Abstract
1-Alpha, 25-dihydroxy vitamin D3 (1α,25(OH)2D3), an active form of vitamin D3, plays a critical role in calcium and bone metabolism. Although 1α,25(OH)2D3 has been used for osteoporosis therapy, the direct role of 1α,25(OH)2D3 on human osteoclastogenesis has not been well characterized. Here we show that 1α,25(OH)2D3 treatment significantly inhibited human osteoclast formation at the early stage of differentiation in a concentration-dependent manner. 1α,25(OH)2D3 inhibited the expression of nuclear factor of activated T cells c1 (NFATc1, also referred as NFAT2), an essential transcription factor for osteoclast differentiation, and upregulated the expression of interferon-β (IFN-β), a strong inhibitor of osteoclastogenesis in osteoclast progenitors. Inhibitory effects of 1α,25(OH)2D3 on osteoclastogenesis and NFATc1 expression were restored by treatment with an antibody against IFN-β, suggesting that upregulation of IFN-β by 1α,25(OH)2D3 treatment results in inhibition of NFATc1 expression, in turn interfering with osteoclast formation. Thus, our study may provide a molecular basis for the treatment of human bone diseases by 1α,25(OH)2D3 through regulation of the IFN-β and NFATc1 axis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
1-Alpha, 25-dihydroxy vitamin D3 (1α,25(OH)2D3) regulates calcium metabolism through the nuclear vitamin D receptor (VDR). 1α,25(OH)2D3 upregulates intestinal calcium absorption and downregulates parathyroid hormone (PTH) mRNA expression in parathyroid cells [1–3], thereby preventing bone mineral density (BMD) reduction and maintaining bone architecture in circumstances of high bone turnover [4, 5]. Therefore, 1α,25(OH)2D3 and its analogues have been used for treatment of postmenopausal, secondary hyperparathyroidism or glucocorticoid-induced osteoporosis [6–8].
Osteoclasts are bone-resorbing multinuclear cells derived from hematopoietic stem cells [9]. Recent studies have shown that the interaction between receptor activator of nuclear factor kappa-B (RANK) and RANK-ligand (RANKL) is essential for osteoclast differentiation and activation [10–12]. RANK signaling stimulates tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6) and c-Fos, a component of the AP-1 transcription factor complex, which in turn activates various downstream molecules such as tartrate-resistant acid phosphatase (TRAP) [13, 14]. c-Fos is an essential transcription factor for osteoclast differentiation, and mice deficient in c-Fos exhibit a complete lack of osteoclast formation and severe osteopetrosis [15]. Nuclear factor of activated T cells (NFAT) 2, also called NFATc1, is a downstream transcription factor of c-Fos in the RANKL/RANK axis and is also essential for osteoclastogenesis [16, 17]. Although c-Fos positively regulates osteoclast differentiation by inducing NFATc1 and Fos-related antigen (Fra)-1, both of which are essential transcription factors for osteoclast differentiation [14, 16, 18], it also induces interferon-beta (IFN-β), a strong inhibitor of osteoclast differentiation, and it negatively regulates osteoclastogenesis through one of the IFN-α/β receptor components, IFNAR1, in a negative feedback manner [19].
Patients with osteoporosis exhibit excessive bone resorption as a consequence of accelerated osteoclast differentiation. Extensive bone resorption brings immature bone formation, which results in decreased BMD and attenuated bone strength. Administration of active vitamin D3 analogues to osteoporosis patients reduces bone resorption and bone fracture frequency [6, 20]. Ovariectomized rodents have been utilized as models of postmenopausal osteoporosis, and treatment with active vitamin D3 analogues has been demonstrated to reduce osteoclast formation and bone resorption, thereby increasing BMD and bone strength in vivo [4, 5]. Interestingly, 1α,25(OH)2D3 has been used to induce osteoclast formation in a coculture system of bone marrow (BM) and osteoblastic cells. It has been shown that 1α,25(OH)2D3 acts on osteoblasts to upregulate the expression of RANKL, an essential transmembrane ligand for osteoclastogenesis, while downregulating the expression of osteoprotegerin (OPG), a decoy receptor of RANKL that prevents osteoclastogenesis [10]. Thus, 1α,25(OH)2D3 has been considered to be an osteoclast-inducing factor, although it inhibits osteoclast formation and increases BMD in osteoporosis patients. Recently, it was shown that 1α,25(OH)2D3 inhibited osteoclast formation by inhibiting c-Fos protein expression in mouse osteoclasts [21]. However, the mechanisms of its inhibition of osteoclast formation remain largely unknown.
In this study, we show that human osteoclastogenesis induced by macrophage colony-stimulating factor (M-CSF) and RANKL from bone marrow-derived osteoclast progenitor cells was strongly inhibited by 1α,25(OH)2D3 treatment at an early stage of differentiation in a concentration-dependent manner. 1α,25(OH)2D3 treatment inhibited expression of NFATc1 and upregulated IFN-β expression in osteoclast progenitor cells. The inhibitory effect of 1α,25(OH)2D3 on human osteoclastogenesis was restored by addition of an antibody against IFN-β, which also restored the expression of NFATc1 downregulated by 1α,25(OH)2D3 in osteoclast progenitor cells. Thus, our data suggest a molecular basis for the treatment of activated osteoclast-induced bone diseases such as osteoporosis by 1α,25(OH)2D3 through the upregulation of IFN-β, which downregulates NFATc1 expression in osteoclast progenitor cells.
Materials and methods
Isolation of human bone marrow CFU-GM cells
The study was approved by an Institutional Ethical Review Board (Keio Hospital #16-17-1), and informed consent was obtained from all study subjects. Human bone marrow-derived colony-forming unit granulocyte macrophage (CFU-GM) cells were generated as follows. Human bone marrow was obtained from patients undergoing routine hip replacement surgery. Cells were diluted 1:1 with Dulbecco’s phosphate-buffered saline (Invitrogen, Carlsbad, CA, USA) and filtered through a 70-μm nylon mesh cell strainer (BD Bioscience, Franklin Lakes, NJ, USA). The cell suspension was carefully layered on histopaque-1077 (Sigma-Aldrich, St. Louis, MO, USA) and centrifuged at 440g for 30 min at room temperature. The cell layer at the interface was transferred into a fresh tube as bone marrow mononuclear cells. Bone marrow mononuclear cells were cultured in methylcellulose semisolid medium (MethoCult H-4534; Stem cell Technologies, Vancouver, BC, Canada) containing 1% methylcellulose, 30% fetal bovine serum (FBS), 1% bovine serum albumin, 10–4 M 2-mercaptoethanol, 2 mM l-glutamine, 50 ng/ml recombinant human (rh) stem cell factor, 10 ng/ml rh GM-CSF, and 10 ng/ml rh IL-3 in 35-mm Petri dishes. Cultures were maintained in a humidified atmosphere at 5% CO2 at 37°C for 2 weeks to obtain CFU-GM cells.
Osteoclast differentiation of human CFU-GM cells
Human CFU-GM cells were seeded in 96-well plates (2.0 × 104 cells/well) and cultured for 6 days in alpha-minimum essential medium (α-MEM) supplemented with 10% FBS, 30 ng/ml M-CSF (Wako, Osaka, Japan) and 30 ng/ml RANKL (Wako) in the absence or presence of 10–10 M, 10–9 M, 10–8 M 1α,25(OH)2D3 (Wako). To examine the role for IFN-β in osteoclastogenesis of human CFU-GM cells, 500 unit/ml sheep polyclonal antibody against human IFN-β (PBL Biomedical Laboratories, New Brunswick, NJ, USA) was added in the culture medium. The culture medium was changed to fresh medium every other day. Osteoclastogenesis was evaluated by TRAP staining as described below or pit formation assay as described elsewhere [22, 23].
Tartrate-resistant acid phosphatase (TRAP) staining
Cultured cells were fixed with 10% formalin in PBS for 10 min at room temperature. After treatment with ethanol/acetone (50:50 vol/vol) for 1 min, the well surface was air dried and incubated for 30 min at room temperature with TRAP-staining solution: 0.1 M sodium acetate (pH 5.0) containing 0.01% naphthol AS-MX phosphate (Sigma-Aldrich) as a substrate, and 0.03% red violet LB salt (Sigma-Aldrich) as a stain for the reaction product in the presence of 50 mM sodium tartrate. TRAP-positive multinuclear cells containing more than three nuclei were counted as osteoclasts.
RNA extraction, RT-PCR, and quantitative real-time PCR analysis
Total RNA was isolated from osteoclast progenitors or osteoclasts using an RNeasy Mini kit (Qiagen, Valencia, CA, USA), and total RNA was reverse transcribed using Reverscript IV (Wako). reverse transcription-polymerase chain reaction (RT-PCR) analysis was performed using the following primer sets: NFATc1, 5′-TGT GCC GGA ATC CTG AAA CT-3′–5′-GGC GGG AAG GTA GGT GAA AC-3′; c-Fos, 5′-GGA CCT TAT CTG TGC GTGAAAC-3′–3′-CAC ACT ATT GCC AGGAACACAG-5′; IFN-β, 5′-TCA TCT AGC ACTGGCTGG AA-3′–3′-TTTCAAAATCTTCTAGTGTCCTTTCA-5′; β-actin, 5′-TCC TGT GGC ATC CAC GAAACTA-3′–5′-CTC GGC CAC ATT GTGAACTTTG-3′.
PCR reactions were performed using TITANIUM Taq PCR Kit (Clontech, Mountain View, CA, USA). To quantify transcripts, cDNA was subjected to real-time PCR using Applied Biosystem 7500 Fastin Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) or a Thermal Cycler Dice Real Time System (Takara Bio, Otsu, Japan). Amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for sample normalization. The probes and primers used were predesigned transcripts (so-called inventoried assays) validated by Applied Biosystems bioinformatics design pipelines. Applied Biosystems assay IDs were Hs00542678_m1 (NFATc1), Hs01077958_s1 (IFNB1), and Hs99999905_m1 (GAPDH). Each sample was analyzed in triplicate.
Flow cytometry
Monoclonal antibodies (mAbs) recognizing the following markers were used for flow cytometric analyses and cell sorting on human CFU-GM cells: CD45 (HI30; BD Pharmingen, Franklin Lakes, NJ, USA), CD44 (DF1485; DAKO, Glostrup, Denmark), and CD11b (ICRF44; BD Pharmingen).
Osteoclast differentiation of mouse bone marrow cells
All mice were maintained under pathogen-free conditions and cared for in accordance with the guidelines of Keio University School of Medicine. Bone marrow cells were isolated from 8- to 12-week-old IFN-α/β receptor 1 (IFNAR1)-deficient or wild-type mice and cultured in α-MEM supplemented with 10% FBS for 5 h. Nonadherent cells were harvested as osteoclast progenitor cells, seeded in 96-well plates (2.0 × 104 cells/well), and cultured for 6 days in α-MEM supplemented with 10% FBS, 30 ng/ml M-CSF, and 30 ng/ml RANKL in the presence or absence of 1α,25(OH)2D3. The culture medium was changed to fresh medium on day 3. Osteoclastogenesis was evaluated by TRAP staining.
Western blot analysis
Cell lysate was collected from the cells derived from IFNARI-deficient or wild-type mice cultured in the presence of M-CSF and RANKL with or without 1α,25(OH)2D3 for the indicated culture period. Western blot analysis was performed as previously described [24] using polyclonal antibodies to detect c-Fos (K-25; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and Actin (A2066; Sigma-Aldrich).
Statistics
P values were calculated by unpaired Student’s t test. P values less than 0.05 were considered significant and are indicated by asterisks.
Results
1α,25(OH)2D3 inhibits human osteoclastogenesis
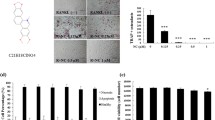
We first asked whether 1α,25(OH)2D3 directly affects human osteoclastogenesis from osteoclast progenitor cells. Human osteoclast progenitor cells were prepared by cultivation of human bone marrow mononuclear cells in methylcellulose semisolid culture medium. Human osteoclastogenesis induced by M-CSF and RANKL in CFU-GM cells was significantly inhibited by 1α,25(OH)2D3 in a concentration-dependent manner (Fig. 1a, b). 1α,25(OH)2D3 also inhibited the bone-resorbing activity, which is the most important function of osteoclasts (Fig. 1c). The inhibitory effect of 1α,25(OH)2D3 on human osteoclast differentiation was also observed for osteoclastogenesis from mononuclear cells isolated from bone marrow (data not shown), indicating that 1α,25(OH)2D3 inhibits osteoclast differentiation in human bone marrow-derived cells.
1-Alpha, 25-dihydroxy vitamin D3 (1α,25(OH)2D3) inhibits osteoclast formation in colony-forming unit granulocyte macrophage (CFU-GM) cells. Human CFU-GM cells were cultured for 6 days with M-CSF (30 ng/ml) and receptor activator of nuclear factor kappa-B ligand (RANKL) (30 ng/ml) in the presence or absence of the indicated concentrations of 1α,25(OH)2D3. Cells were subjected to TRAP staining (a, b) and pit formation assay (c). Multinuclear TRAP-positive cells containing more than three nuclei were scored as osteoclasts (b). Vehicle, 0.1% EtOH. Data are mean number ± SD of osteoclasts (*P < 0.05, ***P < 0.001)
Human osteoclastogenesis is inhibited by 1α,25(OH)2D3 downstream of RANKL at an early stage of differentiation
Next, we investigated whether increased concentrations of M-CSF, RANKL, or both could rescue the osteoclastogenesis inhibited by 1α,25(OH)2D3. High concentrations of RANKL or M-CSF plus RANKL partially but significantly rescued the osteoclast differentiation inhibited by 1α,25(OH)2D3 (Fig. 2a).
1α,25(OH)2D3 inhibits osteoclastogenesis in the RANKL-RANK axis at an early stage of differentiation. Human CFU-GM cells were cultured with the indicated concentrations of M-CSF and RANKL in the presence or absence of 10–8 M 1α,25(OH)2D3 for 6 days and stained with TRAP (a). Multinuclear TRAP-positive cells containing more than three nuclei were scored as osteoclasts. Data are mean number ± SD osteoclasts (*P < 0.05). Human CFU-GM cells were cultured with M-CSF (30 ng/ml) and RANKL (30 ng/ml) for 6 days, and 10–8 M 1α,25(OH)2D3 was added to the culture medium at days 0–2, 2–4, 4–6, or 0–6 (b). 1α,25(OH)2D3 effectively suppressed the formation of osteoclasts at an early stage of osteoclastogenesis. Vehicle, 0.1% EtOH. Data are mean number ± SD osteoclasts (*P < 0.05, **P < 0.01, ***P < 0.001)
To understand the inhibitory mechanism of 1α,25(OH)2D3 on osteoclastogenesis, we subdivided the culture period into three parts (days 0–2, days 2–4, and days 4–6) to assess which stage was critical for inhibition of osteoclastogenesis by 1α,25(OH)2D3. Osteoclastogenesis was strongly inhibited when 1α,25(OH)2D3 was added to the culture medium at the earlier stage of differentiation (Fig. 2b), which suggests that 1α,25(OH)2D3 affects molecules that act at an early rather than a later period of osteoclast differentiation in the presence of M-CSF and RANKL.
NFATc1 expression is reduced in 1α,25(OH)2D3-treated cells during human osteoclastogenesis induced by M-CSF and RANKL
Because osteoclast differentiation was inhibited by 1α,25(OH)2D3, we analyzed the expression of NFATc1, an essential molecule for osteoclast differentiation [16, 17], in cells treated with various concentrations of 1α,25(OH)2D3 (Fig. 3a, b). NFATc1 expression induced by M-CSF and RANKL in osteoclast progenitor cells was significantly downregulated by 1α,25(OH)2D3, which suggests that the inhibitory effects of 1α,25(OH)2D3 on osteoclastogenesis are the result of downregulation of NFATc1 expression.
1α,25(OH)2D3 inhibits NFATc1 expression in human CFU-GM cells. Human CFU-GM cells were cultured with M-CSF (30 ng/ml) and RANKL (30 ng/ml) in the presence or absence of the indicated concentrations of 1α,25(OH)2D3 for 1–2 days (a). Then, total RNA was isolated and reverse transcription-polymerase chain reaction (RT-PCR) analysis was undertaken to detect the expression of NFATc1. 1α,25(OH)2D3 inhibited the expression of NFATc1 in a concentration-dependent manner. β-actin expression is shown as an internal control. Vehicle, 0.1% EtOH. Human CFU-GM cells were cultured with M-CSF (30 ng/ml) and RANKL (30 ng/ml) in the presence or absence of 10–8 M 1α,25(OH)2D3 for 2 days (b). Total RNA was then isolated and real-time PCR analysis performed to detect the expression of NFATc1 relative to GAPDH (**P < 0.01)
As CFU-GM cells may contain several types of cells, the phenotype of CFU-GM cells was analyzed to determine the target cells of 1α,25(OH)2D3 (Fig. 4). Although CFU-GM cells showed single peaks in CD45 and CD44, cells were subdivided into two populations by the expression of CD11b, CD45+CD44+CD11b–, or CD45+CD44+CD11b+ cells (Fig. 4a). Each cell population was sorted and cultured in the presence of M-CSF and RANKL with or without 1α,25(OH)2D3. Compared with CD11+ cells, CD11b– cells showed significantly higher ability to differentiate into osteoclasts in the presence of M-CSF and RANKL (Fig. 4b, c). The osteoclastogenesis induced by M-CSF and RANKL was severely inhibited by 1α,25(OH)2D3 in both CD11b– and CD11b + cells (Fig. 4b, c). The expression of NFATc1 was analyzed by quantitative real-time PCR; the expression of NFATc1 was significantly downregulated by 1α,25(OH)2D3 treatment in both populations, but CD45+CD44+CD11b– cells were more sensitive to 1α,25(OH)2D3 compared with CD45+CD44+CD11b+ cells in inhibiting NFATc1 expression (Fig. 4d).
CD11b– cells in human CFU-GM cells have higher potential to differentiate into osteoclasts. a Human CFU-GM cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD45, CD44, or CD11b antibody and examined by FACS Calibur. b, c CD45+CD44+CD11b– or CD45+CD44+CD11b+ cells were sorted, cultured with M-CSF (30 ng/ml) and RANKL (30 ng/ml) in the presence or absence of 10–8 M 1α,25(OH)2D3 for 6 days, and the number of multinuclear TRAP-positive cells containing more than three nuclei was scored. d CD45+CD44+CD11b– or CD45+CD44+CD11b+ cells were sorted and cultured with M-CSF (30 ng/ml) and RANKL (30 ng/ml) in the presence or absence of 10–8 M 1α,25(OH)2D3 for 2 days. Total RNA was then isolated and real-time PCR analysis was undertaken to detect the expression of NFATc1 relative to GAPDH (*P < 0.05, **P < 0.01)
1α,25(OH)2D3 upregulates IFN-β expression in osteoclast progenitor cells
Next, we tried to elucidate the mechanism underlying inhibition of osteoclastogenesis by 1α,25(OH)2D3. We found that the expression of IFN-β, a strong inhibitor of osteoclastogenesis [19], was significantly upregulated by treatment with 1α,25(OH)2D3 in osteoclast progenitor cells (Fig. 5a, b). To analyze the role of IFN-β in the inhibition of osteoclastogenesis by 1α,25(OH)2D3, an antibody against IFN-β (500 unit/ml) was added to cultures with 1α,25(OH)2D3. Osteoclastogenesis inhibited by 1α,25(OH)2D3 was significantly restored by treatment with the antibody against IFN-β (Fig. 5c). Furthermore, the inhibition of NFATc1 expression by 1α,25(OH)2D3 also recovered after adding an antibody against IFN-β (Fig. 5d), indicating that 1α,25(OH)2D3 negatively regulates osteoclastogenesis through the upregulation of IFN-β, which in turn inhibits NFATc1 expression in osteoclast progenitor cells.
Interferon-beta (IFN-β) induced by 1α,25(OH)2D3 inhibits human osteoclastogenesis. Human CFU-GM cells were cultured with M-CSF (30 ng/ml) and RANKL (30 ng/ml) in the presence or absence of the indicated concentrations of 1α,25(OH)2D3 for 1–2 days. Then, total RNA was isolated and RT-PCR analysis was undertaken to detect the expression of IFN-β (a). β-actin expression is shown as an internal control. IFN-β expression was upregulated by 1α,25(OH)2D3 treatment in human osteoclast progenitor cells. V, vehicle (0.1% EtOH). Human CFU-GM cells were cultured with M-CSF (30 ng/ml) and RANKL (30 ng/ml) in the presence or absence of 10–8 M 1α,25(OH)2D3 for 2 days. Total RNA was then isolated and real-time PCR analysis performed to detect the expression of IFN-β relative to GAPDH (b) (*P < 0.05). Human CFU-GM cells were cultured with M-CSF (30 ng/ml) and RANKL (30 ng/ml) in the presence or absence of 10–8 M 1α,25(OH)2D3 with (black column) or without (white column) antibody against IFN-β for 6 days and stained with TRAP. Multinuclear TRAP-positive cells containing more than three nuclei were scored as osteoclasts (c). Left, representative data; right, data are mean number ± SD osteoclasts (**P < 0.01). Osteoclastogenesis inhibited by 10–8 M 1α,25(OH)2D3 was significantly rescued by the antibody against IFN-β. Vehicle, 0.1% EtOH. Human CFU-GM cells were cultured with M-CSF (30 ng/ml) and RANKL (30 ng/ml) in the presence or absence of 10–8 M 1α,25(OH)2D3 with or without the antibody against IFN-β for 2 days, and RT-PCR analysis was undertaken to detect the expression of NFATc1 (d). β-actin expression is shown as an internal control. NFATc1 expression induced by M-CSF and RANKL was inhibited by 1α,25(OH)2D3 treatment and was rescued by treatment with the antibody against IFN-β in human osteoclast progenitor cells
IFNARI-deficient cells are more resistant to 1α,25(OH)2D3 than wild-type cells in osteoclastogenesis
Finally, we tried to confirm the inhibitory effect of 1α,25(OH)2D3 on osteoclastogenesis through an IFN-β-dependent mechanism. To this end, we utilized an animal model deficient in IFNARI, one of the IFN-α/β receptor components, to inhibit the IFN-β-induced signals (Fig. 6). The inhibition of osteoclast differentiation by 1α,25(OH)2D3 was severe in wild-type cells compared with IFNARI-deficient cells (Fig. 6a). The c-Fos protein was downregulated in wild-type but not IFNARI-deficient cells by 1α,25(OH)2D3 treatment (Fig. 6b), suggesting that downregulation of c-Fos by 1α,25(OH)2D3 also, at least in part, results from the induction of IFN-β by 1α,25(OH)2D3.
IFN-α/β receptor 1-dependent inhibition of osteoclastogenesis by 1α,25(OH)2D3. a Osteoclast progenitor cells isolated from wild-type (white column) or IFNAR-deficient (black column) mice were cultured with M-CSF (30 ng/ml) and RANKL (30 ng/ml) in the presence or absence of the indicated concentrations of 1α,25(OH)2D3 for 5 days and stained with TRAP. Multinuclear TRAP-positive cells containing more than three nuclei were scored as osteoclasts. Data are mean number ± SD of osteoclasts (**P < 0.01, ***P < 0.001). V, vehicle, 0.1% EtOH. b Osteoclast progenitor cells isolated from wild-type (WT) or IFNAR-deficient (IFNARI KO) mice were cultured with M-CSF (30 ng/ml) and RANKL (30 ng/ml) in the presence or absence of 10–8 M 1α,25(OH)2D3 for the indicated period, and Western blot analysis was performed to detect c-Fos and actin protein
Taken together, our results demonstrate a novel mechanism of the direct inhibition of osteoclast differentiation by 1α,25(OH)2D3.
Discussion
The clinical effects of 1α,25(OH)2D3 analogues have been considered to occur via correction of vitamin D deficiency in osteoporotic patients. However, a recent study reported that administration of 1α,25(OH)2D3 analogues to osteoporosis patients under vitamin D supplementation also increased BMD [25], suggesting that 1α,25(OH)2D3 analogues have antiosteoporotic effects in addition to correction of vitamin D deficiency. Here we provide a possible mechanism underlying the antiosteoporotic effect of 1α,25(OH)2D3 in which 1α,25(OH)2D3 inhibits osteoclastogenesis by upregulating IFN-β expression, which in turn inhibits the expression of NFATc1, an essential transcription factor for osteoclast differentiation.
1α,25(OH)2D3 downregulates PTH transcription in parathyroid cells [2, 3] and upregulates intestinal calcium absorption, thereby preventing BMD reduction [1]. In spite of its usage for osteoporosis therapy in humans, it was reported that 1α,25(OH)2D3 is an osteoclast-inducing factor in human cells [26]. 1α,25(OH)2D3 has also been used as an osteoclastogenesis-stimulating agent in cocultures of murine osteoclast precursor cells with calvarial osteoblasts. 1α,25(OH)2D3 upregulates RANKL and downregulates the expression of OPG in osteoblasts [10], and this reciprocal regulation of RANKL and OPG is critical for osteoclastogenesis. Thus, 1α,25(OH)2D3 has been considered to be an osteoclast-inducing factor through osteoblast-mediated activity. The regulation of the balance between direct inhibition of osteoclast progenitor cells by 1α,25(OH)2D3 and indirect stimulation of osteoclastogenesis through osteoblasts is still unclear, but our results suggest that administration of 1α,25(OH)2D3 or its analogues to osteoporosis patients could directly inhibit osteoclastogenesis in circumstances with high bone turnover rates. Because we could not find any putative vitamin D response elements in 5′-flanking region of the IFN-β gene, the regulation of IFN-β expression by 1α,25(OH)2D3 is likely indirect. Further study is needed to elucidate the molecular mechanisms of the regulation of IFN-β expression by 1α,25(OH)2D3.
Several differences have been reported between mouse and human osteoclastogenesis; granulocyte macrophage colony-stimulating factor (GM-CSF) accelerates osteoclast differentiation and osteolytic bone metastasis of human breast cancer [27], but completely inhibits osteoclastogenesis in mouse bone marrow cells through the downregulation of c-Fos [28]. Therefore, analysis in human cells would be required in developing antihuman osteoclast therapies.
c-Fos, a member of the AP-1 transcription factor family, is one of the key osteoclastogenesis molecules induced by M-CSF and RANKL, and c-Fos-deficient mice exhibit osteopetrosis because of a complete lack of osteoclast differentiation. NFATc1 is a downstream target of c-Fos and is also an essential transcription factor for osteoclast differentiation [14, 16, 17], and thus c-Fos and NFATc1 cooperatively regulate osteoclast differentiation. Although several differences in osteoclastogenesis may occur between humans and mice, both c-Fos and NFATc1 might be essential for osteoclastogenesis in both human and mouse systems.
The physiological serum concentration of 1α,25(OH)2D3 has been reported to be approximately 0.1 nM; however, the administration of 1α,25(OH)2D3 or its analogues such as 1α(OH)D3 was reported to increase the local concentration of 1α,25(OH)2D3 in bone [29, 30]. Thus our study may provide, at least in part, the mechanisms of the effects of 1α,25(OH)2D3 and its analogues in treatment for preventing BMD reduction, and therefore inhibitory effects of NFATc1 expression and IFN-β induction levels in osteoclast progenitor cells may be a good index for development of 1α,25(OH)2D3 analogues to provide therapies for osteoclast-activating diseases such as osteoporosis.
References
Gallagher JC, Riggs BL, Eisman J, Hamstra A, Arnaud SB, DeLuca HF (1979) Intestinal calcium absorption and serum vitamin D metabolites in normal subjects and osteoporotic patients: effect of age and dietary calcium. J Clin Invest 64:729–736
Okazaki T, Igarashi T, Kronenberg HM (1988) 5′-Flanking region of the parathyroid hormone gene mediates negative regulation by 1, 25-(OH)2 vitamin D3. J Biol Chem 263:2203–2208
Demay MB, Kiernan MS, DeLuca HF, Kronenberg HM (1992) Sequences in the human parathyroid hormone gene that bind the 1, 25-dihydroxyvitamin D3 receptor and mediate transcriptional repression in response to 1, 25-dihydroxyvitamin D3. Proc Natl Acad Sci USA 89:8097–8101
Shiraishi A, Takeda S, Masaki T, Higuchi Y, Uchiyama Y, Kubodera N, Sato K, Ikeda K, Nakamura T, Matsumoto T, Ogata E (2000) Alfacalcidol inhibits bone resorption and stimulates formation in an ovariectomized rat model of osteoporosis: distinct actions from estrogen. J Bone Miner Res 15:770–779
Uchiyama Y, Higuchi Y, Takeda S, Masaki T, Shira-Ishi A, Sato K, Kubodera N, Ikeda K, Ogata E (2002) ED-71, a vitamin D analog, is a more potent inhibitor of bone resorption than alfacalcidol in an estrogen-deficient rat model of osteoporosis. Bone (NY) 30:582–588
Sairanen S, Kärkkäinen M, Tähtelä R, Laitinen K, Mäkelä P, Lamberg-Allardt C, Välimäki MJ (2000) Bone mass and markers of bone and calcium metabolism in postmenopausal women treated with 1, 25-dihydroxyvitamin D (calcitriol) for four years. Calcif Tissue Int 67:122–127
Lukert BP, Raisz LG (1990) Glucocorticoid-induced osteoporosis: pathogenesis and management. Ann Intern Med 112:352–364
Slatopolsky E, Weerts C, Thielan J, Horst R, Harter H, Martin KJ (1984) Marked suppression of secondary hyperparathyroidism by intravenous administration of 1, 25-dihydroxy-cholecalciferol in uremic patients. J Clin Invest 74:2136–2143
Miyamoto T, Suda T (2003) Differentiation and function of osteoclasts. Keio J Med 52:1–7
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95:3597–3602
Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature (Lond) 397:315–323
Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J (1999) RANK is essential for osteoclast and lymph node development. Genes Dev 13:2412–2424
Naito A, Azuma S, Tanaka S, Miyazaki T, Takaki S, Takatsu K, Nakao K, Nakamura K, Katsuki M, Yamamoto T, Inoue J (1999) Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells 4:353–362
Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3:889–901
Johnson RS, Spiegelman BM, Papaioannou V (1992) Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell 71:577–586
Matsuo K, Galson DL, Zhao C, Peng L, Laplace C, Wang KZ, Bachler MA, Amano H, Aburatani H, Ishikawa H, Wagner EF (2004) Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J Biol Chem 279:26475–26480
Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E, Takayanagi H (2005) Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med 202:1261–1269
Matsuo K, Owens JM, Tonko M, Elliott C, Chambers TJ, Wagner EF (2000) Fosl1 is a transcriptional target of c-Fos during osteoclast differentiation. Nat Genet 24:184–187
Takayanagi H, Kim S, Matsuo K, Suzuki H, Suzuki T, Sato K, Yokochi T, Oda H, Nakamura K, Ida N, Wagner EF, Taniguchi T (2002) RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature (Lond) 416:744–749
Aloia JF, Vaswani A, Yeh JK, Ellis K, Yasumura S, Cohn SH (1988) Calcitriol in the treatment of postmenopausal osteoporosis. Am J Med 84:401–408
Takasu H, Sugita A, Uchiyama Y, Katagiri N, Okazaki M, Ogata E, Ikeda K (2006) c-Fos protein as a target of anti-osteoclastogenic action of vitamin D, and synthesis of new analogs. J Clin Invest 116:528–535
Iwamoto K, Miyamoto T, Sawatani Y, Hosogane N, Hamaguchi I, Takami M, Nomiyama K, Takagi K, Suda T (2004) Ligand-independent dimer formation of receptor activator of nuclear factor kappa B (RANK) induces incomplete osteoclast formation. Biochem Biophys Res Commun 325:229–234
Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K, Oike Y, Takeya M, Toyama Y, Suda T (2005) DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med 202:345–351
Morita K, Miyamoto T, Fujita N, Kubota Y, Ito K, Takubo K, Miyamoto K, Ninomiya K, Suzuki T, Iwasaki R, Yagi M, Takaishi H, Toyama Y, Suda T (2007) Reactive oxygen species induce chondrocyte hypertrophy in endochondral ossification. J Exp Med 204:1613–1623
Matsumoto T, Miki T, Hagino H, Sugimoto T, Okamoto S, Hirota T, Tanigawara Y, Hayashi Y, Fukunaga M, Shiraki M, Nakamura T (2005) A new active vitamin D, ED-71, increases bone mass in osteoporotic patients under vitamin D supplementation: a randomized, double-blind, placebo-controlled clinical trial. J Clin Endocrinol Metab 90:5031–5036
Takahashi N, Kukita T, MacDonald BR, Bird A, Mundy GR, McManus LM, Miller M, Boyde A, Jones SJ, Roodman GD (1989) Osteoclast-like cells form in long-term human bone marrow but not in peripheral blood cultures. J Clin Invest 83:543–550
Park BK, Zhang H, Zeng Q, Dai J, Keller ET, Giordano T, Gu K, Shah V, Pei L, Zarbo RJ, McCauley L, Shi S, Chen S, Wang CY (2007) NF-kappaB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat Med 13:62–69
Miyamoto T, Ohneda O, Arai F, Iwamoto K, Okada S, Takagi K, Anderson DM, Suda T (2001) Bifurcation of osteoclasts and dendritic cells from common progenitors. Blood 98:2544–2554
Kitanaka S, Takeyama K, Murayama A, Sato T, Okumura K, Nogami M, Hasegawa Y, Niimi H, Yanagisawa J, Tanaka T, Kato S (1998) Inactivating mutations in the 25-hydroxyvitamin D3 1α-hydroxylase gene in patients with pseudovitamin D-deficiency rickets. N Engl J Med 338:653–661
Koike N, Ichikawa F, Nishii Y, Stumpf E (1998) Sustained osteoblast nuclear receptor binding of converted 1α, 25-dihydroxyvitamin D3 after administration of 3H–1α-hydroxyvitamin D3: a combined receptor autoradiography and radioassay time course study with comparison to 3H–1α, 25-dihydroxyvitamin D3. Calcif Tissue Int 63:391–395
Acknowledgments
We thank Y. Sato for technical support. T. Miyamoto was supported by Precursory Research for Embryonic Science and Technology, Japan Science and Technology Agency, Japan Society for the Promotion of Science Fujita Memorial Fund for Medical Research, and a grant-in-aid from the Global COE Program of the Ministry of Education, Culture, Sports, Science and Technology, Japan, to Keio University.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sakai, S., Takaishi, H., Matsuzaki, K. et al. 1-Alpha, 25-dihydroxy vitamin D3 inhibits osteoclastogenesis through IFN-beta-dependent NFATc1 suppression. J Bone Miner Metab 27, 643–652 (2009). https://doi.org/10.1007/s00774-009-0084-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-009-0084-4