Abstract.
The present study was designed to examine the effects of median nerve stimulation on motoneurones of remote muscles in healthy subjects using H-reflex, averaged EMG and PSTH methods. Stimulation of the median nerve induced facilitation of soleus H-reflex from about 50 ms and it reached a peak at about 100 ms of conditioning-test interval. Afferents that induced the facilitation consisted of at least two types of fibres, the high-threshold cutaneous fibres and the low-threshold fibres. When the effects were examined by the averaged surface EMG and PSTH, no facilitation but rather inhibition or inhibition-facilitation was induced in all tested muscles except for the upper limb muscles on the stimulated side. The inhibition latency was shortest in masseter muscle and longest in leg muscles, while values for the contralateral upper limb muscles were in the middle, indicating that the onset of inhibition was delayed from rostral to caudal muscles. Inputs from the median nerve converged to inhibitory interneurones, which mediate the masseter inhibitory reflex. Our findings suggested that inputs from the median nerve initially ascend to the brain, at least to the brainstem, and then descend to the spinal cord. Therefore, inhibition induced by median nerve stimulation was not considered as an interlimb reflex mediated by a propriospinal pathway, but long-loop reflex, at least via the pons. The discrepancy between the results of reflex and motor units suggests that facilitation of soleus H-reflex following median nerve stimulation was mainly due to reduced presynaptic inhibition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In decerebrate cats, forelimb afferent nerve stimulation elicits two types of motor responses in bilateral hindlimbs; one is the propriospinal reflex and the other is the spinobulbospinal (SBS) reflex (Shimamura and Livingstone 1963). Electrical stimulation of forelimb afferents induces both excitation and inhibition of hindlimb motoneurones in high spinal cat, and the final effect is dependent on the phase or position of the hindlimb (Schomburg et al. 1978). Propriospinal interlimb connections are thought to coordinate the forelimbs and hindlimbs of the cat during locomotion (Miller et al. 1975). On the other hand, the functional implications of SBS reflex are not known in the cat and human.
Several studies have investigated reflexes from afferents of upper limb muscles onto motoneurones of lower limb muscles (and vice versa) by clinical examination of reflex characteristics and recording of electromyographic (EMG) activity in intact man (Gassel 1970; Meier-Ewert et al. 1972; Delwaide et al. 1977; Piesiur-Strehlow and Meinck 1980; Meink and Piesiur-Strehlow 1981; Kearney and Chan 1981; Delwaide and Crenna 1984; Zehr et al. 2001). Reflex latency in arm muscles by ankle displacement was short and the difference from the latency of stretch reflex in leg muscles was only 12–17 ms (Kearney and Chan 1981). Latency of early response between upper and lower limbs was shorter than the earliest possible latency for transcortical reflex (Zehr et al. 2001). Such results indicate indirectly that these reflexes are interlimb reflexes within the spinal cord and are at least not transcortical reflexes. Although the reflex pathways between upper and lower limbs in normal man are not identified at present, there are two possible reflex pathways; one is interlimb reflex mediated by a propriospinal pathway and another is long-loop reflex via the supraspinal centre, not including the transcortical reflex.
The present study was designed to investigate the reflex responses from arm afferents onto motoneurones of the face, upper and lower limbs in healthy subjects. The long-latency reflex could be evoked on the face and all limb muscles and its reflex pathway was considered not to be interlimb reflex but the long-loop reflex, at least via the pons.
Materials and methods
A total of 32 experiments were carried out in 8 healthy subjects aged between 21 and 47 years. All subjects gave informed consent to the experimental procedure, which had been approved by the local ethics committee.
General experimental arrangement
The subject was seated in an armchair; both lower limbs were semiflexed at the hip (120 deg), the knees flexed to 160 deg, and the ankle in 110 deg of plantarflexion. EMG signals from the test muscles were rectified, integrated, and displayed on an oscilloscope placed in front of the subject.
H-reflex
Surface electrodes were used for both stimulation and recording. Paired electrodes for recording (10 mm in diameter) were placed 2.0 cm apart over the bellies of the soleus muscles and connected to conventional amplifiers (frequency band, 50–3 kHz). The soleus H-reflex was evoked by stimulating the posterior tibial nerve through a monopolar electrode (1 ms rectangular pulse) by isolated constant current stimulation (electric stimulator and isolator, model SS–104 J, Nihon Kohden, Tokyo, Japan). The reflex response was measured as peak-to-peak amplitude of the H-reflex. The size of the maximal motor response (Mmax) was measured at the beginning of each experiment. During the experiment, the stimulus strength of the soleus H-reflex was adjusted to yield an H-reflex that was 15–25% of that value because the sensitivities of small size H-reflexes vary with the amplitude of the unconditioned reflex (Crone et al. 1990).
The conditioning stimulus was delivered to the right median nerve at the wrist joint. Conditioning stimuli usually consisted of trains of three rectangular pulses (pulse duration: 0.5 ms, pulse interval: 4 ms). The intensities of the stimuli were expressed as multiples of the threshold for the direct M response (×MT) of the abductor pollicis brevis (APB) muscle. The stimulus was applied every 3 s in a randomized, alternating conditioned and unconditioned test stimuli sequence. At least ten reflexes (conditioned and unconditioned) were measured in every series for later statistical analysis (mean, standard error of the mean, and differences between groups by Student's paired t-test).
Ischaemia was induced by inflating a tourniquet placed around the forearm above the wrist joint. Series of measurements were then performed until the size of Mmax of APB muscle began to decrease, signifying that the ischaemia affected the transmission in alpha motor axons; the tourniquet was released thereafter. The cuff was inflated to 160 and 180 mmHg, because higher pressure directly pressed the nerve and decreased the size of the M response of the APB muscle immediately after ischaemia. The duration of ischaemia was about 15–20 min depending on the subject. The measurements were then continued for at least another 15 min.
Averaged EMG
In the averaged EMG study, the subjects were instructed to maintain weak steady contractions (about 10–20% of maximal EMG) of both the right and left tested muscles; the integrated EMG was displayed in front of the subject as a feedback. The activities of the following muscles were recorded bilaterally using disposable surface electrodes: masseter, biceps brachii, triceps brachii, extensor carpi radialis (ECR), flexor carpi radialis (FCR), quadriceps (Q), tibialis anterior (TA), peroneous longus (Per), and soleus (Sol) muscles. Conditioning stimuli to the median nerve usually consisted of trains of three rectangular pulses as in the H-reflex study and were applied every 1 s. The rectified EMG triggered by the conditioning stimulus was averaged over a period extending 50 ms prior to and 150 ms after, usually for 100–200 sweeps (model ATAC 3300, Medical Data Processor, Nihon Kohden). The background EMG was measured from the 50-ms period preceding the stimulus.
Poststimulus time histograms
The method used for recording poststimulus time histograms (PSTHs) was described previously by Founier et al. (1986). In this method, the time of stimulation is set with respect to the discharges of the single unit. In this way, a stimulus can be delivered at a time when the probability of a new discharge of the unit is high, thus considerably reducing the number of stimuli necessary to evoke obvious peaks or troughs in the PSTH.
The PSTH of the discharge of voluntarily activated soleus motor units (recorded with monopolar concentric needle electrodes: DANTEC) was constructed for the 40–120 ms following stimulation of the right median nerve. The bin width of the histograms was 1 ms in all cases.
The EMG potentials of the motor units were converted into standard pulses using a spike discriminator with a variable trigger level and were fed into a computer (NEC PC-98XL2), which was then programmed to trigger the stimulator. Stimuli were delivered every 1 s.
Since stimuli at a fixed interval following the previous motor unit discharge were used, the probability of discharge in the PSTH not only reflects the effect of the stimulation but also the membrane trajectory in the motoneurone during the interspike interval. Histograms were therefore constructed for both the occurrence of spikes following the stimulation and for the background firing probability in a control situation without stimulation (see Fig. 6). Measurements with and without stimulation were regularly alternated. To elucidate the effect of stimulation, the background firing probability was subtracted from the firing probability following the stimulus and a new histogram was constructed. Changes in bin counts were determined from inflections in the cumulative curve derived from the histogram (Ellaway 1978).
Results
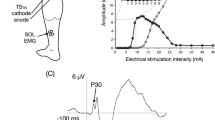
Figure 1A shows the time course of soleus H-reflex following ipsilateral median nerve stimulation at rest. The strength of conditioning stimulus used was 1.9×MT (solid circles) and 1.3×MT (open circles) of APB muscle. In this example, the subject complained of a slight painful sensation at 1.9×MT but did not feel pain at 1.3×MT. Facilitation started at 50 ms and reached a peak at around 100 ms in both situations although the amount of facilitation at 1.9×MT was larger than that at 1.3×MT. Figure 1B, C shows the relation between the amount of facilitation and strength of conditioning stimulus. Data shown in Fig. 1B were from the subject whose data are shown in Fig. 1A. Facilitation started at 0.8×MT and its level increased steeply above 1.6×MT. The subject complained of a slight painful sensation at the strength indicated by the arrow. However, facilitation started above 1.0×MT in most subjects but enhancement of facilitation was seen at a strength above painful sensation as shown in Fig. 1C. Appearance of facilitation evoked painful sensation when the conditioning stimulus was a single shock except for one subject. The time course was investigated in seven subjects and all showed clear facilitation, which started at 50–70 ms and reached a peak at 100–120 ms, by the conditioning stimulus without painful sensation. Facilitation with a similar time course could be induced by stimulation of the contralateral median nerve, and thus median nerve stimulation induced facilitation of the soleus H-reflex on both sides.
Time course of effects of the soleus H-reflex evoked by conditioning stimulus to the ipsilateral median nerve (0.5 ms duration, three shocks) at rest (subject NI). The strength of the conditioning stimulus was 1.9×MT (solid circles) and 1.3×MT (open circles) of the APB muscle. The abscissa and ordinate respectively show the conditioning and test stimulus intervals in milliseconds, and the size of the conditioned reflex, expressed as a percentage of its unconditioned value. Each bar represents 1 SE of the mean. B Relation between the amount of facilitation and strength of conditioning stimulation at a CT interval of 100 ms in the same subject as A. C The same relation is shown for another subject (YK) in B. The abscissa is the strength of the conditioning stimulus, expressed as multiples of the threshold for the direct M response (×MT) of abductor pollicis brevis (APB) muscle. Each bar represents 1 SE of the mean
Next, we investigated the type of afferent fibres involved in the facilitation. Figure 2A shows the time course of soleus H-reflex following ipsilateral median nerve stimulation (three shocks, 1.3×MT) and stimulation of the second fingertip by ring electrodes at 3.0×PT (perceptional threshold). The subject complained of painful sensation by cutaneous stimulation but did not complain of pain by median nerve stimulation. Cutaneous stimulation associated with painful sensation could induce facilitation of soleus H-reflex (open triangles), similar to median nerve stimulation (solid circles). On the other hand, when the second finger was stimulated with 1.5×PT without painful sensation, no significant facilitation was recognized (data not shown). Similar observations were made in three other subjects. These results suggested that high-threshold cutaneous fibres involved in pain sensation act as afferent fibres, contributing to the appearance of facilitation.
Time course of effects of the soleus H-reflex evoked by conditioning stimulus to the median nerve (1.3×MT, 0.5 ms duration, three shocks; solid circles) and the second fingertip by ring electrodes (3.0×PT, 0.5 ms duration, three shocks; open triangles) at rest. Each bar represents 1 SE of the mean. B Effect of ischaemia on facilitation of the soleus H-reflex evoked by conditioning stimulus to the median nerve (1.2×MT) at a conditioning-test interval of 100 ms. Ischaemia was induced by inflating a tourniquet placed just above the wrist. The start and end of the ischaemic period are marked by the dotted line and arrow. The abscissa is the time course of the measurements in relation to the onset of ischaemia. Each bar represents 1 SE of the mean
Figure 2B shows the effect of ischaemia on facilitation following median nerve stimulation. Data are from four experiments on three subjects. The median nerve was stimulated at 1.2×MT, which produced no painful sensation. The conditioning-test interval was chosen at 100 ms. The test H-reflex was maintained at a constant size during all experiments (approximately 20% of Mmax). Facilitation began to decrease gradually from 13 min after inflation of the tourniquet placed above the wrist joint and completely disappeared at 16 min after induction of ischaemia. Since the size of the maximal M wave of APB muscle began to decrease at 19 min after ischaemia, the tourniquet was released. The latter was associated with a gradual recovery of the facilitation. Similar results were observed in the other two subjects. These results suggested that low-threshold afferent fibres are involved in mediating the facilitation observed following median nerve stimulation. The results also suggest that plural types of afferent fibres are involved in facilitation of the soleus H-reflex.
In order to investigate other muscles, we examined the effects of median nerve stimulation by recording the averaged EMG. Data were generated from 12 experiments performed in four subjects. The subjects were instructed to contract the tested muscles bilaterally. Figure 3 shows the results of responses of muscles of the face, and upper and lower limbs on both sides. In these studies, the right median nerve was stimulated at 1.2×MT without painful sensation. This elicited segmental reflex responses in the upper limb muscles on the stimulated side (Fig. 3B–D, E), consisting of facilitation of biceps brachii and FCR muscles and inhibition of triceps brachii and ECR muscles (Cavallari et al. 1992). However, inhibition was seen in the masseter and lower limb muscles on both sides and the upper limb muscles of the contralateral side (Fig. 3). Stimulation strength above 1.0×MT of APB muscle could induce inhibition of the averaged EMG as well as facilitation of soleus H-reflex. Although it is difficult to measure exactly the latency of inhibition from the averaged EMG by visual inspection, it was clear that the onset of inhibition showed gradual prolongation from the rostral to caudal muscles. The onset of inhibition is shown on the right side by the open arrows in Fig. 3. Figure 4A shows the relation between latency of inhibition and tested muscles, and Fig. 4B–D shows motor responses of the masseter, FCR and TA muscles of the contralateral side. The latencies of inhibition were the shortest in the masseter, the longest in TA and in the middle in FCR muscle.
Averaged surface EMGs triggered by a conditioning stimulus to the right median nerve (1.2×MT, 0.5 ms duration, three shocks) during tonic contraction of tested muscles for 200 sweeps. A Masseter; B biceps brachii; C triceps brachii; D extensor carpi radialis (ECR); E flexor carpi radialis (FCR); F quadriceps (Q); G tibialis anterior (TA); H soleus (Sol) muscles. The ordinate shows the amplitude of rectified EMG (µV). The abscissa is the latency after the conditioning stimulation of the median nerve
Latencies of inhibition on averaged EMG in four subjects: (1) masseter; (2) biceps brachii; (3) triceps brachii; (4) extensor carpi radialis (ECR); (5) flexor carpi radialis (FCR); (6) quadriceps (Q); (7) tibialis anterior; (8) peroneus longus; (9) soleus (Sol) muscles. B–D show the results of masseter, FCR and TA muscles. Solid arrows show onset of inhibition. The ordinate shows the amplitude of rectified EMG (µV). The abscissa is the latency after the conditioning stimulation of the median nerve
The masseter muscle is inhibited by stimulation of the mental nerve, a sensory branch of the trigeminal nerve. This inhibition has been known as the masseter inhibitory reflex and the inhibitory interneurones exist in the pons (Ongerboer et al. 1989). Figure 5A shows the averaged EMG of the left masseter muscle following mental nerve stimulation (1.2×PT, three shocks). At this strength, the stimulus could not evoke the masseter inhibitory reflex. Figure 5B shows the response of the same muscle to median nerve stimulation (1.08×MT, three shocks). At this strength, the stimulus evoked a slight inhibition of the masseter muscle. When both the median and mental nerves were stimulated with a delay time of 10 ms, a clear inhibition could be evoked in the masseter muscle (Fig. 5C). These results suggest that inputs from the mental and median nerves converged at the level of pontine inhibitory interneurones, which mediate the masseter inhibitory reflex. These results were confirmed in three subjects.
Motor response of masseter muscle upon stimulation of the mental and median nerves. A Mental nerve stimulation (1.2×PT, 3 shocks, 200 sweeps); B median nerve stimulation (1.08×MT, 3 shocks, 200 sweeps); C stimulation of median and mental nerves with 10 ms delay stimulation (200 sweeps). The ordinate shows the amplitude of rectified EMG (µV). The abscissa is the latency after the conditioning stimulation
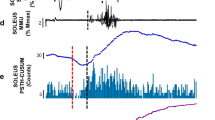
We also investigated the effect of median nerve stimulation on the motor units using PSTH. The experiments were carried in three subjects and the results of ten motor units from the soleus muscle are reported. Inhibition was noted in seven of ten motor units while the other three motor units were equivocal. Figure 6 shows the results of PSTH and averaged EMG in one subject. This motor unit was recruited early during weak tonic plantarflexion, and its mean interspike interval and 1 SD was 150.9±24.2 ms. Stimulation of the median nerve was triggered with a delay time of 70 ms by the previous motor unit discharge. The subject did not complain of painful sensation. Figure 6A, B shows the histograms of the control basal condition without stimulation and during stimulation, respectively. Figure 6C shows the difference between the counts per bin obtained in the stimulation and basal conditions. The histogram shows the appearance of inhibition from about 50 ms. Inhibition was determined from inflections in the cumulative curve derived from the histogram of Fig. 6C (Fig. 6D), which clearly shows the extent of inhibition. In this experiment, rectified surface EMG of the soleus muscle during tonic plantarflexion was averaged at the same time. The result is shown in Fig. 6F, and Fig. 6E shows the averaged EMG between 40 and 120 ms. The profile of averaged EMG (Fig. 6E) was very similar to the cumulative curve derived from PSTH (Fig. 6D). These results indicate that stimulation of the median nerve reduced the firing of motor units in the soleus muscle similar to the averaged EMG.
Effect of conditioning stimulation of the median nerve (1.1×MT, three shocks) on the firing probability of a single voluntary activated soleus motor unit (A–D) and on the averaged EMG (E, F). The abscissa is the latency after the conditioning stimulation of median nerve. The ordinate is the number of counts as a percentage of the number of triggers (A–D) and the amplitude of rectified EMG (µV, E, F). Mean interspike interval, 151 ms, number of counts, 1000. A Histogram obtained during basal condition without stimulation; B histogram obtained following stimulation; C the difference between A and B; D the cumulative curve derived from the histogram shown in D; E, F the averaged surface EMG of soleus muscle
Finally, Fig. 7 shows the time course of H-reflex of soleus muscle and averaged EMG following medial nerve stimulation (Fig. 7A, C) and ipsilateral sural nerve (Fig. 7B, D). The strength of sural nerve stimulation was 2.7×PT (three shocks, 250 Hz) with painful sensation. In the case of sural nerve stimulation, the time course of H-reflex was similar to the averaged EMG, taking account of the latency of H-reflex. However, in the case of median nerve stimulation, the results of H-reflex and averaged EMG were completely different.
Effects of conditioning of median and sural nerve stimulation on the size of the soleus H-reflex and the averaged surface EMGs. A, C Results of median nerve stimulation (1.1×MT, three shocks). B, D Results of sural nerve stimulation (2.7×PT, three shocks). In A and B, each bar represents 1 SE of the mean
Discussion
The present study examined the effects of median nerve stimulation on the motoneurones of remote muscles by examination of the H-reflex, averaged EMG and PSTH. The major findings can be summarized as follows. Stimulation of the median nerve induced facilitation of soleus H-reflex from about 50 ms and it reached a peak at about 100 ms of conditioning-test interval. At least two types of afferent fibres induced the facilitation; one was high-threshold cutaneous fibres with painful sensation and the other was low-threshold fibres compared with motor fibres. When the effect of median nerve stimulation was examined by using averaged EMG and PSTH, no facilitation but inhibition of the face, upper and lower limb muscles was noted, with the exception of the ipsilateral upper limb muscles. The onset of inhibition was prolonged from the rostral to caudal muscles. Furthermore, inputs from the median nerve converged on the brainstem inhibitory interneurones, which mediate the masseter inhibitory reflex. Thus, the reflex from ipsilateral median nerve to remote muscles in this study is not due to interlimb reflex mediated by a propriospinal pathway, but long-loop reflex, probably via the pons.
Previous studies demonstrated that electrical stimulation of the mixed nerve and mechanical stimulation of the upper limb muscles induced facilitation of the tendon reflex and H-reflex in the lower limb muscles (Meinck and Piesiur-Strehlow 1981; Delwaide and Toulouse 1981; Delwaide and Crenna 1984). In the present study, we investigated facilitation in only the soleus H-reflex. However, the time course of facilitation was almost the same as that recorded in the above studies, after taking into account the stimulus position. Based on the results of previous studies and our findings, showing decreased facilitation before reduction of the M response of APB muscle during ischaemia, the afferents for facilitation are probably group Ia fibres. However, since painful stimulation of cutaneous nerve fibres induced facilitation, high-threshold cutaneous fibres could also be involved in inducing facilitation.
The stimulation effects of the mixed nerve and cutaneous nerve in the upper limb on the averaged EMGs in the lower limb muscles have also been reported (Meier-Ewert et al. 1972; Piesiur-Strehlow and Meinck 1980; Meinck and Piesiur-Strehlow 1981; Zehr et al. 2001). Our results are almost similar to those studies, and showed that at least the initial response was inhibition or inhibition-facilitation. So far, these reflexes have been considered as interlimb reflexes, mediated by a propriospinal pathway. Meinck and Piesiur-Strehlow (1981) delivered conditioning stimulus to the dorsal roots of C4 and Th9, and stimulation of dorsal root at Th9 could evoke earlier H-reflex facilitation and inhibition of averaged EMG than stimulation of C4. Based on this finding, they reported that this reflex was mediated via a directly descending, long spinal pathway. Zehr et al. (2001) reported that responses of remote muscles could be evoked by electrical stimulation of cutaneous nerves in the hand and foot. They suggested that these cutaneous reflexes were interlimb reflexes that involve a propriospinal pathway since the latency of early response was shorter than the earliest possible latency for transcortical reflex. On the other hand, Meier-Ewert et al. (1972) reported that electrical stimulation of the sole of foot and fingertips resulted in motor responses from head, neck, upper and lower limb muscles by averaged EMGs. The latencies of inhibitory responses were short in the order of head, neck, upper and lower limb muscles. They mentioned that these inhibitory responses were examples of spino-bulbo-cranial and spino-bulbo-spinal reflexes in man. Our results are similar to those of Meier-Ewert et al. (1972); the responses of the masseter, upper and lower limb muscles, as well as inhibition, could be evoked in all muscles except for the upper limb muscles on the stimulated side, and the latencies of inhibition progressively increased from rostral to caudal muscles (Figs. 3, 4). The latencies of inhibition in this study were within 75 ms in all tested muscles, and these values are in agreement with the early response reported by Zehr et al. (2001).
The masseter muscle is inhibited by mental nerve stimulation, one of the sensory branches of the trigeminal nerve, and this inhibition is known as the masseter inhibitory reflex. Inhibition consists of early (10–15 ms) and late (40–50 ms) phases of electrical silence, interrupting the voluntary EMG activity in the masseter muscles on both sides. Studies in patients with localized brainstem lesions suggested that this reflex is abnormal in patients with lesions involving the pontine tegmentum and represents a brainstem inhibitory reflex (Ongerboer et al. 1989). Other studies reported that the masseter inhibitory reflex could be evoked by median nerve stimulation through the spinotrigeminal reflex (Deriu et al. 2002). In the present study, early and late inhibitions could be evoked following median nerve stimulation in only one subject. Probably two phases of inhibition were fused into a single broad inhibitory phase in other subjects by the long distance between stimulating and recording positions, and conditioning stimulation of three shocks. Stimulation of the median nerve converged to the inhibitory interneurones, which mediated the masseter inhibitory reflex (Fig. 5). The masseter inhibitory reflex induced by median nerve stimulation as well as mental nerve stimulation could not be evoked in patients with pontine lesions (Kagamihara and Hayashi, unpublished). These findings suggest that inputs from the median nerve initially ascend to the brain, at least to the level of the pons, and then descend to the spinal cord, and thus inhibition of the averaged EMG and PSTH was a long-loop reflex via the supraspinal centre.
The discrepancy of the results of H-reflexes and motor units following stimulation of the median nerve might be explained by the differences between rest and tonic voluntary contractions. We compared changes in H-reflex of soleus muscle following median nerve stimulation at rest and during tonic plantarflexion in three subjects. H-reflexes were slightly depressed just before facilitation during tonic plantarflexion (data not shown). However, the size and duration of facilitation was far larger than that of inhibition during tonic plantarflexion. Although descending motor commands, associated with voluntary contraction, might activate the inhibitory circuit, it is difficult to explain entirely the discrepancy of H-reflexes and motor units by this mechanism. Stimulation of sural nerve results in postsynaptic inhibition of soleus motoneurones, mediated by a spinal inhibitory reflex (Manconi et al. 1998; Logigian et al. 1999). The effects of soleus H-reflexes from sural nerve stimulation were similar to those of averaged EMG (Fig. 7B, D). Therefore, facilitation of H-reflex might be induced by a different mechanism with inhibition of motor units, and it is possible that it is mainly reduced presynaptic inhibition of the Ia terminal.
Motor responses such as the facilitation of H-reflex and inhibition of average surface EMG and PSTH following median nerve stimulation noted in the present study are considered to be generated by a long-loop reflex, probably via the pons. So far the existence of interlimb reflex in normal man has not been confirmed. The interlimb reflex has been demonstrated only in patients with chronic cervical spinal cord injury (Calancie 1991). In that study, EMG responses in the hand and forearm could be evoked by electrical stimulation of the mixed nerve in the lower limb. However, this interlimb reflex pathway is reorganized and redistributed by a new synaptic connection within the remaining spinal cord (Calancie et al. 2002). Further studies are necessary to test the existence of interlimb reflex and its physiological role in normal man.
References
Calancie B (1991) Interlimb reflexes following cervical spinal cord injury in man. Exp Brain Res 85:458–469
Calancie B, Molano MR, Broton JG (2002) Interlimb reflexes and synaptic plasticity become evident months after human spinal cord injury. Brain 125:1150–1161
Cavallari P, Katz R, Penicaud A (1992) Pattern of projections of group I afferents from elbow muscles to motoneurones supplying wrist muscles in man. Exp Brain Res 91:311–319
Crone C, Hultborn H, Mazieres L, Morin C, Nielsen J, Pierrot-Deseilligny E (1990) Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res 81:35–45
Delwaide PJ, Crenna P (1984) Cutaneous nerve stimulation and motoneuronal excitability. II: evidence for non-segmental influences. J Neurol Neurosurg Psychiatry 47:190–196
Delwaide PJ, Toulouse P (1981) Facilitation of monosynaptic reflexes by voluntary contraction of muscles in remote parts of the body. Mechanisms involved in the Jendrassik maneuver. Brain 104:701–719
Delwaide PJ, Figiel C, Richelle C (1977) Effects of postural changes of the upper limb on reflex transmission in the lower limb. Cervicolumbar reflex interactions in man. J Neurol Neurosurg Psychiatr 40:616–621
Deriu F, Milia M, Sau G, Podda MV, Ortu E, Chessa G, Aiello I, Tolu E (2002) Non-nociceptive upper limb afferents modulate masseter muscle EMG activity in man. Exp Brain Res 143:286–294
Ellaway PH (1978) Cumulative sum technique and its application to the analysis of peristimulus time histograms. Electroencephalogr Clin Neurophysiol 45:302–304
Fournier E, Meunier S, Pierrot-Deseilligny E, Shindo M (1986) Evidence for interneuronally mediated Ia excitatory effects to human quadriceps motoneurones. J Physiol (Lond) 377:143–169
Gassel MM (1970) A critical review of evidence concerning long-loop reflexes excited by muscle afferents in man. J Neurol Neurosurg Psychiatr 33:358–362
Kearney RE, Chan CWY (1981) Interlimb reflexes evoked in human arm muscles by ankle displacement. Electroencephalogr Clin Neurophysiol 52:65–71
Logigian EL, Plotkin GM, Shefner JM (1999) The cutaneous silent period is mediated by spinal inhibitory pathway. Muscle Nerve 22:467–472
Manconi FM, Syed NA, Floeter MK (1998) Mechanisms underlying spinal motor neuron excitability during the cutaneous silent period in humans. Muscle Nerve 21:1256–1264
Meier-Ewert K, Hümme U, Dahm J (1972) New evidence favouring long loop reflexes in man. Arch Psychiatr Nervenkr 215:121–128
Meinck HM, Piesiur-Strehlow B (1981) Reflexes evoked in leg muscles from arm afferents: a propriospinal pathway in man? Exp Brain Res 43:78–86
Miller S, Van der Burg J, Van der Meche FGA (1975) Coordination of movements of the hindlimbs and forelimbs in different forms of locomotion in normal and decerebrate cat. Brain Res 91:217–237
Ongerboer de Visser BW, Cruccu G, Manfredi M, Koelman JH (1989) Effects of brainstem lesions on the masseter inhibitory reflex. Functional mechanisms of reflex pathways. Brain 113:781–792
Piesiur-Strehlow B, Meinck HM (1980) Response patterns of human lumbo-sacral motoneurone pools to distant somatosensory stimuli. Electroencephalogr Clin Neurophysiol 48:673–682
Schomburg ED, Meinck HM, Haustein J, Roesler J (1978) Functional organization of the spinal reflex pathways from forelimb afferents to hindlimb motoneurones in the cat. Brain Res 139:21–33
Shimamura M, Livingston RB (1963) Longitudinal conduction systems serving spinal and brain-stem coordination. J Neurophysiol 26:258–272
Zehr EP, Collins DF, Chua R (2001) Human interlimb reflexes evoked by electrical stimulation of cutaneous nerves innervating the hand and foot. Exp Brain Res 140:495–504
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kagamihara, Y., Hayashi, A., Masakado, Y. et al. Long-loop reflex from arm afferents to remote muscles in normal man. Exp Brain Res 151, 136–144 (2003). https://doi.org/10.1007/s00221-003-1436-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-003-1436-2