Abstract
Gait coordination is generated by neuronal inter-connections between central pattern generators in the spinal cord governed by cortical areas. Malfunction of central vestibular processing areas generates vestibular symptoms in the absence of an identifiable peripheral vestibular system lesion. Walking in the dark enforces a coordinated afference primarily from the vestibular and somatosensory systems. We hypothesized that patients with aberrant central vestibular processing would demonstrate unique gait characteristics, and have impaired gait coordination compared with those patients with abnormal peripheral vestibular function and healthy controls. One-hundred and eighteen subjects were recruited. Peripheral vestibular function was determined based on laboratory and clinical examinations. Patients with abnormal central vestibular processing had normal peripheral vestibular function. Subjects were instructed to walk at a comfortable pace during three visual conditions; eyes open, eyes open and closed intermittently, and eyes closed. Both patient groups showed a similar spatiotemporal gait pattern, significantly different from the pattern of the healthy controls. However, only the central vestibular patient group had an abnormal coordination of gait as measured by the phase coordination index (PCI). There were no significant interactions between the groups and walking conditions. Peripheral vestibular deficits impair gait though our data suggest that it is the central processing of such peripheral vestibular information that has a greater influence. This impairment may be related to a neural un-coupling between the brain and central pattern generator of the spinal cord based on the abnormal PCI, which seems to be a good indicator of the integrity of this linkage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Healthy human gait is symmetric (Sadeghi et al. 2000) and exhibits anti-phased left–right stepping, ensuring an inter-limb coordination to maximize both balance control (Plotnik et al. 2007) and energy expenditure (Ellis et al. 2013). Neuronal inter-connections between central pattern generators (CPGs) in the spinal cord are the presumed neuronal substrate responsible for inter-limb coordination of muscle activation based on models of fictive locomotion that display reciprocal motor output without descending efferent or ascending afferent information (Dietz 2003; Duysens and Van de Crommert 1998). However, these spinal mechanisms (CPGs) are not the sole provider for motor coordination because complex activities (e.g., locomotion) demand behavioral diversity and require real time (i.e., cycle-by-cycle) sensory feedback to best navigate the environment (Rossignol et al. 2006). This diversity and real-time feedback is assured via mediation from cortical regions (Cramer and Keller 2006) and descending pathways that supply unique neuromodulators to activate the CPG circuits (Marder and Bucher 2001). Specifically, CPGs are activated from the brainstem command centers and the locomotor regions within the mesopontine and diencephalon, primarily via reticulospinal neurons (Grillner et al. 2008).
Inter-limb coordination is a hallmark of healthy motor control. The development of inter-limb coordination is dependent on spatiotemporal coupling of limb motion, as occurs when humans engage in activities such as locomotion or playing a musical instrument. Limb coordination is governed by a process known as neural coupling, which implies neuro-anatomical and neuro-physiological links between the spinal cord and the brain with the purpose of rhythmic motor output (Arya and Pandian 2014). One of the descending tracts critical for mediation of locomotion is the vestibulo-spinal tract, which has been shown to contribute spatiotemporal characteristics during unique components of the gait cycle (i.e., heel strike, mid-stance, toe off). For example, galvanic vestibular stimulation delivered to healthy young adults during heel contact, mid-stance, or toe off leads to significant change in the magnitude and timing of their foot placement in response to the stimulation (Bent et al. 2004). Based on animal models, Shik and Orlovsky (1976) showed that activation within the vestibular nucleus modifies the lower limb muscle activation (i.e., phasic excitation or suppression) during the gait cycle. Evidence for this in patients with impaired vestibular function reveals an abnormal gait manifest as shortened steps and a wider base of support relative to healthy control subjects. This becomes more evident when these same subjects walk in a less common direction (i.e., backward) (Davalos-Bichara et al. 2014). In addition, patients with impaired vestibular function present reduced rhythmicity of gait (Davalos-Bichara et al. 2014; Perring and Summers 2007).
The role of the vestibular system in gait rhythmicity
The vestibular system is critical for rhythmic motion. Recently, Zimmerman and Barlow (2012) showed that preterm infants adapt their respiratory rate due to vestibular stimulation. The authors revealed that exposing infants to a horizontal, linear vestibular stimuli significantly increased the chest wall cycling motion thereby increasing the number of breaths per minute. Interestingly, the respiratory rate was influenced more by the acceleration magnitude and not the frequency of the horizontal, linear stimuli. This effect was presumed to occur via strong otolith output that reset the respiratory CPG via vestibulo-spinal descending input given the correlation between acceleration magnitude and respiration rate—the higher acceleration led to the highest respiratory change (Zimmerman and Barlow 2012). Gait coordination too, like respiration, is a rhythmic, sequential, and simultaneous motion that should be considered a critical parameter of walking performance.
Bilateral coordination of gait (BCG) is a useful measure of gait variability as it considers rhythmicity between the right and left legs as a function of gait control. BCG is measured using the phase coordination index (PCI), which quantifies the long-term consistency and accuracy in generating anti-phased left–right stepping. Lower PCI values reflect a consistent and accurate limb phasing, while higher values indicate an impaired coordination of gait (Plotnik et al. 2007). An abnormally high BCG is implicated in the risk for falling and in cognitive decline, as well as being correlated to unique neurologic pathologies (Plotnik et al. 2007, 2011a, b; Meijer et al. 2011). For example, stroke patients have an impaired BCG based on being 427% less accurate and 209% less consistent in walking coordination with a 314% elevated PCI compared with healthy controls (Meijer et al. 2011). The role of vestibular function on bilateral gait coordination is unknown.
Central processing of vestibular afference
Previous data reveal that patients experiencing the symptom of dizziness without an identifiable vestibular cause have abnormal dynamic visual acuity and abnormal spatiotemporal gait characteristics similar with those that have an identifiable peripheral vestibular pathology (Schubert et al. 2002; Davalos-Bichara et al. 2014). Relative to controls, however, the patients with vestibular symptoms, not due to peripheral end organ disease, have more robust gait differences than those with an identifiable vestibular pathology (Davalos-Bichara et al. 2014). This suggests that central processing of peripheral vestibular input has a greater influence on gait. Others have shown a central inhibition of vestibular afference during fast walking and running, that is instead disinhibited during slow and normal walking (Jahn et al. 2000; Brandt et al. 1999; Brandt 2000).
Several cortical areas process vestibular afference including the vestibular nuclei, thalamus, parieto-insular vestibular cortex (Kirsch et al. 2016), temporo-peri-Sylvian vestibular cortex, and right superior temporal gyrus (Tarnutzer et al. 2013). Many of these regions are congruent with visual information (Frank et al. 2014). Mal-functions within these central vestibular processing areas do generate vestibular symptoms in the absence of evidence for a peripheral vestibular system lesion based on objective clinical and laboratory testing. For example, patients with confirmed vestibular migraine have a false perception of their body orientation in space and are unable to detect their vertical orientation (related to gravity) as quickly as healthy controls (Wang and Lewis 2016), putatively due to underestimating their vestibular input (Crane 2012).
Dynamic sensory reweighting
Vestibular, visual, and proprioceptive inputs represent the primary afferent contributions for posture and gait, integrated in the brain to interpret the complex sensory environments in which we live (Horak et al. 1990; Kavounoudias et al. 1998; Angelaki et al. 2009). Each of these three sensory inputs integrate within the sensorimotor cortex to ensure safe and functional posture. The integration of sensory afference must be modifiable in real time to adjust to a dynamic environment. Postural modulation in healthy subjects is both sensory and context-dependent acting in a linear manner when there is no conflict within or between the sensory inputs. In this situation, the unique sensory afference sums to a single corrective postural torque. In the case of inaccurate information or dynamic conditions, the integration processes become non-linear and the brain shifts its reliance to the most accurate afferent source, which is then used as the primary contributor to postural control (Peterka 2002). In the case of bilateral vestibular loss, patients are unable to maintain their balance in conditions of sensory conflict between vision and proprioception illustrating their “rigid” linear processing of postural integration without an ability to rely on vestibular input to control their balance (Nashner et al. 1982; Horak et al. 1989; Peterka 2002). Interestingly, postural control also is challenged when moving from an unstable to stable context, further indicating the dynamic nature of the sensory reweighting process (Peterka and Loughlin 2004). Recently, Assländer and Peterka (2016) investigated the sensory reweighting process during dynamic conditions of postural control. To do this, they altered both visual and proprioceptive cues by either removing or re-introducing them (lights turned on or off, sway referencing on or off). They found that the dynamic stability (1) was influenced by the contribution of a passive torque generated from abrupt changes in sway referencing (muscles and tendons were not stretched relative to the actual body sway); (2) was impaired during each transition time (on and off was equally disruptive); (3) benefits from anticipation.
In the present study, we explored spatiotemporal characteristics, rhythmicity and bilateral coordination of gait in healthy controls and patients with vestibular symptoms that did/did not have identifiable peripheral or central vestibular pathology. We used three unique visual conditions [eyes open (EO); eyes open and closed (EOEC); eyes closed (EC)], to examine the sensory reweighting process during walking. We defined ‘vestibular patients’ as having an objectively identifiable peripheral vestibular dysfunction and ‘non-vestibular patients’ as having similar symptoms yet no objective, identifiable peripheral or central vestibular pathology (‘non-vestibular’). We hypothesized: (1) both patients groups would show a gait pattern that is less coordinated (i.e., higher PCI values) and less rhythmic with worse spatiotemporal characteristics than healthy controls; (2) non-vestibular patients would be less coordinated and less rhythmic than the vestibular patients but have the same spatiotemporal characteristics; and (3) a positive interaction effect between vestibular function (vestibular, non-vestibular, healthy subjects) and walking conditions (EO, EOEC, EC).
Materials and methods
Subjects
One-hundred and eighteen subjects were recruited from the outpatient otolaryngology clinic at the Johns Hopkins School of Medicine. Eighty-three were patients with symptoms of vestibular pathology that included dizziness, imbalance, and/or nausea. Of the 83, 61 had identifiable peripheral vestibular pathology (14–85 years), and 22 patients had no identifiable peripheral vestibular pathology (12–83 years) based on both clinical and laboratory vestibular function examination. Patients with identifiable vestibular pathophysiology (vestibular) included vestibular hypofunction, benign paroxysmal positional vertigo (BPPV), and Meniere’s disease based on abnormal video head impulse testing, abnormal ocular and cervical vestibular evoked myogenic potential testing, abnormal videonystagmography, or abnormal clinical examination (Table 1).
Of the 22 with no identifiable peripheral vestibular pathology (non-vestibular, normal vestibular function based on clinical and laboratory examination), n = 17 had vestibular migraine based on accepted criteria (Lempert et al. 2012) and n = 5 had no identifiable central or peripheral vestibular cause, only symptoms. Of these five patients, two had a history of migraine headache only, one had sustained a fall and hit her head 12 days prior but MRI and CT scans of the head were normal and no mild traumatic brain injury diagnosis was made; the final two patients had no medical history related to their symptoms. Thirty-five healthy controls were also recruited (21–80 years). Healthy controls had no vestibular symptoms. Subjects with orthopedic, neurological or cognitive impairments were excluded. All study participants gave informed consent, as approved by the Johns Hopkins Institutional Review Board.
Data collection
Subjects walked at their comfortable pace during three visual conditions: eyes open (EO), eyes open and closed intermittently (EOEC), and eyes closed (EC). The instructions for each condition were: (EO)—“walk with your eyes open until you cross the line on the floor”; (EOEC)—“walk one step with your eyes open and two with your eyes closed until you cross the line on the floor”; (EC)—“walk all the way with your eyes closed until we tell you to stop. We will prevent you from falling or walking into any objects”. For safety, one of the investigators walked beside the patient during each condition. In each condition, the subjects performed two walks each, a distance of 10 m. A 6.7-m GAITRite™ electronic walkway system (CIR systems, Inc., Franklin, NJ, USA) was placed in the walking path.
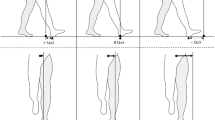
All data were measured and calculated from data obtained using the GAITRite™ system (Fig. 1). Phase coordination index (PCI) and gait asymmetry (GA) were calculated using the raw data of step and stride time, and processed with Matlab software (Math Works, Inc., Cambridge, MA, USA). GA was calculated as a logarithm of the time spent in each step, which considers the effect of any deviation from a straight path:
Raw GAITRite™ data illustrating step and stride of representative individuals during EOEC condition. Purple footprints represent the right leg; green footprints represent the left leg. Blue arrows indicate the walking direction. Note, the patients use more steps, illustrating shorter steps and strides. In addition, the patients display a greater variability in step length and width than the healthy control. The dashed lines help illustrate these differences
The PCI was calculated by determining the left–right stepping phase φ i (ideally φ i = 180°) for each stride (Plotnik et al. 2007). Lower PCI values reflect a more consistent and accurate BCG (phase generation), while higher values indicating an impaired BCG (Plotnik et al. 2007; Meijer et al. 2011).
Statistical analysis
PCI values in patients with vestibular pathology is unknown. Therefore, we estimated sample size based on data published in healthy young and older adults (Plotnik et al. 2007). In that study, the PCI for healthy young adults was 2.47 ± 0.58 (26.3 ± 0.5 years old) and for older adults was 3.30 ± 0.67 (69.1 ± 1.3 years old). Therefore, using the mean difference between groups of 0.83 with a standard deviation 0.6, we would need a minimum of 9 subjects in each group to be able to reject the null hypothesis with probability (power) of 0.8, presuming α < 0.05 (PS Power and Sample Size Calculations, version 3.0).
Statistical analysis was completed using SPSS (version 22, Chicago, IL, USA). Mean and one standard deviation values of the dependent variables were calculated for each of the GAITRite walkway data, measured during each walking condition (EO, EOEC, EC). There was no difference in diagnoses (UVH, BVH, BPPV and Meniere’s) for any of the patients’ data, thus the four sub-groups were combined to one group, the ‘vestibular’ group. There was no difference in left and right foot data and thus the two walking repetitions of each condition were combined and are reported below. All data were normally distributed. A 3 × 3 ANOVA assessed the main and interaction effects of vestibular function (Vestibular, Non-vestibular, Healthy) and walking conditions (EO, EOEC, EC). We controlled for age in all statistical computations, with age being evaluated at 58.6 years. The level of statistical significance was set at P ≤ 0.05. In cases where the model provided a statistically significant effect, a post hoc analysis compared between the different groups and the different walking conditions. The dependent variables were: gait speed, step time, step length, stride time, stride length, stride width, stride time variability, stride length variability, stride width variability PCI, GA and left–right phasing.
Results
There were significant main effects of group and walking condition on the spatiotemporal parameters of gait (Table 2). We also report main effects on gait rhythmicity (Table 3) and gait coordination. There was no significant interaction between groups and walking conditions. We report below, the evidence that the non-vestibular patients alone were significantly less accurate with greater inconsistency in gait coordination than healthy controls.
Spatiotemporal parameters of gait
We found a significant main effect of groups and walking conditions for all spatiotemporal gait parameters excluding the effect of groups on stride width. There were, however, no significant interactions between groups and walking conditions in any of the spatiotemporal parameters. The pattern of spatiotemporal gait behavior to the visual input manipulation is similar across groups (Fig. 2a).
Pattern of gait behavior between the groups to the varied visual conditions. A1–A4—spatiotemporal parameters, B1–B3—rhythmicity parameters, C1, C2—coordination parameters. All three groups display similar spatiotemporal and rhythmicity parameters. Only the non-vestibular patients use a behavioral pattern different from the healthy control or vestibular patient groups with significantly less accuracy and consistency in their BCG
Post hoc analysis for groups revealed that the healthy controls showed significantly larger gait speed, step time, step length, stride time, and stride length compared with both patient groups.
There were, however, no differences in any spatiotemporal gait variables between the two patient groups. Post hoc analysis for walking conditions revealed that each group showed significantly faster gait speed while walking with EO compared with walking either EOEC or EC. On the contrary, stride width was significantly wider only when subjects walked with their eyes closed. All other parameters were significantly different between all walking conditions.
Gait rhythmicity
We found a significant main effect of groups for stride length CV and stride width CV. A significant main effect of walking conditions were found for stride time CV, stride length CV, and stride width CV. No significant interaction for groups and walking conditions was found, the groups show a similar pattern of gait rhythmicity to the visual input manipulation (Fig. 2b).
Post hoc analysis for groups revealed that the healthy controls were more consistent than the two patient groups in stride length CV. However, stride width CV was significantly less consistent for the non-vestibular group than the healthy and vestibular groups. Post hoc analysis for walking conditions revealed that stride time CV and stride width CV were significantly more consistent for the EO condition compared with the EOEC and EC conditions. Stride length CV was significantly different between all conditions.
Gait coordination
A significant main effect of groups were found only for PCI (ANOVA P = 0.042) but not for GA or mean right–left phasing (Fig. 2c). A significant main effect of walking conditions (ANOVA P = 0.036) was also found only for PCI; healthy controls 4.93 ± 5.76 (EO), 5.65 ± 5.35 (EOEC), 4.90 ± 4.98 (EC); vestibular group 4.92 ± 4.24 (EO), 8.72 ± 7.61 (EOEC), 6.78 ± 4.20(EC); non-vestibular 5.94 ± 5.55 (EO), 7.40 ± 6.78 (EOEC), 8.19 ± 4.58 (EC). There was no significant interaction between groups and walking conditions (ANOVA P = 0.291). Post hoc analysis for groups revealed that only the non-vestibular patients were significantly less accurate and less consistent in their BCG than the healthy controls, without any significant differences between the vestibular and control groups (Fig. 3). Post hoc analysis for walking conditions revealed a statistically significant difference between EOEC and EO conditions only.
Post hoc analysis for walking conditions revealed that the EOEC condition was significantly disruptive to accuracy and consistency of gait (relative to the EO condition) for all groups.
Discussion
The critical result of this study is that only those patients with a putative central vestibular cause for their symptoms (non-vestibular patients) were less accurate and less consistence in their BCG than the healthy controls. In contrast, we found no significant differences between the peripheral vestibular and control groups for either GA or BCG. Additionally, we found no significant interaction between the extent of vestibular function and walking during different visual conditions (EO, EOEC, and EC) for any gait parameter, suggesting that altering visual afference is a commonly disruptive condition. Together, our results suggest that the central processing of vestibular information has a more significant role in gait than the peripheral vestibular input.
Vestibular influence on gait
CPGs, as part of the neural control of human gait have a significant role for BCG as can be inferred from patients suffering from spinal cord injury (Duysens and Van de Crommert 1998). Additionally, animal studies confirm that motor cortex mediates the CPGs (Cramer and Keller 2006). Our data (PCI) suggest that the BCG for humans with peripheral vestibular dysfunction is no different than healthy controls, suggesting that in this case—the mediation of BCG is preserved via central and not peripheral vestibular processes. In contrast, when putative central vestibular areas are impaired, the BCG is pathologically affected. Together, this suggests that central vestibular processing is a control parameter regulating gait coordination. Schniepp et al. (2017) in their recent review suggest a topodiagnostic scheme for motor control within the cerebellum. The scheme indicates the Flocculus as an essential structure for afferent vestibular integration and the intermediate cerebellar zones as important mediators of gait coordination. Future investigations of the Flocculus, intermediate and lateral zones of the cerebellum might reveal pathology and related impairments unique to patients with central vestibular pathology (including those with vestibular migraine).
There is a functional risk to having an abnormally increased PCI—an increased risk for falls (Plotnik et al. 2007, 2011a, b; Plotnik and Hausdorff 2008; Meijer et al. 2011). Recent evidence suggests that gait coordination (as measured by the PCI) and gait rhythmicity are modifiable. Using stochastic vestibular stimulation, Wuehr et al. improved gait rhythmicity (stride time and length CV) and BCG (PCI) (reduction of 26 and 8.8%, respectively), presumably via improved peripheral vestibular afference. However, the mean spatiotemporal gait characteristics were unaffected by the same stimulation, suggesting that the improvement is incomplete (Wuehr et al. 2016). This new information, coupled with our data, leads us to propose using PCI as an indicator of a more severe deterioration of gait abilities and therefore a better marker of fall risk.
Vestibular rehabilitation is the standard of care to address the related gait instability for patients that report vestibular symptoms (Hall et al. 2016). Our data suggest that adding coordination exercises during walking may be of value for “non-vestibular” patients, particularly those suffering from vestibular migraine, who may still be at risk for fall at times they are not experiencing active vestibular symptoms. In addition, it would be useful to establish a means to translate our results into a simple clinical screening test. Cohen et al. (2012) showed that although vestibular patients and healthy subjects differed in performance of tandem-walk, walking with head turns, and functional mobility tests; the groups were not sufficiently different on these tests for easy use as screening tests.
Sensory reweighting
Sensory reweighting processes during walking appear to be different than the processes during standing. Describing healthy controls, Peterka (2002) showed an increased reliance on vestibular input when either the visual field or the supporting surface were manipulated. This increased dependence was presumed ‘vestibular’ as the visual and somatosensory contributors for balance were no longer reliable, yet the healthy controls were still able to maintain balance in these conditions. Healthy controls do this by inversely modifying their postural sway amplitudes relative to the stimulus sway amplitude; at lower stimuli (0.5°–2°) they use an amplitude of sway that is greater than the stimulus, during higher frequency sway (4° and 8°) they use a smaller amplitude sway to keep their balance. Therefore, healthy controls are able to ‘reweight’ the incoming sensory information to maintain balance by relying on the most accurate afferent source, which in these conditions is from the vestibular system. Peterka describes this as being a non-linear postural control response. In contrast, patients with bilateral vestibular loss are unable to keep their balance as the magnitude of the visual/somatosensory disruption changes, suggesting that these patients have a “rigid” or linear processing of postural control. The patients are unable to change their behavioral postural response with the change of the stimuli causing a loss of balance and thus are unable to ‘reweight’ the afferent information for functional relevance. Creath et al. (2002) showed similar postural sway behaviors of healthy subjects and patients with vestibular loss. In contrast to the sensory reweighting using altered visual information in standing, our data show that both of the vestibular patient groups and the healthy controls use a similar behavioral pattern (Fig. 2) while walking. This lack of significant interaction between the three groups and visual walking conditions suggests that altered visual information, and in the case of the vestibular group—altered vestibular information, does not appear detrimental to the sensory reweighting process. This suggests sensory reweighting processes are contextually dependent, differing between gait and standing. This may relate to the fact that multiple regions within the central nervous system assimilate visual and vestibular information (Frank et al. 2014). Another reason for the apparent incongruence of sensory integration between gait and posture may relate to directionality. During walking, the brain’s primary gait task involves moving in one direction—forward; whereas during standing, the brain must prepare for multidirectional sway. Recent data reveal balance is optimized when the plane of vestibular stimulation is harmonious with the direction of whole body motion; a strong linear relationship (high coherence) exists between EMG of the soleus muscle and direction of perceived vestibular stimulation (Forbes et al. 2016). Therefore, patients with vestibular dysfunction and healthy subjects alike may use similar processes of sensory integration for gait given the vestibular stimulus (linear acceleration) and engaged muscles (linear forward direction) are congruent.
Limitations
The precise pathophysiology causing vestibular symptoms in the non-vestibular patients is unknown, yet we have presumed the symptoms relate to abnormal central processing of peripheral vestibular information. Secondly, although we statistically controlled for age, the patients within the vestibular group were significantly older than the non-vestibular group and using an age-matched design might reveal larger differences in gait. Finally, our patient groups were combined from several sub-groups, reflective of the true clinical presentation. However, more homogeneous groups with larger sample sizes might better distinguish the unique contribution of the peripheral vestibular labyrinth and central processing of that content on the different measures of gait.
Conclusion
Peripheral vestibular deficits impair gait though our data suggest the central processing of peripheral vestibular information that has a greater influence. Vestibular patients react similarly to healthy subjects during manipulation of visual input during walking, thus sensory reweighting during walking appears different from standing. Impaired central processing of vestibular afference appears related to a neural un-coupling between the brain and CPGs of the spinal cord based on abnormal PCI, which seems to be a good indicator of the integrity of this linkage.
References
Angelaki DE, Gu Y, DeAngelis GC (2009) Multisensory integration: psychophysics, neurophysiology, and computation. Curr Opin Neurobiol 19:452–458
Arya KN, Pandian S (2014) Interlimb neural coupling: implications for poststroke hemiparesis. Ann Phys Rehabil Med 57(9–10):696–713
Assländer L, Peterka RJ (2016) Sensory reweighting dynamics following removal and addition of visual and proprioceptive cues. J Neurophysiol 116(2):272–285
Bent LR, Inglis JT, McFadyen BJ (2004) When is vestibular information important during walking? J Neurophysiol 92(3):1269–1275
Brandt T (2000) Vestibulopathic gait: you’re better off running than walking. Curr Opin Neurol 13(1):3–5
Brandt T, Strupp M, Benson J (1999) You are better off running than walking with acute vestibulopathy. Lancet 354(9180):746
Cohen HS, Mulavara AP, Peters BT, Sangi-Haghpeykar H, Bloomberg JJ (2012) Tests of walking balance for screening vestibular disorders. J Vestib Res 22(2):95–104
Cramer NP, Keller A (2006) Cortical control of a whisking central pattern generator. J Neurophysiol 96(1):209–217
Crane BT (2012) Direction specific biases in human visual and vestibular heading perception. PLoS One 7(12):e51383
Creath R, Kiemel T, Horak F, Jeka JJ (2002) Limited control strategies with the loss of vestibular function. Exp Brain Res 145(3):323–333
Davalos-Bichara M, Zuniga MG, Agrawal Y, Carey JP, Schubert MC (2014) Forward and backward locomotion in individuals with dizziness. Gait Posture 40(4):499–503
Dietz V (2003) Spinal cord pattern generators for locomotion. Clin Neurophysiol 14(8):1379–1389
Duysens J, Van de Crommert HWAA (1998) Neural control of locomotion; Part 1: the central pattern generator from cats to humans. Gait Posture 7:131–141
Ellis RG, Howard KC, Kram R (2013) The metabolic and mechanical costs of step time asymmetry in walking. Proc Biol Sci 280(1756):20122784
Forbes PA, Luu BL, Van der Loos HF, Croft EA, Inglis JT, Blouin JS (2016) Transformation of vestibular signals for the control of standing in humans. J Neurosci 36(45):11510–11520
Frank SM, Baumann O, Mattingley JB, Greenlee MW (2014) Vestibular and visual responses in human posterior insular cortex. J Neurophysiol 112(10):2481–2491
Grillner S, Wallén P, Saitoh K, Kozlov A, Robertson B (2008) Neural bases of goal-directed locomotion in vertebrates-an overview. Brain Res Rev 57(1):2–12
Hall CD, Herdman SJ, Whitney SL, Cass SP, Clendaniel RA, Fife TD, Furman JM, Getchius TS, Goebel JA, Shepard NT, Woodhouse SN (2016) Vestibular rehabilitation for peripheral vestibular hypofunction: an evidence-based clinical practice guideline. J Neurol Phys Ther 40(2):124–155
Horak FB, Diener HC, Nashner LM (1989) Influence of central set on human postural responses. J Neurophysiol 62(4):841–853
Horak FB, Nashner LM, Diener HC (1990) Postural strategies associated with somatosensory and vestibular loss. Exp Brain Res 82(1):167–177
Jahn K, Strupp M, Schneider E, Dieterich M, Brandt T (2000) Differential effects of vestibular stimulation on walking and running. NeuroReport 11(8):1745–1748
Kavounoudias A, Roll R, Roll JP (1998) The plantar sole is a ‘dynamometric map’ for human balance control. NeuroReport 9(14):3247–3252
Kirsch V, Keeser D, Hergenroeder T, Erat O, Ertl-Wagner B, Brandt T, Dieterich M (2016) Structural and functional connectivity mapping of the vestibular circuitry from human brainstem to cortex. Brain Struct Funct 221(3):1291–1308
Lempert T, Olesen J, Furman J, Waterston J, Seemungal B, Carey J, Bisdorff A, Versino M, Evers S, Newman-Toker D (2012) Vestibular migraine: diagnostic criteria. J Vestib Res 22(4):167–172
Marder E, Bucher D (2001) Central pattern generators and the control of rhythmic movements. Curr Biol 11(23):R986–R996
McGarvie LA, MacDougall HG, Halmagyi GM, Burgess AM, Weber KP, Curthoys IS (2015) The video head impulse test (vHIT) of semicircular canal function age dependent normative values of VOR gain in healthy subjects. Front Neurol 8(6):154
Meijer R, Plotnik M, Zwaaftink EG, van Lummel RC, Ainsworth E, Martina JD, Hausdorff JM (2011) Markedly impaired bilateral coordination of gait in post-stroke patients: is this deficit distinct from asymmetry? A cohort study. J Neuroeng Rehabil 8:23
Nashner LM, Black FO, Wall C 3rd (1982) Adaptation to altered support and visual conditions during stance: patients with vestibular deficis. J Neurosci 2(5):536–544
Perring S, Summers T (2007) Laboratory-free measurement of gait rhythmicity in the assessment of the degree of impairment and the effectiveness of rehabilitation in individuals with vertigo resulting from vestibular hypofunction. Physiol Meas 28:697–705
Peterka RJ (2002) Sensorimotor integration in human postural control. J Neurophysiol 88(3):1097–1118
Peterka RJ, Loughlin PJ (2004) Dynamic regulation of sensorimotor integration in human postural control. J Neurophysiol 91(1):410–423
Plotnik M, Hausdorff JM (2008) The role of gait rhythmicity and bilateral coordination of stepping in the pathophysiology of freezing of gait in Parkinson’s disease. Mov Dis 23:444–450
Plotnik M, Giladi N, Hausdorff JM (2007) A new measure for quantifying the bilateral coordination of human gait: effects of aging and Parkinson’s disease. Exp Brain Res 181:561–570
Plotnik M, Dagan Y, Gurevich T, Giladi N, Hausdorff JM (2011a) Effects of cognitive function on gait and dual tasking abilities in patients with Parkinson’s disease suffering from motor response fluctuations. Exp Brain Res 208:169–179
Plotnik M, Giladi N, Dagan Y, Hausdorff JM (2011b) Postural instability and fall risk in Parkinson’s disease: impaired dual tasking, pacing, and bilateral coordination of gait during the “ON” medication state. Exp Brain Res 210:529–538
Rossignol S, Dubuc R, Gossard JP (2006) Dynamic sensorimotor interactions in locomotion. Physiol Rev 86(1):89–154
Sadeghi H, Allard P, Prince F, Labelle H (2000) Symmetry and limb dominance in able-bodied gait: a review. Gait Posture 12:34–45
Schniepp R, Möhwald K, Wuehr M (2017) Gait ataxia in humans: vestibular and cerebellar control of dynamic stability. J Neurol. doi:10.1007/s00415-017-8482-3
Schubert MC, Herdman SJ, Tusa RJ (2002) Vertical dynamic visual acuity in normal subjects and patients with vestibular hypofunction. Otol Neurotol 23(3):372–377
Shik ML, Orlovsky GN (1976) Neurophysiology of locomotor automatism. Physiol Rev 56(3):465–501
Tarnutzer AA, Lasker AG, Zee DS (2013) Continuous theta-burst stimulation of the right superior temporal gyrus impairs self-motion perception. Exp Brain Res 230(3):359–370
Wang J, Lewis RF (2016) Abnormal tilt perception during centrifugation in patients with vestibular migraine. J Assoc Res Otolaryngol 17(3):253–258
Wuehr M, Nusser E, Decker J, Krafczyk S, Straube A, Brandt T, Jahn K, Schniepp R (2016) Noisy vestibular stimulation improves dynamic walking stability in bilateral vestibulopathy. Neurology 86(23):2196–2202
Zimmerman E, Barlow SM (2012) The effects of vestibular stimulation rate and magnitude of acceleration on central pattern generation for chest wall kinematics in preterm infants. J Perinatol 32(8):614–620
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gimmon, Y., Millar, J., Pak, R. et al. Central not peripheral vestibular processing impairs gait coordination. Exp Brain Res 235, 3345–3355 (2017). https://doi.org/10.1007/s00221-017-5061-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-017-5061-x