Abstract
Peripheral inflammation and nerve injury usually accompany each other. However, whether inflammatory and neuropathic pain share similar mechanisms at all stages is unknown. TRPV1 and P2X3 are two major ion channels in dorsal root ganglia (DRGs) and are involved in chronic pain. Here, their function and expression in DRGs at different phases of the two types of pain were investigated. Both the paw withdrawal threshold (PWT) and paw withdrawal latency were decreased in rats injected with complete Freud’s adjuvant (CFA). However, only the PWT was decreased in rats with spared nerve injury (SNI). CFA increased the magnitude of the TRPV1-mediated Ca2+ response but not the P2X3-mediated Ca2+ response 14 days after injection. Consistent with this result, the P2X3 expression level in CFA rats was increased only at 3 days after injection. SNI surgery increased the magnitudes of the TRPV1- and P2X3-mediated Ca2+ responses and upregulated both TRPV1 and P2X3 expression in lumbar DRGs. The distributions of TRPV1 and P2X3 in DRGs after modeling were observed, and TRPV1 was found to be highly expressed mainly in the L4–L5 DRGs in CFA rats and in the L5–L6 DRGs in SNI rats. P2X3 was highly expressed in the L4–L6 DRGs in CFA rats 3 days after injection but was only highly expressed in the L4 DRG 14 days after modeling. On the other hand, SNI promoted the P2X3 expression L4–L5 DRGs 3 days after surgery, but only L6 DRG 14 days after modeling. All the results indicate that P2X3 and TPRV1 are involved in inflammatory and neuropathic pain by different expression levels and distributions in the lumbar DRG in the chronic stage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Generally, a peripheral injury can induce chronic pain, which is usually characterized by pain that persists even when damage is repaired. Chronic pain is one of the biggest threats to quality of live in humans. More than one-fifth of the world’s population, particularly the elderly, suffers from chronic pain (Patel et al. 2013). Based on pathology, chronic pain is divided into two major types: inflammatory pain, and neuropathic pain. The treatment of inflammatory and neuropathic pain remains a significant clinical challenge due to the poor understanding of the pathological mechanisms underlying these types of pain (Cruccu and Truini 2017; Yezierski and Hansson 2018). Furthermore, clearly distinguishing these types of chronic pain and identifying specific treatment for them is challenges to clinical practice. In most cases, swelling may injure nerves (Büttner et al. 2018), and nerve injuries may also accompany inflammation (Sommer et al. 2018). Therefore, finding a way to cure them simultaneously may be necessary. However, little is known about whether inflammatory and neuropathic pain shares similar or the same mechanism throughout their stages of development.
According to previous studies, many receptors are involved in both inflammatory pain and neuropathic pain in the peripheral nervous system. Transient receptor potential cation channel subfamily V member 1 (TRPV1) is a critical nonselective cation channel belonging to the TRP family and is expressed in both the central and peripheral terminals of sensory neurons (Premkumar 2014). A growing amount of evidence suggests that TRPV1 expressed in primary sensory neurons is responsible for mediating nociception in various pain pathologies (Brandt et al. 2012; Hung and Tan 2018). In the periphery, TRPV1 is highly expressed in the dorsal root ganglia (DRGs) after inflammation and nerve injury (Kan et al. 2018; Sondermann et al. 2019). Reducing the expression or blocking the activation of TRPV1 in primary sensory neurons via siRNA transfection, pharmacological intervention, or other methods can dramatically alleviate inflammatory and neuropathic pain (Warwick et al. 2019; Xiang et al. 2017). These findings support the notion that TRPV1 is a potential therapeutic target for chronic pain.
P2X purinoceptor 3 (P2X3) is one of the primary receptors involved in the transmission of nociception information by primary sensory neurons (Burnstock 2000; Magni and Ceruti 2014). P2X3 belongs to the family of ATP purinoceptors. Previous studies have reported that most members of the P2X family can be detected in DRGs (Bernier et al. 2018). Moreover, numerous studies have suggested that P2X3 expressed in peripheral neurons contributes mainly to peripheral pain responses (Teixeira et al. 2017; Zhang et al. 2018; Zhou et al. 2015). Peripheral nerve injuries and inflammation increase the expression of P2X3 in DRGs (Teixeira et al. 2017; Xiang et al. 2008). Local or intra-DRG injection of P2X3 antagonists can produce analgesic effects in animals with both inflammatory and neuropathic pain (Teixeira et al. 2017; Xiong et al. 2017).
However, there are still some discrepancies regarding the pivotal roles that P2X3 and TRPV1 play in chronic pain. Various studies have reported that P2X3 expression increases, decreases, and remains unchanged in the context of pathological pain (Chen et al. 2016; Fukuoka et al. 2002; Kage et al. 2002), and TRPV1 is also variably expressed in the L4–L6 DRGs after peripheral injury (Hudson et al. 2001; Schafers et al. 2003). An interesting question is whether P2X3 expression and TRPV1 expression in the L4–L6 DRGs play different roles in inflammatory and neuropathic pain.
Here, we hypothesized that P2X3 and TRPV1 may play different roles in inflammatory and neuropathic pain and investigated P2X3 and TRPV1 expression in the early phase (3 d) and the chronic phase (14 d) of the two types of pain. We also investigated the magnitudes of P2X3- and TRPV1-mediated Ca2+ responses in DRG neurons. In addition, the expression levels of P2X3 and TRPV1 in the L4, L5 and L6 DRGs were analyzed.
Materials and methods
Animals
Adult male Sprague–Dawley rats (weighing 180–230 g) (animal certificate no. SCXK(Hu)2013–0016, Shanghai Laboratory Animal Center, Chinese Academy of Sciences) were used for this study. The animals were housed five per cage in the laboratory animal center of Zhejiang Chinese Medical University (SYXK(浙)2013–0184) with access to food and water ad libitum in a controlled environment under a 12-h light/dark cycle with a temperature of 25 ± 2 ℃ and a humidity of 55 ± 5%. During the study, all animal manipulation complied with institutional and Chinese governmental regulations regarding the ethical use of animals (approval no. IACUC-20180319–12).
Spared nerve injury
Spared nerve injury (SNI) was used to induce neuropathic pain, as described below. To establish SNI, the rats were first anesthetized by inhalation of 2% isoflurane (Lunambert, China). The left sciatic nerve and its terminal branches were exposed. The common peroneal and tibial nerves were tightly ligated with a 5.0 silk thread and sectioned distal to the ligation by removing 2–4 mm of the nerve stump. Adequate care was taken to avoid any contact with or stretching of the sural nerve. Both the muscle and skin were sutured. Only animals that developed mechanical allodynia were used. For sham-operated rats, the left sciatic nerve and its trifurcation were exposed but not manipulated. After the animals woke up from anesthesia, they were returned to their cages. All rats were administered penicillin (80 U, intramuscularly, Shanghai Yuanye Bio-Technology Co., Ltd., China) for 3 days to prevent bacterial infection.
Peripheral inflammation
Peripheral inflammation was induced by intraplantar injection of complete Freund’s adjuvant (CFA, Sigma-Aldrich, USA). Before CFA injection, the rats were anesthetized by inhalation of 2% isoflurane. Then, the rats received subcutaneous injections of CFA (0.1 ml) into the plantar surface of the left hind paw. For normal rats, 0.1 ml of saline was subcutaneously injected into the plantar surface of the left hind paw.
Paw withdrawal threshold
The paw withdrawal threshold (PWT) was measured by the 50% PWT method as previously described (Chaplan et al. 1994). The rats were placed in transparent organic glass boxes (20 cm × 20 cm × 15 cm) on an elevated metal floor until they adapted. Then, 0.4-, 0.6-, 1-, 2-, 4-, 6-, 8-, 15-, and 26-g Von Frey filaments (Stoelting, IL, USA) were pressed on the lateral plantar surface (sural nerve area) of the hind paw for approximately 6–8 s. The first filament applied corresponded to a force of 2 g. If a negative response (no movement or paw withdrawal) was observed, recorded as O, then a filament exerting a greater force was applied next. If a positive reaction (paw withdrawal or escape) was induced, recorded as X, then a filament of a lesser force was used next. After the first “OX” or “XO” sequence was obtained, another four “O” or “X” responses were obtained, and the 50% PWT was calculated on the basis of the “O” and “X” sequences.
Paw withdrawal latency
To measure the paw withdrawal latency (PWL), radiant heat from a test instrument (37370, Ugo Basile, Italy) was aimed at the lateral plantar surface of the hind paw after the rats adapted to the test chamber, and the elapsed time was recorded as the PWL. The radiant heat was set to 35 °C, and the cutoff time was 20 s. PWL testing was repeated three times with 5-min intervals at each time point, and the mean of these values was used for further calculations.
Western blot analysis
Rats were anesthetized with 2% pentobarbital sodium (Absin, China) and euthanized 3 days or 14 days after modeling. They were quickly perfused with 150 ml of 0.9% NaCl (4 °C). Then, the L4–L6 DRGs were extracted rapidly and stored in a −80 °C freezer for western blot analysis.
RIPA lysis buffer (Beyotime, China) containing 1% PMSF (Beyotime, China) and a protease/phosphatase inhibitor cocktail (Applygen, China) was used to extract the total protein from L4–L6 DRGs. The protein concentration was detected with a BCA protein assay kit. Protein samples (20 μg) were separated on 8% SDS-PAGE gels and electrophoretically transferred to polyvinyl difluoride (PVDF) membranes (Bio-Rad, USA). The membranes were incubated in 5% low-fat milk in TBST for 1 h at room temperature, with a rabbit anti-P2X3 antibody (1:1000 in 5% normal donkey serum, Abcam, USA) and an anti-TRPV1 antibody (1:1000 in 5% normal donkey serum, Abcam, USA) overnight at 4 °C, and with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:10,000, Abcam, USA) for 1 h at room temperature. A rabbit anti-GAPDH antibody (HRP-conjugated) (1:1000, CST, USA) was used as the internal control. The membranes were developed with an ECL kit (Pierce, USA), and the signals were captured with an ImageQuant LAS 4000 system (GE, USA). The density of each band was measured using ImageQuant TL 7.0 analysis software (GE, USA). The mean expression level of the target protein in the normal or sham SNI group was considered to be 1, and the relative expression level of the target protein in all animals was normalized to the level in the normal or sham SNI group.
Immunofluorescence
Rats were anesthetized with 2% pentobarbital sodium and euthanized 3 days or 14 days after modeling. They were quickly perfused with 150 ml of 0.9% NaCl (4 °C) followed by 400 ml of fresh 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). Then, the L4, L5, and L6 DRGs were removed and postfixed in the same fixative for 3 h at 4 °C before serial transfer to 15% and 30% sucrose for dehydration.
The DRGs were sectioned into 14-μm slices. The slices were blocked with 5% normal donkey serum in TBST (1% Tween-20) for 1 h at 37 °C and then incubated with a rabbit anti-P2X3 antibody (1:1000 in 5% normal donkey serum, Abcam, USA) and a rabbit anti-TRPV1 antibody (1:1000 in 5% normal donkey serum, Abcam, USA) overnight at 4 °C. The slices were then incubated in fluorescein isothiocyanate (FITC)-conjugated AffiniPure donkey anti-rat IgG (H + L) (Jackson, USA) for 1 h at 37 °C. Images were captured using an A1R confocal microscope (Nikon, Tokyo, Japan) to visualize the expression of P2X3 and TRPV1 in the L4, L5, and L6 DRGs. We used Image-Pro Plus 6.0 to analyze the results. Five slices from each segment of the L4–L6 DRGs were randomly selected for analysis. Characteristics such as the shape, area, and fluorescence intensity were assessed through negotiation by two experimenters who were blinded to the study groups. Briefly, a nearly circular area with increased fluorescence intensity was considered a neuron. Then, the positive cells in each slice were counted, and the diameter of each neuron was calculated by the software.
DRG culture
DRG neurons were obtained from 6- to 8-week-old rats and short-term cultured. The rats were anesthetized and decapitated. The ipsilateral DRGs were harvested from the L4–L6 level and temporarily placed in cold DMEM. Then, the DRGs were trimmed and placed in a dissociation enzyme solution (1 mg/ml collagenase II (Sigma-Aldrich) and 0.5 mg/ml trypsin (Sigma-Aldrich) in DMEM). After 30 min of digestion at 37 °C, the neurons were mechanically dissociated and filtered. Then, the cells were placed on poly-d-lysine/laminin-coated glass coverslips. The medium contained equal amounts of DMEM and F12 (Gibco, Grand Island, NY, USA), 10% FCS (Gibco) and 1% penicillin and streptomycin (Invitrogen). The cultured neurons were used for Ca2+ imaging 1 h after isolation.
Ca2+ imaging
Ca2+ imaging was performed on small- and medium-diameter DRG neurons. Briefly, DRG neurons were loaded with 1 μM Fura 2-acetoxymethyl ester (Fura 2-AM, Invitrogen) for approximately 45 min at 37 °C in a 5% CO2 humidified incubator. Then, the DRG neurons were washed twice for at least 20 min per wash to remove the extracellular dye from the DMEM. The imaging bath solution consisted of the following (in mM): 130.0 NaCl, 3.0 KCl, 0.6 MgCl2, 2.0 CaCl2, 1.0 NaHCO3, 5.0 glucose, and 10.0 HEPES. The pH was maintained with NaOH at 7.4, and the osmolarity was adjusted to 300–320 mOsm with sucrose.
Ratiometric Ca2+ imaging was performed at a room temperature of 20–22 °C using an inverted Nikon A1 microscope equipped with a DG4 lamp source (Sutter). The neurons were illuminated at wavelengths of 340 nm and 380 nm by the source (the 340- and 380-nm filters were automatically controlled by the lamp source), and the signals at 340-nm and 380-nm excitation were captured at 2-s intervals using a cooled charge-coupled device (CCD) camera (Flash 4.0, Hamamatsu, Japan) controlled by a computer with MetaFluor software. The ratio of 340-nm/380-nm fluorescence intensity (R340/380) within a certain region of interest was used as a relative measure of the intracellular Ca2+ concentration. Capsaicin (CAP, 3 μM) and αβ-meATP (10 μM) were dissolved in the bath solution and applied to the neurons with a solution perfusion system. K+ (50 mM) was applied to distinguish neurons from non-neuronal cells and confirm the viability of the neurons at the end of each experiment. Neurons were considered to respond to a chemical if the R340/380 increased to at least 15% above baseline. Only the neurons that responded to chemical stimulation were used for calculation.
Experimental design
The rats were randomly divided into four groups: the normal (n = 16), CFA (n = 32), sham SNI (n = 27) and SNI groups (n = 32). In the CFA and SNI groups, five randomly selected rats were used for Ca2+ imaging, five randomly selected rats were used for immunofluorescence analysis, and six randomly selected rats were used for western blot analysis at each time point. In the normal group, five randomly selected rats were used for Ca2+ imaging, five randomly selected rats were used for immunofluorescence analysis, and six randomly selected rats were used for western blot analysis. In the sham SNI group, 5 randomly selected rats were used for Ca2+ imaging, 10 randomly selected rats were used for immunofluorescence, and 12 randomly selected rats were used for western blot analysis.
Statistical methods
All data are expressed as the means ± standard errors (SEMs). One-way ANOVA for independent samples followed by Bonferroni post hoc test was used to analyze differences among three or more groups. Independent Student’s t tests were used to analyze differences between the two groups. P < 0.05 was considered to indicate statistical significance.
Results
Peripheral inflammation and nerve injury produce different pain responses
The changes in mechanical and thermal pain thresholds are shown in Fig. 1. Peripheral inflammation significantly decreased the PWT of the rats (P < 0.01, Fig. 1a) as well as the PWL (P < 0.01, Fig. 1b) 3 days and 14 days after CFA injection. In addition, SNI surgery significantly decreased the PWT (P < 0.01, Fig. 1c) but not the PWL (P > 0.05, Fig. 1d) of the rats 3 days and 14 days after the operation. These results indicated that peripheral inflammation induced both mechanical allodynia and thermal hyperalgesia. However, SNI surgery induced only mechanical allodynia.
CFA injection produced chronic mechanical and thermal pain, while SNI surgery induced only chronic mechanical pain. PWTs (a) and PWLs (b) of animals that received CFA injections. PWTs (c) and PWLs (d) of animals that underwent SNI surgery. n = 10. **: compared with the normal group, P < 0.01; ##: compared with the sham SNI group, P < 0.01
CFA and SNI increased the magnitude of TRPV1- and P2X3-mediated Ca2+ responses in DRG neurons
The local application of CAP to the cell bodies significantly increased [Ca2+]i in DRGs in the four different groups (Fig. 2). We also applied KCl to the cell bodies to raise the [Ca2+]i (Fig. 2a-f). Only the neurons that responded to both CAP and KCl were analyzed further. The proportion of neurons that responded to CAP was much higher in CFA rats than in normal rats 3 days and 14 days after injection (Fig. 2g), and CFA increased the magnitude of the CAP-evoked Ca2+ response 3 days and 14 days after injection (P < 0.01, Fig. 2h). SNI surgery also increased the proportion of neurons that responded to CAP 3 days and 14 days after surgery (Fig. 2i). Furthermore, the magnitude of the CAP-evoked Ca2+ response was significantly increased at the same time points (P < 0.01, Fig. 2j).
CFA injection and SNI surgery increased the incidence and magnitude of TRPV1-mediated Ca2+ responses in DRG neurons. DRG neurons isolated from rats in the normal (a), 3-day CFA (b), 14-day CFA (c), sham SNI (d), 3-day SNI (e) and 14-day SNI (f) groups were exposed to CAP (3 μM) followed by KCl (50 mM) and analyzed by Fura 2-AM ratiometric Ca2+ imaging (representative traces are shown). The black bars above the traces show the chemicals applied. Proportion of CAP-responsive DRG neurons (g) and the mean magnitude of TRPV1-mediated Ca2+ responses (h) in CFA rats. Proportion of CAP-responsive DRG neurons (i) and the mean magnitude of TRPV1-mediated Ca2+ responses (j) in SNI rats. A total of 50–75 neurons from five rats were analyzed per group. **: compared with the normal group, P < 0.01; ##: compared with the sham SNI group, P < 0.01
Local application of αβ-meATP to the cell bodies significantly increased [Ca2+]i in DRGs in normal, CFA, sham SNI and SNI rats (Fig. 3). As described above, only the neurons that reacted to both αβ-meATP and KCl were analyzed (Fig. 3a-f). At 3 d, the proportion of neurons that reacted to αβ-meATP was much higher in CFA rats than in normal rats (Fig. 3g). However, there was little difference in the percentage of responding neurons between the CFA group and the normal group 14 days after injection. Furthermore, the mean magnitude of the αβ-meATP-evoked Ca2+ response was significantly higher in neurons from CFA rats than in neurons from normal rats 3 days after injection (P < 0.01, Fig. 3h). In addition, there was no significant difference in the average magnitude between the CFA group and the normal group 14 days after injection (P > 0.05, Fig. 3h). SNI surgery also increased the proportion of neurons that reacted to αβ-meATP (Fig. 3i). Compared to CFA, SNI induced a higher percentage of neurons to respond to the agonist both 3 days and 14 days after surgery. Moreover, the mean magnitude of the αβ-meATP-evoked Ca2+ response was higher in neurons from SNI rats than in neurons from sham SNI rats 3 days and 14 days after surgery (P < 0.01, Fig. 3j).
CFA injection and SNI surgery increased the incidence and magnitude of P2X3-mediated Ca2+ responses in DRG neurons. DRG neurons isolated from rats in the normal (a), 3-day CFA (b), 14-day CFA (c), sham SNI (d), 3-day SNI (e) and 14-day SNI (f) groups were exposed to αβ-meATP (10 μM) followed by KCl (50 mM) and analyzed by Fura 2-AM ratiometric Ca2+ imaging (representative traces are shown). The black bars above the traces show the chemicals applied. Proportion of αβ-meATP-responsive DRG neurons (g) and the mean magnitude of P2X3-mediated Ca2+ responses (h) in CFA rats. Proportion of αβ-meATP-responsive DRG neurons (i) and the mean magnitude of P2X3-mediated Ca2+ responses (j) in SNI rats. A total of 50–75 neurons from five rats were analyzed per group. **: compared with the normal group, P < 0.01; #: compared with the sham SNI group, P < 0.05; ##: compared with the sham SNI group, P < 0.01
All the above data indicate that CFA and SNI changed the Ca2+ influx induced by TRPV1 and P2X3 in the peripheral nervous system. Moreover, the findings suggest that inflammatory and neuropathic pain may not share similar mechanisms in the chronic stage. Therefore, we further investigated the expression of P2X3 and TRPV1 in lumbar DRGs.
CFA and SNI induced different TRPV1 and P2X3 expressions in DRGs
The expression levels of TRPV1 and P2X3 in the L4–L6 DRGs were investigated by western blotting. Peripheral inflammation increased the expression of both TRPV1 and P2X3 3 days after CFA injection (P < 0.05, Fig. 4a-c). Inflammation induced high expression of TRPV1 (P < 0.05, Fig. 4b) but did not affect the expression of P2X3 14 days after CFA injection (P > 0.05, Fig. 4c). Peripheral nerve injury also upregulated TRPV1 and P2X3 expressions in the L4–L6 DRGs 3 days after the operation (P < 0.05, Fig. 4d-f). SNI increased the expression of both TRPV1 and P2X3 in the L4–L6 DRGs 14 days after surgery (P < 0.05, Fig. 4e-f).
CFA injection and SNI surgery increased the expression levels of TRPV1 and P2X3 in DRGs. a Representative western blot showing TRPV1 and P2X3 proteins isolated from DRGs 3 days and 14 days after CFA injection. Quantification results of the western blots of TRPV1 (b) and P2X3 (c) proteins isolated from the DRGs 3 days and 14 days after CFA injection. d Representative western blot showing TRPV1 and P2X3 proteins isolated from DRGs 3 days and 14 days after SNI surgery. Quantification results of the western blots of TRPV1 (e) and P2X3 (f) proteins isolated from the DRG 3 days and 14 days after SNI surgery. n = 6. *: compared with the normal group, P < 0.05; **: compared with the normal group, P < 0.01; #: compared with the sham SNI group, P < 0.05
TRPV1-positive cells in the L4–L6 DRGs of CFA and SNI rats
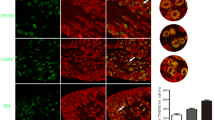
Peripheral inflammation and nerve injury both increased the numbers of TRPV1-immunoreactive (IR) cells in DRGs (P < 0.05, Fig. 5a, b, e). The numbers of TRPV1-IR cells were slightly downregulated over time in the L4–L6 DRGs in both CFA and SNI rats (Fig. 5b, e). Furthermore, CFA significantly increased the numbers of TRPV1-IR cells in the L4 and L5 DRGs from 3 to 14 days after injection (P < 0.05, Fig. 5c). However, CFA increased the number of TRPV1-IR cells in the L6 DRG only 3 days after injection (P < 0.05, Fig. 5c); it failed to affect the number of TRPV1-IR cells in the L6 DRG 14 days after injection (P > 0.05, Fig. 5c). The numbers of TRPV1-IR cells in the L5 and L6 DRGs from 3 to 14 days after surgery were greater in the SNI group than in the sham SNI group (P < 0.05, Fig. 5f). However, the operation only minimally affected the number of positive cells in the L4 DRG at any time point in this study (P > 0.05, Fig. 5f). Finally, TRPV1 was expressed mainly in neurons that were less than 299 pixels in area regardless of the experimental group. Peripheral inflammation and nerve injury did not change this observation (Fig. 5d, g).
CFA injection and SNI surgery increased the numbers of TRPV1-positive neurons in DRGs. a TRPV1 staining in the peripheral nervous system 3 days and 14 days after CFA injection or SNI surgery. Scale bar: 100 mm. b Quantification of TRPV1-IR neurons after CFA injection. c Quantification of TRPV1-IR neurons in the L4, L5 and L6 DRGs after CFA injection. d Areas of TRPV1-IR neurons in normal and CFA rats. e Quantification of TRPV1-IR neurons after SNI surgery. f Quantification of TRPV1-IR neurons in the L4, L5 and L6 DRGs after SNI surgery. g Areas of TRPV1-IR neurons in sham SNI and SNI rats. n = 5. *: compared with the normal group, P < 0.05; **: compared with the normal group, P < 0.01; ##: compared with the sham SNI group, P < 0.01
A specific antagonist of TRPV1, capsazepine (CPZ), was next used to reverse the PWT changes in CFA and SNI rats 14 days after injection or operation. Subcutaneous application of 0.4 mg/25 μl CPZ markedly reversed the PWT alterations in CFA and SNI rats (P < 0.01, Supplementary Fig. 1a).
P2X3-positive cells in the L4–6 DRGs of CFA and SNI rats
Peripheral inflammation significantly increased the numbers of P2X3-IR cells in the L4–L6 DRGs 3 days after CFA injection but not 14 days after CFA injection (P > 0.05, Fig. 6a, b). However, peripheral nerve injury increased the numbers of positive cells in the L4–L6 DRGs from 3 to 14 days after the operation; after 14 d, the numbers gradually decreased (P < 0.05, Fig. 6a, e). CFA significantly increased the numbers of P2X3-IR cells in the L4, L5 and L6 DRGs 3 days after injection (P < 0.05, Fig. 6c) but considerably increased the number of P2X3-IR cells only in the L4 DRG 14 days after injection (P < 0.05, Fig. 6c). The numbers of P2X3-IR cells in the L4 and L5 DRGs in the SNI group were significantly higher than those in the sham SNI group 3 days after the SNI operation (P < 0.05, Fig. 6f). However, there was no significant difference in the number of P2X3-IR cells in the L6 DRG between the two groups at the same time point (P > 0.05, Fig. 6F). In addition, peripheral nerve injury only significantly increased the number of P2X3-IR cells in the L6 DRG 14 days after the operation (P < 0.01, Fig. 6f). There were minimal differences in the numbers of P2X3-IR cells in the L4 and L5 DRGs between the sham and SNI groups (P > 0.05, Fig. 6f). Finally, P2X3, similar to TRPV1, was expressed mainly in neurons with areas less than 299 pixels (Fig. 6d, g).
CFA injection and SNI surgery increased the numbers of P2X3-positive neurons in DRGs. a P2X3 staining in the peripheral nervous system 3 days and 14 days after CFA injection or SNI surgery. Scale bar: 100 mm. b Quantification of P2X3-IR neurons after CFA injection. c Quantification of P2X3-IR neurons in the L4, L5 and L6 DRGs after CFA injection. d Areas of P2X3-IR neurons in normal and CFA rats. e Quantification of P2X3-IR neurons after SNI surgery. f Quantification of P2X3-IR neurons in the L4, L5 and L6 DRGs after SNI surgery. g Areas of P2X3-IR neurons in sham SNI and SNI rats. n = 5. * compared with the normal group, P < 0.05; **: compared with the normal group, P < 0.01; Δ: compared with the CFA 3-day group, P < 0.01; #: compared with the sham SNI group, P < 0.01; ##: compared with the sham SNI group, P < 0.01
A specific antagonist of P2X3, TNP-ATP, was used to reverse the PWT changes in CFA and SNI rats 14 days after injection or surgery. Subcutaneous application of 250 nmol/25 μl TNP-ATP markedly reversed the PWT alterations in SNI rats and CFA rats (both P < 0.01, Supplementary Fig. 1b). These effects may have been due to involvement of P2X3 in the normal pain perception of rats. We will further investigate this possibility in future studies.
Discussion
The results obtained in this work indicate that although inflammation and nerve injury often accompany each other, they may not share a similar mechanism in the peripheral nervous system in some cases. Peripheral inflammation and nerve injury may induce pain through the same mechanism, such as via high expression of P2X3 and TRPV1 in primary sensory neurons in the early phase. However, the mechanisms of inflammatory and neuropathic pain might be slightly different in the chronic stage. In this study, TRPV1 was found to be involved in both the maintenance and development of inflammatory and neuropathic pain in peripheral nerves. However, P2X3 did not appear to contribute to the maintenance of chronic inflammatory pain in primary sensory neurons.
CFA and SNI models were chosen to induce inflammatory pain and neuropathic pain, respectively, in mammals in this study. CFA is a classic chronic inflammatory pain model inducer that is able to elicit mechanical allodynia and thermal hyperalgesia lasting more than 14 days (Liu et al. 2017). SNI is well established as an inducer of animal models of neuropathic pain that only minimally affects sympathetic nerves and can produce profound and reliable pain behaviors (Straube et al. 2013). Here, the SNI model was chosen not only because it is a classic and easily established neuropathic pain model but also because it can elicit considerable mechanical allodynia without inducing thermal hyperalgesia (Shields et al. 2003). In this study, CFA decreased the PWT and PWL 3–14 days after injection, and SNI decreased the PWT without affecting the PWL at the same time points after surgery. These results are consistent with previous reports (Liu et al. 2017; Shields et al. 2003).
Since the two models induce thermal pain with distinct features but mechanical pain with similar characteristics, TRPV1 was chosen for investigation based on its functions in primary sensory neurons. TRPV1 is an ion channel that contributes mainly to allodynia and hyperalgesia (Gonzalez-Rodriguez 2019). It is generally believed that TRPV1 increases sensitivity and decreases the pain threshold associated with thermal hyperalgesia to below pathological levels. High expression of TRPV1 in the peripheral nervous system is involved in various types of pathological pain, including inflammatory pain caused by formalin, CFA or carrageenan and neuropathic pain caused by diabetic peripheral nerve damage diabetic or paclitaxel injection (Fattori et al. 2019; Hirai et al. 2014; Kwon et al. 2014; Liu et al. 2018; Luo et al. 2018). In the current study, CFA enhanced TRPV1-evoked Ca2+ responses and increased TRPV1 expression in the L4–L6 DRGs 3–14 days after treatment while significantly decreasing the threshold of thermal hyperalgesia, consistent with the findings of previous studies (Leisengang et al. 2020; Li et al. 2019). Interestingly, SNI promoted TRPV1-evoked Ca2+ responses and TRPV1 expression in peripheral nerves without changing the threshold of thermal hyperalgesia. Previous studies have indicated that regulation of the expression of this protein even in only one DRG segment, such as L4 or L5, is enough to change the pain threshold in mammals (Du et al. 2017; Souza et al. 2013). Therefore, we hypothesized that TRPV1 plays an important role in hyperalgesia in CFA and SNI rats. However, we did not observe elevated expression of TRPV1 in the L4 DRGs of SNI rats. Whether the lack of change in TRPV1 expression was related to a lack of alteration in the thermal threshold needs further study.
Since CFA and SNI induced similar changes in TRPV1 expression, another ion channel receptor was selected for observation. P2X3 is localized predominantly in peripheral primary sensory neurons and is activated by the ligand ATP. Although it was originally discovered as the main chemical energy substance, the nucleotide ATP is also known to be an essential extracellular signaling molecule for nociception as well as various types of chronic pain (Burnstock 2016; North 2016). P2X3 is expressed mainly in non-peptide-containing small-to-medium-diameter C- and Aδ-fiber sensory neurons and contributes strongly to persistent pain generation (Chen et al. 2005). Many studies have indicated that P2X3, like TRPV1, might be involved in multiple types of chronic pain (Jung et al. 2017; Nunez-Badinez et al. 2018; Wu et al. 2016). However, we found that peripheral inflammation promoted P2X3 expression and P2X3-induced Ca2+ responses in L4–L6 DRGs only over a short period. In contrast, SNI surgery increased P2X3 expression and P2X3-evoked Ca2+ responses in DRG neurons over a long period of time (at least 14 d). Although significant differences in the numbers of P2X3-positive cells in the L4–L5 DRGs were not observed at the 14-day time point, the lack of significance may have been due to the large SDs. Given that the average numbers of P2X3-positive cells were strongly elevated, we hypothesize that P2X3 plays pivotal roles in all phases of neuropathic pain. In CFA rats, although high expression of P2X3 was only observed in the L4–L5 DRGs 14 days after the operation, the P2X3 antagonist still partly reversed the hyperalgesia. It is not clear whether this effect was due to the high expression of P2X3 in the L4 DRG or to the role of P2X3 in normal pain sensation. Based on the results for total protein expression, total P2X3-positive cell numbers and Ca2+ imaging, we hypothesize that P2X3 may not play an important role in the chronic phase of CFA-induced inflammatory pain. Therefore, regarding P2X3 and TRPV1 expression, neuropathic pain and inflammatory pain might have similar mechanisms in the early phase but different mechanisms in the chronic stage.
An interesting phenomenon was observed in this study. Although high expression of TRPV1 was observed in DRGs in the contexts of inflammatory and neuropathic pain, SNI did not produce a noticeable change in the thermal pain threshold. Sensitization and increased expression of TRPV1 are two of the primary mechanisms associated with thermal pain, regardless of whether the pain is due to inflammation or nerve injury (Hung and Tan 2018; Viana 2018). We still do not fully understand the reason for this phenomenon but will investigate it in future studies.
Conclusion
P2X3 and TRPV1 are expressed in the peripheral nervous system and are both involved in inflammatory and neuropathic pain. Based on changes in the timing and distribution of the expression of these proteins, inflammatory and neuropathic pain may share similar neuronal mechanisms during the early phase. However, in the chronic phase, inflammatory and neuropathic pain might be maintained by different neuronal mechanisms.
Code availability
Not applicable.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- P2X3:
-
P2X purinoceptor 3
- DRG:
-
Dorsal root ganglion
- TRPV1:
-
Transient receptor potential cation channel subfamily V member 1
- CAP:
-
Capsaicin
- SNI:
-
Spared nerve injury
- CFA:
-
Complete Freund’s adjuvant
- PWT:
-
Paw withdrawal threshold
- PWL:
-
Paw withdrawal latency
- CPZ:
-
Capsazepine
References
Bernier LP, Ase AR, Seguela P (2018) P2X receptor channels in chronic pain pathways. Br J Pharmacol 175:219–230. https://doi.org/10.1111/bph.13957
Brandt MR, Beyer CE, Stahl SM (2012) TRPV1 Antagonists and chronic pain: beyond thermal perception. Pharmaceuticals (Basel, Switzerland) 5:114–132. https://doi.org/10.3390/ph5020114
Burnstock G (2000) P2X receptors in sensory neurons. Br J Anaest 84:476–488
Burnstock G (2016) Purinergic mechanisms and pain. Adv pharmacol (San Diego, Calif) 75:91–137. https://doi.org/10.1016/bs.apha.2015.09.001
Büttner R et al (2018) Inflammaging impairs peripheral nerve maintenance and regeneration. Aging Cell 17:e12833–e12833. https://doi.org/10.1111/acel.12833
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63. https://doi.org/10.1016/0165-0270(94)90144-9
Chen Y, Li GW, Wang C, Gu Y, Huang LY (2005) Mechanisms underlying enhanced P2X receptor-mediated responses in the neuropathic pain state. Pain 119:38–48. https://doi.org/10.1016/j.pain.2005.09.007
Chen L et al (2016) Differential expression of ATP-gated P2X receptors in DRG between chronic neuropathic pain and visceralgia rat models. Purinergic Signal 12:79–87. https://doi.org/10.1007/s11302-015-9481-4
Cruccu G, Truini A (2017) A Rev Neuro From Guide Clin Pain Thera 6:35–42. https://doi.org/10.1007/s40122-017-0087-0
Du X et al (2017) Local GABAergic signaling within sensory ganglia controls peripheral nociceptive transmission. J Clin Investig 127:1741–1756. https://doi.org/10.1172/jci86812
Fattori V, Pinho-Ribeiro FA, Staurengo-Ferrari L, Borghi SM, Rossaneis AC, Casagrande R, Verri WA (2019) The specialized pro-resolving lipid mediator Maresin-1 reduces inflammatory pain with a long-lasting analgesic effect. Br J Pharmacol. https://doi.org/10.1111/bph.14647
Fukuoka T, Tokunaga A, Tachibana T, Dai Y, Yamanaka H, Noguchi K (2002) VR1, but not P2X in the spared L4 DRG in rats with L5 spinal nerve ligation. Pain 99:111–120
Gonzalez-Rodriguez S (2019) In Vivo methods to study ThermoTRP channels in rodents. Methods Mole Biol (Clifton, NJ) 1987:223–231. https://doi.org/10.1007/978-1-4939-9446-5_14
Hirai T et al (2014) Intrathecal AAV serotype 9-mediated delivery of shRNA against TRPV1 attenuates thermal hyperalgesia in a mouse model of peripheral nerve injury. Mole Ther J Am Soc Gene 22:409–419. https://doi.org/10.1038/mt.2013.247
Hudson LJ, Bevan S, Wotherspoon G, Gentry C, Fox A, Winter J (2001) VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. Eur J Neurosci 13:2105–2114
Hung CY, Tan CH (2018) TRP channels in nociception and pathological pain advances in experimental. Med Biol 1099:13–27. https://doi.org/10.1007/978-981-13-1756-9_2
Jung YH et al (2017) Discovery of potent antiallodynic agents for neuropathic pain targeting P2X3 receptors ACS. Chem Neurosci 8:1465–1478. https://doi.org/10.1021/acschemneuro.6b00401
Kage K, Niforatos W, Zhu CZ, Lynch KJ, Honore P, Jarvis MF (2002) Alteration of dorsal root ganglion P2X3 receptor expression and function following spinal nerve ligation in the rat. Exp Brain Res 147:511–519. https://doi.org/10.1007/s00221-002-1263-x
Kan HW, Chang CH, Lin CL, Lee YC, Hsieh ST, Hsieh YL (2018) Downregulation of adenosine and adenosine A1 receptor contributes to neuropathic pain in resiniferatoxin neuropathy. Pain 159:1580–1591. https://doi.org/10.1016/j.bja.2018.01.016
Kwon SG et al (2014) Blockade of peripheral P2Y1 receptors prevents the induction of thermal hyperalgesia via modulation of TRPV1 expression in carrageenan-induced inflammatory pain rats: involvement of p38 MAPK phosphorylation in DRGs. Neuropharmacology 79:368–379. https://doi.org/10.1016/j.neuropharm.2013.12.005
Leisengang S et al (2020) Effects of gabapentinoids on responses of primary cultures from rat dorsal root ganglia to inflammatory or somatosensory stimuli. J Basic Clin Physiol pharmacol 1:31. https://doi.org/10.1515/jbcpp-2019-0261
Li Y et al (2019) Electroacupuncture alleviates paclitaxel-induced peripheral neuropathic pain in rats via suppressing TLR4 signaling and TRPV1 upregulation in sensory. Neurons Internat J Mole Sci 1:20. https://doi.org/10.3390/ijms20235917
Liu CK, Liao WT, Chu YC, Yang CH, Chen KH, Wu CH, Lin CR (2017) Pulsed radiofrequency attenuates complete Freund’s adjuvant-induced epigenetic suppression of potassium chloride cotransporter two expression pain. Medicine (Malden, Mass) 18:807–813. https://doi.org/10.1093/pm/pnw243
Liu C, Li C, Deng Z, Du E, Xu C (2018) Long non-coding RNA BC168687 is involved in TRPV1-mediated diabetic neuropathic pain in rats. Neuroscience 374:214–222. https://doi.org/10.1016/j.neuroscience.2018.01.049
Luo J et al (2018) Zinc Inhibits TRPV1 to alleviate chemotherapy-induced neuropathic pain the journal of neuroscience: the official. J Soc Neurosci 38:474–483. https://doi.org/10.1523/jneurosci.1816-17.2017
Magni G, Ceruti S (2014) The purinergic system and glial cells: emerging costars in nociception. BioMed Res Internat 4:495789. https://doi.org/10.1155/2014/495789
North RA (2016) P2X receptors philosophical transactions of the royal society of London series B. Biological sciences. https://doi.org/10.1098/rstb.2015.0427
Nunez-Badinez P et al (2018) Variable transcriptional responsiveness of the P2X3 receptor gene during CFA-induced inflammatory hyperalgesia. J Cell Biochem 119:3922–3935. https://doi.org/10.1002/jcb.26534
Patel KV, Guralnik JM, Dansie EJ (2011) Turk DC (2013) Prevalence and impact of pain among older adults in the United States: findings from the. National Health Aging Trends Study Pain 154:2649–2657. https://doi.org/10.1016/j.pain.2013.07.029
Premkumar LS (2014) Transient receptor potential channels as targets for phytochemicals ACS. Chem Neurosci 5:1117–1130. https://doi.org/10.1021/cn500094a
Schafers M, Geis C, Svensson CI, Luo ZD, Sommer C (2003) Selective increase of tumour necrosis factor-alpha in injured and spared myelinated primary afferents after chronic constrictive injury of rat sciatic nerve. Eur J Neurosci 17:791–804
Shields SD, Eckert WA 3rd, Basbaum AI (2003) Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J Pain Off Am Soc 4:465–470
Sommer C, Leinders M, Üçeyler N (2018) Inflammation in the pathophysiology of neuropathic pain. Pain 159:595–602. https://doi.org/10.1097/j.pain.0000000000001122
Sondermann JR et al (2019) Vti1b promotes TRPV1 sensitization during inflammatory pain. Pain 160:508–527. https://doi.org/10.1097/j.pain.0000000000001418
Souza GR, Talbot J, Lotufo CM, Cunha FQ, Cunha TM, Ferreira SH (2013) Fractalkine mediates inflammatory pain through activation of satellite glial cells. Proc Natl Acad Sci USA 110:11193–11198. https://doi.org/10.1073/pnas.1307445110
Straube S, Derry S, Moore RA, Cole P (2013) Cervico-thoracic or lumbar sympathectomy for neuropathic pain and complex regional pain syndrome. Cochrane Database Syst Rev 1:002918. https://doi.org/10.1002/14651858.CD002918.pub3
Teixeira JM, Bobinski F, Parada CA, Sluka KA, Tambeli CH (2017) P2X3 and P2X2/3 receptors play a crucial role in articular hyperalgesia development through inflammatory mechanisms in the knee joint experimental synovitis molecular. Neurobiology 54:6174–6186. https://doi.org/10.1007/s12035-016-0146-2
Viana F (2018) Nociceptors: thermal allodynia and thermal pain. Handbook Clin Neurol 156:103–119. https://doi.org/10.1016/b978-0-444-63912-7.00006-0
Warwick CA, Shutov LP, Shepherd AJ, Mohapatra DP, Usachev YM (2019) Mechanisms underlying mechanical sensitization induced by complement C5a: the roles of macrophages, TRPV1, and calcitonin gene-related peptide receptors. Pain 160:702–711. https://doi.org/10.1097/j.pain.0000000000001449
Wu JX, Yuan XM, Wang Q, Wei W, Xu MY (2016) Rho/ROCK acts downstream of lysophosphatidic acid receptor 1 in modulating P2X3 receptor-mediated bone cancer pain in rats. Mole Pain 12:26–27. https://doi.org/10.1177/1744806916644929
Xiang Z et al (2008) Functional up-regulation of P2X 3 receptors in the chronically compressed dorsal root ganglion. Pain 140:23–34. https://doi.org/10.1016/j.pain.2008.07.006
Xiang H et al (2017) Primary sensory neuron-specific interference of TRPV1 signaling by AAV-encoded TRPV1 peptide aptamer attenuates neuropathic pain. Mole Pain 13:1744806917717040. https://doi.org/10.1177/1744806917717040
Xiong W et al (2017) Inhibitory effects of tetramethylpyrazine on pain transmission of trigeminal neuralgia in CCI-ION rats. Brain Res Bull 134:72–78. https://doi.org/10.1016/j.brainresbull.2017.07.005
Yezierski RP, Hansson P (2018) Inflammatory and neuropathic pain from bench to bedside: what went wrong? J Pain Off Am Pain Soc 19:571–588. https://doi.org/10.1016/j.jpain.2017.12.261
Zhang YL et al (2018) Microencapsulated Schwann cell transplantation inhibits P2X2/3 receptors overexpression in a sciatic nerve injury rat model with neuropathic pain. Neurosci lett 676:51–57. https://doi.org/10.1016/j.neulet.2018.03.063
Zhou YL et al (2015) Enhanced binding capability of nuclear factor-kappaB with demethylated P2X3 receptor gene contributes to cancer pain in rats. Pain 156:1892–1905. https://doi.org/10.1097/j.pain.0000000000000248
Funding
This work was supported by the National Natural Science Foundation of China (8147377, 81603690, 81603692), the Zhejiang Provincial Natural Science Foundation [China] (LY19H270003) and the Talent Project of the Zhejiang Association for Science and Technology (2017YCGC004).
Author information
Authors and Affiliations
Contributions
Ju. F., J. D. and Ji. F. conceived the presented study. Ju. F., Ji. F., J. D., X. X., X. H. and Y. J. designed and carried out the experiments. Y. L. and X. S. verified the analytical methods. B. L. and J. F. supervised the project.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
In the current study, all animal manipulation complied with institutional and Chinese governmental regulations regarding the ethical use of animals (approval no. IACUC-20180319–12).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Sreedharan Sajikumar.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fang, J., Du, J., Xiang, X. et al. SNI and CFA induce similar changes in TRPV1 and P2X3 expressions in the acute phase but not in the chronic phase of pain. Exp Brain Res 239, 983–995 (2021). https://doi.org/10.1007/s00221-020-05988-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-020-05988-4