Abstract
Vomiting and nausea can be elicited by a variety of stimuli, although there is considerable evidence that the same brainstem areas mediate these responses despite the triggering mechanism. A variety of experimental approaches showed that nucleus tractus solitarius, the dorsolateral reticular formation of the caudal medulla (lateral tegmental field), and the parabrachial nucleus play key roles in integrating signals that trigger nausea and vomiting. These brainstem areas presumably coordinate the contractions of the diaphragm and abdominal muscles that result in vomiting. However, it is unclear whether these regions also mediate the autonomic responses that precede and accompany vomiting, including alterations in gastrointestinal activity, sweating, and changes in blood flow to the skin. Recent studies showed that delivery of an emetic compound to the gastrointestinal system affects the processing of vestibular inputs in the lateral tegmental field and parabrachial nucleus, potentially altering susceptibility for vestibular-elicited vomiting. Findings from these studies suggested that multiple emetic inputs converge on the same brainstem neurons, such that delivery of one emetic stimulus affects the processing of another emetic signal. Despite the advances in understanding the neurobiology of nausea and vomiting, much is left to be learned. Additional neurophysiologic studies, particularly those conducted in conscious animals, will be crucial to discern the integrative processes in the brain stem that result in emesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vomiting is a protective reflex to rid the body of ingested toxins. However, this response also occurs following anesthesia or exposure to radiation, during cancer chemotherapy or pregnancy, and even as a consequence of some psychological stimuli (Grelot and Miller 1994; Miller and Grélot 1996). Emesis can also occur as a component of a malady called motion sickness that sometimes accompanies movement (Reason and Brand 1975; Money et al. 1996; Yates et al. 1998).
The motion paradigms that evoke motion sickness can be complex and variable from individual to individual. These paradigms have been thoroughly described in other publications (Tyler and Bard 1949; Money 1970; Reason and Brand 1975; Reason 1978; Oman 1990; Money et al. 1996; Bles et al. 1998, 2000; Yates et al. 1998; Golding and Gresty 2005; Shupak and Gordon 2006; Kennedy et al. 2010) and thus will only be discussed briefly here. The amplitude of movements is not the major factor that triggers motion sickness (Oman 1990; Eyeson-Annan et al. 1996), but a deviation between sensory inputs and those expected based on experience, including a pattern of inputs from sensory receptors that provides ambiguous cues regarding body position in space (Lackner and Dizio 2006; Thornton and Bonato 2013). The evolutionary rationale for motion sickness is unclear, although a variety of hypotheses have been proposed. For example, motion sickness could be protective, as it induces animals to become less active in situations where continued movement might result in postural instability or injury (Riccio and Stoffregen 1991; Knox 2014). However, such “protection theories” are not the only explanations that have emerged for motion sickness (Treisman 1977; Ebenholtz et al. 1994; Balaban 1999), which include the notion that motion sickness is an epiphenomenon, and results from aberrant activation of vestibulo-autonomic pathways that typically serve to maintain homeostasis (Yates et al. 1998; Golding 2006).
The most critical signals required for the generation of motion sickness come from the vestibular system, as evidenced by the fact that individuals with bilateral vestibular dysfunction are usually not susceptible to motion sickness induced by stimuli that are typically provocative (Money 1970; Cheung et al. 1991), although one study reported that visual stimuli could induce motion sickness-like symptoms in subjects with loss of labyrinthine function (Johnson et al. 1999). In addition, conflicting sensory information from different vestibular end organs can induce motion sickness. For example, bilateral galvanic stimulation of the vestibular nerves, which produces a signal indicating that the head is simultaneously moving in many directions, can elicit retching and emesis in cats (Bard et al. 1947; Miller and Wilson 1983; Balaban et al. 2014).
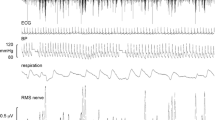
The motor act of vomiting includes complex gastrointestinal (GI) and respiratory components, as well as changes in posture (Miller et al. 1994; Miller and Grélot 1996; Money et al. 1996). The GI components incorporate marked reductions in gastric tone and motility, changes in gastric myoelectric activity, and a retrograde contraction that moves GI contents from the upper part of the small intestine back into the stomach prior to expulsion. However, GI changes are not essential to generate vomiting, as emesis still occurs following vagotomy, which eliminates these responses (Wang et al. 1957; Lang et al. 1999). Retching and expulsion are primarily produced by the powerful and coordinated action of the major respiratory muscles (McCarthy and Borison 1974). These muscles contract in different patterns during respiration and vomiting (Miller and Grélot 1996). In particular, the diaphragm and abdominal muscles, which are activated sequentially during the inspiratory and expiratory phases of respiration, co-contract during retching and expulsion (see Fig. 1).
Electromyographic (EMG) recordings from the diaphragm and abdominal muscles of a feline during vomiting elicited by bilateral sinusoidal polarization of the labyrinth. Two episodes are shown, consisting of co-contractions of the muscles during retching followed by expulsion, when the contraction of the abdominal musculature (blue curve, indicated by gray shaded area) persists longer than that of the diaphragm (red curve). Each emetic episode is preceded by a period of apnea (black arrows), related to the inhibition of the respiratory pattern generator. Also note that the diaphragm EMG activity during breathing after each period of vomiting (red arrows) is much smaller than during emesis
Nausea is a sensation that usually precedes vomiting and is triggered by the same inputs. It thus seems likely that the brainstem regions that receive sensory inputs that elicit emesis also participate in generating nausea. Nonetheless, the neural pathways that produce nausea and vomiting are at least partly separate. Emesis can be evoked in animals with all portions of the nervous system removed except the caudal medulla and spinal cord (Fukuda and Koga 1991; Miller et al. 1994). As such, the critical pattern generator that coordinates the respiratory muscle contractions that generate vomiting must be located in the caudal medulla. In contrast, a variety of experimental approaches have indicated that an ascending pathway from the brain stem through the parabrachial nucleus (PBN) to the hypothalamus, limbic system, and perhaps other cortical areas is responsible for nausea and the affective responses (e.g., stress and discomfort) that precede and accompany emesis (Yamamoto et al. 1992, 1994; Gieroba and Blessing 1994; Scalera et al. 1995; Balaban 1996; Sakai and Yamamoto 1997, 1998; Gallo et al. 1999; Reilly 1999; Ballesteros and Gallo 2000; Snyder et al. 2000; Yamamoto and Sawa 2000; Welzl et al. 2001; Grabus et al. 2004; De Jonghe and Horn 2009).
It is generally assumed that emesis, despite its triggering mechanism, is mediated through a “final common pathway” (Money 1970; Treisman 1977; Money and Cheung 1983; Miller and Leslie 1994; Yates et al. 1998). The existence of broad-spectrum antiemetics such as neurokinin-1 (NK1) receptor antagonists that prevent vomiting induced by a variety of triggers supports the hypothesis that a common output pathway from the brainstem coordinates and controls the respiratory muscle contractions during emesis (Bountra et al. 1993; Watson et al. 1995; Gardner et al. 1996; Gonsalves et al. 1996; Fukuda et al. 1998; Gardner and Perren 1998; Fukuda et al. 1999). Similarly, nausea induced by a variety of triggers is also presumably mediated through the same neural pathways, although there is no definitive evidence to support this hypothesis. This review discusses the experimental findings that provide insights into which neural regions mediate nausea and vomiting, with a particular focus on areas that produce motion-induced nausea and vomiting. It additionally considers recent evidence regarding the effects of delivering one emetic stimulus on the processing of another in these regions. In particular, recent studies reporting the effects of intragastric infusion of copper sulfate (CuSO4) on the processing of vestibular inputs by several brainstem areas believed to participate in producing nausea and vomiting are evaluated. Finally, this manuscript considers deficits in the understanding of neural mechanisms that produce nausea and vomiting, and proposes additional experimental approaches needed to address these shortcomings.
Brainstem regions that participate in producing nausea and vomiting
A variety of different experimental approaches have been used to determine which areas of the brain stem participate in producing nausea and vomiting. Studies conducted in several species determined these regions by mapping the distribution of c-fos protein (Fos)-like immunoreactivity elicited during emesis (Boissonade et al. 1994; Miller and Ruggiero 1994; Boissonade and Davison 1996; Billig et al. 2001b; Ito et al. 2003, 2005; Horn et al. 2007; Onishi et al. 2007; Balaban et al. 2014). Fos is quickly expressed in response to neuronal activation. After being synthesized in the cytoplasm, Fos is rapidly translocated to the nucleus where, with the Jun protein, it forms a heterodimer that regulates the expression of other genes (Morgan and Curran 1991; Herrera and Robertson 1996). Unfortunately, many of the studies that mapped neuronal Fos expression during vomiting only considered circumscribed areas of the caudal medulla (Boissonade et al. 1994; Miller and Ruggiero 1994; Boissonade and Davison 1996; Ariumi et al. 2000; Ito et al. 2003, 2005; Onishi et al. 2007), and not the entirety of the brain.
Since emesis is produced by the powerful co-contractions of the diaphragm and abdominal musculature, other studies conducted in emetic species mapped the distribution of brainstem neurons that control these muscles with the use of the transneuronal transport of two neurotropic viruses: pseudorabies (Billig et al. 1999, 2000, 2001a, 2003; Yates et al. 1999) and rabies (Lois et al. 2009). Transneuronal tracing techniques offer a powerful tool to map the polysynaptic pathways providing inputs to a particular target, as the viruses move progressively through neural circuits in a time-dependent retrograde manner (Kelly and Strick 2000; Ugolini 2008). These studies identified bulbospinal neurons that regulate the activity of the respiratory muscles and additionally revealed the locations of cells that provide inputs to these bulbospinal neurons.
Lesion and neurophysiologic techniques have also been used to determine the areas of the brain stem that mediate nausea and vomiting in cats and dogs. For example, neurophysiological studies have localized neurons whose activity is correlated with respiratory muscle contractions during vomiting (Miller et al. 1987, 1990, 1996; Bianchi and Grelot 1989; Fukuda and Koga 1992; Miller and Ezure 1992; Grelot and Miller 1994; Fukuda and Koga 1997). In addition, lesion studies ascertained which regions of the brain stem must remain intact for vomiting to occur (Wang and Borison 1951; Fukuda and Koga 1991; Miller et al. 1994; Koga et al. 1998). Other studies determined which brain regions induce vomiting when activated using electrical stimulation (Borison and Wang 1949; Fukuda and Koga 1991, 1992; Miller et al. 1994).
Bulbospinal pathways
Most neurophysiological studies focused on the control of vomiting hypothesized that neurons in the dorsal and ventral respiratory groups of the caudal medulla coordinate the contractions of respiratory muscles during all behaviors, including emesis. However, in contradiction to expectations, these studies revealed that respiratory group neurons are insufficient to elicit the respiratory muscle activity that produces vomiting. Although the firing of bulbospinal expiratory neurons in the caudal portion of the ventral respiratory is correlated with abdominal muscle contractions during retching and expulsion (Miller et al. 1987), most bulbospinal inspiratory neurons are actively inhibited and mainly silent during emetic responses (Bianchi and Grelot 1989; Miller et al. 1990). Recordings from interneurons in the respiratory groups revealed that the firing of these cells is profoundly altered during emesis, such that they mainly act to suppress the output of the respiratory pattern generator (Miller and Ezure 1992; Grelot and Miller 1994; Fukuda and Koga 1997). These findings explain an observation in Fig. 1: a period of apnea precedes retching and vomiting, presumably as the respiratory pattern generator is inhibited before the vomiting pattern generator is activated.
Since respiratory group neurons, particularly inspiratory neurons, are inhibited during emesis, other bulbospinal neurons must play a primary role in regulating the respiratory muscle contractions that produce vomiting. Injection of pseudorabies virus into the diaphragm or abdominal muscles of ferrets (Billig et al. 1999, 2000, 2001a, 2003; Yates et al. 1999) or rabies virus into the diaphragm of cats (Lois et al. 2009) demonstrated that in addition to cells in the respiratory groups, neurons in the medial medullary reticular formation [region labeled as the magnocellular tegmental field in Berman’s atlas (Berman 1968)] provide direct inputs to respiratory motoneurons. Figure 2 illustrates the locations of neurons infected at short and intermediate times following the injection of rabies virus into the diaphragm. Moreover, the use of two recombinants of pseudorabies virus showed that individual medial reticular formation neurons supply projections to both diaphragm and abdominal motoneurons (Billig et al. 2000). As such, medial medullary reticular formation neurons have the proper connectivity to elicit the simultaneous contractions of the diaphragm and abdominal musculature that occur during retching and the initial phase of expulsion. In addition, neurons in this region fire in synchrony with the co-contractions of respiratory muscles during emesis, and lesions of the medial medullary reticular formation prevent emesis (Miller et al. 1996).
Maps of sections through the medulla in two felines with early (a, b) and one feline with intermediate (c) infection of brainstem neurons following the injection of rabies virus into the diaphragm. Each dot represents a single infected neuron. The dorsal and ventral respiratory groups are depicted as dashed areas on each map. Blue shaded areas designate nucleus tractus solitarius. Orange-pink shaded areas highlight labeling in the magnocellular tegmental field; neurons in this region and the respiratory groups make direct connections with respiratory motoneurons. Numbers to the left of each row of sections indicate the approximate distance (in mm) from the sections to stereotaxic zero, based on Berman’s atlas (Berman 1968). Abbreviations: 5SP spinal trigeminal nucleus, cc central canal, DMV dorsal motor nucleus of the vagus, FTG gigantocellular tegmental field, FTM magnocellular tegmental field, GR gracile nucleus, IO inferior olivary complex, IVN inferior vestibular nucleus, LTF lateral tegmental field, LRN lateral reticular nucleus, mNTS medial nucleus of the solitary tract, MVN medial vestibular nucleus, NC cuneate nucleus, PR paramedian reticular nucleus, RB restiform body, RO raphe obscurus, RP raphe pallidus, S solitary tract, SFN subretrofacial nucleus, v5SP subtrigeminal nucleus. Adapted from (Lois et al. 2009)
Nucleus tractus solitarius (NTS)
Emesis elicited by GI inputs results in Fos expression in NTS, particularly in the medial portion of the nuclear complex (Boissonade et al. 1994; Boissonade and Davison 1996; Onishi et al. 2007). This is not surprising, since visceral afferents terminate in NTS. Fos expression in NTS was also produced by the injection of the cancer chemotherapeutic agent cisplatin (Reynolds et al. 1991; Ariumi et al. 2000; Horn et al. 2007; De Jonghe and Horn 2009) or by irradiation (Ito et al. 2003); both treatments are believed to increase the activity of GI afferents (Horn et al. 2004). Injection of emetic drugs such as apomorphine (Miller and Ruggiero 1994), whose action occurs centrally at area postrema (Borison 1959; Borison et al. 1975), additionally induced Fos expression in NTS. Since NTS receives a large fraction of the efferent projections from area postrema (Leslie and Gwyn 1984; Knox et al. 1994; Miller and Leslie 1994), this observation is also not unexpected. Fos expression increases in NTS of shrews during emesis provoked by shaking, which presumably is associated with motion sickness (Ito et al. 2003, 2005). In addition, cats that exhibited symptoms of motion sickness during galvanic vestibular stimulation expressed considerable Fos labeling in NTS (Balaban et al. 2014). Injection of transneuronal tracers into respiratory muscles resulted in infection of NTS neurons at intermediate survival times (Lois et al. 2009), as illustrated in Fig. 2. Cumulatively, these data raise the possibility that NTS serves as a major integrative site for signals that induce emesis.
Lateral tegmental field (LTF)
A high density of neurons expressing Fos during vomiting was observed between the ventral respiratory group and NTS, in the area referred to as the LTF in the cat (Miller and Ruggiero 1994; Billig et al. 2001b; Ito et al. 2003, 2005; Onishi et al. 2007). Transneuronal tracing studies in cats confirmed that this region is polysynaptically connected with respiratory muscles (Lois et al. 2009) (see Fig. 2). In a series of classical experiments, Borison and Wang (Borison and Wang 1949) demonstrated that stimulation of the dorsolateral LTF within the caudal medulla of cats produces vomiting. Others (Fukuda and Koga 1991, 1992) subsequently confirmed these findings in dogs. In addition, extensive lesions of the dorsolateral reticular formation of the caudal medulla eliminated emetic responses (Wang and Borison 1951; Koga et al. 1998). Furthermore, electrophysiological studies demonstrated that neurons in the dorsolateral medullary reticular formation have appropriate firing patterns to coordinate the respiratory muscle contractions that result in vomiting elicited by electrical stimulation of GI afferents (Fukuda and Koga 1992). Consequently, the dorsolateral region of the caudal medullary reticular formation is often referred to as the “vomiting center” in textbooks.
However, other investigators claimed that there is not a compact vomiting center present in the dorsolateral medullary reticular formation of the caudal medulla. Instead, they suggested that a larger network of cells distributed through the lateral medullary reticular formation coordinates emesis. This view was based on the experiments showing that stimulation in the LTF failed to produce vomiting in cats (Miller et al. 1994), as was previously demonstrated by others (Borison and Wang 1949; Fukuda and Koga 1991, 1992). In addition, although large chemical lesions of the lateral reticular formation prevented vomiting, more focal lesions of the LTF did not abolish the response, albeit the patterning of the respiratory muscle contractions during emesis was altered. The apparent discrepancies in the studies are likely related to the size of lesions and the magnitude of stimulus currents that were employed. The “vomiting center” may in fact be a “vomiting region” distributed over several mm of the dorsolateral medullary reticular formation. Nonetheless, there appears to be general agreement in the literature that neurons located in the LTF play an important role in regulating the respiratory muscle discharges that generate vomiting, and as such are a component of the pattern generator that coordinates the response.
Since LTF neurons do not project to the spinal cord, other brainstem regions must also participate in regulating the activity of respiratory motoneurons during vomiting. As noted above, neurons in the medial medullary reticular formation (magnocellular tegmental field) supply projections to both diaphragm and abdominal motoneurons (Billig et al. 2000), and are candidates for regulating the co-contractions of respiratory muscles during retching and the initial portion of expulsion. In addition, bulbospinal expiratory neurons in the caudal ventral respiratory group fire in synchrony with the abdominal muscle contractions during both retching and expulsion (Miller et al. 1987). It seems likely that these two regions participate in conveying signals from the emetic pattern generator in the LTF to respiratory motoneurons.
Ascending pathways from the brain stem that produce nausea
The sensation of nausea is complex, and the neural pathways that mediate the response are largely unknown. In addition to activating medullary neurons, emetic stimuli induce Fos expression in the lateral PBN, the paraventricular and supraoptic nuclei of the hypothalamus, the central nucleus of the amygdala, and bed nucleus of the stria terminalis (Billig et al. 2001b; De Jonghe and Horn 2009). As such, neurons in multiple regions are candidates for generating nausea. Nonetheless, since the PBN serves as the primary relay of visceral signals from NTS to the hypothalamus, amygdala, and other forebrain regions (King 1980; King and Knox 1982; Cechetto and Calaresu 1983; Fulwiler and Saper 1984; Kobashi and Adachi 1986; Portillo et al. 1994; Saleh and Cechetto 1994, 1995; Halsell et al. 1996; Rinaman and Schwartz 2004), this brain region must play some role in producing the sensation. PBN also receives descending signals from insular and prefrontal cortex (Saper 1982) and provides reciprocal connections to LTF (Herbert et al. 1990), and thus could be involved in triggering vomiting in response to psychological stimuli.
Brain areas that participate in generating motion sickness
Motion sickness is a complex malady and, in humans, includes signs and symptoms in addition to nausea and vomiting, such as cold sweating, and pallor (Reason and Brand 1975; Money et al. 1996; Yates et al. 1998). In addition, motion sickness is often linked with the Sopite syndrome, whose symptoms include lethargy and drowsiness (Graybiel and Knepton 1976). As noted in the introduction, motion-induced nausea and vomiting occur most often when sensory feedback related to movement deviates from that which is expected, which requires a comparison of the sensory signals with the motor plan (Lackner and Dizio 2006; Thornton and Bonato 2013). Hence, the neural pathways that trigger emesis during motion are likely more complex than those that elicit vomiting following exposure to toxins or stimulation of GI afferents.
Early lesion studies suggested that the area postrema chemoreceptive trigger zone was essential for eliciting vomiting during motion sickness. More recent lesion experiments, however, showed that motion-induced vomiting was prevented only in those cases where the area postrema lesions also extended into the underlying NTS (Borison and Borison 1986; Wilpizeski et al. 1986; Brizzee 1990; Fox et al. 1990). Anatomical experiments demonstrated that the caudal medial and inferior vestibular nuclei project directly to NTS (Balaban and Beryozkin 1994; Yates et al. 1994; Porter and Balaban 1997; Aleksandrov et al. 1998; Cai et al. 2007). In addition, physiological experiments showed that NTS neurons respond to electrical stimulation of the VIIIth nerve (Yates et al. 1994) and to tilts of the body that activate labyrinthine receptors (Sugiyama et al. 2011). These data support the notion that NTS plays an important role in relaying labyrinthine signals to the emesis pattern generator.
Physiological experiments showed that cells in LTF are activated by electrical stimulation of the vestibular nerve, mainly at latencies suggesting that the labyrinthine inputs were multisynaptic (Yates et al. 1995). Studies in decerebrate and conscious cats indicated that the responses of many LTF neurons to whole-body rotations in vertical planes are highly complex and do not reflect a simple integration of signals from otolith organs and semicircular canals (Moy et al. 2012; McCall et al. 2013). Such integration of sensory signals would be expected in a brainstem region that mediates motion sickness-related vomiting, since neurons that receive inputs from only one vestibular end organ are unlikely to encode head movements that deviate from expectancy. These findings, along with the data from Fos-mapping studies (Ito et al. 2003, 2005), suggest that the LTF serves as a pattern generator for motion-induced emesis, as it does for vomiting elicited by other stimuli.
The vestibular nuclei receive virtually all labyrinthine inputs and thus must participate in generating motion-induced emesis. It has been suggested that relatively direct connections from the caudal medial and inferior vestibular nuclei to NTS (Yates et al. 1994) and LTF (Yates et al. 1995) are responsible for eliciting vomiting during motion sickness. It is yet to be established whether integration of vestibular signals in other brain regions is also critical for producing motion-induced emesis. Several classical studies reported that destruction of a region of the posterior cerebellar vermis (nodulus and uvula, lobules IX and X), which constitutes a portion of the vestibulocerebellum, abolishes the capacity for an animal to vomit during provocative motion (Bard et al. 1947; Tyler and Bard 1949; Wang and Chinn 1956). These observations are supported by the results from physiological experiments (Cohen et al. 2003, 2008). The inputs to the posterior cerebellar vermis are appropriate to detect deviations of sensory inputs from expectancy, which results in motion sickness (Barmack 2003).
Other studies indicated that a deep cerebellar nucleus, the fastigial nucleus, participates in generating motion sickness (Pyykko et al. 1984; Denise and Darlot 1993; Catanzaro et al. 2014). The fastigial nucleus receives a considerable fraction of the output from the vestibulocerebellum (Ruggiero et al. 1977) and projects to the caudal aspect of the vestibular nucleus complex (Carleton and Carpenter 1983; Andrezik et al. 1984), so there is good reason to expect that the vestibulocerebellum and fastigial nuclei act in concert in triggering motion sickness.
There is also evidence that the activity of neurons in the vestibulocerebellum is affected by the stimulation of visceral afferents (Okahara and Nisimaru 1991; Tong et al. 1993; Saab and Willis 2001), which is reinforced by observations that the posterior cerebellar vermis receives projections from both NTS (Somana and Walberg 1979) and area postrema (Shapiro and Miselis 1985). The fastigial nucleus has also been reported to receive inputs from brainstem areas that process visceral signals, including the dorsal motor nucleus of the vagus (Zheng et al. 1982) and the area postrema (Shapiro and Miselis 1985). These findings raise the possibility that the cerebellum might additionally participate in generating nausea and vomiting induced by the consumption of toxic substances, although one classical report indicated that cerebellar lesions do not prevent vomiting produced by the administration of CuSO4 (Wang and Chinn 1956). Moreover, the cerebellum is not required to produce vomiting and related prodromal activity in response to galvanic stimulation of vestibular afferents (Miller and Wilson 1983). It is certainly feasible that several brain regions that process vestibular inputs can independently activate the LTF emetic pattern generator and generate motion sickness. For example, motion sickness elicited solely by labyrinthine inputs could in some cases be due to signal integration within the brain stem, whereas motion sickness triggered by conflicting visual and vestibular signals may require sensory processing in the cerebellum.
Parabrachial nucleus (PBN) neurons in both monkeys (Balaban et al. 2002) and felines (Suzuki et al. 2012) respond to passive translations or rotations of the head that activate labyrinthine receptors. The caudal aspect of PBN receives direct inputs from the vestibular nuclei (Balaban 1996; Balaban et al. 2002), as well as inputs from the vestibulocerebellum (Paton et al. 1991). As noted above, the PBN plays a fundamental role in transmitting visceral signals received by brainstem neurons to the limbic system (King 1980; King and Knox 1982; Cechetto and Calaresu 1983; Fulwiler and Saper 1984; Kobashi and Adachi 1986; Portillo et al. 1994; Saleh and Cechetto 1994, 1995; Halsell et al. 1996; Rinaman and Schwartz 2004). PBN neurons express Fos in animals that exhibit symptoms of motion sickness during galvanic vestibular stimulation (Balaban et al. 2014). In combination, these observations provide strong evidence that PBN neurons participate in generating the nausea and affective responses that occur during motion sickness. To further test this premise, it would be interesting to compare PBN neurons to active and passive head rotations, since actively controlled movements do not result in motion sickness (Rolnick and Lubow 1991; Golding et al. 2003).
A recent study correlated Fos expression during motion sickness elicited by galvanic vestibular stimulation in felines with the severity of observed symptoms (Balaban et al. 2014). A principal component analysis was used to identify the networks of neurons activated during this stimulus paradigm from functional correlations between Fos labeling in different nuclei. Five neural networks were identified, with labeling in two networks being prominent in the animals with the most severe motion sickness symptoms (e.g., retching and salivation). The brain regions containing the most labeled neurons in the animals with indicators of nausea and vomiting included those described above (e.g., the vestibular nuclei, NTS, and PBN). However, other brainstem areas, including the periaqueductal gray and raphe nuclei, contained the preponderance of Fos-labeled neurons in animals lacking overt motion sickness symptoms. These findings underscore the complexity of motion sickness and the variety of symptoms in addition to nausea and vomiting (e.g., changes in blood flow, discomfort, and stress) that are associated with the syndrome (Reason and Brand 1975; Money et al. 1996; Yates et al. 1998). It is likely that the brain areas containing labeling in animals that did not explicitly become sick mediate prodromal signs of motion sickness.
Summary
The use of a variety of experimental approaches has identified a subset of brainstem regions that participate in generating nausea and vomiting, which are summarized in Fig. 3. These regions are divided into areas that receive signals that trigger nausea and vomiting (area postrema, NTS, vestibular nuclei), areas that integrate the signals and regulate the activity of diaphragm motoneurons during retching and emesis (LTF, caudal portion of the ventral respiratory group, medial medullary reticular formation), and areas that mediate nausea by relaying visceral signals to the hypothalamus and limbic system (PBN). Undoubtedly, the neural circuit outlined in Fig. 3 is an oversimplification and likely omits regions responsible for affective and prodromal responses that precede emesis. Nonetheless, the neural connections outlined in Fig. 3 are key contributors to emetic responses and should be the focus of additional studies considering the signal integration responsible for the generation of nausea and vomiting.
Brainstem regions that play a primary role in producing nausea and vomiting. Brainstem sections are from a feline and were obtained from (Berman 1968). Numbers adjacent to a section indicate the distance (in mm) posterior to stereotaxic zero. Red areas receive emetic signals from the periphery and presumably participate in eliciting both vomiting and nausea. Blue areas are part of the vomiting pattern generator and relay emetic motor commands to respiratory motoneurons. Green areas participate in the viscerosensory processing that results in nausea. Black arrows designate connections between the cerebellar fastigial nucleus and caudal cerebellar vermis (nodulus and uvula) and brainstem areas that participate in generating vomiting. Abbreviations: AP area postrema, cVRG caudal portion of the ventral respiratory group, LTF lateral tegmental field, MRF medial medullary reticular formation, NTS nucleus tractus solitarius, PBN parabrachial nuclei, VN vestibular nuclei
Integration of labyrinthine and nonlabyrinthine inputs by brainstem regions that participate in producing nausea and vomiting
Several recent studies considered the processing of labyrinthine inputs by brainstem regions that mediate nausea and vomiting, particularly NTS (Sugiyama et al. 2011), LTF (Moy et al. 2012), PBN (Suzuki et al. 2012), and the caudal aspect of the vestibular nucleus complex (Arshian et al. 2013). It is likely that processing of vestibular inputs by these regions contributes to the triggering or generation of motion-related nausea or vomiting, although the particular role of vestibular signal processing in each area is unknown. These studies also determined whether the responses to vestibular stimulation of neurons in these areas were altered when the emetic compound CuSO4 was injected into the stomach. All of the experiments particularly focused on neurons whose spontaneous firing rate increased or decreased substantially following the intragastric infusion of CuSO4, as illustrated in Fig. 4. Neurons whose spontaneous firing rate was affected by the delivery of CuSO4 likely received inputs from GI receptors activated by the emetic compound and thus were the best candidates for having altered responses to vestibular stimulation.
Effect of intragastric copper sulfate administration (indicated by arrow) on arterial blood pressure (top) and NTS neuronal activity (bottom). Injecting copper sulfate resulted in a transient increase in blood pressure and a sustained increase in unit firing. Adapted from (Sugiyama et al. 2011)
Responses to vestibular stimulation of brainstem neurons that coordinate nausea and vomiting
One goal of the experiments discussed above was to ascertain the fraction of neurons in brainstem areas that generate nausea and vomiting whose responses to vestibular stimulation were complex, and not the simple summation of inputs from semicircular canals or otolith organs. Specifically, these studies determined whether the neurons exhibited spatiotemporal convergence (STC) behavior, which reflects the convergence of labyrinthine inputs with different spatial and temporal properties (e.g., inputs from otolith organs activated by ear-down rotations and semicircular canals activated by nose-up or nose-down rotations) (Baker et al. 1984; Schor et al. 1984; Schor and Angelaki 1992). The expression of STC responses by a large fraction of neurons in a particular brain region is consistent with that region participating in producing motion sickness, because motion sickness may occur when inputs reflecting body position in space deviate from those expected based on experience. Since STC neurons integrate signals from a variety of vestibular end organs, they are better candidates for encoding a movement that deviates from expectation than neurons that receive inputs from only one end organ (e.g., neurons that respond only to horizontal semicircular canal stimulation). Examples of STC responses of an LTF neuron are shown in Fig. 5.
Averaged responses of an LTF neuron to constant amplitude tilts whose direction rotates about the head at constant speed (wobble stimulus). Each panel shows unit activity, with a superimposed sine wave fit to the response. Stimulus amplitudes were 7.5° at 0.2 Hz and 5° at 0.5 Hz. The neuron responded to rotations in the clockwise direction, but not the counterclockwise direction. Such response characteristics are typical for STC neurons. Abbreviations: CED contralateral ear-down roll, IED ipsilateral ear-down roll, ND nose-down pitch, NU nose-up pitch. Adapted from (McCall et al. 2013)
The fraction of neurons with STC responses has been determined in conscious cats for three brainstem areas that participate in generating nausea and vomiting: LTF (McCall et al. 2013), the caudal aspect of the vestibular nuclei (Miller et al. 2008a), and the rostral fastigial nucleus (Miller et al. 2008b). Less than 10 % of neurons in the rostral fastigial and caudal vestibular nuclei of conscious cats exhibited STC responses, whereas 25 % of neurons in the LTF had such complex responses to vestibular stimulation. These differences were statistically significant (χ2 test). A similar comparison was conducted for data collected in decerebrate cats for the key brainstem regions implicated in generating vomiting: NTS (Sugiyama et al. 2011), LTF (Moy et al. 2012), and the caudal vestibular nuclei (Arshian et al. 2013). Whereas only one of 47 vestibular nucleus neurons (2 %) exhibited strong STC behavior, 18 % of NTS neurons and 31 % of LTF neurons had STC responses. These differences were statistically significant (χ2 test). Thus, it appears that STC responses emerge as vestibular signals are transmitted through the neural pathways that produce vomiting, from the vestibular nuclei to NTS to LTF.
Effects of CuSO4 administration on the responses of brainstem neurons to vestibular stimulation
The studies discussed above also ascertained whether intragastric administration of CuSO4 affected the processing of labyrinthine inputs by neurons in NTS, PBN, LTF, and the caudal vestibular nuclei (Sugiyama et al. 2011; Moy et al. 2012; Suzuki et al. 2012; Arshian et al. 2013). The notion underlying these studies was that an emetic stimulus affects motion sickness susceptibility, which is reflected in altered processing of labyrinthine inputs by brainstem areas that mediate nausea and vomiting. These studies showed that the spatial and temporal properties of neuronal responses to vestibular stimulation were relatively unaffected when CuSO4 was placed into the stomach. For example, the median change in response vector orientation (the direction of head movement that elicited a maximal change in neuronal activity) was <25° (out of a possible 365°) for neurons in each of the brain areas considered. However, the magnitudes of responses to vestibular stimulation of many NTS, PBN, LTF, and caudal vestibular nucleus neurons were affected by the administration of CuSO4, as indicated in Fig. 6. Some of the responses were enhanced, and others were diminished, such that the median changes in response gains across the neuronal populations were negligible. Figure 7 shows examples of responses of PBN neurons to vestibular stimulation that were profoundly altered when CuSO4 was infused into the stomach.
Effects of intragastric copper sulfate administration of the gains of responses to vestibular stimulation. Each symbol designates the effects intragastric copper sulfate on the gain of averaged responses to vestibular stimulation of an individual neuron. Red horizontal lines show median percentage changes in gain. Abbreviations: LTF lateral tegmental field, NTS nucleus tractus solitarius, PBN parabrachial nucleus, VN caudal aspect of vestibular nucleus complex. LTF data from (Moy et al. 2012), NTS data from (Sugiyama et al. 2011), PBN data from (Suzuki et al. 2012), VN data from (Arshian et al. 2013)
Effects of copper sulfate administration on the averaged responses of two PBN units to 7.5° tilts whose direction was rotated about the animal at 0.2 Hz (wobble stimuli). Each histogram contains 500 bins; a gray waveform superimposed on each trace indicates tilt table position, whereas a red waveform is a sine wave fit to the response. In each panel, the top waveform indicates the response prior to intragastric copper sulfate, whereas the bottom waveform indicates the response after the compound was delivered. The shapes of five overlapped action potentials recorded from the units whose activity was binned in these histograms are provided to the right of each response. The spike shape was similar throughout the recording period, indicating that the same unit was sampled both before and after intragastric copper sulfate. a Responses of a neuron that lacked a response to vestibular stimulation prior to copper sulfate administration, although a strong response was present afterward. b Responses of a neuron whose activity was robustly modulated by rotations before intragastric CuSO4, but not afterward. Abbreviations: CED contralateral ear-down roll, IED ipsilateral ear-down roll, ND nose down, NU nose up. Adapted from (Suzuki et al. 2012)
The effects of CuSO4 administration on responses to vestibular stimulation were larger in some of the areas considered than others. Delivery of CuSO4 caused a >50 % change in response gain for 55 % PBN neurons, 36 % LTF neurons, 33 % caudal vestibular nucleus neurons, but just 18 % NTS neurons. These proportions were shown to be significantly different via a χ2 test. When the analysis was limited to the subset of neurons whose spontaneous activity increased or decreased following CuSO4 delivery, the differences were even more pronounced: the gains of responses to vestibular stimulation of 67 % PBN and LTF neurons, 50 % caudal vestibular nucleus neurons, but just 15 % NTS neurons were altered over 50 % when the compound was provided (significantly different, χ2 test). These data support the hypothesis that an emetic GI stimulus affects the processing of labyrinthine inputs in brainstem pathways that mediate nausea and vomiting. However, the effects are most pronounced in integrative regions such as PBN and LTF, and not areas such as NTS and the vestibular nuclei that directly receive emetic inputs from peripheral receptors.
Summary and conclusions
Recent studies demonstrated that neurons in brainstem areas that mediate nausea and vomiting receive convergent inputs from GI receptors activated by emetic compounds and labyrinthine receptors (Sugiyama et al. 2011; Moy et al. 2012; Suzuki et al. 2012; Arshian et al. 2013). Such converging inputs were particularly common for LTF and PBN neurons, whose responses to vestibular stimulation were altered when CuSO4 was present in the stomach. These data extend the “final common pathway” hypothesis by suggesting that not only is nausea and vomiting elicited by different triggers mediated by the same pathways, but that one emetic signal can affect the processing of another within those pathways. However, a limiting factor in interpreting these findings is that intragastric infusion of CuSO4 enhanced the responses of some neurons to vestibular stimulation, but attenuated the responses of other neurons. It is possible that these diverging effects could be related to functional differences between the neurons. For example, some PBN neurons have ascending projections to the hypothalamus, thalamus, limbic system, and forebrain structures (Takeuchi et al. 1982; Cechetto et al. 1983; Fulwiler and Saper 1984; Cechetto and Calaresu 1985; Berkley and Scofield 1990), whereas others have descending projections to NTS and the medullary reticular formation (Fulwiler and Saper 1984; Herbert et al. 1990). It is feasible that the effects of CuSO4 administration on the responses of PBN neurons to vestibular stimulation are related to which brain region a particular cell provides outputs. Additional studies are warranted to investigate the integration of labyrinthine and nonlabyrinthine emetic inputs by brainstem neurons that mediate nausea and vomiting, with a particular focus on the neurochemical and neuroanatomical characteristics of each cell examined.
Neural pathways that mediate nausea and vomiting: gaps in knowledge
As discussed above, there has been considerable progress in discerning the neural pathways that mediate nausea and vomiting. Key regions of the brain that coordinate these responses have been identified, and there is some information about the integration of neuronal signals in these regions. However, much is yet to be learned.
Although some studies have incorporated recordings of neuronal activity during vomiting, all such experiments were conducted in decerebrate or anesthetized animals (Miller et al. 1987; Bianchi and Grelot 1989; Miller et al. 1990, 1996; Fukuda and Koga 1992, 1997; Miller and Ezure 1992; Grelot and Miller 1994). Responses of brainstem neurons to labyrinthine and other inputs can be exaggerated in such preparations (DeStefino et al. 2011), and thus, future neurophysiological studies related to the mechanisms producing nausea and vomiting should be conducted on conscious animals. In addition, previous work was limited to discerning the neural pathways that regulate the respiratory muscle contractions that result in vomiting and not those that mediate the autonomic responses that accompany emesis (alterations in GI activity, pallor, sweating, etc.). The bulbospinal pathways that mediate these autonomic responses are yet to be identified. Furthermore, vomiting is an “all or nothing” response, whereas the autonomic changes that precede and accompany vomiting vary considerably, and can persist for a considerable period before emesis occurs (Reason and Brand 1975; Grelot and Miller 1994; Miller and Grélot 1996; Money et al. 1996; Yates et al. 1998). It is thus unclear whether the same brainstem areas coordinate emesis-related respiratory muscle contractions and the accompanying autonomic responses. The studies needed to provide critical insights into the neural underpinnings of nausea and vomiting will be very difficult, particularly since these responses do not occur instantaneously following the presentation of emetic stimuli. Furthermore, it may be difficult to differentiate primary autonomic responses associated with emesis with secondary autonomic responses related to anxiety and stress accompanying the response. Nonetheless, neurophysiological studies of neural pathways that mediate nausea and vomiting are critical to provide the needed insights for developing the next generation of anti-emetic drugs. Because nausea and vomiting are mediated in part through separate neural circuits, there is a potential for pharmaceutical agents to suppress vomiting, but not nausea. Since the act of vomiting can temporarily alleviate nausea, drugs that abolish emesis but not nausea would not be beneficial to patients, and thus, a thorough discrimination of the mechanisms of action of such drugs is needed prior to the initiation of clinical trials (Yates et al. 1998).
Determining the neurophysiological basis of motion sickness will be particularly daunting, as there are individual differences in motion sickness susceptibility between individuals, and prolonged exposure to provocative motion is needed to generate the syndrome. A potential key to performing these studies is that motion sickness typically occurs when vestibular stimuli occur during unexpected movements, but not those that are voluntary (Rolnick and Lubow 1991; Golding et al. 2003). Thus, comparing in conscious animals, the responses to voluntary and unexpected movements of neurons in brainstem areas that produce nausea and vomiting could be particularly enlightening. It would also be useful to train animals to expect a particular movement on the basis of a cue, but in some trials produce a movement that is not aligned with the cue. Neurons in brainstem areas that coordinate nausea and vomiting that respond only to erroneous movement cues could play a particularly salient role in triggering motion sickness (Yates et al. 1998).
References
Aleksandrov VG, Bagaev VA, Nozdrachev AD (1998) Gastric related neurons in the rat medial vestibular nucleus. Neurosci Lett 250:66–68
Andrezik JA, Dormer KJ, Foreman RD, Person RJ (1984) Fastigial nucleus projections to the brain stem in beagles: pathways for autonomic regulation. Neurosci 11:497–507
Ariumi H, Saito R, Nago S, Hyakusoku M, Takano Y, Kamiya H (2000) The role of tachykinin NK-1 receptors in the area postrema of ferrets in emesis. Neurosci Lett 286:123–126
Arshian MS, Puterbaugh SR, Miller DJ, Catanzaro MF, Hobson CE, McCall AA, Yates BJ (2013) Effects of visceral inputs on the processing of labyrinthine signals by the inferior and caudal medial vestibular nuclei: ramifications for the production of motion sickness. Exp Brain Res 228:353–363
Baker J, Goldberg J, Hermann G, Peterson B (1984) Spatial and temporal response properties of secondary neurons that receive convergent input in vestibular nuclei of alert cats. Brain Res 294:138–143
Balaban CD (1996) Vestibular nucleus projections to the parabrachial nucleus in rabbits: implications for vestibular influences on the autonomic nervous system. Exp Brain Res 108:367–381
Balaban CD (1999) Vestibular autonomic regulation (including motion sickness and the mechanism of vomiting). Curr Opin Neurol 12:29–33
Balaban CD, Beryozkin G (1994) Vestibular nucleus projections to nucleus tractus solitarius and the dorsal motor nucleus of the vagus nerve: potential substrates for vestibulo-autonomic interactions. Exp Brain Res 98:200–212
Balaban CD, McGee DM, Zhou J, Scudder CA (2002) Responses of primate caudal parabrachial nucleus and Kolliker-fuse nucleus neurons to whole body rotation. J Neurophysiol 88:3175–3193
Balaban CD, Ogburn SW, Warshafsky SG, Ahmed A, Yates BJ (2014) Identification of neural networks that contribute to motion sickness through principal components analysis of fos labeling induced by galvanic vestibular stimulation. PLoS ONE 9:e86730
Ballesteros MA, Gallo M (2000) Bilateral tetrodotoxin blockade of the rat vestibular nuclei substitutes the natural unconditioned stimulus in taste aversion learning. Neurosci Lett 279:161–164
Bard P, Woolsey CN, Snider RS, Mountcastle VB, Bromiley RB (1947) Delimitation of central nervous mechanisms involved in motion sickness. Fed Proc 6:72
Barmack NH (2003) Central vestibular system: vestibular nuclei and posterior cerebellum. Brain Res Bull 60:511–541
Berkley KJ, Scofield SL (1990) Relays from the spinal cord and solitary nucleus through the parabrachial nucleus to the forebrain in the cat. Brain Res 529:333–338
Berman AI (1968) The brain stem of the cat. University of Wisconsin Press, Madison
Bianchi AL, Grelot L (1989) Converse motor output of inspiratory bulbospinal premotoneurones during vomiting. Neurosci Lett 104:298–302
Billig I, Foris JM, Card JP, Yates BJ (1999) Transneuronal tracing of neural pathways controlling an abdominal muscle, rectus abdominis, in the ferret. Brain Res 820:31–44
Billig I, Foris JM, Enquist LW, Card JP, Yates BJ (2000) Definition of neuronal circuitry controlling the activity of phrenic and abdominal motoneurons in the ferret using recombinant strains of pseudorabies virus. J Neurosci 20:7446–7454
Billig I, Hartge K, Card JP, Yates BJ (2001a) Transneuronal tracing of neural pathways controlling abdominal musculature in the ferret. Brain Res 912:24–32
Billig I, Yates BJ, Rinaman L (2001b) Plasma hormone levels and central c-Fos expression in ferrets after systemic administration of cholecystokinin. Am J Physiol Regul Integr Comp Physiol 281:R1243–R1255
Billig I, Card JP, Yates BJ (2003) Neurochemical phenotypes of MRF neurons influencing diaphragm and rectus abdominis activity. J Appl Physiol 94:391–398
Bles W, Bos JE, de Graaf B, Groen E, Wertheim AH (1998) Motion sickness: only one provocative conflict? Brain Res Bull 47:481–487
Bles W, Bos JE, Kruit H (2000) Motion sickness. Curr Opin Neurol 13:19–25
Boissonade FM, Davison JS (1996) Effect of vagal and splanchnic nerve section on fos expression in ferret brain stem after emetic stimuli. Am J Physiol Regul Integr Comp Physiol 40:R228–R236
Boissonade FM, Sharkey KA, Davison JS (1994) Fos expression in ferret dorsal vagal complex after peripheral emetic stimuli. Am J Physiol 266:R1118–R1126
Borison HL (1959) Effect of ablation of medullary emetic chemoreceptor trigger zone on vomiting responses to cerebral intraventricular injection of adrenaline, apomorphine and pilocarpine in the cat. J Physiol 147:172–177
Borison HL, Borison R (1986) Motion sickness reflex arc bypasses the area postrema in cats. Exp Neurol 92:723–737
Borison HL, Wang SC (1949) Functional localization of central coordinating mechanism for emesis in cat. J Neurophysiol 12:305–313
Borison HL, Hawken MJ, Hubbard JI, Sirett NE (1975) Unit activity from cat area postrema influenced by drugs. Brain Res 92:153–156
Bountra C, Bunce K, Dale T, Gardner C, Jordan C, Twissell D, Ward P (1993) Anti-emetic profile of a non-peptide neurokinin NK1 receptor antagonist, CP-99,994, in ferrets. Eur J Pharmacol 249:R3–R4
Brizzee K (1990) The central nervous system connections involved in motion induced emesis. In: Crampton GH (ed) Motion and space sickness. CRC Press, Boca Raton, pp 9–27
Cai YL, Ma WL, Li M, Guo JS, Li YQ, Wang LG, Wang WZ (2007) Glutamatergic vestibular neurons express Fos after vestibular stimulation and project to the NTS and the PBN in rats. Neurosci Lett 417:132–137
Carleton SC, Carpenter MB (1983) Afferent and efferent connections of the medial, inferior and lateral vestibular nuclei in the cat and monkey. Brain Res 278:29–51
Catanzaro MF, Miller DJ, Cotter LA, McCall AA (2014) Integration of gastrointestinal and vestibular inputs by neurons in the cerebellar rostral fastigial nucleus. Exp Brain Res. doi:10.1007/s00221-014-3898-9
Cechetto DF, Calaresu FR (1983) Parabrachial units responding to stimulation of buffer nerves and forebrain in the cat. Am J Physiol 245:R811–R819
Cechetto DF, Calaresu FR (1985) Central pathways relaying cardiovascular afferent information to amygdala. Am J Physiol 248:R38–R45
Cechetto DF, Ciriello J, Calaresu FR (1983) Afferent connections to cardiovascular sites in the amygdala: a horseradish peroxidase study in the cat. J Auton Nerv Syst 8:97–110
Cheung BS, Howard IP, Money KE (1991) Visually-induced sickness in normal and bilaterally labyrinthine-defective subjects. Aviat Space Environ Med 62:527–531
Cohen B, Dai M, Raphan T (2003) The critical role of velocity storage in production of motion sickness. Ann N Y Acad Sci 1004:359–376
Cohen B, Dai M, Yakushin SB, Raphan T (2008) Baclofen, motion sickness susceptibility and the neural basis for velocity storage. Prog Brain Res 171:543–553
De Jonghe BC, Horn CC (2009) Chemotherapy agent cisplatin induces 48 h Fos expression in the brain of a vomiting species, the house musk shrew (Suncus murinus). Am J Physiol Regul Integr Comp Physiol 296:R902–R911
Denise P, Darlot C (1993) The cerebellum as a predictor of neural messages—II. Role in motor control and motion sickness. Neurosci 56:647–655
DeStefino VJ, Reighard DA, Sugiyama Y et al (2011) Responses of neurons in the rostral ventrolateral medulla to whole body rotations: comparisons in decerebrate and conscious cats. J Appl Physiol 110:1699–1707
Ebenholtz SM, Cohen MM, Linder BJ (1994) The possible role of nystagmus in motion sickness: a hypothesis. Aviat Space Environ Med 65:1032–1035
Eyeson-Annan M, Peterken C, Brown B, Atchison D (1996) Visual and vestibular components of motion sickness. Aviat Space Environ Med 67:955–962
Fox RA, Corcoran M, Brizzee KR (1990) Conditioned taste aversion and motion sickness in cats and squirrel monkeys. Can J Physiol Pharmacol 68:269–278
Fukuda H, Koga T (1991) The Botzinger complex as the pattern generator for retching and vomiting in the dog. Neurosci Res 12:471–485
Fukuda H, Koga T (1992) Non-respiratory neurons in the Botzinger complex exhibiting appropriate firing patterns to generate the emetic act in dogs. Neurosci Res 14:180–194
Fukuda H, Koga T (1997) Most inspiratory neurons in the pre-Botzinger complex are suppressed during vomiting in dogs. Brain Res 763:30–38
Fukuda H, Koga T, Furukawa N, Nakamura E, Shiroshita Y (1998) The tachykinin NK1 receptor antagonist GR205171 prevents vagal stimulation-induced retching but not neuronal transmission from emetic vagal afferents to solitary nucleus neurons in dogs. Brain Res 802:221–231
Fukuda H, Koga T, Furukawa N, Nakamura E, Shiroshita Y (1999) The tachykinin NK1 receptor antagonist GR205171 abolishes the retching activity of neurons comprising the central pattern generator for vomiting in dogs. Neurosci Res 33:25–32
Fulwiler CE, Saper CB (1984) Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res Rev 7:229–259
Gallo M, Marquez SL, Ballesteros MA, Maldonado A (1999) Functional blockade of the parabrachial area by tetrodotoxin disrupts the acquisition of conditioned taste aversion induced by motion-sickness in rats. Neurosci Lett 265:57–60
Gardner C, Perren M (1998) Inhibition of anaesthetic-induced emesis by a NK1 or 5-HT3 receptor antagonist in the house musk shrew, Suncus murinus. Neuropharmacol 37:1643–1644
Gardner CJ, Armour DR, Beattie DT et al (1996) GR205171: a novel antagonist with high affinity for the tachykinin NK1 receptor, and potent broad-spectrum anti-emetic activity. Regul Pept 65:45–53
Gieroba ZJ, Blessing WW (1994) Fos-containing neurons in medulla and pons after unilateral stimulation of the afferent abdominal vagus in conscious rabbits. Neurosci 59:851–858
Golding JF (2006) Motion sickness susceptibility. Auton Neurosci 129:67–76
Golding JF, Gresty MA (2005) Motion sickness. Curr Opin Neurol 18:29–34
Golding JF, Bles W, Bos JE, Haynes T, Gresty MA (2003) Motion sickness and tilts of the inertial force environment: active suspension systems vs. active passengers. Aviat Space Environ Med 74:220–227
Gonsalves S, Watson J, Ashton C (1996) Broad spectrum antiemetic effects of CP-122,721, a tachykinin NK1 receptor antagonist, in ferrets. Eur J Pharmacol 305:181–185
Grabus SD, Glowa JR, Riley AL (2004) Morphine- and cocaine-induced c-Fos levels in Lewis and Fischer rat strains. Brain Res 998:20–28
Graybiel A, Knepton J (1976) Sopite syndrome: a sometimes sole manifestation of motion sickness. Aviat Space Environ Med 47:873–882
Grelot L, Miller A (1994) Vomiting—its ins and outs. News Physiol Sci 9:142–147
Halsell CB, Travers SP, Travers JB (1996) Ascending and descending projections from the rostral nucleus of the solitary tract originate from separate neuronal populations. Neurosci 72:185–197
Herbert H, Moga MM, Saper CB (1990) Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol 293:540–580
Herrera DG, Robertson HA (1996) Activation of c-fos in the brain. Prog Neurobiol 50:83–107
Horn CC, Richardson EJ, Andrews PL, Friedman MI (2004) Differential effects on gastrointestinal and hepatic vagal afferent fibers in the rat by the anti-cancer agent cisplatin. Auton Neurosci 115:74–81
Horn CC, Ciucci M, Chaudhury A (2007) Brain Fos expression during 48 h after cisplatin treatment: neural pathways for acute and delayed visceral sickness. Auton Neurosci 132:44–51
Ito H, Nishibayashi M, Kawabata K, Maeda S, Seki M, Ebukuro S (2003) Induction of Fos protein in neurons in the medulla oblongata after motion- and X-irradiation-induced emesis in musk shrews (Suncus murinus). Auton Neurosci 107:1–8
Ito H, Nishibayashi M, Maeda S, Seki M, Ebukuro S (2005) Emetic responses and neural activity in young musk shrews during the breast-feeding/weaning period: comparison between the high and low emetic response strains using a shaking stimulus. Exp Anim 54:301–307
Johnson WH, Sunahara FA, Landolt JP (1999) Importance of the vestibular system in visually induced nausea and self-vection. J Vestib Res 9:83–87
Kelly RM, Strick PL (2000) Rabies as a transneuronal tracer of circuits in the central nervous system. J Neurosci Meth 103:63–71
Kennedy R, Drexler J, Kennedy R (2010) Research in visually induced motion sickness. Appl Ergonomics 41:494–503
King GW (1980) Topology of ascending brainstem projections to nucleus parabrachialis in the cat. J Comp Neurol 191:615–638
King GW, Knox CK (1982) An electrophysiological study of medullary neurons projecting to nucleus parabrachialis of the cat. Brain Res 236:27–33
Knox GW (2014) Motion sickness: an evolutionary and genetic basis for the negative reinforcement model. Aviat Space Environ Med 85:46–49
Knox AP, Strominger NL, Battles AH, Carpenter DO (1994) The central connections of the vagus nerve in the ferret. Brain Res Bull 33:49–63
Kobashi M, Adachi A (1986) Projection of nucleus tractus solitarius units influenced by hepatoportal afferent signal to parabrachial nucleus. J Auton Nerv Syst 16:153–158
Koga T, Qu R, Fukuda H (1998) The central pattern generator for vomiting may exist in the reticular area dorsomedial to the retrofacial nucleus in dogs. Exp Brain Res 118:139–147
Lackner JR, Dizio P (2006) Space motion sickness. Exp Brain Res 175:377–399
Lang IM, Sarna SK, Shaker R (1999) Gastrointestinal motor and myoelectric correlates of motion sickness. Am J Physiol 277:G642–G652
Leslie RA, Gwyn DG (1984) Neuronal connections of the area postrema. Fed Proc 43:2941–2943
Lois JH, Rice CD, Yates BJ (2009) Neural circuits controlling diaphragm function in the cat revealed by transneuronal tracing. J Appl Physiol 106:138–152
McCall AA, Moy JD, DeMayo WM, Puterbaugh SR, Miller DJ, Catanzaro MF, Yates BJ (2013) Processing of vestibular inputs by the medullary lateral tegmental field of conscious cats: implications for generation of motion sickness. Exp Brain Res 225:349–359
McCarthy LE, Borison HL (1974) Respiratory mechanics of vomiting in decerebrate cats. Am J Physiol 226:738–743
Miller AD, Ezure K (1992) Behavior of inhibitory and excitatory propriobulbar respiratory neurons during fictive vomiting. Brain Res 578:168–176
Miller AD, Grélot L (1996) The neural basis of nausea and vomiting. In: Yates BJ, Miller AD (eds) Vestibular autonomic regulation. CRC Press, Boca Raton, pp 85–94
Miller AD, Leslie RA (1994) The area postrema and vomiting. Front Neuroendocrinol 15:301–320
Miller AD, Ruggiero DA (1994) Emetic reflex are revealed by expression of the Immediate-Early gene C-Fos in the cat. J Neurosci 14:871–888
Miller AD, Wilson VJ (1983) Vestibular-induced vomiting after vestibulocerebellar lesions. Brain Behav Evol 23:26–31
Miller AD, Tan LK, Suzuki I (1987) Control of abdominal and expiratory intercostal muscle activity during vomiting: role of ventral respiratory group expiratory neurons. J Neurophysiol 57:1854–1866
Miller AD, Nonaka S, Lakos SF, Tan LK (1990) Diaphragmatic and external intercostal muscle control during vomiting: behavior of inspiratory bulbospinal neurons. J Neurophysiol 63:31–36
Miller AD, Nonaka S, Jakus J (1994) Brain areas essential or non-essential for emesis. Brain Res 647:255–264
Miller AD, Nonaka S, Jakus J, Yates BJ (1996) Modulation of vomiting by the medullary midline. Brain Res 737:51–58
Miller DM, Cotter LA, Gandhi NJ et al (2008a) Responses of caudal vestibular nucleus neurons of conscious cats to rotations in vertical planes, before and after a bilateral vestibular neurectomy. Exp Brain Res 188:175–186
Miller DM, Cotter LA, Gandhi NJ et al (2008b) Responses of rostral fastigial nucleus neurons of conscious cats to rotations in vertical planes. Neurosci 155:317–325
Money KE (1970) Motion sickness. Physiol Rev 50:1–39
Money KE, Cheung BS (1983) Another function of the inner ear: facilitation of the emetic response to poisons. Aviat Space Environ Med 54:208–211
Money KE, Lackner JR, Cheung RSK (1996) The autonomic nervous system and motion sickness. In: Yates BJ, Miller AD (eds) Vestibular autonomic regulation. CRC Press, Boca Raton, pp 147–173
Morgan JI, Curran T (1991) Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci 14:421–451
Moy JD, Miller DJ, Catanzaro MF et al (2012) Responses of neurons in the caudal medullary lateral tegmental field to visceral inputs and vestibular stimulation in vertical planes. Am J Physiol Regul Integr Comp Physiol 303:R929–R940
Okahara K, Nisimaru N (1991) Climbing fiber responses evoked in lobule VII of the posterior cerebellum from a vagal nerve in rabbits. Neurosci Res 12:232–239
Oman CM (1990) Motion sickness: a synthesis and evaluation of the sensory conflict theory. Can J Physiol Pharmacol 68:294–303
Onishi T, Mori T, Yanagihara M, Furukawa N, Fukuda H (2007) Similarities of the neuronal circuit for the induction of fictive vomiting between ferrets and dogs. Auton Neurosci 136:20–30
Paton JF, La Noce A, Sykes RM, Sebastiani L, Bagnoli P, Ghelarducci B, Bradley DJ (1991) Efferent connections of lobule IX of the posterior cerebellar cortex in the rabbit–some functional considerations. J Auton Nerv Syst 36:209–224
Porter JD, Balaban CD (1997) Connections between the vestibular nuclei and brain stem regions that mediate autonomic function in the rat. J Vestib Res 7:63–76
Portillo F, Grelot L, Milano S, Bianchi AL (1994) Brainstem neurons with projecting axons to both phrenic and abdominal motor nuclei: a double fluorescent labeling study in the cat. Neurosci Lett 173:50–54
Pyykko I, Schalen L, Jantti V, Magnusson M (1984) A reduction of vestibulo-visual integration during transdermally administered scopolamine and dimenhydrinate. A presentation of gain control theory in motion sickness. Acta Otolaryngol Suppl 406:167–173
Reason J (1978) Motion sickness: some theoretical and practical considerations. Appl Ergon 9:163–167
Reason JT, Brand JJ (1975) Motion sickness. Academic Press, London
Reilly S (1999) The parabrachial nucleus and conditioned taste aversion. Brain Res Bull 48:239–254
Reynolds DJ, Barber NA, Grahame-Smith DG, Leslie RA (1991) Cisplatin-evoked induction of c-fos protein in the brainstem of the ferret: the effect of cervical vagotomy and the anti-emetic 5-HT3 receptor antagonist granisetron (BRL 43694). Brain Res 565:231–236
Riccio GE, Stoffregen TA (1991) An ecological theory of motion sickness and postural instability. Ecol Psychol 3:195–240
Rinaman L, Schwartz G (2004) Anterograde transneuronal viral tracing of central viscerosensory pathways in rats. J Neurosci 24:2782–2786
Rolnick A, Lubow RE (1991) Why is the driver rarely motion sick? The role of controllability in motion sickness. Ergonomics 34:867–879
Ruggiero D, Batton RR 3rd, Jayaraman A, Carpenter MB (1977) Brain stem afferents to the fastigial nucleus in the cat demonstrated by transport of horseradish peroxidase. J Comp Neurol 172:189–209
Saab CY, Willis WD (2001) Nociceptive visceral stimulation modulates the activity of cerebellar Purkinje cells. Exp Brain Res 140:122–126
Sakai N, Yamamoto T (1997) Conditioned taste aversion and c-fos expression in the rat brainstem after administration of various USs. NeuroReport 8:2215–2220
Sakai N, Yamamoto T (1998) Role of the medial and lateral parabrachial nucleus in acquisition and retention of conditioned taste aversion in rats. Behav Brain Res 93:63–70
Saleh TM, Cechetto DF (1994) Neurotransmitters in the parabrachial nucleus mediating visceral input to the thalamus in rats. Am J Physiol 266:R1287–R1296
Saleh TM, Cechetto DF (1995) Neurochemical interactions in the parabrachial nucleus mediating visceral inputs to visceral thalamic neurons. Am J Physiol 268:R786–R795
Saper CB (1982) Reciprocal parabrachial-cortical connections in the rat. Brain Res 242:33–40
Scalera G, Spector AC, Norgren R (1995) Excitotoxic lesions of the parabrachial nuclei prevent conditioned taste aversions and sodium appetite in rats. Behav Neurosci 109:997–1008
Schor RH, Angelaki DE (1992) The algebra of neural response vectors. Ann New York Acad Sci 656:190–204
Schor RH, Miller AD, Tomko DL (1984) Responses to head tilt in cat central vestibular neurons. I. Direction of maximum sensitivity. J Neurophysiol 51:136–146
Shapiro RE, Miselis RR (1985) The central neural connections of the area postrema of the rat. J Comp Neurol 234:344–364
Shupak A, Gordon CR (2006) Motion sickness: advances in pathogenesis, prediction, prevention, and treatment. Aviat Space Environ Med 77:1213–1223
Snyder DJ, Jahng JW, Smith JC, Houpt TA (2000) c-Fos induction in visceral and vestibular nuclei of the rat brain stem by a 9.4 T magnetic field. NeuroReport 11:2681–2685
Somana R, Walberg F (1979) Cerebellar afferents from the nucleus of the solitary tract. Neurosci Lett 11:41–47
Sugiyama Y, Suzuki T, DeStefino VJ, Yates BJ (2011) Integrative responses of neurons in nucleus tractus solitarius to visceral afferent stimulation and vestibular stimulation in vertical planes. Am J Physiol Regul Integr Comp Physiol 301:R1380–R1390
Suzuki T, Sugiyama Y, Yates BJ (2012) Integrative responses of neurons in parabrachial nuclei to a nauseogenic gastrointestinal stimulus and vestibular stimulation in vertical planes. Am J Physiol Regul Integr Comp Physiol 302:R965–R975
Takeuchi Y, McLean JH, Hopkins DA (1982) Reciprocal connections between the amygdala and parabrachial nuclei: ultrastructural demonstration by degeneration and axonal transport of horseradish peroxidase in the cat. Brain Res 239:583–588
Thornton WE, Bonato F (2013) Space motion sickness and motion sickness: symptoms and etiology. Aviat Space Environ Med 84:716–721
Tong G, Robertson LT, Brons J (1993) Climbing fiber representation of the renal afferent nerve in the vermal cortex of the cat cerebellum. Brain Res 601:65–75
Treisman M (1977) Motion sickness: an evolutionary hypothesis. Science 197:493–495
Tyler DB, Bard P (1949) Motion sickness. Physiol Rev 29:311–369
Ugolini G (2008) Use of rabies virus as a transneuronal tracer of neuronal connections: implications for the understanding of rabies pathogenesis. Dev Biol (Basel) 131:493–506
Wang SC, Borison HL (1951) The vomiting center; its destruction by radon implantation in dog medulla oblongata. Am J Physiol 166:712–717
Wang SC, Chinn HI (1956) Experimental motion sickness in dogs; importance of labyrinth and vestibular cerebellum. Am J Physiol 185:617–623
Wang SC, Chinn HI, Renzi AA (1957) Experimental motion sickness in dogs: role of abdominal visceral afferents. Am J Physiol 190:578–580
Watson JW, Gonsalves SF, Fossa AA, McLean S, Seeger T, Obach S, Andrews PL (1995) The anti-emetic effects of CP-99,994 in the ferret and the dog: role of the NK1 receptor. British J Pharmacol 115:84–94
Welzl H, D’Adamo P, Lipp HP (2001) Conditioned taste aversion as a learning and memory paradigm. Behav Brain Res 125:205–213
Wilpizeski CR, Lowry LD, Goldman WS (1986) Motion-induced sickness following bilateral ablation of area postrema in squirrel monkeys. Laryngoscope 96:1221–1225
Yamamoto T, Sawa K (2000) c-Fos-like immunoreactivity in the brainstem following gastric loads of various chemical solutions in rats. Brain Res 866:135–143
Yamamoto T, Shimura T, Sako N, Azuma S, Bai WZ, Wakisaka S (1992) C-fos expression in the rat brain after intraperitoneal injection of lithium chloride. NeuroReport 3:1049–1052
Yamamoto T, Shimura T, Sako N, Yasoshima Y, Sakai N (1994) Neural substrates for conditioned taste aversion in the rat. Behav Brain Res 65:123–137
Yates BJ, Grelot L, Kerman IA, Balaban CD, Jakus J, Miller AD (1994) Organization of vestibular inputs to nucleus tractus solitarius and adjacent structures in cat brain stem. Am J Physiol 267:R974–R983
Yates BJ, Balaban CD, Miller AD, Endo K, Yamaguchi Y (1995) Vestibular inputs to the lateral tegmental field of the cat: potential role in autonomic control. Brain Res 689:197–206
Yates BJ, Miller AD, Lucot JB (1998) Physiological basis and pharmacology of motion sickness: an update. Brain Res Bull 47:395–406
Yates BJ, Smail JA, Stocker SD, Card JP (1999) Transneuronal tracing of neural pathways controlling activity of diaphragm motoneurons in the ferret. Neurosci 90:1501–1513
Zheng ZH, Dietrichs E, Walberg F (1982) Cerebellar afferent fibres from the dorsal motor vagal nucleus in the cat. Neurosci Lett 32:113–118
Acknowledgments
Funding was provided by Grant R01-DC003732 from the National Institutes of Health (USA). Michael Catanzaro was supported by an American Physiological Society Undergraduate Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yates, B.J., Catanzaro, M.F., Miller, D.J. et al. Integration of vestibular and emetic gastrointestinal signals that produce nausea and vomiting: potential contributions to motion sickness. Exp Brain Res 232, 2455–2469 (2014). https://doi.org/10.1007/s00221-014-3937-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-014-3937-6