Abstract
This study investigated the effects of postural set on the cortical response evoked by an external perturbation to human upright stance. Postural set was manipulated by providing either predictable or unpredictable whole body perturbations which required balance corrections to maintain upright stability. Unpredictable perturbations evoked a large negative potential (e.g., CZ: −19.9±5.1 μV) that was similar in timing (e.g., CZ: 98.9±5.5 ms) and shape to that reported in previous studies. This large negative potential was not discernable for perturbations with predictable onset timing and direction in spite of the presence of significant compensatory balance reactions. Importantly, when a surprise perturbation was presented following a series of predictable perturbations, the large negative potential occurred on this trial even though subjects expected a predictable stimulus onset. This suggests that the large negative potential was dependent on a dissociation between expected and actual stimuli rather than on a tonic central state defined by task conditions. These results suggest that cortical events may be linked to error detection that is independent of sensory or motor events associated with evoked balance reactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human central nervous system (CNS) must process and integrate sensory information from a variety of sources to generate precise motor adjustments to recover balance. Evidence suggests that the cortex, in some way, plays a role in modifying this control (Ackermann et al. 1986; Dietz et al. 1984, 1985a, b; Dimitrov et al. 1996; Duckrow et al. 1999; Quant et al. 2004a, b; Staines et al. 2001). However, the understanding of the involvement of the cortex in the initiation and execution of peripherally triggered compensatory balance reactions remains unclear.
Research suggests that changes in postural set can alter balance recovery strategies. For example, the predictability associated with the perturbation has been shown to modify peripherally triggered balance adjustments (Badke et al. 1987; Beckley et al. 1991; Gilles et al. 1999; Horak et al. 1989; Maki and Whitelaw 1993; Maki et al. 1994). In these studies, it is possible that the contribution of the cortex to balance recovery is altered by changes in postural set. Thus, a possible way to investigate the role of the cortex in balance control is to manipulate the postural set associated with the impending balance perturbation.
While balance recovery strategies have been well described for predictable and unpredictable balance perturbations, only the cortical activity evoked by unpredictable perturbations has been thoroughly documented. Following an unpredictable perturbation to stability, previous work has revealed multi-component cortical responses involving a small variable positive potential, termed the P1 response, followed by a large stable negative potential, termed the N1 response. The N1 response is widely distributed with maximal responses at fronto-central electrode sites and occurs at approximately 100–200 ms after the onset of the disturbance (Dietz et al. 1984, 1985a, b; Dimitrov et al. 1996; Duckrow et al. 1999; Quant et al. 2004a, b; Staines et al. 2001). Previous work has suggested that the P1 response represents the earliest non-specific cortical response to instability (Dietz et al. 1984) and that the N1 response reflects the processing of the balance disturbance at the level of the cortex (Dietz et al. 1984, 1985a, b). Unlike the P1 response, the N1 response can be modified by subject age (Duckrow et al. 1999), stance width (Dimitrov et al. 1996), perturbation magnitude (Staines et al. 2001), a concurrent peripheral stimulus (Staines et al. 2001), and a concurrent cognitive task (Quant et al. 2004a). These changes in N1 magnitude may reflect differences in the cortical processing of sensory information related to instability.
In contrast to studies that have noted stimulus dependence, Dietz et al. (1985b) observed that N1 magnitudes were reduced up to one-third for self-induced balance perturbations (when the subject was allowed to initiate the onset of the perturbation) compared to experimenter-induced balance perturbations (when the experimenter randomly initiated the onset of the perturbation). In their study, Dietz and colleagues also reported that N1 magnitudes were re-established when a series of experimenter-induced perturbations were again presented after the series of self-induced perturbations. Since their study focused on self-induced perturbations, it is possible that this attenuation was associated with a state dependent change in cognitive state (Quant et al. 2004a). However, it is also possible that these results provide indirect support for the idea that predictability of perturbation onset is an important determinant of N1 amplitude.
The view that N1 may be dependent on stimulus predictability is important since it appears to contrast the apparent role of stimulus characteristics on N1 response features. It is proposed that a possible explanation for the influence of stimulus predictability is the role of N1 in error detection rather than as simply a scaled response to sensory and motor events. As a result of previous work, the current study set out to address two questions: (1) is the N1 response attenuated or removed when the stimulus associated with an external perturbation is entirely predictable and (2) does this modulation reflect a response that is dependent on error detection or merely a tonic inhibition associated with central state conditions, such as those introduced by task instructions.
The first goal of the study was to examine the role of postural set on N1 and balance responses evoked by predictable and unpredictable perturbations that were delivered by an experimenter. The temporal and directional predictability associated with the impending perturbation were manipulated. The timing and direction of the perturbation was known in advance in the predictable case but not known in the unpredictable case. In light of the work by Dietz et al. (1985b), we hypothesized that stimulus predictability would result in attenuated cortical responses. Specifically, the N1 magnitude would be smaller in response to predictable compared to unpredictable perturbations, with no change in the timing of the response.
The second goal of the study was to examine whether any modulation of the N1 response was the result of tonic central state changes (as defined by task conditions) or was dependent on the relationship between the expected and actual stimulus (e.g., the error signal). We proposed that a tonic central state effect, like the attenuation observed by Quant et al. (2004a), would have an effect on the amplitude of the N1 response when subjects expected a predictable stimulus. This would suggest that a single “surprise trial” performed after a series of predictable stimuli would also be characterized by an inhibited N1 response due to the tonic central state at the time of the unpredictable stimulation. In contrast, if the N1 amplitude was determined by the association between the signal and the expectation (e.g., error signal), then the surprise trial would be characterized by a large N1 response due to the mismatch between the central state expectation and the actual stimulus. To examine this, a surprise unpredictable perturbation was presented after the series of cued predictable perturbations. It was hypothesized that the N1 magnitude for this surprise trial would not be attenuated supporting the idea that the N1 was a reflection of a comparison between anticipated and actual states rather than the result of tonic modulation linked to pre-perturbation postural set. This result may reflect a more general role for the N1 response with certain parallels to the error-related negativity responses observed for incorrect responses in decision-making paradigms (see, for example, Pailing and Segalowitz 2004; Yasuda et al. 2004).
Materials and methods
Subjects
Eight healthy young adults (age range 17–32 years; five females) were recruited for the study. Exclusion criteria included any neurological, musculoskeletal, or cardiorespiratory conditions that could influence balance or mobility. Prior to the experiment, all subjects provided informed written consent. This study was approved by the local ethics review board.
Protocol
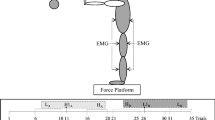
Subjects were instructed to maintain upright stance while standing with their eyes closed and their feet placed shoulder-width apart. Single transient horizontal perturbations to the trunk were applied to the subject using a padded customized device by the same experimenter. Forward trunk perturbations were applied between the scapulas, backward trunk perturbations were applied at the sternum, and sideways (left or right) trunk perturbations were applied at the middle of the upper arm. The timing and magnitude of the perturbation was recorded using a force-sensitive resistor (FSR) that was attached to the end of the padded customized device. Perturbations were comparable between predictable and unpredictable conditions as there were no significant differences in the initial rise in force (measured within the first 20 ms) (t(7)=1.96, P=0.1070), the timing of peak force (t(7)=1.02, P=0.3567), or the magnitude of peak force (t(7)=2.09, P=0.0906) of the FSR. The magnitude of the perturbation was strong enough to evoke compensatory feet-in-place reactions but was not large enough to evoke compensatory stepping responses. The consistency of the applied perturbation was reflected, in part, by the consistent activation of automatic postural reactions from ankle muscles.
Subjects were exposed to three task conditions: (1) a series of perturbations that were unpredictable in their timing and direction (forward, backward, left or right) for 100% of the time (UNPREDICT), (2) a series of perturbations that were predictable in their timing and direction (always forward) for 100% of the time (PREDICT), and (3) a single forward perturbation that occurred at the end of a series of predictable perturbations which was unpredictable in onset timing (SURPRISE). To provide advanced warning of the impending perturbation during the PREDICT condition, an auditory cue was sounded immediately prior to the delivery of the perturbation. The SURPRISE perturbation was applied when subjects did not receive this auditory cue.
Two blocks of UNPREDICT AND PREDICT trials were performed. In order to average cortical activity, a minimum of 30 perturbations for the UNPREDICT condition (each block had a minimum of 15 forward, 5 backward, 5 left, and 5 right perturbations) and a minimum of 30 perturbations for the PREDICT condition (each block had a minimum of 15 forward perturbations) were collected for each subject. In some subjects, additional trials were included under some task conditions to ensure unpredictability and/or to make up for trials that we suspected during collection may need to be excluded. In total, 304 UNPREDICT trials and 338 PREDICT trials were collected from all subjects. Of these trials, 64 UNPREDICT trials (21% of trials) and 81 PREDICT trials (24% of trials) were excluded prior to statistical analyses. Trials were excluded based on the following criteria: (1) EMG latencies below 50 ms or above 300 ms, with the exception of two subjects whose EMG recordings were unreliable due to noise artifact and (2) if noise artifact appeared in any of the EEG, COP or EMG recordings. In addition to UNPREDICT and PREDICT conditions, seven of the eight subjects were exposed to a SURPRISE condition which consisted of a single forward perturbation that occurred following the last block of PREDICT trials. To minimize any order effect or fatigue, the presentation of PREDICT and UNPREDICT blocks was counterbalanced amongst the subjects and rest breaks were provided after each block of trials.
The focus of the study was directed to the comparison of only forward trunk perturbations between UNPREDICT, PREDICT, and SURPRISE conditions. The backward, left, and right trunk perturbations were only included in the UNPREDICT condition to prevent anticipatory postural responses.
Data collection and analysis
A scalp electrode cap (Quick-Cap, Neuromedical Supplies, Texas, USA) was used to record cortical activity. Analysis focused on midline electrode sites based on the International 10–20 system (FCZ, CZ, and CPZ). However, in order to determine the scalp distribution of task differences across multiple sites, other standard sites were also recorded (AFZ, FZ, F3, F4, FC3, FC4, C3, C4, CP3, CP4, PZ, P3, P4, and OZ). All electrodes were referenced to linked mastoids. Impedances were below 10 kohms for all cortical sites, and cortical data were amplified (×2,500), filtered (bandpass of 0.0001–30 Hz), and sampled at 500 Hz (Neuroscan v4.0, Neuromedical Supplies, Texas, USA). In order to use EMG onset latencies as the basis for excluding trials in six of the eight subjects, onset latencies were calculated for each trial. After the exclusion of trials based on EMG onset latencies, the remaining trials were visually inspected for any artefact. Trials with artifact in EEG, EMG or COP recordings were excluded. Dependent measures of N1 (Fig. 1) were the latency (time measured between perturbation onset and timing of peak amplitude) and the peak magnitude with respect to pre-perturbation baseline activity. Pre-perturbation cortical activity was calculated by averaging cortical data within a 200-ms time interval prior to perturbation onset. Dependent measures of N1 were determined by visual inspection of averaged data for each subject for the UNPREDICT condition and visual inspection of single trials for the SURPRISE condition. In most subjects, latencies of N1 during the PREDICT condition were not distinguishable from background cortical activity. As such, N1 peak magnitudes during the PREDICT condition were based on latencies for the UNPREDICT condition. P1 responses were not considered a part of the main analysis because of their inconsistent occurrence during the UNPREDICT condition; only three of the eight subjects had a detectable P1 response during this condition.
Grand average cortical activity recorded at CZ, anterior–posterior centre-of-pressure (AP COP) displacements, and left (L) and right (R) medial gastrocnemius (MG) electromyographic activity during unpredictable (UNPREDICT, solid black line) and predictable perturbations (PREDICT, solid grey line) (n=8 subjects). The N1 response is noted and the vertical grey dashed line indicates the onset of perturbation
To characterize postural responses, anterior–posterior (AP) centre-of-pressure (COP) displacements and bilateral medial gastrocnemius (MG) surface electromyographic (EMG) activity were recorded. AP COP displacements were recorded using an AMTI forceplate (AMTI model 0R6-5) on which subjects stood. Force-plate data were amplified (×4,000), low-pass filtered (cut-off at 10 Hz), and sampled at a rate of 500 Hz (Labview v6.0, National Instruments, Texas, USA). After data were visually inspected to be free of artefact, specific time points (start of first COP peak excursion and time to the first COP peak displacement) were systematically selected by visual inspection of averaged COP data. Initial velocities of the first COP peak displacement were calculated within the first 200 ms that followed the onset of the first COP peak excursion, and the magnitude of the first COP peak displacement was calculated with respect to the pre-perturbation COP position. The pre-perturbation COP position was calculated by averaging COP data within a 200-ms time interval prior to perturbation onset. To record MG activity, Ag/AgCl electrodes were placed 2 cm apart and aligned longitudinally in parallel with the muscle fibres of muscle bellies. The ground electrode was placed on the tibial shaft and impedances were less than 10 kohms. EMG data were amplified (×2,500), filtered (bandpass of DC-100 Hz), and sampled at 500 Hz (Neuroscan v4.0, Neuromedical Supplies). For each subject, a custom-built computer program (Labview v6.0, National Instruments) selected the onset latencies of EMG responses when full-wave rectified EMG activity exceeded a level equivalent to three standard deviations above pre-perturbation baseline activity. Pre-perturbation MG activity was calculated by averaging full-wave rectified MG activity within a 200-ms time interval prior to perturbation onset. Initial magnitudes of MG activity were also obtained by calculating the area of rectified EMG activity within the first 100 ms of muscle activation (reported as differences from pre-perturbation MG activity). Due to technical problems, EMG activity was only analysed in six of the eight subjects.
To determine if task differences were statistically different for N1, COP, and EMG measures, analyses involved paired t-tests with the alpha level set at 0.05. Only descriptive statistics for P1 responses were reported due to the low occurrence of this response.
Results
Characteristics of the N1 response
For all subjects, unpredictable forward perturbations applied to the trunk evoked large N1 responses (Fig. 1) that were maximal at CZ and FCZ cortical sites (Fig. 2). During unpredictable perturbations, average N1 latencies (± standard error, SE) with respect to perturbation onset were 98.9±5.5 ms (CZ), 98.9±6.6 ms (FCZ), and 99.3±7.5 ms (CPZ), and average N1 peak magnitudes (± SE) with respect to pre-perturbation activity were −19.9±5.1 μV (CZ), −22.1±5.0 μV (FCZ), and −7.6±2.8 μV (CPZ). When subjects were exposed to predictable forward perturbations, the average N1 peak magnitude was significantly reduced during the PREDICT condition when compared to the UNPREDICT condition [CZ: t(7)=5.73, P=0.0007; FCZ: t(7)=4.95, P=0.0016; CPZ: t(7)=8.28, P<0.0001; Figs. 1, 2]. Indeed, during the PREDICT condition, N1 peak magnitudes had a positive polarity instead of a negative polarity; average N1 peak magnitudes (± SE) were 6.4±2.5 μV (CZ), 5.0±2.9 μV (FCZ), and 9.3±2.0 μV (CPZ). The SURPRISE condition produced a large N1 magnitude that was not similar to the PREDICT condition, but more similar to the UNPREDICT condition (Fig. 3). Average N1 peak magnitudes (± SE) with respect to pre-perturbation activity (n=7 subjects) were −28.0±7.0 μV (CZ), −28.7±6.7 μV (FCZ), and −19.7±6.9 μV (CPZ). N1 latencies also appeared to be similar between SURPRISE and UNPREDICT conditions (Fig. 3). Average N1 latencies (± SE) with respect to perturbation onset (n=7 subjects) were 101.3±6.9 ms (CZ), 103.6±8.2 ms (FCZ), and 112.9±13.6 ms (CPZ).
Average perturbation-evoked cortical responses recorded from cortical sites of the International 10–20 system during unpredictable (UNPREDICT, solid black line) and predictable (PREDICT, solid grey line) perturbations for one subject. The N1 response was maximal over fronto-central electrode sites. The vertical grey dashed lines represent the onset of perturbation
Cortical activity (recorded at CZ) of all trials in one subject during predictable (top panel A, solid grey lines) and unpredictable (bottom panel B, solid grey lines) perturbations and cortical activity of a single trial during the SURPRISE condition (top and bottom panels, solid black tracing). The vertical grey dashed line indicates the onset of perturbation
Characteristics of the P1 response
As mentioned previously, during the UNPREDICT condition, P1 responses for FCZ, CZ, and CPZ sites were detectable in three of the eight subjects (Fig. 4). For those subjects, there were no observable task-related differences in the amplitude or timing of the P1 response. For the UNPREDICT condition, average P1 latencies (± SE) with respect to perturbation onset were 29.5±2.3 ms (CZ), 28.2±5.9 ms (FCZ), and 61.7±12.3 ms (CPZ), and average P1 peak magnitudes (± SE) with respect to pre-perturbation activity were 5.8±0.4 μV (CZ), 6.7±0.9 μV (FCZ), and 5.4±0.9 μV (CPZ). For the PREDICT condition, average P1 peak magnitudes (± SE) were 4.2±0.9 μV (CZ), 4.8±1.8 μV (FCZ), and 5.5±3.7 μV (CPZ).
Characteristics of the balance response: COP data
In contrast to task-related changes in N1 peak magnitudes, few differences were observed in the AP COP data. The pre-perturbation COP position was statistically different between UNPREDICT and PREDICT conditions (t(7)=2.43, P=0.0455; Fig. 1). The average (± SE) pre-perturbation COP position was −5.8±1.6 mm for the UNPREDICT condition and −9.8±1.9 mm for the PREDICT condition. The initial velocity of the first AP COP excursion after the perturbation was not statistically different between UNPREDICT and PREDICT conditions (t(7)=0.22, P=0.8305; Fig. 1). Furthermore, the peak magnitude of the first AP COP excursion was not statistically different between PREDICT and UNPREDICT conditions. However, this peak was delayed during PREDICT compared to UNPREDICT conditions (t(7)=2.52, P=0.0396; Fig. 1). Average COP peak latencies (± SE) with respect to perturbation onset were 469.0±8.1 ms (UNPREDICT) and 543.0±6.1 ms (PREDICT).
Characteristics of the balance response: EMG data
There were no task differences in EMG responses between UNPREDICT and PREDICT conditions (Fig. 1). Pre-perturbation EMG activity was not statistically different between UNPREDICT and PREDICT conditions (left MG: t(5)= 0.78, P=0.4714; right MG: t(5)=0.2, P=0.8497). There were no statistically significant differences in initial magnitudes of left MG activity (t(5)=1.22, P=0.2784) or right MG activity (t(5)=0.66, P=0.5382) after the perturbation between UNPREDICT and PREDICT conditions. Furthermore, there were no statistically significant differences in onset latencies of left MG (t(5)=1.82; P=0.1288) and right MG (t(5)=1.84, P=0.1252) between PREDICT and UNPREDICT conditions. Average left MG onset latencies (± SE) with respect to perturbation onset were 91.7±1.7 ms (UNPREDICT) and 122.3±4.5 ms (PREDICT), and average right MG onset latencies (± SE) with respect to perturbation onset were 89.4±1.7 ms (UNPREDICT) and 117.3±4.1 ms (PREDICT).
Discussion
This study examined the effects of postural set on the cortical activity observed in response to an external perturbation to human upright stance. Postural set was manipulated by providing either predictable or unpredictable whole-body perturbations that evoked feet-in-place balance corrections. Unpredictable perturbations evoked a large negative potential that was not discernible for predictable perturbations. When a surprise perturbation followed a series of predictable perturbations, the large negative potential was re-established. It is suggested that these findings indicate that the role of postural set is to inform the CNS of the forthcoming perturbation and N1 represents an “error signal” that may be used to influence subsequent control.
Characteristics of the cortical response
Effects of postural set: unpredictable compared to predictable perturbations
Cortical responses evoked by predictable compared to unpredictable external trunk perturbations were significantly different. Unpredictable perturbations evoked multi-component cortical responses that included an N1 response. This negative potential was maximal at CZ and FCZ cortical sites, but was also observed across the range of measured cortical sites (see Fig. 2). This response was comparable in magnitude to that reported for support surface perturbations (Quant et al. 2004a). In contrast, predictable perturbations did not evoke a discernible N1 response. In other words, a negative potential of similar latency to that observed for unpredictable perturbations was not detected for predictable perturbations (Figs. 1, 2). Dietz et al. (1985b) observed a reduction in N1 magnitude of approximately one-third for self-induced compared to experimenter-induced perturbations. In the current study, a postural set, wherein the direction and timing of the perturbation were known, generated a greater change with no N1 response discernable during predictable perturbations.
Although our analyses of cortical activity focused on the N1 response, it is noteworthy to mention the effects of postural set on the P1 response. First, the appearance of the P1 response was more variable than the N1 response as P1 responses were only observed for three of the eight subjects. For these subjects, the P1 response was similar in magnitude for predictable compared to unpredictable perturbations which suggests a similar primary sensory representation of the balance disturbance between the two conditions.
It is possible that prior knowledge of the characteristics associated with the perturbation might alter the role of the cortex in balance control. It has been suggested that the N1 response, following unpredictable balance perturbations, represents cortical processing of the balance disturbance in supra-spinal motor centres (Dietz et al. 1984, 1985a, b). More specifically, Dietz and colleagues suggest that the N1 response reflects sensory inflow and further processing of this information for the coordination of complex balance responses. Evidence of specific cortical involvement during periods of instability is illustrated by several studies. For example, Beloozerova et al. (2003), in an animal model, demonstrated that several neurons in the motor cortex were involved in maintaining balance on a tilting board. The work of Solopova et al. (2003), who applied transcranial magnetic stimulation to the motor cortex in humans during balance tasks on a normal support surface and a rocking platform, suggests that the role of the motor cortex in balance control changes with increased instability. Furthermore, Dimitrov et al. (1996) demonstrated increased N1 magnitudes during narrow stance when compared to normal or wide stance conditions. These findings suggest that the cortex, in some way, is involved in balance control and that this involvement may depend on the challenge to stability. Processing at the level of the cortex may change or be considered unnecessary especially if the characteristics associated with an impending balance perturbation are known. This may be especially true if actual perturbation characteristics match expected perturbation characteristics.
Effects of postural set: surprise perturbations
The N1 response evoked by a “surprise” perturbation was similar to that observed for unpredictable perturbations. Although, on average, slightly larger in magnitude, the N1 response to the surprise perturbation had a latency that was similar to the N1 response observed for unpredictable perturbations. Furthermore, this response was observed on a single trial for each subject and could be readily distinguished from the remainder of the predictable trials (Fig. 3).
In the predictable case, the CNS prepares for and expects the impending balance perturbation. It could be argued that sensory processing of the balance perturbation at the cortex is unnecessary as the characteristics of the disturbance are known and the system is pre-set to respond. In the surprise case, differences in the actual and expected consequences of the perturbation exist. If the system was operating in a state that had pre-planned the balance response to the perturbation, the system would need to be informed of these differences. A large N1 magnitude for this surprise trial would support the idea that the N1 was a reflection of a comparison between anticipated and actual states rather than the result of tonic modulation linked to pre-perturbation postural set.
Characteristics of the balance response
Effects of postural set: unpredictable compared to predictable perturbations
Balance responses evoked by both predictable and unpredictable perturbations were similar with two exceptions. Pre-perturbation COP data revealed a small but significant backward shift of 4.0 mm and the time to reach peak AP COP magnitude following the perturbation revealed a significant delay of 75 ms for predictable compared to unpredictable conditions. Significant shifts in pre-perturbation position have been observed in other studies (Maki and Whitelaw 1993; Maki et al. 1994). However, in the current study there were no significant differences in pre-perturbation EMG measures that accompanied this observation. Important to the interpretation of the present study was that the significant and rapid balance reactions evoked in the predictable perturbation conditions, in spite of the potential differences in pre-perturbation state, reflected the perturbation-evoked processing of the afferent signal arising from the perturbation. There was clearly a profound sensory input that was sufficient to drive large amplitude EMG activity and COP responses in both conditions. While previous studies have revealed some scaling of response amplitude over a broader range of stimulus intensity (Staines et al. 2001), the more subtle differences that may have occurred in sensory discharge are unlikely to have accounted for the profound attenuation (complete absence) of the N1 response in the current study.
Conclusion
The results of this study suggest that postural set influences the characteristics of the cortical contributions to the control of balance. The current findings, comparing responses to predictable and unpredictable stimuli and the reoccurrence of the stimulus in the surprise trial, reveal the link between the evoked negativity (N1) and unpredictable ‘errors’ in stability. Such a link between errors and evoked negativity may have parallels to the error negativity observed in other behavioural paradigms (Pailing and Segalowitz 2004; Yasuda et al. 2004). The modulating influence of central state that is linked to response predictability is especially relevant considering the large body of research that has shown differences in balance control with changes in postural set. For example, the predictability associated with the balance disturbance (Badke et al. 1987; Beckley et al. 1991; Gilles et al. 1999; Horak et al. 1989; Maki and Whitelaw 1993) or an anxiety or fear concerning falling (Brown and Frank 1997; Carpenter et al. 2004) has been shown to modify peripherally triggered balance adjustments. Furthermore, changes in central set or behavioural state have also been shown to modify anticipatory balance adjustments (Adkin et al. 2002; Brown and Frank 1987) and balance corrections during quiet standing tasks (Adkin et al. 2000; Carpenter et al. 1999, 2001). Future research will investigate the specific role of these cortical responses as a possible link to sensorimotor and/or cognitive events linked to the control of stability. Such understanding may be important to inform about the nature of the problem and possible interventions for individuals with balance problems, especially those individuals with cortical deficits (Perennou et al. 2000; Wolfson 2001).
References
Ackermann H, Diener HC, Dichgans J (1986) Mechanically evoked cerebral potentials and long-latency muscle responses in the evaluation of afferent and efferent long-loop pathways in humans. Neurosci Lett 66:233–238
Adkin AL, Frank JS, Carpenter MG, Peysar GW (2000) Postural control is scaled to level of postural threat. Gait Posture 12:87–93
Adkin AL, Frank JS, Carpenter MG, Peysar GW (2002) Fear of falling modifies anticipatory postural control. Exp Brain Res 143:160–170
Badke MB, Duncan PW, Di Fabio RP (1987) Influence of prior knowledge on automatic and voluntary postural adjustments in healthy and hemiplegic subjects. Phys Ther 67:1495–1500
Beckley DJ, Bloem BR, Remler MP, Roos RA, Van Dijk JG (1991) Long latency postural responses are functionally modified by cognitive set. Electroencephalogr Clin Neurophysiol 81:353–358
Beloozerova IN, Sirota MG, Swadlow HA, Orlovsky GN, Popova LB, Deliagina TG (2003) Activity of different classes of neurons of the motor cortex during postural corrections. J Neurosci 23:7844–7853
Brown JE, Frank JS (1987) Influence of event anticipation on postural actions accompanying voluntary movement. Exp Brain Res 67:645–650
Brown LA, Frank JS (1997) Postural compensations to the potential consequences of instability: kinematics. Gait Posture 6:89–97
Carpenter MG, Frank JS, Silcher CP (1999) Surface height effects on postural control: a hypothesis for a stiffness strategy for stance. J Vestib Res 9:277–286
Carpenter MG, Frank JS, Silcher CP, Peysar GW (2001) The influence of postural threat on the control of upright stance. Exp Brain Res 138:210–218
Carpenter MG, Frank JS, Adkin AL, Paton A, Allum JH (2004) Influence of postural anxiety on postural reactions to multi-directional surface rotations. J Neurophysiol 92(6):3255–3265
Dietz V, Quintern J, Berger W (1984) Cerebral evoked potentials associated with the compensatory reactions following stance and gait perturbation. Neurosci Lett 50:181–186
Dietz V, Quintern J, Berger W (1985a) Afferent control of human stance and gait: evidence for blocking of group I afferents during gait. Exp Brain Res 61:153–163
Dietz V, Quintern J, Berger W, Schenck E (1985b) Cerebral potentials and leg muscle e.m.g. responses associated with stance perturbation. Exp Brain Res 57:348–354
Dimitrov B, Gavrilenko T, Gatev P (1996) Mechanically evoked cerebral potentials to sudden ankle dorsiflexion in human subjects during standing. Neurosci Lett 208:199–202
Duckrow RB, Abu-Hasaballah K, Whipple R, Wolfson L (1999) Stance perturbation-evoked potentials in old people with poor gait and balance. Clin Neurophys 110:2026–2032
Gilles M, Wing AM, Kirker SG (1999) Lateral balance organisation in human stance in response to a random or predictable perturbation. Exp Brain Res 124:137–144
Horak FB, Diener HC, Nashner LM (1989) Influence of central set on human postural responses. J Neurophysiol 62:841–853
Maki BE, Whitelaw RS (1993) Influence of expectation and arousal on center-of-pressure responses to transient postural perturbations. J Vestib Res 3:25–39
Maki BE, McIlroy WE, Perry SD (1994) Compensatory responses to multi-directional perturbations. In: Taguchi K, Igarashi M, Mori S (eds) Vestibular and neural front: proceedings of the XIIth International Symposium on Posture and Gait, Matsumoto 3–7 May 1994. International Congress Series No. 1070. Elsevier, Amsterdam, pp 437–440
Pailing PE, Segalowitz SJ. (2004) The effects of uncertainty in error monitoring on associated ERPs. Brain Cogn 56:215–233
Perennou DA, Leblond C, Amblard B, Micallef JP, Rouget E, Pelissier J (2000) The polymodal sensory cortex is crucial for controlling lateral postural stability: evidence from stroke patients. Brain Res Bull 53:359–365
Quant S, Adkin AL, Staines WR, Maki BE, McIlroy WE (2004a) The effect of a concurrent cognitive task on cortical potentials evoked by unpredictable balance perturbations. BMC Neurosci 5:18
Quant S, Adkin AL, Staines WR, McIlroy WE (2004b) Cortical activation following a balance disturbance. Exp Brain Res 155:393–400
Solopova IA, Kazennikov OV, Deniskina NB, Levik YS, Ivanenko YP (2003) Postural instability enhances motor responses to transcranial magnetic stimulation in humans. Neurosci Lett 337:25–28
Staines WR, McIlroy WE, Brooke JD (2001) Cortical representation of whole-body movement is modulated by proprioceptive discharge in humans. Exp Brain Res 138:235–242
Wolfson L (2001) Gait and balance dysfunction: a model of the interaction of age and disease. Neuroscientist 7:178–183
Yasuda A, Sato A, Miyawaki K, Kumano H, Kuboki T (2004) Error-related negativity reflects detection of negative reward prediction error. Neuroreport 15:2561–2565
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adkin, A.L., Quant, S., Maki, B.E. et al. Cortical responses associated with predictable and unpredictable compensatory balance reactions. Exp Brain Res 172, 85–93 (2006). https://doi.org/10.1007/s00221-005-0310-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-005-0310-9