Abstract
Visually induced motion sickness (VIMS) is a common phenomenon when using visual devices such as smartphones and virtual reality applications, with symptoms including nausea, fatigue, and headache. To date, the neuro-cognitive processes underlying VIMS are not fully understood. Previous studies using electroencephalography (EEG) delivered mixed findings, with some reporting an increase in delta and theta power, and others reporting increases in alpha and beta frequencies. The goal of the study was to gain further insight into EEG correlates for VIMS. Participants viewed a VIMS-inducing visual stimulus, composed of moving black-and-white vertical bars presented on an array of three adjacent monitors. The EEG was recorded during visual stimulation and VIMS ratings were recorded after each trial using the Fast Motion Sickness Scale. Time–frequency analyses were conducted comparing neural activity of participants reporting minimal VIMS (n = 21) and mild–moderate VIMS (n = 12). Results suggested a potential increase in delta power in the centro-parietal regions (CP2) and a decrease in alpha power in the central regions (Cz) for participants experiencing mild–moderate VIMS compared to those with minimal VIMS. Event-related spectral perturbations (ERSPs) suggested that group differences in EEG activity developed with increasing duration of a trial. These results support the hypothesis that the EEG might be sensitive to differences in information processing in VIMS and minimal VIMS contexts, and indicate that it may be possible to identify neurophysiological correlate of VIMS. Differences in EEG activity related to VIMS may reflect differential processing of conflicting visual and vestibular sensory information.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of visual displays is a crucial part of our daily lives, with smartphones, tablets, TV/PC screens, and virtual reality applications being the most prominent examples of such devices. Unfortunately, the use of these displays can cause unwanted side effects in some users, including nausea, dizziness, fatigue, headache, and eyestrain (Rebenitsch and Owen 2016; Caserman et al. 2021). Visually induced motion sickness (VIMS) is a sensation similar to traditional motion sickness, with the difference that symptoms are not caused by actual, physical movement, but are triggered by stimulation of the visual system (Cha et al. 2020; Keshavarz and Golding 2022). According to a recent online survey with more than 300 participants (Keshavarz et al. 2021), the percentage of users who experienced nausea at least occasionally when using VR technologies or smartphones was approximately 40% and 20%, respectively. Dizziness was even higher for VR usage (45%), and eyestrain was most the most prevalent symptom for smartphone users (70%). These findings demonstrate that experiences with VIMS are highly prevalent in the population.
Theorized causes of VIMS include sensory conflict between the visual, vestibular, and proprioceptive systems (Reason and Brand 1975; Reason 1978; Oman 1990), the inability to maintain postural control (Riccio and Stoffregen 1991), or the contribution of eye movements (Ebenholtz et al. 1994). It has been proposed that VIMS and traditional motion sickness may represent stress responses (Gianaros et al. 2003; Muth 2006) characterized by an increased release of stress hormones (Drummer et al. 1990; Otto et al. 2006; but see Jacob et al. 2023). Although changes in cardiovascular activity (Cowings et al. 1986; Hu et al. 1991), skin temperature (Cobb 1999; Min et al. 2004), breathing (Kim et al. 2005), electrodermal activity (Warwick-Evans et al. 1987; Golding 1992), and gastric activity (Muth et al. 1996; Cheung and Vaitkus 1998) have been noted in various studies, a coherent pattern that allows to objectively quantify and potentially predict VIMS has yet to emerge (Dennison et al. 2016; Keshavarz et al. 2022).
Neurophysiological correlates of VIMS
Our knowledge on the neurophysiological processes involved during VIMS is limited. Over the course of the last decades, insights into the neural basis of general nausea and vomiting have significantly improved (Andrews 2013; Yates et al. 2014), but understanding of the neural correlates of nausea and other symptoms that are specifically related to VIMS or traditional motion sickness remains vague. Studies using electroencephalography (EEG) to identify the neuro-cortical processes underlying VIMS have led to mixed findings (see Chang et al. 2023 for an overview). For instance, Kim et al. (2005) asked their participants to complete a virtual navigation task for approximately 10 min while they recorded EEG data from 9 electrodes (frontal, temporal, central, parietal, occipital) and measured the severity of VIMS after stimulus exposure using the Simulator Sickness Questionnaire (SSQ; Kennedy et al. 1993). Results showed a significant increase in delta power and a significant decrease in relative beta power of the EEG signal at the frontal (F3) and temporal (T3) electrode sites during the navigation task compared to baseline. The changes in the relative delta and beta power were significantly correlated with the level of subjectively reported VIMS measured via the SSQ. That is, participants who reported more severe VIMS showed a stronger increase in relative delta power (F3, T3, O1) and a stronger decrease in relative beta power (F3, T3, T4, O1). Additionally, a significant positive correlation was found between VIMS severity and theta power in the parietal region (P3). The authors speculate that the overall transient EEG slowing might reflect a general stress response. An increase in the delta band in fronto-temporal and central regions during motion sickness has also been reported by various studies using visual stimuli (Hu et al. 1999; Min et al. 2004; Nürnberger et al. 2021) or cross-coupled stimulation to induce motion sickness (Wood et al. 1990; Chelen et al. 1993). Similarly, an increase in theta power over the fronto-central and/or parietal regions was also observed by Wu (1992), Wood et al. (1990), Chelen et al. (1993), and Henry et al. (2022). In contrast, other studies reported a decrease in the alpha band power accompanying the onset of VIMS (Chen et al. 2010; Lin et al. 2013; Naqvi et al. 2015), but an increase in alpha power was also reported (Chuang et al. 2016; Henry et al. 2022).
In another study, Krokos and Varshney (2022) had their participants engage in a VR task using a head mounted display where they were passively moved through a VR scene. Through independent component analysis (ICA) and time–frequency analyses, the authors found strong positive correlations between the subjectively reported level of VIMS and changes in the delta, theta, alpha, and beta band. In contrast, Tauscher et al. (2020) exposed their participants to a moving scene using immersive VR dome projection system while recording EEG data from 16 electrodes distributed over the scalp. The authors then compared the power spectral density of the alpha, beta, gamma, delta, and theta waves between sick and non-sick participants at different points in time (e.g., the moment when VIMS was reported or before stimulus termination). Although the raw data suggested various differences between sick and non-sick participants (e.g., delta and theta waves were more prevalent in the sick group), none of these differences were statistically significant. Overall, these mixed findings demonstrate that the neurophysiological correlates and processes associated with VIMS are not well understood. The aim of the present study was to address this gap in knowledge.
The present study
The main goal of the present study was to explore the neurophysiological correlates of VIMS using EEG measures to deliver additional insights into the neuro-cortical changes associated with VIMS. Given the mixed results found in previous studies, we aimed to further enhance the existing knowledge on how EEG patterns change as a function of VIMS. To achieve this, we exposed stationary participants to a series of moving visual stimuli and recorded their level of VIMS after each trial while recording EEG. We then compared EEG responses for participants who experienced no/minimal VIMS to those who experienced mild/moderate VIMS. Given the mixed findings from previous EEG studies, the current study took an exploratory approach. However, based on previous studies, we were particularly interested in testing whether an increase in power in the slower EEG bands, for instance in the fronto-central and parietal areas, would emerge. We applied time–frequency analysis and event-related spectral analysis to identify EEG activity correlated with VIMS experience.
Methods
Participants
Data were collected from a total of 38 participants. Of those, 5 participants were removed from the data analysis due to poor/incomplete EEG data, resulting in a final sample size of 33 (19 women, 14 men) with an age range from 19 to 44 years (M = 26.70, SD = 6.32 years).Footnote 1 All participants reported being healthy (i.e., no recent history of stroke, traumatic brain injury, vestibular disorders) and having normal or corrected-to-normal visual acuity. The study complied with the American Psychological Association Code of Ethics and was approved by the Research Ethics Boards of the University Health Network and of Toronto Metropolitan University. Participants provided written consent prior to the study and were free to terminate the study at any time without negative consequences. All participants received a $45 gift card of their choice as a token of appreciation for their time commitment.
Stimuli and apparatus
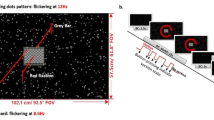
Participants were exposed to a visual stimulus consisting of alternating black-and-white vertical bars that moved to the right or left, inducing the sensation of self-motion (or vection) along the yaw axis. The stimuli were shown on three Lenovo ThinkVision monitors (each 24 inches), arranged at 120° between monitors (see Keshavarz et al. 2017). Display resolution was 1920 × 1200 pixels for each monitor and participants were seated 32 cm from the central screen, resulting in a field-of-view of 228° horizontally and 48° vertically (Fig. 1). Participants were asked to fixate on a red cross presented in the center of the screen during stimulus presentation.
Stimulus speed was set to either 0.5 cycles per second (slow) or 2 cycles per second (fast), with one cycle defined as the combination of one black bar and one white bar. Participants were exposed to a total of 80 trials, including 40 trials at slow speed and 40 trials at fast speed. However, as the average VIMS scores for the fast (M = 1.59, SD = 1.80) and slow (M = 1.36, SD = 1.59) trials did not differ substantially, we averaged VIMS scores across all trials and did not consider an effect of stimulus speed for the data analysis. Similarly, the direction of stimulus motion (left vs. right) was counterbalanced but not further considered for data analysis (D’Amour et al. 2021). The total of 80 trials was divided into 5 blocks of 16 trials each, with a single trial duration of 40 s (2.5-s acceleration, 30-s constant motion, 2.5-s deceleration, 5-s static). Fast and slow trials were mixed within each blocks, with 8 fast and 8 trials shown per block in randomized order. Short rest breaks were provided between blocks.
Dependent measures
VIMS and vection intensity
The Fast Motion Sickness scale (FMS; Keshavarz and Hecht 2011) is a verbal rating scale ranging from 0 (no sickness at all) to 20 (severe/frank sickness). The FMS was designed to inquire the aspects of VIMS related to nausea, general discomfort, and stomach awareness, but does not capture oculomotor issues or symptoms related to spatial disorientation or dizziness. Various studies (D’Amour et al. 2017; Peck et al. 2020) demonstrated strong correlations between the FMS and the Simulator Sickness Questionnaire (Kennedy et al. 1993), one of the most common measurement tools for VIMS. Vection intensity was measured using a rating scale ranging from 0 (no vection at all) to 10 (very intense vection). Both the FMS and the vection intensity ratings were applied after each trial.
EEG measures
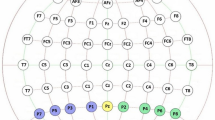
Participants were fitted with a 32-channel EEG headset (ANT Neuro eego™ sports) following the International 10–10 system. The following electrode positions were recorded: Fp1, Fpz, Fp2, AFz, F7, F3, Fz, F4, F8, FC5, FC1, FC2, FC6, T7, C3, Cz, C4, T8, CP5, CP1, CP2, CP6, P7, P3, Pz, P4, P8, POz, O1, Oz, O2 plus the two reference electrodes M1 and M2. The scalp EEG was referenced against CPz and AFz was used as the ground electrode. EOG was recorded using a single electrode attached below to the left eye, in line with the pupil. The EEG was recorded using an eego™ sports (ANT Neuro) amplifier in DC-mode with an online 70Hz low-pass filter and an A/D-rate of 500Hz. The signal impedance was set to below 10kΩ.
Study design and procedure
Participants were post hoc assigned to two different VIMS groups based on average VIMS ratings provided after each trial: participants with minimal VIMS (i.e., average FMS < 1.5) or mild–moderate VIMS (i.e., average FMS score > 1.5). The FMS threshold for dividing the two groups was chosen to separate participants who reported no or only minimal VIMS for the majority of trials from those who reported notable VIMS at least at some point during the experiment. Based on this re-grouping, our sample consisted of 21 participants (13 women) with minimal VIMS and 12 participants (6 women) with mild–moderate VIMS.
A single test session lasting approximately 3 h was conducted. After providing written consent, demographic information (e.g., age, biological sex) was recorded and participants completed a series of baseline measures, including a computerized rod-and-frame test as well as questionnaires on state/trait anxiety, depersonalization, and their susceptibility to VIMS. Note that these baseline measures are not of relevance for the present study and will not be further discussed. Participants were then fitted with the EEG cap and prepared for the presentation of the visual stimulus. Before the beginning of the experiment, the phenomenon of vection was described to the participants using the train illusion (see Kooijman et al. 2022) and two practice trials were presented to familiarize the participants with the experimental protocol and the sensation of vection and VIMS. Participants were then exposed to the visual stimuli separated into 5 blocks of 16 trials each. During each trial, participants were asked to press the left button of a wireless mouse upon perceiving vection and to release it after cessation (see Andrievskaia et al. 2023, for details on the vection onset, vection duration, and EEG results). After each trial, participants had to indicate their level of vection intensity (0–10) and VIMS (0–20) using the buttons of the computer mouse. A short rest break was provided after each block. After completion of the experimental task, participants were debriefed and received their compensation.
EEG data processing
EEG data processing was completed using EEGLab (Delorme and Makeig 2004) for MATLAB (v2021b). A bandpass filter of 1–30 Hz was applied to remove noise. Subsequently, EEG data were down-sampled to 250 Hz (from 500 Hz). Channel information was imported, and the eye electrode was removed from further analyses to avoid any computational problems. Independent Components Analysis (ICA) was then used to identify and automatically reject eye and muscle artifacts (Delorme and Makeig 2004). The continuous EEG data were divided into epochs of 35 s length adjusted to the onset of the motion of the visual input. In other words, each EEG epoch ranged from 0 to 35 s relative to visual motion onset, with the 0–500 ms segment serving as the baseline.Footnote 2 With these settings, the post-baseline EEG within each epoch (i.e., 2.5–32.5 s) included the EEG recorded during constant motion only. EEG epochs containing additional artifacts (threshold limit > ± 120 mv) were identified and removed (Poonian et al. 2015).
Time–frequency decomposition was conducted using a 3-cycle (0.8-s window length) Morlet-wavelet analysis with a Hanning-tapered window applied. To generate the grand-average scalp topographies to compare the two VIMS groups, the 30 s with constant stimulus speed from the original epochs was extracted and compared (i.e., 2.5–32.5 s post-motion onset). The topography of the EEG activity during this epoch is depicted for five frequency bands (i.e., the delta, theta, lower alpha, higher alpha and beta bands) and averaged for the entire epoch. Group comparisons of the distribution of EEG activity within each EEG band during visual motion presentation between the minimal VIMS vs. the mild–moderate VIMS group were computed by means of permutation tests (2000 permutations). Electrodes that depicted significant differences in EEG activity were selected for further exploration. Based on this, separate event-related spectral perturbations (ERSPs) were generated for each electrode of interest. For the ERSP analyses, the time period analyzed included the acceleration and deceleration of the stimulus, and therefore group differences for each electrode investigated variation across the full 35 s of the stimulus exposure.
Results
Behavioral data
The average FMS and vection scores reported by each of the two VIMS groups are presented in Fig. 2. Non-parametric Wilcoxon tests were calculated to compare the average FMS and vection intensity scores between the two different VIMS groups (minimal VIMS, mild–moderate VIMS). Results showed significantly higher FMS scores in the mild–moderate VIMS group (p < 0.001, r = 0.87) and significantly higher vection intensity ratings in the mild–moderate VIMS group (p = 0.016, r = 0.42). Further, the averaged FMS scores and vection intensity showed a moderately strong positive correlation, Spearman’s r(32) = 0.46, p = 0.007.
EEG results
Figure 3 shows the topographical maps for the power distribution in the EEG signal across the scalp for participants with minimal VIMS and mild–moderate VIMS. Results suggested a difference in delta power between the groups, such that participants experiencing mild-moderate VIMS showed an increase in delta power in the right centro-parietal region (CP2) compared to individuals experiencing minimal VIMS. However, this difference was not significant after correction of the alpha level using the False Discovery Rate (FDR) method. In addition, a difference between the two groups was found in the lower alpha band (see Fig. 3C electrodes Cz); again, after FDR correction, this difference was no longer significant.
Grand average topographical maps, showing mean changes in the A delta, B theta, C low alpha, D high alpha, and E beta frequency bands, 2.5–32.5 s after stimulus onset. The maps on the right indicate regions that significantly differ in frequency band activity within select the time period. Note that p-values shown here are not corrected; after FDR correction, no significant differences showed. The baseline ranges from 0 to 500 ms
To further examine potential differences between the minimal VIMS group and the mild-moderate VIMS group, we conducted time–frequency analyses for selected electrodes.Footnote 3 That is, we included electrodes that suggested differences between the two groups in the topographical maps (CP2, Cz) and also added central electrodes (C3, C4) that have linked to changes in motion sickness in previous studies (Hu et al. 1999; Haile et al. 2022). As a result, in the right centro-parietal (CP2) region, greater activity in the lower frequencies (delta and theta bands) showed for the mild-moderate VIMS group compared to the minimal VIMS group, matching the findings from the topographical maps (Fig. 4A). In the midline central region (Cz, Fig. 4B), differences between the two groups appeared to be clustered in the alpha and theta frequency ranges, with participants in the mild-moderate VIMS group showing a decrease in alpha and an increase in theta activity towards the end of the trial. In the other central sites (C3 and C4, Fig. 4C and D, there appeared to be greater activity in the lower frequencies in the moderate VIMS group compared to the minimal VIMS group, particularly in the C4 electrode, although these differences seem rather weak.
Discussion
In the present study, we investigated the neural activity associated with VIMS by comparing EEG measures for participants who experienced minimal VIMS at all to those who experienced mild–moderate VIMS during stimulus presentation. Although the differences between the minimal VIMS group and the mild–moderate VIMS group were statistically not significant after FDR correction, our results point towards likely candidates in the EEG signal that may reflect differences between the two groups. That is, an overall increase in delta power in the mild–moderate VIMS group was observed, particularly in the central-parietal region. This finding is comparable to previous work reporting an increase in delta power; however, previous studies reported an increase in delta power specifically in the frontal, temporal, and occipital regions during more severe VIMS, which we did not find (Kim et al. 2005; Chen et al. 2010; Nürnberger et al. 2021). In addition, we also noted a decrease in alpha activity in the central brain regions in the mild–moderate VIMS group, contrasting results from previous studies that found an increase in alpha activity with more severe levels of motion sickness (Chuang et al. 2016; Henry et al. 2022). Although previous work also suggested changes in the beta band (Kim et al. 2005; Krokos and Varshney 2022) associated with increased VIMS severity, we were not able to identify any meaningful differences in this frequency band.
Further investigation of the electrodes at the central and centro-parietal sites solidified the differences found in the topographical maps, indicating an increase in activity across the lower frequencies and a decrease in the alpha band in the mild-moderate VIMS group. Interestingly, the time–frequency analyses at selected recording sites indicated that the development of differences in neural activity in mild–moderate VIMS compared to minimal VIMS is an important factor, because earlier stages of visual motion processing are qualitatively different from later stages. This does not come as a surprise as VIMS symptoms tend to develop gradually with increasing exposure to visual motion. It is worth noting that in our data a qualitative shift is observable between 10 and 15 s of motion perception, suggesting that VIMS may be triggered between 10 and 15 s after stimulus onset. The significance of this temporal signature should be investigated in future studies.
The difference between the two groups in the delta wavelength may have been due to overall greater desynchronization within this frequency range in the individuals reporting minimal VIMS. The variation in the lower (delta and theta) wavelengths seen in participants with more severe VIMS may reflect the processing of a sensory conflict, which is often considered the main cause for VIMS (Reason 1978; Oman 1990). For instance, Nürnberger et al. (2021) found that increasing a sensory conflict between visual and vestibular information resulted in increased power in the lower frequency bands below 10 Hz, suggesting that this shift in power towards delta and theta power may reflect the processing of the sensory conflict. In addition, previous studies investigating the integration of incongruent multisensory information have also found an increase in both delta and theta activity unrelated to VIMS. Delta power was found to increase during a multimodal speech perception task (Pan et al. 2019), or during a Stroop task (Haciahmet et al. 2023) when the sensory information provided were incongruent. Similarly, theta power has been found to increase during incongruent trials of a various Simon tasks (Cohen and Donner 2013; van Driel et al. 2015). Thus, it is possible that changes in delta and theta power reflect the processing of visual–vestibular incongruency. As this congruency is thought to elicit VIMS, it seems plausible that participants who experienced more VIMS also perceived a more pronounced sensory conflict which might be reflected in the increase in delta and theta power. Support for this assumption can also be found in the vection ratings provided by our participants. That is, in the mild–moderate VIMS group, vection ratings were significantly higher compared to the minimal VIMS group, suggesting that this group perceived a stronger conflict between the visual (i.e., suggesting self-motion) and the vestibular and somatosensory (i.e., suggesting stasis) senses.
While similar work has reported an increase in alpha power during periods of reported motion sickness (Chuang et al. 2016; Henry et al. 2022), we found the opposite pattern in our analyses. Alpha power increased later in the trial for participants reporting minimal VIMS compared to individuals experiencing mild–moderate VIMS (however, note that this was not significant when averaged across 30 s of the trial). This may be attributed to the role of alpha band oscillations in sensory suppression. Specifically, alpha band activity has been associated with an inhibition of neural activity linked to task-irrelevant information (Neuper et al. 2005; Foxe and Snyder 2011; Klimesch 2012; Zumer et al. 2014), suggesting that when an individual watches a moving stimulus while stationary, variation in alpha band activity may be measured. Specifically, greater alpha oscillations may be associated with the suppression of contradictory information (in our study design, a lack of supporting vestibular cues that the body is moving) to promote the processing of the moving visual stimulation.
Lastly, across the midline central region, participants with moderate VIMS showed overall greater variability across all frequency bands compared to the minimal VIMS group. This may have been due to the greater range of sickness scores, given that moderate VIMS scores were as high as 11 out of 20, while the highest FMS score in the minimal VIMS group was 5. As a result, it is recommended that future work should consider a narrower definition of moderate motion sickness and identify if that may reduce variability in the EEG responses.
Limitations and future directions
One of the main limitations of the present study is the small sample size. Although our sample size is common in EEG studies (and even exceeds them) (Chen et al. 2010; Henry et al. 2022), the null findings in the different EEG frequencies between the two VIMS groups must be interpreted with caution, as the power to detect large effects was considerably low (1-beta = 0.58).
Additionally, we found a positive correlation between vection and VIMS in the present study. Although vection alone is considered neither sufficient nor necessary to cause VIMS (Lawson and Riecke 2014; Keshavarz et al. 2015), our results suggests that the two are indeed tightly linked to each other. As such, it is possible that the differences in the EEG signal between the two different VIMS groups might represent the different vection ratings in these two groups. Previous studies have in fact reported that vection leads to changes in the EEG signal (see Berti and Keshavarz 2020, for an overview), although these changes typically occurred in the alpha band. To detangle VIMS and vection, future studies should use stimuli that generate vection and VIMS separately. Vection can easily be generated without VIMS (as shown in our minimal VIMS group), but creating stimuli that induced VIMS without vection are more challenging to create.
Furthermore, our study did not create strong sensations of VIMS across all participants, with the average FMS scores being rather low. To maximize the chance to find potential EEG differences, it is recommended to use stimuli that can create stronger VIMS, for instance by applying a different stimulus and setup (e.g., tilting rotating stimulus).
Additionally, in the present study, we compared EEG responses on a group level. That is, we post hoc split our sample into those who, in general, reported minimal VIMS or mild-moderate VIMS and compared their EEG measures across all trials. A different approach for future studies could be to use a within-subjects design comparing trials that elicited minimal VIMS or mild–moderate VIMS. For the present study, we tried to apply this logic; however, only a few participants reported both trials with no VIMS at all and mild–moderate VIMS. Due to the small sample size, we were not able to conduct this analysis in a meaningful way. For future studies, we recommend to design the visual stimuli in a way that they will contain trials that generate no VIMS or moderate VIMS. An alternative approach was applied in studies by Wei et al. (2019, 2023) who compared EEG differences in individuals with a general higher or lower motion sickness susceptibility. This procedure does not require the actual induction of VIMS, which allows more flexibility with regards to the experimental settings (e.g., duration of stimulus, number of trial repetitions).
Finally, it would be interesting for future studies to integrate findings from various imaging techniques such as fMRI (Napadow et al. 2013; Miyazaki et al. 2015) or fNIRS (Zhang et al. 2020). This allows to combine the strengths of each of the different approaches (e.g., high spatial resolution for fMRI, high temporal resolution for EEG) to gain a more complete picture of the neuro-cognitive processes during VIMS.
Conclusion
In the present study, we compared the neural responses associated with VIMS by comparing participants who experienced minimal VIMS or mild–moderate VIMS. Results pointed towards an increase in delta power in participants who experienced mild–moderate VIMS compared to those with minimal VIMS, particularly in the central-parietal region. Interestingly, an increase in alpha synchronization later in the study trial (15 s onwards) was measured in individuals reporting minimal VIMS in the midline central region. No other notable differences in the EEG signal were found between the two groups. Our results suggest that identifying the neural activity associated with VIMS can offer insight in sensory processing underlying or presumably evoking VIMS. Identifying EEG correlates of VIMS may help to distinguish between sick and non-sick states in future studies.
Data availability
Anonymized data may be made available by the authors upon request.
Notes
No sex-related differences showed with respect to VIMS severity or vection intensity. Thus, biological sex will not be considered for further discussion.
Note that we deliberately chose a baseline that contained visual motion. If a baseline with a static visual scene was applied, differences in the EEG recordings would likely be dominated by the processing of visual motion in general (and not specific to VIMS). In other words, a baseline with visual motion may help to control at least partially the portion of the EEG associated with the processing of motion.
Note that no statistical tests are reported for the ERSP analysis as these analysis were purely exploratory and no hypotheses were generated a priori. The purpose of these analyses were to deliver insights into the dynamics of potential differences between the two VIMS groups.
References
Andrews PLR (2013) Nausea, vomiting, and the autonomic nervous system. In: Mathias CJ, Bannister SR (eds) Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System. Oxford University Press, p 0
Andrievskaia P, Spaniol J, Berti S, Keshavarz B (2023) Influence of stimulus speed and cognitive factors on cortical responses to vection-inducing stimuli. Poster presented at the 1st International Conference on Ageing, Innovation, and Rehabilitation (iCAIR). Toronto, ON.
Berti S, Keshavarz B (2020) Neuropsychological approaches to visually-induced vection: an overview and evaluation of neuroimaging and neurophysiological studies. Multisens Res 34(2):153–186. https://doi.org/10.1163/22134808-bja10035
Caserman P, Garcia-Agundez A, Gámez Zerban A, Göbel S (2021) Cybersickness in current-generation virtual reality head-mounted displays: systematic review and outlook. Virtual Reality. https://doi.org/10.1007/s10055-021-00513-6
Cha Y-H, Baloh RW, Cho C et al (2020) Mal de débarquement syndrome diagnostic criteria: Consensus document of the Classification Committee of the Bárány Society. J Vestib Res 30:285–293. https://doi.org/10.3233/VES-200714
Chang E, Billinghurst M, Yoo B (2023) Brain activity during cybersickness: a scoping review. Virtual Reality. https://doi.org/10.1007/s10055-023-00795-y
Chelen WE, Kabrisky M, Rogers SK (1993) Spectral analysis of the electroencephalographic response to motion sickness. Aviat Space Environ Med 64:24–29
Chen Y-C, Duann J-R, Chuang S-W et al (2010) Spatial and temporal EEG dynamics of motion sickness. Neuroimage 49:2862–2870. https://doi.org/10.1016/j.neuroimage.2009.10.005
Cheung B, Vaitkus P (1998) Perspectives of electrogastrography and motion sickness. Brain Res Bull 47:421–431
Chuang S-W, Chuang C-H, Yu Y-H et al (2016) EEG Alpha and Gamma Modulators Mediate Motion Sickness-Related Spectral Responses. Int J Neural Syst 26:1650007. https://doi.org/10.1142/S0129065716500076
Cobb SV (1999) Measurement of postural stability before and after immersion in a virtual environment. Appl Ergon 30:47–57
Cohen MX, Donner TH (2013) Midfrontal conflict-related theta-band power reflects neural oscillations that predict behavior. J Neurophysiol 110:2752–2763. https://doi.org/10.1152/jn.00479.2013
Cowings PS, Suter S, Toscano WB et al (1986) General Autonomic Components of Motion Sickness. Psychophysiology 23:542–551. https://doi.org/10.1111/j.1469-8986.1986.tb00671.x
D’Amour S, Bos JE, Keshavarz B (2017) The efficacy of airflow and seat vibration on reducing visually induced motion sickness. Exp Brain Res 235:2811–2820. https://doi.org/10.1007/s00221-017-5009-1
D’Amour S, Harris LR, Berti S, Keshavarz B (2021) The role of cognitive factors and personality traits in the perception of illusory self-motion (vection). Atten Percept Psychophys 83:1804–1817. https://doi.org/10.3758/s13414-020-02228-3
Delorme A, Makeig S (2004) EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134:9–21. https://doi.org/10.1016/j.jneumeth.2003.10.009
Dennison MS, Wisti AZ, D’Zmura M (2016) Use of physiological signals to predict cybersickness. Displays 44:42–52. https://doi.org/10.1016/j.displa.2016.07.002
Drummer C, Stromeyer H, Riepl RL et al (1990) Hormonal changes after parabolic flight: implications on the development of motion sickness. Aviat Space Environ Med 61:821–828
Ebenholtz SM, Cohen MM, Linder BJ (1994) The possible role of nystagmus in motion sickness: a hypothesis. Aviat Space Environ Med 65:1032–1035
Foxe JJ, Snyder AC (2011) The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention. Front Psychol. https://doi.org/10.3389/fpsyg.2011.00154
Gianaros PJ, Quigley KS, Muth ER et al (2003) Relationship between temporal changes in cardiac parasympathetic activity and motion sickness severity. Psychophysiology 40:39–44
Golding JF (1992) Phasic skin conductance activity and motion sickness. Aviat Space Environ Med 63:165–171
Haciahmet CC, Frings C, Beste C et al (2023) Posterior delta/theta EEG activity as an early signal of Stroop conflict detection. Psychophysiology. https://doi.org/10.1111/psyp.14195
Haile A, Watts M, Aichner S et al (2022) Central correlates of placebo effects in nausea differ between men and women. Brain Behav. https://doi.org/10.1002/brb3.2685
Henry EH, Bougard C, Bourdin C, Bringoux L (2022) Changes in Electroencephalography Activity of Sensory Areas Linked to Car Sickness in Real Driving Conditions. Front Hum Neurosci. https://doi.org/10.3389/fnhum.2021.809714
Hu S, Grant WF, Stern RM, Koch KL (1991) Motion sickness severity and physiological correlates during repeated exposures to a rotating optokinetic drum. Aviat Space Environ Med 62:308–314
Hu S, McChesney KA, Player KA et al (1999) Systematic investigation of physiological correlates of motion sickness induced by viewing an optokinetic rotating drum. Aviat Space Environ Med 70:759–765
Jacob C, Olliges E, Haile A et al (2023) Placebo effects on nausea and motion sickness are resistant to experimentally-induced stress. Sci Rep 13:9908. https://doi.org/10.1038/s41598-023-36296-w
Kennedy RS, Lane NE, Berbaum KS, Lilienthal MG (1993) Simulator Sickness Questionnaire: An Enhanced Method for Quantifying Simulator Sickness. Int J Aviat Psychol 3:203–220. https://doi.org/10.1207/s15327108ijap0303_3
Keshavarz B, Golding JF (2022) Motion sickness: current concepts and management. Curr Opin Neurol 35:107–112. https://doi.org/10.1097/WCO.0000000000001018
Keshavarz B, Hecht H (2011) Validating an Efficient Method to Quantify Motion Sickness. Human Factors: the Journal of the Human Factors and Ergonomics Society 53:415–426. https://doi.org/10.1177/0018720811403736
Keshavarz B, Riecke BE, Hettinger LJ, Campos JL (2015) Vection and visually induced motion sickness: how are they related? Front Psychol. https://doi.org/10.3389/fpsyg.2015.00472
Keshavarz B, Speck M, Haycock B, Berti S (2017) Effect of Different Display Types on Vection and Its Interaction With Motion Direction and Field Dependence. Iperception. https://doi.org/10.1177/2041669517707768
Keshavarz B, Murovec B, Mohanathas N, Golding JF (2021) The Visually Induced Motion Sickness Susceptibility Questionnaire (VIMSSQ): Estimating Individual Susceptibility to Motion Sickness-Like Symptoms When Using Visual Devices. Hum Factors. https://doi.org/10.1177/00187208211008687
Keshavarz B, Peck K, Rezaei S, Taati B (2022) Detecting and predicting visually induced motion sickness with physiological measures in combination with machine learning techniques. Int J Psychophysiol 176:14–26. https://doi.org/10.1016/j.ijpsycho.2022.03.006
Kim YY, Kim HJ, Kim EN et al (2005) Characteristic changes in the physiological components of cybersickness. Psychophysiology 42:616–625. https://doi.org/10.1111/j.1469-8986.2005.00349.x
Klimesch W (2012) Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci 16:606–617. https://doi.org/10.1016/j.tics.2012.10.007
Kooijman L, Berti S, Asadi H, et al (2022) Measuring vection: A review and critical evaluation of different methods for quantifying illusory self-motion. PsyArXiv
Krokos E, Varshney A (2022) Quantifying VR cybersickness using EEG. Virtual Reality 26:77–89. https://doi.org/10.1007/s10055-021-00517-2
Lawson BD, Riecke BE (2014) The Perception of Body Motion. In: Hale KS, Stanney KM (eds) Handbook of Virtual Environments: Design, Implementation, and Applications, 2nd edn. CRC Press, pp 163–195
Lin C-T, Tsai S-F, Ko L-W (2013) EEG-based learning system for online motion sickness level estimation in a dynamic vehicle environment. IEEE Trans Neural Netw Learn Syst 24:1689–1700. https://doi.org/10.1109/TNNLS.2013.2275003
Min B-C, Chung S-C, Min Y-K, Sakamoto K (2004) Psychophysiological evaluation of simulator sickness evoked by a graphic simulator. Appl Ergon 35:549–556. https://doi.org/10.1016/j.apergo.2004.06.002
Miyazaki J, Yamamoto H, Ichimura Y et al (2015) Inter-hemispheric desynchronization of the human MT+ during visually induced motion sickness. Exp Brain Res 233:2421–2431. https://doi.org/10.1007/s00221-015-4312-y
Muth ER (2006) Motion and space sickness: intestinal and autonomic correlates. Auton Neurosci 129:58–66. https://doi.org/10.1016/j.autneu.2006.07.020
Muth ER, Stern RM, Koch KL (1996) Effects of vection-induced motion sickness on gastric myoelectric activity and oral-cecal transit time. Dig Dis Sci 41:330–334
Napadow V, Sheehan JD, Kim J et al (2013) The brain circuitry underlying the temporal evolution of nausea in humans. Cereb Cortex 23:806–813. https://doi.org/10.1093/cercor/bhs073
Naqvi SAA, Badruddin N, Jatoi MA et al (2015) EEG based time and frequency dynamics analysis of visually induced motion sickness (VIMS). Australas Phys Eng Sci Med 38:721–729. https://doi.org/10.1007/s13246-015-0379-9
Neuper C, Scherer R, Reiner M, Pfurtscheller G (2005) Imagery of motor actions: Differential effects of kinesthetic and visual–motor mode of imagery in single-trial EEG. Cogn Brain Res 25:668–677. https://doi.org/10.1016/j.cogbrainres.2005.08.014
Nürnberger M, Klingner C, Witte OW, Brodoehl S (2021) Mismatch of visual-vestibular information in virtual reality: is motion sickness part of the brains attempt to reduce the prediction error? Front Hum Neurosci 15. https://doi.org/10.3389/fnhum.2021.757735
Oman CM (1990) Motion sickness: a synthesis and evaluation of the sensory conflict theory. Can J Physiol Pharmacol 68:294–303
Otto B, Riepl RL, Klosterhalfen S, Enck P (2006) Endocrine correlates of acute nausea and vomiting. Auton Neurosci 129:17–21. https://doi.org/10.1016/j.autneu.2006.07.010
Pan F, Zhang L, Ou Y, Zhang X (2019) The audio-visual integration effect on music emotion: Behavioral and physiological evidence. PLoS ONE. https://doi.org/10.1371/journal.pone.0217040
Peck K, Russo F, Campos JL, Keshavarz B (2020) Examining potential effects of arousal, valence, and likability of music on visually induced motion sickness. Exp Brain Res 238:2347–2358. https://doi.org/10.1007/s00221-020-05871-2
Poonian SK, McFadyen J, Ogden J, Cunnington R (2015) Implicit Agency in Observed Actions: Evidence for N1 Suppression of Tones Caused by Self-made and Observed Actions. J Cogn Neurosci 27:752–764. https://doi.org/10.1162/jocn_a_00745
Reason JT (1978) Motion sickness adaptation: a neural mismatch model. J R Soc Med 71:819–829
Reason JT, Brand JJ (1975) Motion sickness. Academic Press, London, New York
Rebenitsch L, Owen C (2016) Review on Cybersickness in Applications and Visual Displays. Virtual Real 20:101–125. https://doi.org/10.1007/s10055-016-0285-9
Riccio GE, Stoffregen TA (1991) An ecological Theory of Motion Sickness and Postural Instability. Ecol Psychol 3:195–240. https://doi.org/10.1207/s15326969eco0303_2
Tauscher J-P, Witt A, Bosse S et al (2020) Exploring neural and peripheral physiological correlates of simulator sickness. Comput Animat Virtual Worlds. https://doi.org/10.1002/cav.1953
van Driel J, Swart JC, Egner T et al (2015) (No) time for control: Frontal theta dynamics reveal the cost of temporally guided conflict anticipation. Cogn Affect Behav Neurosci 15:787–807. https://doi.org/10.3758/s13415-015-0367-2
Warwick-Evans LA, Church RE, Hancock C et al (1987) Electrodermal activity as an index of motion sickness. Aviat Space Environ Med 58:417–423
Wei Y, Okazaki YO, So RHY et al (2019) Motion sickness-susceptible participants exposed to coherent rotating dot patterns show excessive N2 amplitudes and impaired theta-band phase synchronization. Neuroimage. https://doi.org/10.1016/j.neuroimage.2019.116028
Wei Y, Wang Y, Okazaki YO et al (2023) Motion sickness resistant people showed suppressed steady-state visually evoked potential (SSVEP) under vection-inducing stimulation. Cogn Neurodyn. https://doi.org/10.1007/s11571-023-09991-7
Wood CD, Stewart JJ, Wood MJ et al (1990) Therapeutic effects of antimotion sickness medications on the secondary symptoms of motion sickness. Aviat Space Environ Med 61:157–161
Wu JP (1992) EEG changes in man during motion sickness induced by parallel swing. Space Med Med Eng 5(3):200–205
Yates BJ, Catanzaro MF, Miller DJ, McCall AA (2014) Integration of vestibular and emetic gastrointestinal signals that produce nausea and vomiting: potential contributions to motion sickness. Exp Brain Res 232:2455–2469. https://doi.org/10.1007/s00221-014-3937-6
Zhang C, Li S, Li Y et al (2020) Analysis of Motion Sickness Associated Brain Activity Using fNIRS: A Driving Simulator Study. IEEE Access 8:207415–207425. https://doi.org/10.1109/ACCESS.2020.3038039
Zumer JM, Scheeringa R, Schoffelen J-M et al (2014) Occipital Alpha Activity during Stimulus Processing Gates the Information Flow to Object-Selective Cortex. PLoS Biol. https://doi.org/10.1371/journal.pbio.1001965
Acknowledgements
We thank Bruce Haycock and Robert Shewaga for technical assistance and programming of the visual stimulus.
Funding
Funding for this study was awarded by the Natural Sciences and Engineering Research Council of Canada (NSERC RGPIN-2017-04387) to Behrang Keshavarz.
Author information
Authors and Affiliations
Contributions
All authors contributed to study conceptualization and design. Material preparation, data collection and analysis were performed by PA, BK, and SB. The first draft of the manuscript was written by PA and BK, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
The study complied with the American Psychological Association Code of Ethics and was approved by the Institutional Review Boards at the University Health Network and Toronto Metropolitan University.
Consent to participate and publish
Informed consent was obtained from all individuals included in the study. Only anonymized and de-identifiable data are presented in the manuscript.
Additional information
Communicated by Bill J Yates.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Andrievskaia, P., Berti, S., Spaniol, J. et al. Exploring neurophysiological correlates of visually induced motion sickness using electroencephalography (EEG). Exp Brain Res 241, 2463–2473 (2023). https://doi.org/10.1007/s00221-023-06690-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-023-06690-x