Abstract
Osteoarthritis (OA) is a degenerative and progressive disease characterized by cartilage breakdown and by synovial membrane inflammation, which results in disability, joint swelling, and pain. The purinergic P2X3 and P2X2/3 receptors contribute to development of inflammatory hyperalgesia, participate in arthritis processes in the knee joint, and are expressed in chondrocytes and nociceptive afferent fibers innervating the knee joint. In this study, we hypothesized that P2X3 and P2X2/3 receptors activation by endogenous ATP (adenosine 5′-triphosphate) induces articular hyperalgesia in the knee joint of male and female rats through an indirect sensitization of primary afferent nociceptors dependent on the previous release of pro-inflammatory cytokines and/or on neutrophil migration. We found that the blockade of articular P2X3 and P2X2/3 receptors significantly attenuated carrageenan-induced hyperalgesia in the knee joint of male and estrus female rats in a similar manner. The carrageenan-induced knee joint inflammation increased the expression of P2X3 receptors in chondrocytes of articular cartilage. Further, the blockade of articular P2X3 and P2X2/3 receptors significantly reduced the increased concentration of TNF-α, IL-6, and CINC-1 and the neutrophil migration induced by carrageenan. These findings indicate that P2X3 and P2X2/3 receptors activation by endogenous ATP is essential to hyperalgesia development in the knee joint through an indirect sensitization of primary afferent nociceptors dependent on the previous release of pro-inflammatory cytokines and/or on neutrophil migration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is the most common form of arthritis occurring more frequently in women than men. It is a progressive and degenerative disease with a higher prevalence in women than in men [1–3]. OA is characterized by inflammation of the synovial membrane (synovitis), cartilage degradation, and pain that interferes with function [4, 5]. A better understanding of the peripheral processes linking inflammatory pain with OA is necessary for the improvement of the analgesic treatments of this disease [6].
There are seven P2X purinergic receptor subtypes (P2X1–P2X7), which are ligand-gated ionotropic channels that open in response to the binding of extracellular ATP (adenosine 5′-triphosphate) [7] and form as both homomers and heteromers in sensory fibers. P2X3 forms as a homomer or can form as a heteromer with P2X2 [8], and both forms are involved in articular pain and hyperalgesia [9–11]. Specifically, P2X3 and P2X2/3 receptors are localized on peripheral and central terminals of unmyelinated C-fiber and thinly myelinated Aδ sensory afferents [12–14]. Thus, P2X3 and P2X2/3 receptors play a significant role in articular pain and hyperalgesia based on their location on afferent fibers.
Purinergic receptors are also found on non-neuronal cells including those involved in the inflammatory process. In particular, human epidermal keratinocytes express P2X3 receptor mRNA [15] and endothelial cells of thymus [16], urothelial cells [17], and chondrocytes [18] express P2X3 receptors. Further, activation of P2X3 receptors in chondrocytes induced nitric oxide and PGE2 release [18], suggesting that P2X3 receptors on non-neuronal joint cells may enhance nociception through modulating the inflammatory process. However, it is not known whether the expression of P2X3 receptors on chondrocytes is increased during inflammation or whether inflammatory mechanisms such as pro-inflammatory cytokines and neutrophil migration are involved in the contribution of P2X3 receptors to articular pain.
Although the prevalence of articular pain conditions is higher in women than in men [1, 3], and the involvement of some receptors in pain and analgesia processes is sex-dependent [19–21], it is not known whether P2X3 and P2X2/3 receptors contribute to articular hyperalgesia in a sex-dependent manner.
Therefore, in this study, we used the carrageenan-induced knee joint inflammation model in rats [22–24] to test the hypothesis that: (I) the P2X3 and P2X2/3 receptors contribute to carrageenan-induced articular hyperalgesia in a sex-dependent manner, (II) the carrageenan-induced articular inflammation increases expression of P2X3 receptors in the chondrocytes of knee joint articular cartilage, and that (III) the contribution of P2X3 and P2X2/3 receptors activation to carrageenan-induced articular hyperalgesia involves a previous release of pro-inflammatory cytokines TNF-α, IL-1β, IL-6, and chemokine-induced chemoattractant-1 (CINC-1, analog to IL-8 in rats) and/or the migration of neutrophils to the inflamed knee joint.
Materials and Methods
Animals
Male and female Wistar rats (200–250 g) obtained from the Multidisciplinary Center for Biological Research (CEMIB - UNICAMP, Campinas, SP, Brazil) and from Harlan Laboratories (Madison, WI, USA) were used in this study. The animals (specific pathogen free, SPF) were housed in plastic cages with soft bedding (five/cage) on a 12:12 light–dark cycle (lights on at 06 a.m.), with food (commercial chow for rodents) and filtered water available ad libitum, in a temperature-controlled room (±23 °C). Testing sessions took place during light phase (09:00 a.m.–5:00 p.m.) in a quiet room maintained at 23 °C [25]. During the tests, the animals had no access to water or food.
Each animal was used once, and the number of animals per group was kept to a minimum. Experimental protocols were approved by the Committee on Animal Research of the State University of Campinas (protocol number: 2049-1) and by the Animal Care and Use Committee at the University of Iowa and were carried out in accordance with the IASP guidelines for the study of the pain in animals [26]. The sample size of this study was determined and calculated in accordance with [27]. The group size (n) for each experimental group is showed in “Results” and between parentheses in all the figures. Animals were divided randomly into the groups. The experimenter blinded to the experimental groups made all analyses.
Carrageenan-Induced Knee Joint Inflammation (Synovitis)
Under brief inhalation of isoflurane anesthesia, the skin around the knee joints was shaved and treated with an antiseptic solution of iodine alcohol. Using a 26-gauge needle connected to a polyethylene catheter and also to a Hamilton syringe (50 μL), rats were subjected to intra-articular (i.a.) injection of λ-carrageenan dissolved in 25 μL sterile 0.9 % saline solution into their right knee joints [23, 28]. The other drugs were injected intra-articularly in the same manner that carrageenan and the control animals received vehicle or sterile 0.9 % saline solution.
Drugs and Doses
The following drugs were used: λ-carrageenan (Cg; 300 μg/knee, i.a., [23, 29, 30]) and 5-([(3-phenoxybenzyl) [(1S)-1,2,3,4-tetrahydro-1-naphthalenyl]amino]carbonyl)-1,2,4-benzenetricarboxylic acid (A-317491 - the selective P2X3 and P2X2/3 receptor antagonist [31]: 20, 60, 180, 540 μg/knee, i.a., [32]). The drugs were obtained from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in 25 μL sterile 0.9 % saline solution.
Estrus Phase Determination of Estrous Cycle
Because female rats with lower levels of ovarian hormones, such as estrus females, are the most responsive to some analgesic drugs [19–21] and presented an articular hyperalgesic response of the same magnitude than males rats, they were used in this study.
Estrus phase in female rats was determined by daily microscope examination (9:00–10:00 a.m.) of vaginal smears taken by gentle lavage. Estrus phase was identified by the predominance (80 %) of anucleated cornified cells in rats with at least two consecutive regular 4-day cycles [33, 34]. This phase was chosen because it represents the phase of low ovarian hormonal level, 17β-estradiol and progesterone [35, 36].
Gait Disturbance—Rat Knee Joint Incapacitation Test
We used the rat knee joint incapacitation test (Insight, Ribeirão Preto, SP, Brazil), as described previously [23]. Briefly, 3 h after drugs injection into their right knee joints, rats were put to walk on a steel rotary cylinder (30-cm wide × 50-cm diameter), covered with a fine-mesh non-oxidizable wire screen, which rotates at 3 rpm. Designed metal gaiters were wrapped around both hind paws. After placement of the gaiters, rats were placed in the cylinder to walk and the right paw was connected via a simple circuit to microcomputer data input/output port. The paw elevation time (PET) is the total time that rats walk failing to touch the cylinder surface with the injected hind paw, during a 60-s period, which is directly proportional to the gait disturbance. Incapacitation (articular hyperalgesia) was quantified as an increase in the PET. To minimize variations in PET, all rats were introduced to the experimental environment and trained on the apparatus to habituation into the equipment before the testing sessions. To confirm the local effect of A-317491, it was injected into the contralateral rat’s knee joint and the test was performed on the ipsilateral knee joint.
Tissue Preparation
Three hours after carrageenan (300 μg/knee) or sterile 0.9 % saline solution injection (when carrageenan-induced articular hyperalgesia reaches its maximum), rats were anesthetized with sodium pentobarbital (120 mg/kg i.p.) and transcardially perfused with 4 % paraformaldehyde (PFA, in 0.1 M phosphate buffer (PB), pH 7.4). Whole knee joints were rapidly removed and kept in the same fixative for 24 h at 4 °C. The fixed specimens were decalcified for 8 weeks with 10 % ethylenediaminetetraacetic acid (EDTA) in 0.01 M phosphate buffered saline (PBS) at 4 °C with three fresh solution changes per week [37]. After the complete demineralization, the decalcified specimens were then rinsed thoroughly in PBS, placed in 30 % sucrose overnight, and embedded in OCT compound (Sakura Finetek, Torrance, CA, USA). All samples were rapidly frozen and stored at −80 °C until being cut on cryostat. Serial sections were then cryosectioned at 20 μm using a cryostat, which was obtained at the medial midcondylar region in sagittal plane [38, 39].
Immunohistochemistry
Immunohistochemistry labeling was performed using standard immunofluorescence techniques. Nonspecific binding sites were blocked with 10 % normal goat serum (NGS) for 30 min. Sections were then rinsed twice for 5 min in 0.1 M PBS and incubated in primary antibody (1:1000, guinea pig Anti-P2X3 Receptor, AB5896, Merck Millipore, Billerica, MA, USA) diluted in 1 % NGS and 0.05 % Triton X-100 in 0.1 M PBS and applied to the tissues overnight (4 °C) in a humid atmosphere. Sections were then rinsed twice for 5 min in 0.1 M PBS and incubated in secondary antibody (1:1000, goat anti-guinea pig IgG-Alexa488, Life Technologies, Grand Island, NY, USA) diluted in 1 % NGS and 0.05 % Triton X-100 in 0.1 M PBS for 1 h at room temperature. Sections were then rinsed twice for 5 min in 0.1 M PBS and incubated with TO-PRO3 (1:4000, 30 min; Invitrogen, Carlsbad, CA, USA) for nuclear staining. After a final washing, the sections were cover slipped using Vectashield (Vector Laboratories, Burlingame, CA, USA). Negative controls were prepared without incubation in primary antibody to confirm that there was no non-specific binding of the secondary antibody.
After staining, sections were examined with the Confocal Bio-Rad MRC 1024 Microscope and images were taken with a ×20 objective lens of three specific regions of the knee joint: the articular cartilage covering the femoral condyle, the articular cartilage covering the tibial plateau, and the meniscus. Five randomly selected knee sections were chosen for each sample (six samples per group) and were digitally imaged and stored for later analysis. The density (mean, arbitrary units) of each section and the number of positive cells (chondrocytes) were quantified by manually counting total numbers in a given area using Image J (National Institutes of Health). Specifically, a standard size (average area of 75,190 μm2 for the layer of cartilage covering the femoral condyle, 62,410 μm2 for the layer of cartilage covering the tibial plateau, and 93,630 μm2 for meniscus) was applied to each section. Chondrocytes were counted if they were positively stained for P2X3 receptor.
Synovial Lavage Fluid
Under deep anesthesia (80 mg/kg ketamine and 20 mg/kg xylazine, i.p.), rats were killed by cervical dislocation, the skin overlying the knee was excised, the patellar ligament was dissected, and a 26-gauge needle connected to a Hamilton syringe (100 μL) was inserted through the joint capsule. The knee joint cavity was washed twice by injecting and immediately aspirating 100 μL of phosphate-buffered saline solution (PBS) containing 4 mM EDTA. The resulting synovial lavages (total volume of 100 μL) were combined and immediately stored at −80 °C for further analysis [28].
Enzyme-Linked Immunosorbent Assay Procedure
An adaptation of enzyme-linked immunosorbent assay (ELISA) [40] was used to quantify the cytokines of the rat’s knee joint. Briefly, 3 h after the drug injections, the synovial lavage fluid (100 μL) was collected and homogenized in 500 μL of a solution of PBS containing 0.4 M NaCl, 0.05 % Tween 20, 0.5 % bovine serum albumin (BSA), 0.1 mM phenyl-methylsulfonyl-fluoride, 0.1 mM benzotonic-chloride, 10 mM EDTA, and 2 ng/mL aprotinin (Sigma-Aldrich, St. Louis, MO, USA). The samples were centrifuged (10,000 rpm, 15 min, 4 °C), and the supernatants were used to evaluate the protein levels of TNF-α, IL-1β, IL-6, and CINC-1 in the rat’s knee joint. The cytokines were quantified by the following DuoSet ELISA Kits: TNF-α: Rat TNF-α/TNFSF1A (DY510); IL-1β: Rat IL-1β/IL-1F2 (DY501), IL-6: Rat IL-6 (DY506), and CINC-1: Rat CXCL1/CINC-1 (DY515). All procedures followed the instructions of the manufacturer (R&D Systems, Minneapolis, MN, USA). All procedures were repeated twice to guarantee the accuracy of the results.
Measurement of Myeloperoxidase Activity
Myeloperoxidase (MPO) is one of the enzymes released from neutrophils and associated with tissue injury and is used as a marker of neutrophilic activity in peripheral tissues [41]. Briefly, 3 h after the drug injections, synovial lavage fluid was collected and homogenized in 500 μL of buffer 1 (0.1 M NaCl, 0.02 M NaPO4, 1.015 M NaEDTA) followed by centrifugation (3000 rpm, 15 min, 4 °C). The pellet was resuspended in 500 μL of buffer 1 and subjected to hypotonic lyses by the addition of 500 μL of 0.2 % NaCl followed 30 s later by addition of 500 μL of 1.6 % NaCl in 5 % glucose. After further centrifugation, the pellet was resuspended in 0.05 M NaPO4 buffer (pH 5.4) containing 0.5 % hexadecyl-trimethylammonium bromide (HTAB). Samples were then snap-frozen in liquid nitrogen three times and centrifuged (10,000 rpm, 15 min, 4 °C). The supernatant was used in the assay.
The MPO kinetic-colorimetric assay was conducted as previously described [42]. Fifty microliters of each sample and 0.08 M NaPO4 were dropped into wells of a 96-well microplate. Twenty-five microliters of 1.6 mM 3,3′,3,3′-tetramethylbenzidine (TMB) was added in each well, and the reaction was initiated by the addition of 100 μL of 0.5 mM H2O2. The reaction was stopped 5 min later by the addition of 50 μL of 4 M H2SO4. The optical density was read at 450 nm using an Asys UVM340. After that, the results were calculated by comparing the optical density of rat’s knee joint synovial lavage fluid supernatant with a standard curve of neutrophil (>95 % purity), as previously described [43]. The results were presented as number of neutrophils × 104/knee. All procedures were repeated twice to guarantee the results authenticity.
Statistical Analysis
Statistical analysis was performed using Prism v5 (GraphPad, La Jolla, CA, USA). To determine if there were significant differences (p < 0.05) between treatment groups for all measures, one-way ANOVA was performed. If there was a significant between-subjects main effect for treatment group, Tukey’s test examined for individual differences between groups. For PET data shown in Fig. 1b, c, a two-way repeated measures ANOVA with one between subjects factor (i.e., treatment) and one within-subjects factor (i.e., time) were used to determine whether there were significant (p < 0.05) differences among the groups. If there was a significant between-subjects main effect of treatment group, post hoc contrasts using the Bonferroni test were performed to determine the basis of the significant difference. Data are expressed in figures as means ± S.E.M.
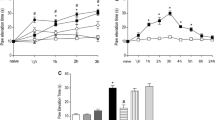
Effect of articular blockade of P2X3 and P2X2/3 receptors on carrageenan-induced hyperalgesia in the knee joint of males and estrus females. Co-administration of the P2X3 and P2X2/3 receptors antagonist A-317491 (60, 180, and 540 μg/knee) with carrageenan (300 μg/knee) significantly reduced carrageenan-induced articular hyperalgesia in males (a) and estrus females (b), as indicated by the symbol “#” (P < 0.05, Tukey test) in a similar manner (P > 0.05, Bonferroni test, C). The highest dose of A-317491 (540 μg/knee) injected in the contralateral knee joint (ct) did not affect the carrageenan-induced articular hyperalgesia (P > 0.05, Tukey test, a and b). A-317491 injected only with 0.9 % NaCl had no effect by itself (P > 0.05, Tukey test, a and b). The symbol “*” indicates a response significantly greater than that induced by 0.9 % NaCl (a and b; P < 0.05, Tukey test). In this and in the subsequent figures, the articular hyperalgesia was measured 3 h after the intra-articular (i.a.) drugs administration and the number of rats or samples used are in parentheses
Results
P2X3 and P2X2/3 Receptors Contribute to Carrageenan-Induced Articular Hyperalgesia in Males and Females
In the current study, we show that 300 μg of intra-articular injection of carrageenan increases PET during walking to a similar extent for both male and estrus female rats (Fig. 1a, b, second bar, P > 0.05, one-way ANOVA, Tukey test, n = 6 per group). The increased PET begins within 1 h after carrageenan injection, remains elevated through 6 h (Fig. 2b, P < 0.05, two-way ANOVA, Bonferroni test, n = 6 per group), and returns to baseline 24 h later (Fig. 2b, P > 0.05, two-way ANOVA, Bonferroni test, n = 6 per group).
Temporal analysis of the effect of the articular blockade of P2X3 and P2X2/3 receptors on carrageenan-induced articular hyperalgesia. a Co-administration (0 h) of the P2X3 and P2X2/3 receptors antagonist A-317491 (540 μg/knee) with carrageenan (Cg, 300 μg/knee), but not its administration ½, 1, 2, or 3 h after the carrageenan administration (P > 0.05, Tukey test) significantly reduced carrageenan-induced articular hyperalgesia, as indicated by the symbol “#” (P < 0.05, Tukey test). The symbol “*” indicates a response significantly greater than that induced by 0.9 % NaCl (P < 0.05, Tukey test). b The temporal analysis of the effect of the co-administration of A-317491 (540 μg/knee) with carrageenan showed that A-317491 blocked the hyperalgesic response 1, 2, 3, 4, 5, and 6 h after its co-administration, as indicated by the symbol “#” (P < 0.05, Bonferroni test)
To determine if P2X3 and P2X2/3 receptors contributed to the enhanced PET, the selective antagonist A-317491 was co-administrated with carrageenan (300 μg/knee) into the knee joint. A-317491 at doses of 60, 180, and 540 μg/knee, but not 20 μg/knee (Fig. 1a, b, P > 0.05, one-way ANOVA, Tukey test, n = 6 per group), significantly attenuated the carrageenan-induced increase in PET (Fig. 1a, b, P < 0.05, one-way ANOVA, Tukey test, n = 6 per group) in males and estrus females knee joint when compared to vehicle controls. The highest dose of A-317491 (540 μg/knee) did not affect the carrageenan-induced elevated PET when applied on the contralateral knee joint (Fig. 1a, b, P > 0.05, one-way ANOVA, Tukey test, n = 6 per group) of males and estrus females, confirming its local peripheral action. There were no significant differences in the anti-hyperalgesic effect of the different doses of A-317491 on carrageenan-induced articular hyperalgesia between males and estrus females (Fig. 1c, P > 0.05, two-way ANOVA, Bonferroni test, n = 6 per group). Therefore, either males or females could be used to perform the subsequent experiments and we chose to perform them only in male rats due to methodological facilities.
P2X3 and P2X2/3 Receptors Are Crucial to the Development of Carrageenan-Induced Articular Hyperalgesia
To characterize the period of time at which the activation of P2X3 and P2X2/3 receptors contributes to the development of carrageenan-induced hyperalgesia in the knee joint, A-317491 was co-administrated (0 h) with carrageenan or administrated ½, 1, 2, or 3 h after injection of carrageenan. Co-administration of A-317941 (540 μg/knee) with carrageenan (300 μg/knee) (Fig. 2a, P < 0.05, one-way ANOVA, Tukey test, n = 6 per group), but not its administration ½, 1, 2, or 3 h after the carrageenan administration (Fig. 2a, P > 0.05, one-way ANOVA, Tukey test, n = 6 per group) significantly prevented the carrageenan-induced PET.
In another set of experiments, A-317491 (540 μg/knee) was co-administrated with carrageenan (300 μg/knee) and the measurements were taken ½, 1, 2, 3, 4, 5, 6, and 24 h later. The carrageenan-induced articular hyperalgesia reached its maximum 3 h after injection (Fig. 2b). A-317491 prevented the development of the carrageenan-induced PET from 1 to 6 h (Fig. 2b, P < 0.05, two-way ANOVA, Bonferroni test, n = 6 per group).
Carrageenan Increased Expression of the P2X3 Receptor on the Chondrocytes in the Knee Joint
To test if intra-articular carrageenan injection increases P2X3 receptors on chondrocytes in the knee joint, we examined P2X3 receptors expression after induction of inflammation by immunofluorescence. Intra-articular administration of carrageenan (300 μg/knee) significantly increased the number of chondrocytes that express P2X3 receptor in the articular cartilage covering the femoral condyle (Fig. 3, P < 0.05, one-way ANOVA, Tukey test, n = 6 per group), the tibial plateau (Fig. 4, P < 0.05, one-way ANOVA, Tukey test, n = 6 per group), and on the meniscus (Fig. 5, P < 0.05, one-way ANOVA, Tukey test, n = 6 per group) when compared to saline or naive controls. The expression of P2X3 receptors on the chondrocytes after the intra-articular injection of 0.9 % NaCl (50 μL/knee) was similar to that of naive rats in all three regions analyzed (P > 0.05, one-way ANOVA, Tukey test, n = 6 per group).
Effect of intra-articular injection of carrageenan on P2X3 receptors expression in the chondrocytes of the articular cartilage of the femoral condyle of the rats knee joint. Induction of knee joint inflammation with carrageenan (300 μg/knee) significantly increased expression of P2X3 receptor in the chondrocytes of the articular cartilage of the femoral condyle (a and b). The symbol “*” indicates an expression significantly greater than that of 0.9 % NaCl and naïve groups (P < 0.05, Tukey test). Immunofluorescence confocal micrographs of the articular cartilage (white arrows) of the femoral condyle after carrageenan (c, d and e) and 0.9 % NaCl injection (f, g and h) and of untreated (naive, i, j and k) groups. Sections show immunohistochemical labeling for P2X3 receptor (green, c, f and i) and labeling for nuclear staining with TOPRO-3 (red, d, g and j). Merged pictures in e, h and k show co-localization of P2X3 receptor and nucleus cells (yellow). BM bone marrow. Scale bars = 100 μm
Effect of intra-articular injection of carrageenan on P2X3 receptors expression in the chondrocytes of the articular cartilage of the tibial plateau of the rat’s knee joint. Induction of joint inflammation with carrageenan (300 μg/knee) significantly increased the expression of P2X3 receptor in the chondrocytes of the articular cartilage covering the tibial plateau (a and b). The symbol “*” indicates an expression significantly greater than that of 0.9 % NaCl and naïve groups (P < 0.05, Tukey test). Immunofluorescence confocal micrographs of the articular cartilage (white arrows) covering the tibial plateau after carrageenan (c, d and e) and 0.9 % NaCl (f, g and h) injection and of untreated (naive, i, j and k) groups. Sections show immunohistochemical labeling for P2X3 receptor (green, c, f and i) and labeling for nuclear staining with TOPRO-3 (red, d, g and j). Merged pictures in e, h and k show co-localization of P2X3 receptor and nucleus cells (yellow). BM bone marrow. Scale bars = 100 μm
Effect of intra-articular injection of carrageenan on P2X3 receptors expression in the chondrocytes of the cartilage that forms the meniscus of the rat’s knee joint. Induction of joint inflammation with carrageenan (300 μg/knee) significantly increased expression of P2X3 receptor in the chondrocytes of the cartilage that forms the meniscus (a and b). The symbol “*” indicates an expression significantly greater than that of 0.9 % NaCl and naïve groups (P < 0.05, Tukey test). Immunofluorescence confocal micrographs of the meniscus after carrageenan (c, d and e) and 0.9 % NaCl (f, g and h) injection and of untreated (naive, i, j and k) groups. Sections show immunohistochemical labeling for P2X3 receptor (green, c, f and i) and labeling for nuclear staining with TOPRO-3 (red, d, g and j). Merged pictures in e, h and k show co-localization of P2X3 receptor and nucleus cells (yellow). Scale bars = 100 μm
P2X3 and P2X2/3 Receptors Contribute to Carrageenan-Induced Increase in Pro-inflammatory Cytokines
To verify if P2X3 and P2X2/3 receptors contribute to the release of pro-inflammatory cytokines in the inflamed joint, we examined the cytokines pro-inflammatory concentrations in the inflamed knee joint from animals injected with A-317491 (540 μg/knee) and compared to vehicle controls.
Intra-articular injection of carrageenan (300 μg/knee) significantly increased concentrations of TNF-α (Fig. 6a), IL-1β (Fig. 6b), IL-6 (Fig. 6c), and CINC-1 (Fig. 6d) in the knee joint 3 h after carrageenan injection (P < 0.05, one-way ANOVA, Tukey test, n = 8 per group). Co-administration of A-317491 (540 μg/knee) with carrageenan significantly prevented increases in TNF-α (Fig. 6a), IL-6 (Fig. 6c), and CINC-1 (Fig. 6d) (P < 0.05, one-way ANOVA, Tukey test, n = 8 per group), but not IL-1β concentration in the inflamed knee joint (Fig. 6b) (P > 0.05, one-way ANOVA, Tukey test, n = 8 per group). The endogenous concentration of TNF-α, IL-1β, IL-6, and CINC-1 after the intra-articular injection of 0.9 % NaCl was not significantly different from that of naive rats (Fig. 6, P > 0.05, one-way ANOVA, Tukey test, n = 8 per group).
Effect of the articular blockade of P2X3 and P2X2/3 receptors on carrageenan-induced pro-inflammatory cytokine release. Induction of joint inflammation with carrageenan (Cg, 300 μg/knee) significantly increased local concentrations of TNF-α (a), IL-1β (b), IL-6 (c), and CINC-1 (d) 3 h after carrageenan injection. The co-administration of the P2X3 and P2X2/3 receptors antagonist A-317491 (540 μg/knee) with carrageenan significantly reduced the local concentration of TNF-α (a), IL-6 (c), and CINC-1 (d), as indicated by the symbol “#” (P < 0.05, Tukey test), but not the local concentration of IL-1β (b) (P > 0.05, Tukey test). The intra-articular injection of 0.9 % NaCl alone did not significantly affect the local endogenous concentration of TNF-α, IL-1β, IL-6, and CINC-1 when compared with naive rats (P > 0.05, Tukey test). The symbol “*” indicates a response significantly greater than that of 0.9 % NaCl and naive groups (P < 0.05, Tukey test)
P2X3 and P2X2/3 Receptors Contribute to Carrageenan-Induced Knee Joint Neutrophil Infiltration
To test if P2X3 and P2X2/3 receptors contribute to the neutrophilic infiltration induced by carrageenan, we examined myeloperoxidase enzyme (MPO) activity after co-administration of A-317491 (540 μg/knee) with carrageenan and compared to vehicle-controls.
Intra-articular injection of carrageenan (300 μg/knee) significantly increased MPO activity 3 h after injection (Fig. 7, P < 0.05, one-way ANOVA, Tukey test, n = 8 per group). Co-administration of A-317491 (540 μg/knee) with carrageenan showed significantly lower MPO activity when compared to vehicle-controls (Fig. 7, P < 0.05, one-way ANOVA, Tukey test, n = 8 per group). The intra-articular injection of 0.9 % NaCl showed similar levels in MPO activity when compared to naive rats (Fig. 7, P > 0.05, one-way ANOVA, Tukey test, n = 8 per group).
Effect of the articular blockade of P2X3 and P2X2/3 receptors on carrageenan-induced neutrophil migration. Induction of joint inflammation with carrageenan (Cg, 300 μg/knee) significantly increased the neutrophil migration into the rat’s knee joint 3 h after carrageenan injection. The co-administration of the P2X3 and P2X2/3 receptors antagonist A-317491 (540 μg/knee) with carrageenan significantly reduced the carrageenan-induced increase of neutrophil migration, as indicated by the symbol “#” (P < 0.05, Tukey test). The symbol “*” indicates a response significantly greater than that of 0.9 % NaCl and naive groups (P < 0.05, Tukey test)
Discussion
In this study, we showed for the first time that P2X3 and P2X2/3 receptors contribute to carrageenan-induced articular hyperalgesia in the knee joint of both male and female rats. Uniquely, we showed that carrageenan-induced articular inflammation increases the expression of P2X3 receptors on chondrocytes of the articular cartilage of the knee joint, and that blockade of P2X3 and P2X2/3 receptors prevents carrageenan-induced increases in pro-inflammatory cytokines, and prevents neutrophilic migration to the knee joint. Together these data suggest that P2X3 and P2X2/3 receptors contribute to carrageenan-induced articular inflammatory hyperalgesia indirectly through enhanced release of the pro-inflammatory cytokines (TNF-α, IL-6, and CINC-1) and stimulation of neutrophilic infiltration to the injured joint.
We showed that the blockade of P2X3 and P2X2/3 receptors in the knee joint significantly reduced carrageenan-induced articular hyperalgesia from 1 to 6 h. Although spinal P2X3 and P2X2/3 receptors also contribute to hyperalgesia [44], this anti-hyperalgesic effect was arguably the result of blockade of P2X3 and P2X2/3 receptors local to the site of inflammation since A-317941 injection into the contralateral joint did not reproduce the effect.
Likewise in the subcutaneous tissue of rat paw [32], the P2X3 and P2X2/3 receptors antagonist only reduced carrageenan-induced articular hyperalgesia when it was co-administered with carrageenan, but not when it was administered ½, 1, 2, or 3 h after the carrageenan administration in the knee joint. Therefore, these purinergic receptors in the peripheral tissue seem to be essential to the development of hyperalgesic response, but not for its maintenance. These results may suggest that in this carrageenan-induced synovitis model, ATP may be released only immediately after the carrageenan administration. However, many inflammatory articular conditions (e.g., osteoarthritis) are in a progressive development and ATP may be continuously released, sustaining the articular hyperalgesia in these pathologies. Therefore, P2X3 and P2X2/3 receptor blockers may be effective in ongoing articular inflammatory conditions, although this possibility needs further confirmation by clinical studies in patients with arthropathies.
Our findings are consistent with animal studies showing that during inflammation ATP is released from macrophages, platelets, neutrophils, and dying cells [45–51] to the extracellular milieu, where it contributes to the development of inflammatory hyperalgesia in the subcutaneous tissue [32] and in the articular tissue such as the TMJ [11] and the knee joint [9] of rats via P2X3 and P2X2/3 receptors activation. Further, they are also consistent with human studies showing that extracellular ATP is often found in the synovial fluid of patients with arthropathies [52, 53].
The chondrocytes, the unique cell type residing in cartilage [54, 55], can release PGE2 [56], a major contributor of inflammatory pain in arthritis conditions, that subsequently acts through a variety of prostanoids receptors expressed in peripheral sensory neurons and spinal cord [57]. A previous in vitro study showed that ATP and α,β-meATP (P2X3 agonist, [14] increase PGE2 production, an effect that was blocked by the selective P2X3 and P2X2/3 receptors antagonist A317491, indicating a role of P2X3 receptors in PGE2 release by chondrocytes [18]. Therefore, in addition to the P2X3 and P2X2/3 receptors expressed on the peripheral terminals of primary afferent nociceptors that innervate the knee joint [58], the P2X3 and P2X2/3 receptors expressed in chondrocytes may also contribute to carrageenan-induced articular hyperalgesia.
In this study, we showed for the first time that the local inflammation induced by carrageenan in the rat’s knee joint increases the expression of the P2X3 receptor in the chondrocytes of the articular cartilage. Specifically, we show increases in the cartilage covering the femoral condyle and the tibial plateau and the meniscus of the rat’s knee joint. However, it is important to point out that the increased P2X3 receptor expression on the chondrocytes was observed 3 h after the intra-articular administration of carrageenan. Because, at this time, the administration of the P2X3 and P2X2/3 receptors antagonist A-317491 had no effect on carrageenan-induced articular hyperalgesia, the increased P2X3 receptor expression on the chondrocytes may not contribute to carrageenan-induced articular hyperalgesia. Alternatively, it may contribute to carrageenan-induced inflammation associated with cartilage degradation [59], which is consistent with the demonstration that ATP can promote cartilage resorption [60].
Several studies suggest the involvement of pro-inflammatory cytokines in the articular inflammatory processes [61–63]. For example, the concentration of IL-1β, TNF-α, IL-6, and IL-8 is significantly increased in the experimental OA knee joint synovial fluid of rats [64, 65] and in the synovial fluid of patients with OA [66]. The importance of TNF-α and IL-6 to the development of arthropathies was demonstrated by the ability of anti-TNF-α and anti-IL-6 antibodies to prevent the progression of bone and cartilage damage, relieving pain symptoms in patients [67–70] and by their ability in the prevention of collagen-induced arthritis in mice [71, 72]. In this study, we showed that the pro-inflammatory cytokines TNF-α, IL-6, and CINC-1 mediates, at least in part, the essential role played by articular P2X3 and P2X2/3 receptors in the development of carrageenan-induced hyperalgesia in the knee joint. As shown in the subcutaneous tissue [32], IL-1β is not involved in the contribution of P2X3 and P2X2/3 receptor activation in carrageenan-induced articular hyperalgesia. Thus, we suggest that the activation of P2X3 receptors expressed on chondrocytes may contribute to the release of pro-inflammatory cytokines, which subsequently enhance inflammation and activate nociceptors.
The pro-inflammatory cytokines TNF-α, IL-6, and CINC-1 could contribute to the essential role played by articular P2X3 and P2X2/3 receptors in the development of carrageenan-induced hyperalgesia in the knee joint by stimulating the release of PGE2 from chondrocytes. Consistent to this idea, these cytokines can act on chondrocytes [73, 74] and can release PGE2 [56]. These pro-inflammatory cytokines could also contribute to the essential role played by articular P2X3 and P2X2/3 receptors in the development of carrageenan-induced hyperalgesia in the knee joint by other mechanisms. For example, TNF-α and IL-6 could direct activate the primary afferent nociceptor [75, 76] and CINC-1 could stimulate release of sympathomimetic amines [77, 78] from sympathetic nerves that innervate the synovial tissue [79].
In patients with OA, there is an intense neutrophil migration [80] into synovial tissues. Neutrophils are involved in the genesis of inflammatory hyperalgesia [81], and their infiltration into synovial tissues can cause proteolytic enzymes release which contributes significantly to the tissue damage [82]. Neutrophil migration can be induced by carrageenan [28] and by the pro-inflammatory chemokine CINC-1 [83]. In this study, we showed that blockade of P2X3 and P2X2/3 receptors reduces carrageenan-induced neutrophilic migration. This finding highlights an important difference between the pathophysiology of inflammatory hyperalgesia in the articular and subcutaneous tissues because the neutrophil migration does not mediate the contribution of P2X3 and P2X2/3 receptors to the development of carrageenan-induced hyperalgesia in the subcutaneous tissue [32]. It further suggests that the inflammatory process is unique to tissue type and may depend on the cell types located in the tissue.
Finally, although sex differences in the action of opioids agonists [19, 20, 84], β-adrenoceptor antagonist [21], and P2X7 receptor antagonist (unpublished data) have been reported, we showed that there is no sex difference in the anti-hyperalgesic effect induced by the blockade of the P2X3 and P2X2/3 receptors in the rat’s knee joint. Similarly, the mechanical analgesia induced by kappa opioid agonist asimadoline is not sex dependent [85]. Therefore, taken together, these findings reinforce our previous suggestion that sex differences in pain and analgesia depend, at least in part, on the particular receptor type under study.
In summary, we have shown in the current study that in the carrageenan-induced knee joint synovitis model, the endogenous ATP through P2X3 and P2X2/3 receptors activation plays a crucial role in the development of carrageenan-induced articular hyperalgesia in the knee joint of males and estrus females in a similar manner. Moreover, during knee joint inflammation, the expression of P2X3 receptors is increased in chondrocytes, suggesting that their activation may contribute to the inflammatory process. The essential role played by P2X3 and P2X2/3 receptors in the development of carrageenan-induced articular hyperalgesia is dependent on neutrophil migration and on previous pro-inflammatory cytokines release in the knee joint. Taken together, these findings suggest that selective antagonists for the P2X3 and P2X2/3 receptors could be potential targets for drug development for the symptoms treatment of inflammatory joint diseases.
References
Felson DT (2006) Clinical practice. Osteoarthritis of the knee. N Engl J Med 354(8):841–848. doi:10.1056/NEJMcp051726
Nevitt MC, Felson DT (1996) Sex hormones and the risk of osteoarthritis in women: epidemiological evidence. Ann Rheum Dis 55(9):673–676
Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G (2005) A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr Cartil 13(9):769–781. doi:10.1016/j.joca.2005.04.014
Hunter DJ (2009) Insights from imaging on the epidemiology and pathophysiology of osteoarthritis. Radiol Clin N Am 47(4):539–551
Rocha FA, Aragao AG Jr, Oliveira RC, Pompeu MM, Vale MR, Ribeiro RA (1999) Periarthritis promotes gait disturbance in zymosan-induced arthritis in rats. Inflamm Res 48(9):485–490
Berenbaum F (2011) Osteoarthritis year 2010 in review: pharmacological therapies. Osteoarthr Cartil 19(4):361–365. doi:10.1016/j.joca.2011.01.019
Buell G, Collo G, Rassendren F (1996) P2X receptors: an emerging channel family. Eur J Neurosci 8(10):2221–2228
Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A (1995) Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature 377(6548):432–435
Seino D, Tokunaga A, Tachibana T, Yoshiya S, Dai Y, Obata K, Yamanaka H, Kobayashi K, Noguchi K (2006) The role of ERK signaling and the P2X receptor on mechanical pain evoked by movement of inflamed knee joint. Pain 123(1–2):193–203
Shinoda M, Ozaki N, Asai H, Nagamine K, Sugiura Y (2005) Changes in P2X3 receptor expression in the trigeminal ganglion following monoarthritis of the temporomandibular joint in rats. Pain 116(1–2):42–51
Teixeira JM, Oliveira MC, Nociti FH Jr, Clemente-Napimoga JT, Pelegrini-da-Silva A, Parada CA, Tambeli CH (2010) Involvement of temporomandibular joint P2X3 and P2X2/3 receptors in carrageenan-induced inflammatory hyperalgesia in rats. Eur J Pharmacol 645(1–3):79–85. doi:10.1016/j.ejphar.2010.06.008
Bradbury EJ, Burnstock G, McMahon SB (1998) The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci 12(4–5):256–268. doi:10.1006/mcne.1998.0719
Burnstock G, Knight GE (2004) Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol 240:31–304. doi:10.1016/S0074-7696(04)40002-3
Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford AP (2006) Pharmacology of P2X channels. Pflugers Arch 452(5):513–537
Inoue K, Denda M, Tozaki H, Fujishita K, Koizumi S (2005) Characterization of multiple P2X receptors in cultured normal human epidermal keratinocytes. J Invest Dermatol 124(4):756–763. doi:10.1111/j.0022-202X.2005.23683.x
Glass R, Townsend-Nicholson A, Burnstock G (2000) P2 receptors in the thymus: expression of P2X and P2Y receptors in adult rats, an immunohistochemical and in situ hybridisation study. Cell Tissue Res 300(2):295–306
Sun Y, Chai TC (2004) Up-regulation of P2X3 receptor during stretch of bladder urothelial cells from patients with interstitial cystitis. J Urol 171(1):448–452. doi:10.1097/01.ju.0000099660.46774.3c
Varani K, De Mattei M, Vincenzi F, Tosi A, Gessi S, Merighi S, Pellati A, Masieri F, Ongaro A, Borea PA (2008) Pharmacological characterization of P2X1 and P2X3 purinergic receptors in bovine chondrocytes. Osteoarthr Cartil 16(11):1421–1429. doi:10.1016/j.joca.2008.03.016
Clemente JT, Parada CA, Veiga MC, Gear RW, Tambeli CH (2004) Sexual dimorphism in the antinociception mediated by kappa opioid receptors in the rat temporomandibular joint. Neurosci Lett 372(3):250–255. doi:10.1016/j.neulet.2004.09.048
Clemente-Napimoga JT, Pellegrini-da-Silva A, Ferreira VH, Napimoga MH, Parada CA, Tambeli CH (2009) Gonadal hormones decrease temporomandibular joint kappa-mediated antinociception through a down-regulation in the expression of kappa opioid receptors in the trigeminal ganglia. Eur J Pharmacol 617(1–3):41–47. doi:10.1016/j.ejphar.2009.06.036
Favaro-Moreira NC, Parada CA, Tambeli CH (2012) Blockade of beta(1)-, beta(2)- and beta(3)-adrenoceptors in the temporomandibular joint induces antinociception especially in female rats. Eur J Pain 16(9):1302–1310. doi:10.1002/j.1532-2149.2012.00132.x
Gomis A, Meini S, Miralles A, Valenti C, Giuliani S, Belmonte C, Maggi CA (2013) Blockade of nociceptive sensory afferent activity of the rat knee joint by the bradykinin B2 receptor antagonist fasitibant. Osteoarthr Cartil 21(9):1346–1354. doi:10.1016/j.joca.2013.03.013
Tonussi CR, Ferreira SH (1992) Rat knee-joint carrageenin incapacitation test: an objective screen for central and peripheral analgesics. Pain 48(3):421–427
Valenti C, Giuliani S, Cialdai C, Tramontana M, Maggi CA (2010) Anti-inflammatory synergy of MEN16132, a kinin B(2) receptor antagonist, and dexamethasone in carrageenan-induced knee joint arthritis in rats. Br J Pharmacol 161(7):1616–1627. doi:10.1111/j.1476-5381.2010.00995.x
Rosland JH (1991) The formalin test in mice: the influence of ambient temperature. Pain 45(2):211–216
Zimmermann M (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16(2):109–110
Eng J (2003) Sample size estimation: how many individuals should be studied? Radiology 227(2):309–313. doi:10.1148/radiol.2272012051
Ekundi-Valentim E, Santos KT, Camargo EA, Denadai-Souza A, Teixeira SA, Zanoni CI, Grant AD, Wallace J, Muscara MN, Costa SK (2010) Differing effects of exogenous and endogenous hydrogen sulphide in carrageenan-induced knee joint synovitis in the rat. Br J Pharmacol 159(7):1463–1474. doi:10.1111/j.1476-5381.2010.00640.x
De-Melo JD, Tonussi CR, D'Orleans-Juste P, Rae GA (1998) Articular nociception induced by endothelin-1, carrageenan and LPS in naive and previously inflamed knee-joints in the rat: inhibition by endothelin receptor antagonists. Pain 77(3):261–269
Tonussi CR, Ferreira SH (1999) Tumour necrosis factor-alpha mediates carrageenin-induced knee-joint incapacitation and also triggers overt nociception in previously inflamed rat knee-joints. Pain 82(1):81–87
Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, Subieta A, Van Biesen T, Cartmell J, Bianchi B, Niforatos W, Kage K, Yu H, Mikusa J, Wismer CT, Zhu CZ, Chu K, Lee CH, Stewart AO, Polakowski J, Cox BF, Kowaluk E, Williams M, Sullivan J, Faltynek C (2002) A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci U S A 99(26):17179–17184
Oliveira MC, Pelegrini-da-Silva A, Tambeli CH, Parada CA (2009) Peripheral mechanisms underlying the essential role of P2X3,2/3 receptors in the development of inflammatory hyperalgesia. Pain 141(1–2):127–134. doi:10.1016/j.pain.2008.10.024
Marcondes FK, Bianchi FJ, Tanno AP (2002) Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol 62(4A):609–614
Smith MS, Freeman ME, Neill JD (1975) The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 96(1):219–226. doi:10.1210/endo-96-1-219
Butcher RL, Collins WE, Fugo NW (1974) Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology 94(6):1704–1708. doi:10.1210/endo-94-6-1704
Spornitz UM, Socin CD, Dravid AA (1999) Estrous stage determination in rats by means of scanning electron microscopic images of uterine surface epithelium. Anat Rec 254(1):116–126. doi:10.1002/(SICI)1097-0185(19990101)254:1<116::AID-AR15>3.0.CO;2-X
Ando A, Hagiwara Y, Onoda Y, Hatori K, Suda H, Chimoto E, Itoi E (2010) Distribution of type a and B synoviocytes in the adhesive and shortened synovial membrane during immobilization of the knee joint in rats. Tohoku J Exp Med 221(2):161–168
Ando A, Hagiwara Y, Tsuchiya M, Onoda Y, Suda H, Chimoto E, Itoi E (2009) Increased expression of metalloproteinase-8 and -13 on articular cartilage in a rat immobilized knee model. Tohoku J Exp Med 217(4):271–278
Hagiwara Y, Saijo Y, Chimoto E, Akita H, Sasano Y, Matsumoto F, Kokubun S (2006) Increased elasticity of capsule after immobilization in a rat knee experimental model assessed by scanning acoustic microscopy. Ups J Med Sci 111(3):303–313
Safieh-Garabedian B, Poole S, Allchorne A, Winter J, Woolf CJ (1995) Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br J Pharmacol 115(7):1265–1275
Everse J, Everse KE, Grisham MB (1991) Peroxidases in chemistry and biology. CRC Press, Boca Raton
Bradley PP, Priebat DA, Christensen RD, Rothstein G (1982) Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 78(3):206–209
Torres-Chavez KE, Sanfins JM, Clemente-Napimoga JT, Pelegrini-Da-Silva A, Parada CA, Fischer L, Tambeli CH (2012) Effect of gonadal steroid hormones on formalin-induced temporomandibular joint inflammation. Eur J Pain 16(2):204–216. doi:10.1016/j.ejpain.2011.06.007
McGaraughty S, Wismer CT, Zhu CZ, Mikusa J, Honore P, Chu KL, Lee CH, Faltynek CR, Jarvis MF (2003) Effects of A-317491, a novel and selective P2X3/P2X2/3 receptor antagonist, on neuropathic, inflammatory and chemogenic nociception following intrathecal and intraplantar administration. Br J Pharmacol 140(8):1381–1388
Beigi R, Kobatake E, Aizawa M, Dubyak GR (1999) Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am J Phys 276(1 Pt 1):C267–C278
Campwala H, Fountain SJ (2013) Constitutive and agonist stimulated ATP secretion in leukocytes. Communicative & integrative biology 6(3):e23631. doi:10.4161/cib.23631
Dubyak GR, el-Moatassim C (1993) Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Phys 265(3 Pt 1):C577–C606
Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, Di Virgilio F (1997) Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol 159(3):1451–1458
Filippini A, Taffs RE, Agui T, Sitkovsky MV (1990) Ecto-ATPase activity in cytolytic T-lymphocytes. Protection from the cytolytic effects of extracellular ATP. J Biol Chem 265(1):334–340
Mizumoto N, Mummert ME, Shalhevet D, Takashima A (2003) Keratinocyte ATP release assay for testing skin-irritating potentials of structurally diverse chemicals. J Invest Dermatol 121(5):1066–1072. doi:10.1046/j.1523-1747.2003.12558.x
Sikora A, Liu J, Brosnan C, Buell G, Chessel I, Bloom BR (1999) Cutting edge: purinergic signaling regulates radical-mediated bacterial killing mechanisms in macrophages through a P2X7-independent mechanism. J Immunol 163(2):558–561
Kumahashi N, Naitou K, Nishi H, Oae K, Watanabe Y, Kuwata S, Ochi M, Ikeda M, Uchio Y (2011) Correlation of changes in pain intensity with synovial fluid adenosine triphosphate levels after treatment of patients with osteoarthritis of the knee with high-molecular-weight hyaluronic acid. Knee 18(3):160–164. doi:10.1016/j.knee.2010.04.013
Ryan LM, Rachow JW, McCarty DJ (1991) Synovial fluid ATP: a potential substrate for the production of inorganic pyrophosphate. J Rheumatol 18(5):716–720
Maldonado M, Nam J (2013) The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed Res Int 2013:284873. doi:10.1155/2013/284873
Picher M, Graff RD, Lee GM (2003) Extracellular nucleotide metabolism and signaling in the pathophysiology of articular cartilage. Arthritis Rheum 48(10):2722–2736. doi:10.1002/art.11289
Chowdhury TT, Akanji OO, Salter DM, Bader DL, Lee DA (2008) Dynamic compression influences interleukin-1beta-induced nitric oxide and prostaglandin E2 release by articular chondrocytes via alterations in iNOS and COX-2 expression. Biorheology 45(3–4):257–274
Dray A, Read SJ (2007) Arthritis and pain. Future targets to control osteoarthritis pain. Arthritis Res Ther 9(3):212. doi:10.1186/ar2178
Dowd E, McQueen DS, Chessell IP, Humphrey PP (1998) P2X receptor-mediated excitation of nociceptive afferents in the normal and arthritic rat knee joint. Br J Pharmacol 125(2):341–346. doi:10.1038/sj.bjp.0702080
Lowther DA, Gillard GC (1976) Carrageenin-induced arthritis. I. The effect of intraarticular carrageenin on the chemical composition of articular cartilage. Arthritis Rheum 19(4):769–776
Leong WS, Russell RG, Caswell AM (1994) Stimulation of cartilage resorption by extracellular ATP acting at P2-purinoceptors. Biochim Biophys Acta 1201(2):298–304
Fiorito S, Magrini L, Adrey J, Mailhe D, Brouty-Boye D (2005) Inflammatory status and cartilage regenerative potential of synovial fibroblasts from patients with osteoarthritis and chondropathy. Rheumatology (Oxford) 44(2):164–171. doi:10.1093/rheumatology/keh431
Pearle AD, Scanzello CR, George S, Mandl LA, DiCarlo EF, Peterson M, Sculco TP, Crow MK (2007) Elevated high-sensitivity C-reactive protein levels are associated with local inflammatory findings in patients with osteoarthritis. Osteoarthr Cartil 15(5):516–523. doi:10.1016/j.joca.2006.10.010
Smith MD, Triantafillou S, Parker A, Youssef PP, Coleman M (1997) Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol 24(2):365–371
Gong K, Shao W, Chen H, Wang Z, Luo ZJ (2011) Rat model of lumbar facet joint osteoarthritis associated with facet-mediated mechanical hyperalgesia induced by intra-articular injection of monosodium iodoacetate. Journal of the Formosan Medical Association = Taiwan yi zhi 110(3):145–152. doi:10.1016/S0929-6646(11)60024-7
Rocha FA, Silva FS Jr, Leite AC, Leite AK, Girao VC, Castro RR, Cunha FQ (2011) Tadalafil analgesia in experimental arthritis involves suppression of intra-articular TNF release. Br J Pharmacol 164(2b):828–835. doi:10.1111/j.1476-5381.2011.01469.x
Orita S, Koshi T, Mitsuka T, Miyagi M, Inoue G, Arai G, Ishikawa T, Hanaoka E, Yamashita K, Yamashita M, Eguchi Y, Toyone T, Takahashi K, Ohtori S (2011) Associations between proinflammatory cytokines in the synovial fluid and radiographic grading and pain-related scores in 47 consecutive patients with osteoarthritis of the knee. BMC Musculoskelet Disord 12:144. doi:10.1186/1471-2474-12-144
Garnero P, Thompson E, Woodworth T, Smolen JS Rapid and sustained improvement in bone and cartilage turnover markers with the anti-interleukin-6 receptor inhibitor tocilizumab plus methotrexate in rheumatoid arthritis patients with an inadequate response to methotrexate: results from a substudy of the multicenter double-blind, placebo-controlled trial of tocilizumab in inadequate responders to methotrexate alone. Arthritis Rheum 62(1):33–43. doi:10.1002/art.25053
Grunke M, Schulze-Koops H (2006) Successful treatment of inflammatory knee osteoarthritis with tumour necrosis factor blockade. Ann Rheum Dis 65(4):555–556. doi:10.1136/ard.2006.053272
Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, Smolen JS, Weisman M, Emery P, Feldmann M, Harriman GR, Maini RN (2000) Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study group. N Engl J Med 343(22):1594–1602. doi:10.1056/NEJM200011303432202
Tanaka Y, Takeuchi T, Amano K, Saito K, Hanami K, Nawata M, Fukuyo S, Kameda H, Kaneko Y, Kurasawa T, Nagasawa H, Hoshi D, Sato E, Yamanaka H (2014) Effect of interleukin-6 receptor inhibitor, tocilizumab, in preventing joint destruction in patients with rheumatoid arthritis showing inadequate response to TNF inhibitors. Mod Rheumatol 24(3):399–404. doi:10.3109/14397595.2013.843757
Takagi N, Mihara M, Moriya Y, Nishimoto N, Yoshizaki K, Kishimoto T, Takeda Y, Ohsugi Y (1998) Blockage of interleukin-6 receptor ameliorates joint disease in murine collagen-induced arthritis. Arthritis Rheum 41(12):2117–2121. doi:10.1002/1529-0131(199812)41:12<2117::AID-ART6>3.0.CO;2-P
Williams RO, Feldmann M, Maini RN (1992) Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci U S A 89(20):9784–9788
Guerne PA, Carson DA, Lotz M (1990) IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J Immunol 144(2):499–505
Lotz M, Terkeltaub R, Villiger PM (1992) Cartilage and joint inflammation. Regulation of IL-8 expression by human articular chondrocytes. J Immunol 148(2):466–473
Hakim AW, Dong XD, Svensson P, Kumar U, Cairns BE (2009) TNFalpha mechanically sensitizes masseter muscle afferent fibers of male rats. J Neurophysiol 102(3):1551–1559. doi:10.1152/jn.00326.2009
Obreja O, Biasio W, Andratsch M, Lips KS, Rathee PK, Ludwig A, Rose-John S, Kress M (2005) Fast modulation of heat-activated ionic current by proinflammatory interleukin 6 in rat sensory neurons. Brain 128(Pt 7):1634–1641. doi:10.1093/brain/awh490
Cunha FQ, Lorenzetti BB, Poole S, Ferreira SH (1991) Interleukin-8 as a mediator of sympathetic pain. Br J Pharmacol 104(3):765–767
Lorenzetti BB, Veiga FH, Canetti CA, Poole S, Cunha FQ, Ferreira SH (2002) Cytokine-induced neutrophil chemoattractant 1 (CINC-1) mediates the sympathetic component of inflammatory mechanical hypersensitivitiy in rats. Eur Cytokine Netw 13(4):456–461
Weidler C, Holzer C, Harbuz M, Hofbauer R, Angele P, Scholmerich J, Straub RH (2005) Low density of sympathetic nerve fibres and increased density of brain derived neurotrophic factor positive cells in RA synovium. Ann Rheum Dis 64(1):13–20. doi:10.1136/ard.2003.016154
Jones AK, al-Janabi MA, Solanki K, Sobnack R, Greenwood A, Doyle DV, Britton KE, Huskisson EC (1991) In vivo leukocyte migration in arthritis. Arthritis Rheum 34(3):270–275
Cunha TM, Verri WA Jr, Schivo IR, Napimoga MH, Parada CA, Poole S, Teixeira MM, Ferreira SH, Cunha FQ (2008) Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukoc Biol 83(4):824–832. doi:10.1189/jlb.0907654
Edwards SW, Hallett MB (1997) Seeing the wood for the trees: the forgotten role of neutrophils in rheumatoid arthritis. Immunol Today 18(7):320–324
Moon SJ, Park MK, Oh HJ, Lee SY, Kwok SK, Cho ML, Ju JH, Park KS, Kim HY, Park SH (2010) Engagement of toll-like receptor 3 induces vascular endothelial growth factor and interleukin-8 in human rheumatoid synovial fibroblasts. Korean J Intern Med 25(4):429–435. doi:10.3904/kjim.2010.25.4.429
Cai BB, Cairns BE, Sessle BJ, Hu JW (2001) Sex-related suppression of reflex jaw muscle activity by peripheral morphine but not GABA. Neuroreport 12(16):3457–3460
Binder W, Carmody J, Walker J (2000) Effect of gender on anti-inflammatory and analgesic actions of two kappa-opioids. J Pharmacol Exp Ther 292(1):303–309
Acknowledgments
The authors thank the Central Microscopy Research Facility (CMRF) and Jessica Danielson, Sandra J. Kolker, and Lynn Rasmussen (University of Iowa, Iowa City, IA, USA) for technical assistance. This work was supported by Grants from Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP, Brazil (2009/16854-3 and 2010/05381-4) and from National Institutes of Health - NIH, USA (AR053509, AR052316, and AR061371).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Teixeira, J.M., Bobinski, F., Parada, C.A. et al. P2X3 and P2X2/3 Receptors Play a Crucial Role in Articular Hyperalgesia Development Through Inflammatory Mechanisms in the Knee Joint Experimental Synovitis. Mol Neurobiol 54, 6174–6186 (2017). https://doi.org/10.1007/s12035-016-0146-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0146-2