous basic and clinical studies regarding the effect of hyperhomocysteinemia on bone fragility suggests that hyperhomocysteinemia may affect bone quality and bone quantity. The mechanisms of deterioration of collagen cross-link formation induced by hyperhomocysteinemia are not clear at the present time. A possible explanation for cross-link deterioration in hyperhomocysteinemia may be due to an adverse effect of Hcys on the cross-link formation pathway. In terms of enzymatic cross-link formation, Hcys is thought to interfere with enzymatic cross-link formation via a reduction in gene expression and enzymatic activity of lysyl oxidase [47]. Kang et al. [48] demonstrated that Hcys binds to the aldehydic groups of allysine and hydroxyallysine, which are the precursors of enzymatic cross-links, and results in a marked decrease in total enzymatic cross-links.
The other candidate is AGEs formation. AGEs are thought to be formed by lysine residues, which are essential sites of enzymatic cross-linking in collagen, and results in competitively inhibiting formation of enzymatic cross-links [49]. However, the possibility of a relationship between the accumulation of AGEs and enzymatic cross-link formation remains hypothetical at the present time. AGEs cross-links, Pen, are formed spontaneously by nonenzymatic glycation [4] or oxidation reactions [17]. The amount of nonenzymatic cross-links in bone is mainly regulated by three factors: bone turnover, the degree of oxidation, or glycation. In this study, we show an excessive formation of Pen in Met-rich diet groups, although there was no difference in a marker of bone turnover, uDPD. Also, Met diet groups such as the Met and Met/OVX groups did not show hyperglycemia in this study. Since hyperhomocysteinemia is thought to increase oxidative stress [50], the high accumulation of Pen in our study may be due to increased oxidation reaction. In preliminary study, we confirmed that no significant increase in the amount of AGEs, Pen, in skin type I collagen from Met and Met/OVX groups in spite of higher accumulation in bone type I collagen. Since Herrmann et al. [45] demonstrated using hyperhomocysteinemia-induced rat model that Hcys seemed to accumulate specifically (1,300% to 2,000% of control rat) and bind to collagen in bone, the adverse effect of hyperhomocysteinemia on collagen enzymatic and nonenzymatic cross-link formation may be predominant in bone collagen.
Raloxifene and cross-links
Based on previous studies using estrogen-deficient rabbits, we treated rabbits with RLX for 4 months [27, 51]. In the present study, we demonstrated that RLX treatment has a beneficial effect on enzymatic immature lysinonorleucine-type and mature pyridinium-type of cross-links and nonenzymatic AGEs cross-link formations in bone.
Positive changes in toughness would suggest that RLX has a positive effect on bone quality and that this effect is likely to be mediated independently of BMD change and bone turnover suppression (Tables 1 and 4). Allen et al. [23] showed that 1 year treatment with RLX did not show any changes in enzymatic mature pyridinium cross-links in bone from a non-OVX beagle dog model. However, there was no information on immature enzymatic cross-link formation, which is the predominant form in bone. In this study, we found that treatment with RLX increased the content of both immature and mature enzymatic cross-links in bone from the OVX and Met/OVX groups. This discrepancy may be due to species differences. Interestingly, Khastgir et al. [52] showed that estrogen replacement therapy (HRT) increased enzymatic cross-links in bone from elderly postmenopausal women with osteoporosis. Paschalis et al. [53] and Faibish et al. [54] also demonstrated the beneficial effects of HRT and RLX on enzymatic cross-link ratios assessed by FTIRI when paired biopsy specimens were compared before and after 2 years of HRT. Such positive effects of HRT and RLX on enzymatic cross-link ratios may be a possible explanation for the increased amount of enzymatic cross-links detected in the RLX-treated OVX and Met/OVX groups.
Yang et al. [55] demonstrated that RLX and estrogen increased the expression of transforming growth factor-beta (TGF-beta) through the interaction of the estrogen receptor in vitro. It is thought that TGF-beta acts as a positive regulator of lysyl oxidase in osteoblasts [56]. Additionally, because TGF-beta increased collagen accumulation in the extracellular matrix [57], collagen content in bone from the RLX-treated Met/OVX group might be restored to a similar level as the sham group. Based on these reports, the positive effect of RLX on enzymatic cross-link formation and collagen accumulation might be mainly attributed to an antagonistic action on the estrogen receptor. Moreover, Helvering et al. [58] proposed a distinctive action of RLX, which is different from estrogen, based on gene expression profiles after the treatment of RLX and estrogen in OVX rat models.
Another candidate for the protective effect of RLX on enzymatic cross-link formation may be lowering of plasma Hcys levels by RLX treatment. Since Hcys itself acts as a negative regulator of lysyl oxidase [47, 48], lowering Hcys levels in plasma may be beneficial for lysyl oxidase expression. Reduction in circulating Hcys levels by RLX treatment of postmenopausal women was also confirmed in three randomized controlled studies [24, 25], although the mechanism by which this occurs is not clear at the present time. However, we cannot conclude from this study which mechanism, that is, the speculated estrogen agonistic action or decrease in plasma Hcys levels or both, is crucial for ameliorating impaired enzymatic cross-link formation in the Met/OVX group. To address this issue, further investigation of the effect of circulating Hcys-lowering therapies on cross-link formation without estrogen deficiency is required.
In this study, we found that the RLX-treated Met/OVX group showed a positive effect on enzymatic cross-link formation and also inhibited excessive accumulation of AGEs cross-linking compared with the nontreated Met/OVX group (−69%, p < 0.0001). This also correlated with improvement of toughness (Table 4). Allen et al. [23] demonstrated that a 1-year treatment of RLX inhibits age-related accumulation of AGEs cross-link, Pen, using non-OVX female dogs. Additionally, as described above, it is assumed that a significant increase in AGEs cross-link formation in the Met/OVX group may be attributed to elevated oxidation reactions induced by hyperhomocysteinemia [50]. Interestingly, Mann et al. [59] showed that RLX decreased circulating Hcys levels and also oxidative stress resulting in the inhibition of osteocyte apoptosis via reduction in oxygen species. Therefore, we suggest that RLX may ameliorate excessive accumulation of AGEs regardless of estrogen and circulating Hcys status.
Limitations exist for the interpretation of the results of this study. First, we have assessed only the femoral cortex. Therefore, we cannot definitively state whether the observed changes in bone quality are applicable to other clinically relevant bone sites such as vertebra. Second, enzymatic mature pyrrole cross-links may be equally important as pyridinium cross-links [8, 60]. Pyrrole cross-links are unstable during acid hydrolysis and, therefore, we cannot measure them using our HPLC method. Since the major determinant of the total amount of immature lysinonorleucine-type and their mature forms, such as pyridinium and pyrrole, is lysyl oxidase activity, overall formation of pyrrole cross-links might be similar to other lysyl oxidase-controlled cross-linking. Third, Pen is just one of many AGEs in bone. The reasons we elected to quantify Pen are that Pen can be quantified easily and precisely in small (less than 1 mg) bone specimens [29], Pen is covalently bonded to adjacent collagen fibers, and Pen content is often widely used in mechanical studies [4–6, 18, 36]. Another AGEs-type cross-link, vesperlysine, can be detected by immunohistochemistry and the fluorescence method of Vashishth [4] presumably detects all AGEs, including noncross-links and cross-link types in combination. Recently, Vashishth et al. [4] reported a significant relationship between the content of Pen and bulk fluorescence for detection of all AGEs, suggesting that the pathway of their formation may be similar and that Pen may be used as a biomarker of AGEs.
In conclusion, the enzymatic and nonenzymatic cross-link deterioration induced by hyperhomocysteinemia may lead to an accelerated increase in bone fragility independent of BMD. RLX treatment may improve bone strength through the amelioration of both impaired enzymatic cross-link formation and excessive formation of nonenzymatic AGEs cross-link, Pen, without affecting BMD in hyperhomocysteinemia-induced rabbits with estrogen deficiency.
References
Seeman E, Delmas PD (2006) Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med 354:2250–2261
Burr DB (2002) Bone material properties and mineral matrix contributions to fracture risk or age in women and men. J Musculoskelet Neuronal Interact 2:201–204
Paschalis EP, Shane E, Lyritis G et al (2004) Bone fragility and collagen cross-links. J Bone Miner Res 19:2000–2004
Vashishth D (2007) The role of the collagen matrix in skeletal fragility. Curr Osteoporos Rep 5:62–66
Wang X, Shen X, Li X et al (2002) Age-related changes in the collagen network and toughness of bone. Bone 31:1–7
Saito M, Fujii K, Mori Y et al (2006) Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporos Int 17:1514–1523
Oxlund H, Mosekilde L, Ortoft G (1996) Reduced concentration of collagen reducible crosslinks in human trabecular bone with respect to age and osteoporosis. Bone 19:479–484
Banse X, Sims TJ, Bailey AJ (2002) Mechanical properties of adult vertebral cancellous bone: correlation with collagen intermolecular cross-links. J Bone Miner Res 17:1621–1628
Paschalis EP, Recker R, Dicarlo E et al (2003) Distribution of collagen cross-links in normal human trabecular bone. J Bone Miner Res 18:1942–1946
Saito M, Soshi S, Fujii K (2003) Effect of hyper- and microgravity on collagen post-translational controls of MC3T3-E1 osteoblasts. J Bone Miner Res 18:1695–1705
Uzawa K, Grzesik WJ, Nishiura T et al (1999) Differential expression of human lysyl hydroxylase genes, lysyl hydroxylation, and cross-linking of type I collagen during osteoblastic differentiation in vitro. J Bone Miner Res 14:1272–1280
McLean RR, Jacques PF, Selhub J et al (2004) Homocysteine as a predictive factor for hip fracture in older persons. N Engl J Med 350:2042–2049
van Meurs JB, Dhonukshe-Rutten RA, Pluijm SM et al (2004) Homocysteine levels and the risk of osteoporotic fracture. N Engl J Med 35:2033–2041
McKusick VA (1966) Heritable disorders of connective tissue, 3rd edn. C.V. Mosby, St. Louis, p 155
Lubec B, Fang-Kircher S, Lubec T et al (1996) Evidence for McKusick's hypothesis of deficient collagen cross-linking in patients with homocystinuria. Biochim Biophys Acta 13:159–162
Saito M, Sohsi S, Tanaka T et al (2004) Intensity-related differences in collagen post-translational modification in MC3T3-E1 osteoblasts after exposure to low and high intensity pulsed ultrasound. Bone 35:644–655
McCarthy AD, Etcheverry SB, Bruzzone L et al (2001) Non-enzymatic glycation of a type I collagen matrix: effect on osteoblastic development and oxidative stress. BMC Cell Biol 2:16
Garnero P, Borel O, Gineyts E et al (2006) Extracellular post-translational modifications of collagen are major determinants of biomechanical properties of fetal bovine cortical bone. Bone 38:300–309
Blouin S, Thaler HW, Korninger C et al (2009) Bone matrix quality and plasma homocysteine levels. Bone 44:959–964
Saito M, Fujii K, Marumo K (2006) Degree of mineralization-related collagen crosslinking in the femoral neck cancellous bone in cases of hip fracture and controls. Calcif Tissue Int 79:160–168
Delmas PD, Li Z, Cooper C (2004) Relationship between changes in bone mineral density and fracture risk reduction with antiresorptive drugs: some issues with meta-analyses. J Bone Miner Res 19:330–337
Recker RR, Kendler D, Recknor CP et al (2007) Comparative effects of raloxifene and alendronate on fracture outcomes in post-menopausal women with low bone mass. Bone 40:843–851
Allen MR, Gynets E, Leeming DJ et al (2008) Bisphosphonates alter trabecular bone collagen cross-linking and isomerization in beagle dog vertebra. Osteoporos Int 19:329–337
Walsh BW, Paul S, Wild RA et al (2000) The effects of hormone replacement therapy and raloxifene on C-reactive protein and homocysteine in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab 85:214–218
De Leo V, la Marca A, Morgante G et al (2001) Randomized control study of the effects of raloxifene on serum lipids and homocysteine in older women. Am J Obstet Gynecol 84:350–353
Toborek M, Kopieczna-Grzebieniak E, Drozdz M et al (1995) Increases lipid peroxidation as a mechanism of methionine-induced atherosclerosis in rabbit. Atherosclerosis 115:217–224
Bjarnason NH, Haarbo J, Byrjalsen I et al (2000) Raloxifene reduces atherosclerosis: studies of optimized raloxifene doses in ovariectomized, cholesterol-fed rabbits. Clin Endocrinol 52:225–233
Araki A, Sako Y (1987) Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr 422:43–52
Saito M, Marumo K, Fujii K et al (1997) Single column high-performance liquid chromatographic-fluorescence detection of immature, mature and senescent cross-links of collagen. Anal Biochem 253:26–32
Nonaka K, Fukuda S, Aoki K et al (2006) Regional distinctions in cortical bone mineral density measured by pQCT can predict alterations in material properties at the tibial diaphysis of the Cynomolgus monkey. Bone 38:265–272
Manabe T, Mori S, Mashiba T et al (2007) Human parathyroid hormone (1–34) accelerates natural fracture healing process in the femoral osteotomy model of cynomolgus monkeys. Bone 40:1475–1482
Turner CH, Akhter MP, Heaney RP (1992) The effects of fluoridated water on bone strength. J Orthop Res 10:581–587
Castaneda S, Calvo E, Largo R et al (2008) Characterization of a new experimental model of osteoporosis in rabbits. J Bone Miner Metab 26:53–59
Shukla N, Koupparis A, Jones RA et al (2006) Penicillamine administration reverses the inhibitory effect of hyperhomocysteinaemia on endothelium-dependent relaxation and superoxide formation in the aorta of the rabbit. Eur J Pharmacol 531:201–208
Narin F, Narin N, Akcakus M et al (2002) The effect of folic acid, vitamin B6 and vitamin B12 on the homocysteine levels in rabbits fed by methionine-enriched diets. Tohoku J Exp Med 198:99–105
Saito M, Mori S, Mashiba T et al (2008) Collagen maturity, glycation induced-pentosidine, and mineralization are increased following 3-year treatment with incadronate in dogs. Osteoporos Int 19:1343–1354
Bellino FL (2000) Nonprimate animal models of menopause: workshop report. Menopause 7:14–24
Silva MJ, Brodt MD, Wopenka B et al (2006) Decreased collagen organization and content are associated with reduced strength of demineralized and intact bone in the SAMP6 mouse. J Bone Miner Res 21:78–88
Saito M, Fujii K, Soshi S et al (2006) Reductions in degree of mineralization and enzymatic collagen cross-links and increases in glycation induced pentosidine in the femoral neck cortex in cases of femoral neck fracture. Osteoporos Int 17:986–995
Shiraki M, Urano T, Kuroda T et al (2008) The synergistic effect of bone mineral density and methylenetetrahydrofolate reductase (MTHFR) polymorphism (C677T) on fracture. J Bone Miner Metab 26:595–602
Turecek C, Fratzl-Zelman N, Rumpler M (2008) Collagen cross-linking influences osteoblastic differentiation. Calcif Tissue Int 82:392–400
Ozasa H, Tominaga T, Nishimura T et al (1981) Lysyl oxidase activity in the mouse uterine cervix is physiologically regulated by estrogen. Endocrinology 109:618–621
Hak AE, Polderman KH, Westendorp IC et al (2000) Increased plasma homocysteine after menopause. Atherosclerosis 149:163–168
Russo GT, Di Benedetto A, Alessi E et al (2008) Menopause modulates homocysteine levels in diabetic and non-diabetic women. J Endocrinol Invest 31:546–551
Herrmann M, Tami A, Wildemann B et al (2008) Hyperhomocysteinemia induces a tissue specific accumulation of homocysteine in bone by collagen binding and adversely affects bone. Bone 44:467–475
Herrmann M, Peter Schmidt J, Umanskaya N et al (2007) The role of hyperhomocysteinemia as well as folate, vitamin B(6) and B(12) deficiencies in osteoporosis: a systematic review. Clin Chem Lab Med 45:1621–1632
Raposo B, Rodriguez C, Martinez-Gonzalez J et al (2004) High levels of homocysteine inhibit lysyl oxidase (LOX) and down regulate LOX expression in vascular endothelial cells. Atherosclerosis 177:1–8
Kang HA, Trelstad RL (1973) A collagen defect in homocystinuria. J Clin Invest 52:2571–2578
Reiser KM (1991) Nonenzymatic glycation of collagen in aging and diabetes. Proc Soc Exp Biol Med 196:17–29
Eberhardt RT, Forgione MA, Cap A et al (2000) Endothelial dysfunction in a murine model of mild hyper homocyst(e) inemia. J Clin Invest 106:483–491
Castelo-Branco C, Sanjuan A, Casals E et al (2004) Raloxifene inhibits cholesterol aortic content but not atherosclerotic plaque size in oophorectomised cholesterol-fed rabbits. J Obstet Gynaecol 24:47–51
Khastgir G, Studd J, Holland N et al (2001) Anabolic effect of long-term estrogen replacement on bone collagen in elderly postmenopausal women with osteoporosis. Osteoporos Int 12:465–470
Paschalis EP, Boskey AL, Kassem M et al (2003) Effect of hormone replacement therapy on bone quality in early postmenopausal women. J Bone Miner Res 18:955–959
Faibish D, Ott SM, Bosky AL (2006) Mineral changes in osteoporosis—a review. Clin Orthop Relat Res 443:28–38
Yang NN, Bryant HU, Hardikar S et al (1996) Estrogen and raloxifene stimulate transforming growth factor-beta 3 gene expression in rat bone: a potential mechanism for estrogen- or raloxifene-mediated bone maintenance. Endocrinology 137:2075–2084
Feres-Filho EJ, Choi YJ, Han X et al (1995) Pre- and post-translational regulation of lysyl oxidase by transforming growth factor-beta 1 in osteoblastic MC3T3-E1 cells. J Biol Chem 270:30797–30803
Shibata Y, Abiko Y, Moriya Y et al (1993) Effects of transforming growth factor-beta on collagen gene expression and collagen synthesis level in mineralizing cultures of osteoblast-like cell line, MC3T3-E1. Int J Biochem 25:239–245
Helvering LM, Liu R, Kulkarni N et al (2005) Expression profiling of rat femur revealed suppression of bone formation genes by treatment with alendronate and estrogen but not raloxifene. Mol Pharmacol 68:1225–1238
Mann V, Huber C, Kogianni G et al (2007) The antioxidant effect of estrogen and selective estrogen receptor modulators in the inhibition of osteocyte apoptosis in vitro. Bone 40:674–684
Brady JD, Robins SP (2001) Structural characterization of pyrrolic cross-links in collagen using a biotinylated Ehrlich's reagent. J Biol Chem 276:18812–18818
Acknowledgements
The authors are grateful to Dr. Azusa Seki, DVM (Hamri, Japan), Ms. Mika Imamura, and Ms. Kazumi Hirakawa (Research Assistants, Jikei University School of Medicine, Japan) for aiding in the specimen preparation and testing. This study was funded by a competitive research foundation grant from the Osteoporosis Society, Japan and The Nakatomi Foundation, Japan.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saito, M., Marumo, K., Soshi, S. et al. Raloxifene ameliorates detrimental enzymatic and nonenzymatic collagen cross-links and bone strength in rabbits with hyperhomocysteinemia. Osteoporos Int 21, 655–666 (2010). https://doi.org/10.1007/s00198-009-0980-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-009-0980-4