Abstract
The purpose of this study is to estimate the efficacy of eldecalcitol (1α, 25-Dihydroxy-2β- (3-hydroxypropyloxy) vitamin D3; ELD) on bone metabolism after long-term administration. Six-month-old Wistar–Imamichi rats were ovariectomized (OVX) and administered ELD orally at doses of 7.5, 15, or 30 ng/kg daily. Bone mineral density (BMD), urinary excretion of deoxypyridinoline (DPD), a bone resorption marker, and serum total alkaline phosphatase (ALP), a surrogate marker of bone formation, were assessed after 3, 6, and 12 months of treatment. After 12 months of treatment, the biomechanical strength of the L4 lumbar vertebra and femoral shaft was measured, and bone histomorphometry was performed on the L3 lumbar vertebra and the tibia diaphysis. ELD prevented OVX-induced decreases in BMD of the lumbar vertebrae and femur throughout the treatment period. ELD significantly suppressed OVX-induced increases in urinary DPD excretion throughout the treatment period with minimal effects on ALP. OVX resulted in significant decreases in ultimate load and stiffness of the L4 lumbar vertebra and femoral shaft, and ELD significantly prevented the reduction in these biomechanical parameters. Bone histomorphometry at the L3 lumbar vertebra revealed that OVX induced increases in bone resorption parameters (osteoclast surface and osteoclast number) and bone formation parameters (osteoblast surface, osteoid surface, and bone formation rate), and ELD suppressed these parameters after 12 months treatment. Activation frequency, which was elevated in the OVX/vehicle group, was significantly suppressed to baseline levels in ELD-treated groups, indicating that ELD maintained bone turnover at a normal level. ELD also prevented OVX-induced deterioration of microstructure in trabecular and cortical bone. These results indicated that long-term treatment of OVX rats with ELD suppressed bone turnover, and prevented OVX-induced bone loss, deterioration of bone microstructure, and reduction in bone biomechanical strength.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eldecalcitol (1α, 25-Dihydroxy-2β- (3-hydroxypropyloxy)vitamin D3; ELD), an active vitamin D3 analog bearing a hydroxypropoxy residue at the 2β position [1–3], is used in Japan for the treatment of patients with osteoporosis. ELD possesses higher binding affinity to the vitamin D-binding protein and lower binding affinity to the vitamin D receptor (VDR) compared to 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3) [4, 5]. A 3-year, randomized, double-blind, clinical trial in patients with osteoporosis revealed that ELD treatment significantly decreased the incidences of vertebral fractures and wrist fractures compared to alfacalcidol, a prodrug of the active form of vitamin D3 [6]. ELD also more potently increased bone mineral density (BMD) of the lumbar spine and total hip and reduced bone turnover markers compared to alfacalcidol in osteoporotic patients [6, 7]. The effects of ELD on bone metabolism have also been shown in non-clinical studies using animal models of osteoporosis [8–11]. In ovariectomized (OVX) rats, ELD inhibited osteoclastic bone resorption and increased BMD more effectively than alfacalcidol [8]. ELD reduced the number of osteoclasts in trabecular bone of OVX rats and also promoted bone minimodeling, a type of focal bone formation [10, 11]. These reports suggested that ELD regulates both bone resorption and bone formation and acts to maintain bone mass.
Because drug treatment of osteoporosis is carried out over a long period, it is important to assess how long-term treatment influences bone quality, including bone turnover. There is growing concern that chronic administration of bisphosphonates, the most commonly used drugs in the treatment of osteoporosis, might lead to oversuppression of bone turnover and increase the risk of unusual fractures [12–17]. Histomorphometric analyses have revealed that bisphosphonates lowered activation frequency (Ac.f), a bone turnover parameter, to below the normal level in OVX rats and also in osteoporotic patients after long-term administration [18–20]. Furthermore, animal studies have shown that long-term treatment with bisphosphonates suppresses bone remodeling and causes microcrack accumulation, resulting in a decline in the material properties of bone [21–24]. Although it is not yet clear whether long-term therapy with bisphosphonate causes the accumulation of microcracks in osteoporotic patients [20, 25], it has been suggested that the oversuppression of bone turnover might have relevance to unusual fractures.
It is not well understood how long-term treatment with ELD affects bone metabolism including bone turnover. In the present study, to assess the effects of long-term ELD administration on bone metabolism, we treated OVX rats with ELD for 12 months and examined bone mass, bone biomechanical strength, bone turnover, and bone microstructure.
Materials and Methods
Reagents
ELD was synthesized by Chugai Pharmaceutical Co., Ltd. ELD was dissolved in ethanol and diluted to a given concentration with medium chain triglyceride.
Animals
Female Wistar–Imamichi rats were obtained from the Institute for Animal Reproduction (Ibaraki, Japan) and acclimated for 4 weeks. Animals were housed individually in stainless steel wire mesh floor cages under standard laboratory conditions: 22 ± 3 °C, humidity of 50 ± 20 %, and a 12:12 h light/dark cycle. All animals had free access to tap water and a standard commercial laboratory diet (PMI Certified CLE Rodent Rat and Mouse 25 % Diet; PMI Nutrition International Inc., St. Louis, MO, USA).
Experimental Design
At the start of the treatment, animals were approximately 6 months of age. Before ovariectomy, BMD of the lumbar vertebrae (L2–L5) was measured in vivo by dual-energy X-ray absorptiometry (DXA) using a Discovery A Hologic densitometer (Hologic, Inc. Marlborough, MA, USA). Using a randomization procedure stratified according to lumbar spine BMD, rats were divided into five groups of 15 females/group. An additional group of ten females was sacrificed by exsanguinations from the abdominal aorta under isoflurane anesthesia at the beginning of the experiment as baseline controls for the histomorphometry and biomechanical testing. In the remaining groups of rats, bilateral ovariectomy or sham operation was performed under anesthesia with isoflurane/oxygen gas. From the day following surgery, OVX animals were orally administered ELD (7.5, 15, 30 ng/kg of body weight) for 12 months. In the sham and OVX control groups, rats received the vehicle at a dose of 1 mL/kg of body weight. At 10 and 3 days prior to necropsy, calcein (8 mg/kg of body weight) was injected subcutaneously to label bone-forming surfaces. After 12 months of treatment, animals were euthanized by exsanguination from the abdominal aorta under isoflurane anesthesia. The lumbar vertebra (L3) and tibia were removed, cleaned of excess soft tissue, retained in 10 % neutral buffered formalin for 3 days and then transferred to 70 % ethanol for histomorphometric analysis. A femur and lumbar vertebra (L4) were collected, cleaned of soft tissue, wrapped in saline-soaked gauze and stored at −20 °C prior to densitometry and biomechanical testing. All animal procedures were approved by the Institutional Animal Care and Use Committee at Charles River Laboratories Preclinical Services Montreal and were performed in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility. The study was performed in accordance with the Good Laboratory Practice Regulations of the US Food and Drug Administration.
Measurement of Bone Mineral Density
Animals were anesthetized using isoflurane prior to measurement. BMD of the lumbar vertebrae (L2–L5) and right femur were measured in vivo by DXA using a Discovery A Hologic densitometer with small animal high-resolution software before surgery and after 3, 6, and 12 months of treatment.
Biochemical Analysis
Blood was collected via the jugular vein before surgery and after 3, 6, and 12 months of treatment. Urine was collected over a 24-h period before surgery and after 3, 6, and 12 months of treatment. Animals were fasted overnight prior to blood and urine collection. Serum calcium (Ca) concentration, serum total alkaline phosphatase (ALP) activity, urinary Ca, and urinary creatinine (Cr) were measured with a Modular Analytics ISE1800 and P800 (Roche Diagnostics, Laval, QC, Canada). Urinary deoxypyridinoline (DPD) was measured using a gamma-BCT DPD RIA kit (Immunodiagnostic Systems Ltd, Fountain Hills, AZ, USA). Urinary DPD values were corrected for urinary Cr concentration.
Biomechanical Testing
Biomechanical testing was performed using an 858 Mini Bionix Servohydraulic Test System Model 242.03 (MTS System Corporation, Eden Prairie, MN, USA). All data were collected using TestWorks (version 3.8A) for TestStar software (version 4.0C) to produce load–displacement curves. For the femur 3-point bending test, the upper loading device was aligned at the expected breaking point of the femoral shaft on the anterior side. The load was applied at a rate of 1 mm/s until failure occurred. Prior to the compression test of the L4 vertebral body, the vertebral arch and end plates were removed to obtain a specimen with planoparallel ends. The loading rate of the compression test was set at 20 mm/min. Ultimate load and work to failure were determined from the load–displacement curve, and ultimate stress and toughness were calculated. Stiffness was determined from the slope of the linear elastic region of the load–displacement curve, and modulus was calculated [26]. Prior to the 3-point bending test of the femoral shaft, moment of inertia and bone mineral content (BMC) were measured at the expected breaking point by peripheral quantitative computed tomography (pQCT; XCT Research SA, Stratec Medizintechnik, Pforzheim, Germany) using Cortmode 2. Cross-sectional area and BMC at the mid-section of the L4 vertebra were quantified by pQCT using Contmode 2, Peelmode 20 (trabecular area 45 %), voxel size 0.1 mm.
Bone Histomorphometry
Bone histomorphometry was performed on the L3 vertebra and tibial diaphysis. Specimens were dehydrated and then infiltrated and embedded in methyl methacrylate. Goldner’s trichrome-stained sections were prepared to evaluate the cancellous bone in the L3 vertebra. Stevenel’s blue-stained ground sections were prepared for evaluation of the cortical bone at the tibio-fibular junction. Measurements of static and dynamic parameters were collected with a Bioquant image analyzer (Bioquant Image Analysis Corp., Nashville, TN, USA) linked to a microscope equipped with bright and epifluorescence illumination. The following variables were measured: bone volume (BV/TV, %), trabecular thickness (Tb.Th, μm), trabecular number (Tb.N,/mm), trabecular separation (Tb.Sp, μm), osteoclast surface (Oc.S/BS, %), osteoclast number (N.Oc/BS,/mm), osteoblast surface (Ob.S/BS, %), osteoid surface (OS/BS, %), mineral apposition rate (MAR, μm/day), bone formation rate (BFR/BS, μm3/μm2/year), activation frequency (Ac.f,/year), tissue area (T.Ar, mm2), medullary area (Me.Ar, mm2), cortical area (Ct.Ar, mm2), cortical width (Ct.Wi, μm), endocortical perimeter (Ec.Pm, mm), and periosteal perimeter (Ps.Pm, mm). Nomenclature and units used in this study follow the report of the American Society for Bone and Mineral Research Histomorphometry Nomenclature Committee [27].
Statistical Analysis
Data are expressed as the mean ± SEM. For each dataset, the homogeneity of group variances was evaluated using Levene’s test. If group variances were homogenous, multiple comparisons among the groups were analyzed by 1-way analysis of variance (ANOVA) followed by Dunnett’s test to determine significance. If group variances were found to be heterogeneous, the Kruskal–Wallis test was employed, and statistical differences were determined by Dunn’s test. Statistical comparisons between the Sham/vehicle group and the OVX/vehicle group were performed by t test on the least square means for the parametric dataset and by Wilcoxon rank-sum test for the non-parametric dataset. P ≤ 0.05 was considered statistically significant. Correlation analysis of biomechanical strength and BMC was performed using a Pearson correlation test. All statistical analyses were performed using SRS version 1.4, which is a Charles River Laboratories Preclinical Services Montreal Inc. in-house application built with SAS release 8.2.
Results
Bone Mineral Density
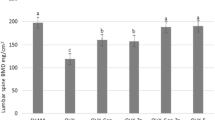
Lumbar vertebral BMD in the OVX/vehicle group was significantly decreased 3 months after OVX compared to the Sham/vehicle group and continued to decline over the study period (Fig. 1a). Treatment with ELD dose-dependently prevented OVX-induced decreases in BMD of the lumbar vertebrae throughout the treatment period. BMD was significantly higher in each of the ELD-treated groups compared to the OVX/vehicle group. BMD in the 15 ng/kg ELD-treated group was maintained at the sham control level, and BMD in the 30 ng/kg ELD-treated group was increased above the sham control level.
Effects of 12-month ELD treatment on BMD in the lumbar spine (L2-L5) (a) and femur (b) of OVX rats. OVX rats were given the indicated doses of ELD daily. BMD of the lumbar spine and femur was measured in vivo by DXA at 0, 3, 6, and 12 months of treatment. Data are presented as mean ± SEM (n = 12–15 for each group). a P ≤ 0.05 versus Sham/vehicle group by t test; b P ≤ 0.05 versus Sham/vehicle group by Wilcoxon rank-sum test; c P ≤ 0.05 versus OVX/vehicle group by Dunnett’s test; d P ≤ 0.05 versus OVX/vehicle group by Dunn’s test
Similar to the lumbar vertebral BMD, OVX resulted in a significant decrease in femur BMD throughout the study period (Fig. 1b). Treatment with 15 and 30 ng/kg of ELD significantly prevented the OVX-induced decrease in femur BMD at 12 months.
Bone Turnover Markers
Urinary DPD excretion, a bone resorption marker, was significantly increased in the OVX/vehicle group compared to the Sham/vehicle group throughout the study period (Fig. 2a). Treatment with 15 and 30 ng/kg of ELD significantly suppressed the OVX-induced increase in urinary DPD excretion after 3 months of treatment, which was sustained throughout the 12 months of treatment. The 7.5 ng/kg dose significantly suppressed the increase in urinary DPD excretion at 12 months. Serum ALP activity in the OVX/vehicle group was significantly increased compared to the Sham/vehicle group (Fig. 2b). No significant change in serum ALP activity was observed in any of the ELD-treated groups compared to the OVX/vehicle group.
Effects of 12-month ELD treatment on urinary DPD excretion (a) and serum ALP activity (b). OVX rats were given the indicated doses of ELD daily. Urinary DPD and serum ALP activities were measured at 0, 3, 6, and 12 months of treatment. Data are presented as mean ± SEM (n = 9–10 for each group). a P ≤ 0.05 versus Sham/vehicle group by t test; b P ≤ 0.05 versus Sham/vehicle group by Wilcoxon rank-sum test; c P ≤ 0.05 versus OVX/vehicle group by Dunnett’s test; d P ≤ 0.05 versus OVX/vehicle group by Dunn’s test
Bone Biomechanical Strength
At the end of the treatment period, OVX resulted in significant decreases in ultimate load, stiffness, ultimate stress, modulus, and toughness of the L4 vertebral body (Table 1). OVX did not alter work to failure of the L4 vertebral body. Treatment with ELD significantly and dose-dependently prevented the OVX-induced decrease in ultimate stress of the L4 vertebral body compared to the OVX/vehicle group. The 15 and 30 ng/kg doses of ELD significantly prevented the decreases in stiffness, modulus, and toughness, and the 30 ng/kg dose also prevented the decrease in ultimate load of the L4 vertebral body. Work to failure in the 30 ng/kg ELD-treated group was significantly higher than in the OVX/vehicle group. Similar to BMD, values for all biomechanical strength parameters of the L4 vertebral body in the 15 ng/kg ELD-treated group were maintained at the sham control levels and increased above the sham control levels in the 30 ng/kg ELD-treated group.
In the 3-point bending test of the femoral shaft, OVX resulted in significant decreases in ultimate load, stiffness, ultimate stress, and modulus compared to the Sham/vehicle group and did not change work to failure or toughness (Table 1). Compared to the OVX/vehicle group, treatment with 15 and 30 ng/kg of ELD significantly prevented the OVX-induced decreases in ultimate load and stiffness of the femoral shaft. Work to failure and toughness of the femoral shaft in the 30 ng/kg ELD-treated group were significantly lower than in the OVX/vehicle group.
Correlation analyses between BMC as assessed by pQCT and ultimate load in the L4 vertebral body and femoral shaft showed significant positive correlations between BMC and ultimate load at both sites (L4 vertebral body: r = 0.94, P < 0.001; femoral shaft: r = 0.77, P < 0.001) (Fig. 3).
Correlation between ultimate load and BMC in the L4 vertebral body (a) and femur (b). Ultimate load for the L4 vertebral body and mid-femoral shaft were determined by compression test and 3-point bending test, respectively. BMC of the L4 vertebral body and mid-femoral shaft was measured using pQCT. a: r = 0.94, P ≤ 0.001 (n = 57); b: r = 0.77, P ≤ 0.001 (n = 60)
Bone Histomorphometry
OVX induced a significant reduction in BV/TV of the L3 vertebra, accompanied by a significant decrease in Tb.N and a significant increase in Tb.Sp (Table 2). Increased bone turnover induced by OVX was confirmed as evidenced by significant increases in Oc.S/BS, N.Oc/BS, Ob.S/BS, OS/BS, and BFR/BS.
Treatment with 15 and 30 ng/kg of ELD significantly prevented the OVX-induced decrease in BV/TV. The increase in BV/TV observed in those ELD-treated groups was accompanied by a significant increase in Tb.N and a significant decrease in Tb.Sp. The 30 ng/kg dose of ELD also significantly increased Tb.Th. Treatment with all doses of ELD significantly suppressed the OVX-induced increases in Ob.S/BS and OS/BS. The 15 and 30 ng/kg dose of ELD suppressed the OVX-induced increases in BFR/BS, and the 30 ng/kg dose of ELD also suppressed Oc.S/BS and N.Oc/BS. ELD did not change MAR in the trabecular bone of the L3 vertebra. Ac.f, a bone turnover marker, which was elevated in the OVX/vehicle group, was significantly lower in the 15 and 30 ng/kg ELD-treated groups than in the OVX/vehicle group (Fig. 4).
Effect of 12-month ELD treatment on Ac.f in the trabecular bone of the L3 vertebra. OVX rats were given the indicated doses of ELD daily. After 12 months of treatment, histomorphometric analysis of the trabecular bone of the L3 vertebral body was performed. Data are presented as mean ± SEM (n = 9–10 for each group). b P ≤ 0.05 versus Sham/vehicle group by Wilcoxon rank-sum test; d P ≤ 0.05 versus OVX/vehicle group by Dunn’s test
At the cortical bone in the tibial diaphysis, OVX significantly increased T.Ar, Me.Ar, Ec.Pm, and Ps.Pm compared to the Sham/vehicle group, but did not change Ct.Ar or Ct.Wi (Table 3). Treatment with 15 and 30 ng/kg of ELD resulted in significantly higher Ct.Ar, Ct.Wi, and Ps.Pm compared to the OVX/vehicle group. T.Ar was significantly higher and Me.Ar was significantly lower in the 30 ng/kg ELD-treated group than in the OVX/vehicle group. OVX elevated endocortical MAR and endocortical BFR/BS as well as periosteal BFR/BS compared to the Sham/vehicle group. Treatment with all doses of ELD significantly suppressed OVX-induced increases in endocortical MAR and endocortical BFR/BS, but did not alter periosteal BFR/BS. OVX and treatment with ELD did not change endocortical Oc.S/BS, endocortical N.Oc/BS, or periosteal MAR.
Serum Calcium and Urinary Calcium Excretion
Serum Ca concentration was significantly lower in the OVX/vehicle group than in the Sham/vehicle group at 6 and 12 months after OVX (Fig. 5a). In the 15 and 30 ng/kg ELD-treated groups, serum Ca concentrations were significantly higher than in the OVX/vehicle group. The value in the 15 ng/kg ELD-treated group was at the level of the sham control group. Urinary Ca excretion was significantly lower in the OVX/vehicle group than in the Sham/vehicle group at 12 months after OVX (Fig. 5b). Treatment with 15 and 30 ng/kg of ELD significantly increased urinary Ca excretion compared to the OVX/vehicle group.
Effects of 12-month ELD treatment on serum Ca (a) and urinary Ca excretion (b). OVX rats were given the indicated doses of ELD daily. Serum Ca and urinary Ca excretion were measured at 0, 3, 6, and 12 months of treatment. Data are presented as mean ± SEM (n = 9–10 for each group). a P ≤ 0.05 versus Sham/vehicle group by t test; c P ≤ 0.05 versus OVX/vehicle group by Dunnett’s test; d P ≤ 0.05 versus OVX/vehicle group by Dunn’s test
Discussion
In this study, OVX induced a significant decrease in BMD and consequently bone biomechanical strength. Bone turnover markers, urinary DPD excretion and serum ALP activity, were increased in the OVX/vehicle group at 3, 6, and 12 months after OVX, indicating that animals undergoing OVX surgery were in a high-bone-turnover state throughout the study period. This high-bone-turnover state was also confirmed by histomorphometric analysis of trabecular bone in the lumbar vertebral body, in which bone resorption (Oc.S/BS and N.Oc/BS) and bone formation (Ob.S/BS, OS/BS, and BFR/BS) parameters were elevated. Bone histomorphometric analysis also revealed that BV/TV was decreased by OVX, resulting from decreases in Tb.N associated with increased Tb.Sp. This deterioration of trabecular architecture and the decrease in BMD resulted in a reduction in bone biomechanical strength after OVX.
Treatment with ELD resulted in a dose-dependent increase in ultimate load and stiffness of the lumbar vertebral body and femoral shaft. ELD at 15 ng/kg maintained these biomechanical parameters at sham control level, and ELD at 30 ng/kg not only prevented the OVX-induced decrease in bone biomechanical strength but also increased strength above the level of the sham controls. These dose-related effects on bone biomechanical strength were consistent with the effects on BMD. Treatment with ELD also increased material properties of the lumbar vertebral body as indicated by significant increases in ultimate stress, modulus, and toughness. On the other hand, ELD did not alter ultimate stress and modulus of the femoral shaft. Decreases in work to failure and toughness of the femoral shaft in the 30 ng/kg ELD-treated group may be associated with increased stiffness by ELD. Treatment with ELD increased BV/TV by increases in Tb.N and Tb.Th associated with decreased Tb.Sp, indicating that ELD prevented OVX-induced deterioration of trabecular architecture. It has been reported that BV/TV is strongly related with yield strength in the vertebral body [28]. The increase in bone biomechanical strength in lumbar vertebrae by ELD treatment can be attributed to improved trabecular microstructure and increased BMD.
OVX significantly changed the microstructure of the cortical bone in the tibial diaphysis. The most evident structural change related to OVX was an expansion of the marrow cavity as indicated by significant increases in Me.Ar and Ec.Pm. This medullary expansion was compensated for with an expansion at the periosteal surface as indicated by significant increases in T.Ar and Ps.Pm. ELD prevented the OVX-related expansion of the marrow cavity of the tibial diaphysis. ELD also increased cortical bone accretion at the periosteal surface as evidenced by an increase in Ps.Pm. These effects of ELD acted synergistically to increase the cortical bone compartment as shown by significant increases in T.Ar, Ct.Ar, and Ct.Wi, contributing to increase in the biomechanical strength of cortical bone.
Positive correlations between BMC and bone biomechanical strength were observed in the lumbar vertebrae and femoral shaft. The linear regression in the ELD-treated groups was consistent with that in the sham and the OVX control groups, suggesting that increased bone mass by ELD was accompanied by appropriate increases in bone strength.
The suppressive effect of ELD on urinary DPD excretion was observed throughout the treatment period. Histomorphometric analysis of the trabecular bone demonstrated that ELD decreased Oc.S/BS and N.Oc/BS at the end of the treatment. Uchiyama et al. reported that treatment with ELD for 3 months in OVX rats inhibited osteoclastic bone resorption more potently than did treatment with alfacalcidol [8]. ELD was shown to suppress bone resorption markers in osteoporosis patients [6]. In the present study, it was demonstrated that long-term treatment with ELD suppressed bone resorption throughout the 12-month treatment period.
The mechanism by which ELD inhibits bone resorption in vivo remains to be fully clarified. In rats, it has been shown that ELD has a greater activity than alfacalcidol in increasing bone mass with no difference in the effects on serum Ca and serum parathyroid hormone (PTH) [8], indicating that the effects of active vitamin D3 on bone can take place, at least in part, independent of the effects on intestinal Ca absorption and on PTH suppression. Harada et al. reported that ELD decreases the number of osteoclasts in vivo through the suppression of receptor activator of NF-κB ligand (RANKL) expression in trabecular bone [29]. A recent study showed that ELD suppresses expression of sphingosine-1-phosphate receptor 2 (S1PR2), a chemorepulsive receptor for S1P, in osteoclast precursor cells and inhibits osteoclastic bone resorption by mobilizing osteoclast precursor cells from bone to the blood [30]. These reports indicate that ELD acts directly on bone and inhibits bone resorption by regulating osteoclastogenesis. Furthermore, an in vitro study has shown that active vitamin D3 acts directly on osteoclast precursors and inhibits their differentiation into mature osteoclasts through suppressing expression of c-Fos protein [31]. Additionally, active vitamin D3 inhibits human osteoclastogenesis in vitro through upregulating IFN-β expression, which in turn suppresses expression of nuclear factor of activated T cells c1 (NFATc1) [32]. In addition to the above-mentioned direct action of ELD on bone, ELD may also act on osteoclast precursors, resulting in inhibition of osteoclastogenesis, although further studies are required to fully elucidate the mechanism.
Ac.f was increased in the OVX/vehicle group at 12 months after OVX. Treatment with ELD inhibited the OVX-induced increase in Ac.f and maintained it at baseline level. A previous report showed that treatment with ELD did not lower Ac.f in normal rats and in OVX rats [33]. There are some differences in dosing regimen between that previous study and the current study. In the previous study, ELD was given 2 times a week at a dose of 10–100 ng/kg for 12 weeks, while in this study, we treated the rats daily for 12 months. The effect of ELD on bone turnover may be affected by frequency, dose, and period of treatment. Alfacalcidol suppressed bone resorption and maintained or stimulated bone formation in OVX rats and in intact rats [34–36]. While ELD and 1α,25(OH)2D3 suppressed bone resorption, they stimulated focal bone formation (minimodeling), which was induced independent of bone resorption in OVX rats [10, 11]. These reports indicate that although vitamin D3 compounds suppress bone resorption, they do not depress bone formation excessively. In this study, ELD treatment did not alter MAR in trabecular bone or at the periosteal surface of cortical bone and did not affect serum ALP activity, indicating that the osteoblastic function of mineralization was preserved on ELD. Taken together, long-term treatment with ELD may not excessively inhibit bone turnover.
It is reported that the effect of ELD on urinary Ca excretion was comparable with that of alfacalcidol in osteoporotic patients [7]. In this study, urinary Ca excretion and serum Ca concentration in the OVX/vehicle group were significantly decreased compared with the sham/vehicle group, and ELD increased urinary Ca excretion and serum Ca concentration throughout the treatment period. These increases in urinary Ca excretion and serum Ca by ELD plateaued at 3 months after ELD treatment began. While OVX induces calcium efflux from bone, estrogen deficiency reduces intestinal calcium absorption by suppressing transient receptor potential vanilloid subfamily member 6 (TRPV6), plasma membrane Ca ATPase 1b (PMCA1b), and VDR expression at the duodenum [37–39]. Totally, OVX leads to a negative calcium balance, resulting in decreased serum calcium concentration. ELD stimulates intestinal calcium absorption, resulting in increases in serum calcium as well as urinary calcium excretion [40]. Taken together, these results indicate that ELD improved the Ca balance that declined negatively in OVX rats, and that a positive Ca balance may be maintained at a certain constant level, even if ELD is administered over a long period.
In conclusion, long-term treatment of OVX rats with ELD suppressed increased bone turnover and prevented OVX-induced bone loss, deterioration of microstructure, and reduction in bone biomechanical strength, suggesting that ELD may be well tolerated and useful for long-term treatment of postmenopausal osteoporosis.
References
Miyamoto K, Murayama E, Ochi K, Watanabe H, Kubodera N (1993) Synthetic studies of vitamin D analogues. XIV. Synthesis and calcium regulating activity of vitamin D3 analogues bearing a hydroxyalkoxy group at the 2 beta-position. Chem Pharm Bull (Tokyo) 41:1111–1113
Ono Y, Watanabe H, Shiraishi A, Takeda S, Higuchi Y, Sato K, Tsugawa N, Okano T, Kobayashi T, Kubodera N (1997) Synthetic studies of vitamin D analogs. XXIV. Synthesis of active vitamin D3 analogs substituted at the 2 beta-position and their preventive effects on bone mineral loss in ovariectomized rats. Chem Pharm Bull (Tokyo) 45:1626–1630
Ono Y, Kawase A, Watanabe H, Shiraishi A, Takeda S, Higuchi Y, Sato K, Yamauchi T, Mikami T, Kato M, Tsugawa N, Okano T, Kubodera N (1998) Syntheses and preventive effects of analogues related to 1α,25-dihydroxy-2β-(3-hydroxypropoxy)vitamin D3 (ED-71) on bone mineral loss in ovariectomized rats. Bioorg Med Chem 6:2517–2523
Okano T, Tsugawa N, Masuda S, Takeuchi A, Kobayashi T, Takita Y, Nishii Y (1989) Regulatory activities of 2β-(3-hydroxypropoxy)-1α, 25-dihydroxyvitamin D3, a novel synthetic vitamin D3 derivative, on calcium metabolism. Biochem Biophys Res Commun 163:1444–1449
Hatakeyama S, Nagashima S, Imai N, Takahashi K, Ishihara J, Sugita A, Nihei T, Saito H, Takahashi F, Kubodera N (2007) Synthesis and biological evaluation of a 3-positon epimer of 1α,25-dihydroxy-2β-(3-hydroxypropoxy) vitamin D3 (ED-71). J Steroid Biochem Mol Biol 103:222–226
Matsumoto T, Ito M, Hayashi Y, Hirota T, Tanigawara Y, Sone T, Fukunaga M, Shiraki M, Nakamura T (2011) A new active vitamin D3 analog, eldecalcitol, prevents the risk of osteoporotic fractures—a randomized, active comparator, double-blind study. Bone 49:605–612
Matsumoto T, Takano T, Yamakido S, Takahashi F, Tsuji N (2010) Comparison of the effects of eldecalcitol and alfacalcidol on bone and calcium metabolism. J Steroid Biochem Mol Biol 121:261–264
Uchiyama Y, Higuchi Y, Takeda S, Masaki T, Shira-ishi A, Sato K, Kubodera N, Ikeda K, Ogata E (2002) ED-71, a vitamin D analog, is a more potent inhibitor of bone resorption than alfacalcidol in an estrogen-deficient rat model of osteoporosis. Bone 30:582–588
Tanaka Y, Nakamura T, Nishida S, Suzuki K, Takeda S, Sato K, Nishii Y (1996) Effects of a synthetic vitamin D analog, ED-71, on bone dynamics and strength in cancellous and cortical bone in prednisolone-treated rats. J Bone Miner Res 11:325–336
de Freitas PH, Hasegawa T, Takeda S, Sasaki M, Tabata C, Oda K, Li M, Saito H, Amizuka N (2011) Eldecalcitol, a second-generation vitamin D analog, drives bone minimodeling and reduces osteoclastic number in trabecular bone of ovariectomized rats. Bone 49:335–342
Saito H, Takeda S, Amizuka N (2013) Eldecalcitol and calcitriol stimulates ‘bone minimodeling’, focal bone formation without prior bone resorption, in rat trabecular bone. J Steroid Biochem Mol Biol 136:178–182
Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY (2005) Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab 90:1294–1301
Schneider JP (2009) Bisphosphonates and low-impact femoral fractures: current evidence on alendronate-fracture risk. Geriatrics 64:18–23
Armamento-Villareal R, Napoli N, Diemer K, Watkins M, Civitelli R, Teitelbaum S, Novack D (2009) Bone turnover in bone biopsies of patients with low-energy cortical fractures receiving bisphosphonates: a case series. Calcif Tissue Int 85:37–44
Lenart BA, Neviaser AS, Lyman S, Chang CC, Edobor-Osula F, Steele B, van der Meulen MC, Lorich DG, Lane JM (2009) Association of low-energy femoral fractures with prolonged bisphosphonate use: a case control study. Osteoporos Int 20:1353–1362
Visekruna M, Wilson D, McKiernan FE (2008) Severely suppressed bone turnover and atypical skeletal fragility. J Clin Endocrinol Metab 93:2948–2952
Park-Wyllie LY, Mamdani MM, Juurlink DN, Hawker GA, Gunraj N, Austin PC, Whelan DB, Weiler PJ, Laupacis A (2011) Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 305:783–789
Ma YL, Bryant HU, Zeng Q, Schmidt A, Hoover J, Cole HW, Yao W, Jee WS, Sato M (2003) New bone formation with teriparatide [human parathyroid hormone-(1-34)] is not retarded by long-term pretreatment with alendronate, estrogen, or raloxifene in ovariectomized rats. Endocrinology 144:2008–2015
Chavassieux PM, Arlot ME, Reda C, Wei L, Yates AJ, Meunier PJ (1997) Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J Clin Invest 100:1475–1480
Chapurlat RD, Arlot M, Burt-Pichat B, Chavassieux P, Roux JP, Portero-Muzy N, Delmas PD (2007) Microcrack frequency and bone remodeling in postmenopausal osteoporotic women on long-term bisphosphonates: a bone biopsy study. J Bone Miner Res 22:1502–1509
Allen MR, Iwata K, Phipps R, Burr DB (2006) Alterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1-year treatment with clinical treatment doses of risedronate or alendronate. Bone 39:872–879
Komatsubara S, Mori S, Mashiba T, Ito M, Li J, Kaji Y, Akiyama T, Miyamoto K, Cao Y, Kawanishi J, Norimatsu H (2003) Long-term treatment of incadronate disodium accumulates microdamage but improves the trabecular bone microarchitecture in dog vertebra. J Bone Miner Res 18:512–520
Mashiba T, Turner CH, Hirano T, Forwood MR, Johnston CC, Burr DB (2001) Effects of suppressed bone turnover by bisphosphonates on microdamage accumulation and biomechanical properties in clinically relevant skeletal sites in beagles. Bone 28:524–531
Allen MR, Burr DB (2007) Three years of alendronate treatment results in similar levels of vertebral microdamage as after one year of treatment. J Bone Miner Res 22:1759–1765
Stepan JJ, Burr DB, Pavo I, Sipos A, Michalska D, Li J, Fahrleitner-Pammer A, Petto H, Westmore M, Michalsky D, Sato M, Dobnig H (2007) Low bone mineral density is associated with bone microdamage accumulation in postmenopausal women with osteoporosis. Bone 41:378–385
Turner CH, Burr DB (1993) Basic biomechanical measurements of bone: a tutorial. Bone 14:595–608
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610
Ito M, Nishida A, Koga A, Ikeda S, Shiraishi A, Uetani M, Hayashi K, Nakamura T (2002) Contribution of trabecular and cortical components to the mechanical properties of bone and their regulating parameters. Bone 31:351–358
Harada S, Mizoguchi T, Kobayashi Y, Nakamichi Y, Takeda S, Sakai S, Takahashi F, Saito H, Yasuda H, Udagawa N, Suda T, Takahashi N (2012) Daily administration of eldecalcitol (ED-71), an active vitamin D analog, increases bone mineral density by suppressing RANKL expression in mouse trabecular bone. J Bone Miner Res 27:461–473
Kikuta J, Kawamura S, Okiji F, Shirazaki M, Sakai S, Saito H, Ishii M (2013) Sphingosine-1-phosphate-mediated osteoclast precursor monocyte migration is a critical point of control in antibone-resorptive action of active vitamin D. Proc Natl Acad Sci USA 110:7009–7013
Takasu H, Sugita A, Uchiyama Y, Katagiri N, Okazaki M, Ogata E, Ikeda K (2006) c-Fos protein as a target of anti-osteoclastogenic action of vitamin D, and synthesis of new analogs. J Clin Invest 116:528–535
Sakai S, Takaishi H, Matsuzaki K, Kaneko H, Furukawa M, Miyauchi Y, Shiraishi A, Saito K, Tanaka A, Taniguchi T, Suda T, Miyamoto T, Toyama Y (2009) 1-Alpha, 25-dihydroxy vitamin D3 inhibits osteoclastogenesis through IFN-beta-dependent NFATc1 suppression. J Bone Miner Metab 27:643–652
Tsurukami H, Nakamura T, Suzuki K, Sato K, Higuchi Y, Nishii Y (1994) A novel synthetic vitamin D analogue, 2 beta-(3-hydroxypropoxy)1 alpha, 25-dihydroxyvitamin D3 (ED-71), increases bone mass by stimulating the bone formation in normal and ovariectomized rats. Calcif Tissue Int 54:142–149
Shiraishi A, Takeda S, Masaki T, Higuchi Y, Uchiyama Y, Kubodera N, Sato K, Ikeda K, Nakamura T, Matsumoto T, Ogata E (2000) Alfacalcidol inhibits bone resorption and stimulates formation in an ovariectomized rat model of osteoporosis: distinct actions from estrogen. J Bone Miner Res 15:770–779
Li M, Healy DR, Simmons HA, Ke HZ, Thompson DD (2003) Alfacalcidol restores cancellous bone in ovariectomized rats. J Musculoskelet Neuronal Interact 3:39–46
Liu XQ, Chen HY, Tian XY, Setterberg RB, Li M, Jee WS (2008) Alfacalcidol treatment increases bone mass from anticatabolic and anabolic effects on cancellous and cortical bone in intact female rats. J Bone Miner Metab 26:425–435
Colin EM, Van Den Bemd GJ, Van Aken M, Christakos S, De Jonge HR, Deluca HF, Prahl JM, Birkenhäger JC, Buurman CJ, Pols HA, Van Leeuwen JP (1999) Evidence for involvement of 17beta-estradiol in intestinal calcium absorption independent of 1,25-dihydroxyvitamin D3 level in the Rat. J Bone Miner Res 14:57–64
Dong XL, Zhang Y, Wong MS (2014) Estrogen deficiency-induced Ca balance impairment is associated with decrease in expression of epithelial Ca transport proteins in aged female rats. Life Sci 96:26–32
Liel Y, Shany S, Smirnoff P, Schwartz B (1999) Estrogen increases 1,25-dihydroxyvitamin D receptors expression and bioresponse in the rat duodenal mucosa. Endocrinology 140:280–285
Brown AJ, Ritter CS (2011) The vitamin D analog 1α,25-Dihydroxy-2β-(3-Hydroxypropyloxy) vitamin D(3) (Eldecalcitol) is a potent regulator of calcium and phosphate metabolism. Calcif Tissue Int 89:372–378
Acknowledgments
The authors gratefully acknowledge the technical support of the technical staff and research scientists at Charles River Laboratories.
Conflict of interest
Satoshi Takeda, Susan Y Smith, Tatsuya Tamura, Hitoshi Saito, Fumiaki Takahashi, Rana Samadfam, Solomon Haile, Nancy Doyle, Koichi Endo have no conflicts of interest.
Human and Animal Rights and Informed Consent
All animal procedures in this study were ethically approved by the Institutional Animal Care and Use Committee at Charles River Laboratories Preclinical Services Montreal.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takeda, S., Smith, S.Y., Tamura, T. et al. Long-Term Treatment with Eldecalcitol (1α, 25-Dihydroxy-2β- (3-hydroxypropyloxy) Vitamin D3) Suppresses Bone Turnover and Leads to Prevention of Bone Loss and Bone Fragility in Ovariectomized Rats. Calcif Tissue Int 96, 45–55 (2015). https://doi.org/10.1007/s00223-014-9937-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-014-9937-5