Abstract
To date, little work has focused on whether cognitive-task interference during postural response execution is influenced by the direction and/or magnitude of the perturbation applied. Hypothetically, the increased difficulty associated with a backward loss of balance could necessitate increased allocation of cognitive resources to counteract destabilizing forces. The current study investigated these relationships using a paradigm in which individuals performed a cognitive task (auditory Stroop task during quiet stance; baseline condition). In certain trials, a translation of the support surface was concurrently evoked (magnitude: small or large; direction: forward or backward) which required a postural response to maintain balance. Ten healthy young adults completed four blocks of these experimental trials (26 randomized trials/block). Postural stability during balance recovery was evaluated using the margin of stability (MoS), while Stroop task performance was based on reaction time cost (RTC) and differences between experimental conditions. Results showed no effect of perturbation direction on RTC, but there was an observed MoS increase at peak extrapolated center of mass excursion following a small perturbation evoked concurrently with the cognitive task. No effect of cognitive-task performance was detected for MoS during stepping strategies (followed large perturbations). Instead, increased RTC were observed relative to the fixed base of support responses. In general, young adults adopted a “posture-first” strategy, regardless of perturbation direction, reinforcing the importance of cognition in the maintenance of upright balance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When an external perturbation to upright balance is applied (e.g. support-surface translations, lean-and release method, etc.) the central nervous system (CNS) must initiate postural response(s) to correct for displacement of the center of mass (CoM) with respect to the boundaries of the base of support (BoS; see review by Maki and McIlroy 2007). These responses vary in complexity and typically involve either a fixed base of support (fBoS) or a change in support (∆BoS). To maintain a fixed base of support, muscle activation around the ankle and/or hip joints is required to generate a moment of sufficient magnitude to maintain equilibrium (Horak and Nashner 1986; Nashner et al. 1989). A change in support strategy may involve rapid execution of a step to alter the BoS (Maki and McIlroy 1997). The type of response evoked is dependent on numerous factors including the magnitude of the perturbation and environmental or physical constraints, which may restrict the size of the BoS. Recently emerging evidence has also suggested the importance of executive function in proper execution of these postural responses (see reviews by Maki and McIlroy 2007 and Bolton 2015).
Aside from direct methods of measuring cortical activity during perturbation-evoked postural responses, dual-task paradigms offer a means by which allocation of cognitive resources may be inferred. Cognitive tasks used within dual-task paradigms range from relatively simple, (probe reaction time tests: Chen et al. 1996; Lajoie et al. 1993; Wellmon et al. 2013) to attentionally demanding (Stroop tests, working memory tasks: Kerr et al. 1985; Schaefer et al. 2008; Siu et al. 2008; Worden and Vallis 2014). Unfortunately, observed dual-task effects vary in part due to the different cognitive tasks chosen in these paradigms (Ebersbach et al. 1995; Lajoie et al. 1993; Schaefer et al. 2008; Weerdesteyn et al. 2003; Worden and Vallis 2014). Furthermore, some studies have used experimental methods that result in a cognitive interference at the perceptual level (e.g. task requires visual attention to be divided between the motor and cognitive tasks) as well as the central response selection stages of dual-task performance (Chen et al. 1996; Worden et al. 2016). Other dual-task studies (usual versus narrow-base walking, Kelly et al. 2010; obstacle avoidance in young adults; Worden and Vallis 2014, 2016; obstacle avoidance in older adults during treadmill walking; Weerdesteyn et al. 2003; Potocanac et al. 2015) have used an auditory version of the Stroop task to examine dual task interference, although this cognitive paradigm has been used less often in perturbation-based studies (Etemadi et al. 2016). The auditory Stroop task still necessitates cognitive inhibition for correct performance, thus involving higher-order executive processes for correct performance. However, unlike many vision-based cognitive tasks, it does so without causing structural interference at the perceptual level (Kahneman and Chajczyk 1983; Morgan and Brandt 1989). Given the role of vision in quiet standing and reactive postural control (Dijkstra et al. 1994; Joseph Jilk et al. 2014), it is important to avoid the possibility of such interference during phases of experimental design.
Many previous studies have examined simultaneous performance of a cognitive task and postural response execution using perturbations in a single direction. Those utilizing multiple directions have either included them as a “catch trial” to reduce proactive changes in postural control or focused their analyses on only fBoS responses (Redfern et al. 2002; Norrie et al. 2002; Etemadi et al. 2016). In general, healthy young adults have been suggested to alter both cognitive task performance and postural control when executing a fBoS response; however, a ∆BoS requires additional cognitive input reflected by further reductions in cognitive performance despite slight changes in postural control (Brown et al. 1999; Brauer et al. 2002; Norrie et al. 2002; Little and Woollacott 2015; Etemadi et al. 2016). Whether these effects are dependent on perturbation direction is not entirely clear. Norrie et al. (2002) suggested that dual task interference affected postural control following only forward perturbations evoking a fBoS response to correct for the backward loss of balance. Given a backward loss of balance is a more difficult to counteract due to lack of visual input and reduced functional BoS (Maki et al. 1996), it is possible that they require greater allocation of cognitive resources towards the generation of appropriate postural responses. Extrapolating from this logic, it is possible that generation of a backward recovery step requires greater cognitive input relative to a forward recovery step, however, the influence of perturbation direction on dual-task interference is inconsistent within the literature (Norrie et al. 2002; Redfern et al. 2002).
Researchers interested in examining the mechanisms behind a loss of balance (fall) often use kinematic variables to quantify dynamic stability (Curtze et al. 2010; Hasson et al. 2008). One line of questioning in this area of study is whether we can identify if certain individuals are theoretically less stable and more likely to fall while dual-tasking (Bernard-Demanze et al. 2014; Lamoth et al. 2011). A method used to quantify dynamic stability in several gait-based dual-task studies (Bohm et al. 2012; Worden et al. 2016) was proposed by Hof et al. (2005). The stability metric (i.e. margin of stability; MoS) accounts for both CoM position and velocity with respect to the boundaries of the BoS. Given the dynamic nature of the aforementioned postural responses, this measure provides a robust quantification of an individual’s stability over the course of balance recovery.
Our lab is interested in exploring the attentional requirements of human movement; we are specifically interested in how executive function mental processes (necessary for central planning and cognitive processing) influence motor performance. To this end, we designed a challenging experimental task that would allow us to explore cognitive task performance during a reactive balance task that presented multi-directional support-surface perturbations. Any changes in cognitive or motor performance would be indicative of the important role that frontal and prefrontal lobes play in the generation of these postural response strategies. Thus, the purpose of the current study was to investigate how the following were affected in a challenging perturbation-based paradigm: (1) performance of the auditory Stroop task; (2) postural stability following a perturbation. Perturbations were altered in magnitude and direction to trigger postural responses of different complexity and difficulty (e.g. fBoS vs. ∆BoS). We hypothesized that cognitive task performance when responding to a small perturbation (fBoS) would reduce the MoS and increase Stroop response reaction time (Norrie et al. 2002; Etemadi et al. 2016). Conversely, responding to a large perturbation (i.e. reactive/recovery step or a ∆BoS) would result in greater reductions of cognitive task performance (compared to fBoS) with no changes in postural stability (Brauer et al. 2002). We further hypothesized that within each level of response (fBoS and ∆BoS), forward perturbations would result in larger decrements in task performance than the backward counterpart (Norrie et al. 2002). Please note, for this study we will use the term ‘perturbation direction’ to refer to the direction of support-surface translation. Secondary analysis focused on trunk pitch to explore the possibility of individuals altering trunk control and their postural strategy during the experimental trials.
Methods
Participants
Ten healthy young adults (5 male, 5 female; age 22.5 ± 1.78 years; height 1.71 ± 0.09 m; weight 72.4 ± 12.0 kg), free from self-reported musculoskeletal or neurological disorders that could impact postural control, participated in the current study. Individuals were also screened for potential hearing impairments that could influence their ability to perform the auditory Stroop task proficiently. Each individual provided informed, written consent to participate within the protocol as approved by the University of Guelph Research Ethics Board.
Perturbation and cognitive task
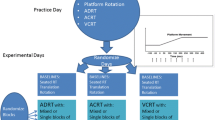
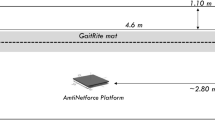
The perturbation task was designed to evoke either fBoS or ∆BoS postural responses via linear support-surface translations in one of four possible directions (forward, backward, left and right; Fig. 1). For clarity, we chose to focus this paper on responses following perturbations evoked in the sagittal plane (forward or backward) within the current study. Perturbations within each direction were of either a small or large magnitude based on values reported by Maki et al. (1996) and were produced using a 5 × 2 m robotic motion platform (Shelley Automation, Cambridge, ON, Canada). In brief, perturbations were a 0.30 s acceleration pulse (square-wave) and 0.30 s deceleration pulse of equivalent shape in succession (Maki et al. 1996). Small perturbations were used to evoke a fBoS response; large magnitudes evoked a ∆BoS via a single step. At the start of each trial, participants were asked to “stand comfortably with your feet hip width apart; respond as quickly and accurately as possible”. They were also informed that the floor may or may not move and were instructed to maintain their upright balance without stepping. These instructions were provided to encourage participants to maintain a fBoS following a small perturbation.
The cognitive task used was the auditory Stroop task (Worden et al. 2016; Fig. 1). Here, participants were instructed to identify the pitch of an audio cue (the word “high” or “low”) emitted from subject-facing speakers as quickly and accurately as possible. They were not to identify the word itself. Cues were either congruent (pitch matched the cue, e.g. word “high” spoken in high pitch) or incongruent (pitch did not match cue).
Experimental protocol
Participants were first provided with demonstrations of each perturbation and practice for the auditory Stroop task to ensure they understood the instructions provided. They then completed a series of four blocks, each consisting of 26 trials presented in randomized order; an outline of block structure is provided in Fig. 1. In short, each trial was either a perturbed stance trial with no Stroop task (‘catch trials’), a baseline (quiet stance + auditory Stroop task) or baseline + perturbation. Though evidence suggesting the role of cognition in quiet stance implies that our (quiet stance + auditory Stroop) trials are not a true single-task trials (Melzer et al. 2001; Redfern et al. 2017), we considered it Baseline task performance within the current paradigm (Patel and Bhatt 2015). This enabled us to examine whether the cognitive load associated with our experimental conditions was greater than that during quiet standing. For all trials that included the auditory Stroop task participants were instructed to not prioritize one task over the other, but rather to “Complete the cognitive task to the best of their ability while maintaining their balance”. Eight possible perturbations were used for these scenarios and were triggered simultaneously with the onset of the auditory Stroop audio cue.
During testing, the positions of retro-reflective markers affixed to the individual according to Winter et al. (1998) were captured using a 12-camera motion capture system (100 Hz; Optitrack, NaturalPoint, Corvallis, OR, USA). Markers were either fixed to anatomical landmarks or located (as a quartet) on a rigid body affixed to the following segments: feet, thighs, and pelvis. Relevant anatomical positions (e.g. heels, toes, metatarsals) were digitized relative to these rigid bodies in post-processing. Audio signals were recorded using a custom lapel microphone (1000 Hz) to quantify Stroop task response time. Response accuracy was tracked by a secondary researcher present during testing.
Postural stability and trunk/step parameters
Analyses of kinematic data were completed within Visual3D software (Version 6, C-Motion Inc., Germantown, MD, USA). Data were interpolated and low-pass filtered using a zero-lag, fourth-order, dual-pass filter (Butterworth; cutoff = 6 Hz). Position of the whole-body CoM was estimated using a modified 13-segment version of the anthropometric model provided by Winter et al. (1998). Extrapolated CoM position (xCoM) was then calculated using the method described in Hof et al. (2005). In brief, the position of the xCoM was considered the position of the CoM extrapolated according to the direction and magnitude of its velocity at that corresponding instance. For trials containing a perturbation, the sagittal and frontal MoS was calculated within the lab Cartesian coordinate system as
where BoSmax and BoSmin are, respectively, the maximum and minimum boundaries of the BoS within the relevant plane. Using this method, a negative MoS would indicate position of the xCoM had exceeded either BoS boundary. In this scenario, postural strategies not involving center of pressure manipulation were executed in the recovery of upright balance (Hof et al. 2005).
Following small perturbations, sagittal MoS was calculated at the instant of peak xCoM excursion (MoSpeak; Fig. 2a). This event occurred approximately 250–300 ms following perturbation onset. Minimum frontal MoS was calculated within the 2 s following perturbation onset (MoSmin; Fig. 2a). A secondary calculation of MoS concerned with the late-phase corrections of CoM following the initial postural disturbance was made. Here, the minimum MoS with respect to the BoS boundary opposite the direction of balance loss (e.g. backward perturbation, posterior boundary) was calculated within a window from peak excursion to the end of the aforementioned 2 s period (MoScorr; Fig. 2a).
a Representative sagittal extrapolated center of mass (xCoM) trajectory following onset of a small, backward perturbation (P) that evoked a fixed base of support response. Sagittal margin of stability (MoS) was calculated at the peak excursion (MoSpeak) and as the minimum value in the frontal plane in the 2 s window (MoSmin) starting from perturbation onset (P). Additionally, the minimum sagittal MoS with respect to the base of support boundary opposite the direction of balance-loss was calculated (MoScorr). b Representative sagittal xCoM trajectory following a large, backward perturbation that evoked a change in support response. Sagittal and frontal MoS were calculated at foot-off (O; MoSoff) and foot contact (C; MoScon) as estimated by foot angular and linear velocity
For large perturbations evoking a ∆BoS reactive step response, sagittal and frontal MoS were calculated at two events: foot-off (MoSoff), and foot contact (MoScon; Fig. 2b). The latter was determined using the sagittal velocity of the 5th metatarsal marker regardless of step direction. However, the instant of foot-off was observed to be direction-dependent. Foot-off for a forward step (following backward perturbations) was identified using the peak vertical velocity of the estimated foot CoM position, similar to that observed with toe-off during gait (O’Connor et al. 2007). Backward foot-off was identified as the instant the plantarflexion (angular) velocity of the foot exceeded a threshold, suggesting the “heel-off” phase of the gait cycle; this event followed the initial dorsiflexion (“toe-rise”) induced by the backward loss of balance. All events were visually inspected to ensure consistent and correct identification. Additional analyses included quantifying the following step parameters: step onset (time from perturbation onset to foot-off); step duration (time from foot-off to foot contact); step size (norm of step foot horizontal displacement from initial position). Peak trunk flexion and extension were calculated using pelvis and superior trunk segment CoM following backward and forward perturbation, respectively, within the aforementioned 2 s window.
Auditory Stroop task performance
Recorded audio signals were analyzed using a custom-written script written within MATLAB software (Version 9.2; MathWorks, Natick, MA, USA). Data were band-pass filtered (40–200 Hz) and liner enveloped (low-pass cutoff 4 Hz); filters used were second-order, dual-pass Butterworth filter. Response time was determined by identifying the onset of the audio cue and participant’s response within the approximated time-derivative of the processed signal. Each onset was considered the instant the signal consistently exceeded a threshold (sum of mean and twice the standard deviation of the quiet signal) and verified by visual inspection. The reaction time cost (RTC) associated with mean response time for each scenario was calculated with reference to the baseline task (quiet standing with Stroop) as shown in Eq. 2.
A positive RTC in the current paradigm would indicate an increased response time and a reduction in task performance (relative to quiet standing) when correctly responding to the Stroop task and executing a specific postural response (Kelly et al. 2010; Worden et al. 2016).
Statistical analyses
Statistical analyses were completed within SPSS software (Version 24.0, IBM Corp., North Castle, NY). The first trials associated with each condition were excluded from all analyses, to ensure the removal of any learning effect. Prior to investigating cognitive task effects, we investigated whether congruency was associated with Stroop response accuracy (%) via Wilcoxin signed-rank tests. The effect of congruency on the average Stroop response time (excluding incorrect trials) was further investigated by performing paired t tests with Bonferroni adjustments for multiple comparisons. If congruency effects were not observed, incorrect trials were excluded and congruent/incongruent trials were grouped into a single condition: “Stroop”. This analysis reflected no congruency effect (see “Results”).
The effect of task complexity on Stroop RTC was next analyzed by performing one-sample t tests (two-tailed) on aggregated trial data to examine whether RTCs under each condition (perturbation + Stroop) were different from 0 (with Bonferroni adjustments for multiple comparisons). Two-way repeated measures ANOVAs with perturbation direction and magnitude as independent factors were used to investigate the effects of task complexity on RTCs (in relation to perturbation parameters). Here, a significant interaction would provide insight as to whether the effects of cognitive task performance are influenced by the direction of the perturbation. Additional two-way repeated measures ANOVAs were performed to investigate effects of perturbation direction and cognitive task performance on the MoS and step parameters. A final set of two-way repeated measures ANOVAs were used to investigate the effects of perturbation magnitude and cognitive task performance on peak trunk flexion and extension within backward and forward perturbation conditions. Cognitive task performance in these scenarios was considered either with Stroop (baseline + perturbation) or no-Stroop (perturbation only). Post-hoc multiple comparisons were made, where appropriate. Statistical significance was set to p < 0.05 and partial eta squared (ηp2) was used as a measure of effect size for the ANOVAs.
Results
Three trials in which participants performed an incorrect postural response (e.g. evoked a step with a small perturbation) were excluded from analyses. Interaction effects were reported only if statistically significant. If not, main effects were reported for the given independent variables (e.g. task, perturbation direction). Note that prior to averaging outcome measures for each participant, extreme outliers were removed to mitigate data skewing. These outliers represented data from approximately 2.6% of all trials within the raw data set.
Congruency effects on Stroop response accuracy and time
For one participant, Stroop response accuracy for incongruent trials was 55%. This poor accuracy reflected a prioritization of response time as opposed to correctly performing the task as instructed (recall, participants were instructed to not prioritize one task over the other), thus data from this participant was excluded from subsequent analyses. The remaining sample size was, therefore, N = 9. For the remaining subjects, there was no association between congruent and incongruent trials in terms of response accuracy within any condition (p > 0.05). Average response accuracy across conditions was 97.7 ± 2.2%. With incorrect trials excluded from analyses, no effect of congruency was detected for response times (p > 0.05), thus congruent and incongruent trials were collapsed into a single condition (“Stroop”).
Simultaneous perturbation and Stroop task: response time reaction time costs
Stroop response times are displayed in Table 1. For small backward [t(9) = − 0.998, p = 1.00] and forward perturbations [t(9) = − 0.655, p = 1.00] RTC was not significantly different from 0. Similar observations were made for large backward [t(9) = 1.771, p = 0.46] and forward perturbations [t(9) = 1.541, p = 0.65]. Despite these findings, results from the ANOVA analysis indicated large perturbations produced significantly larger Stroop response time RTCs compared to small perturbations [F(1, 8) = 12.907, p = 0.007, ηp2 = 0.617; Fig. 3] though no main effect of perturbation direction was observed [F(1, 8) = 0.086, p = 0.777, ηp2 = 0.011].
Auditory Stroop response time reaction task cost (RTC; mean ± SE) for both backward and forward perturbations at the small and large magnitude. Large perturbations that evoked a change in support response resulted in significantly greater reaction time costs compared to the small perturbations that evoked a fixed base of support response (*p < 0.05)
Simultaneous perturbation and Stroop task: postural stability and control
A main effect of cognitive task performance was observed for sagittal MoSpeak [F(1, 8) = 12.319, p = 0.008, ηp2 = 0.606], MoScorr [F(1, 8) = 10.168, p = 0.013, ηp2 = 0.560] and frontal MoSmin [F(1, 8) = 7.557, p = 0.025, ηp2 = 0.486; Table 2]. When the auditory Stroop task was performed simultaneously with an fBoS postural response (baseline + perturbation), sagittal MoSpeak and frontal MoSmin were greater than perturbation only values. Conversely, sagittal MoScorr was reduced in baseline + perturbation conditions. A main effect of perturbation direction was observed for sagittal MoSpeak [F(1, 8) = 110.418, p < 0.001, ηp2 = 0.932] and MoScorr [F(1, 8) = 12.907, p < 0.001, ηp2 = 0.811]. Briefly, backward perturbations resulted in lower values of MoSpeak and higher MoScorr.
No effect of combining the baseline and perturbation condition was observed for sagittal MoSoff [F(1, 8) = 0.250, p = 0.630, ηp2 = 0.030] or MoScon [F(1, 8) = 0.564, p = 0.474, ηp2 = 0.066]. Likewise, no main effect of baseline + perturbation was detected for frontal MoSoff [F(1, 8) = 0.945, p = 0.359, ηp2 = 0.106] or MoScon [F(1, 8) = 3.499, p = 0.098, ηp2 = 0.304]. Note that no main effect of direction was observed for either of these variables (p > 0.05). A main effect of perturbation direction was observed for sagittal MoSoff [F(1, 8) = 222.584, p < 0.001, ηp2 = 0.965] and MoScon [F(1, 8) = 100.803, p < 0.001, ηp2 = 0.926]. Sagittal MoSoff was higher for forward perturbations; though, sagittal MoScon was higher following backward perturbations.
Regarding step parameters, no main effect of (baseline + perturbation) was evident for any step parameters: step size [F(1, 8) = 0.313, p = 0.591, ηp2 = 0.038], step duration [F(1, 8) = 0.008, p = 0.933, ηp2 = 0.001] and onset [F(1, 8) = 0.375, p = 0.557, ηp2 = 0.045]. A main effect of perturbation direction was observed for both step size [F(1, 8) = 34.778, p < 0.001, ηp2 = 0.813] and duration [F(1, 8) = 24.099, p = 0.001, ηp2 = 0.751]. Forward recovery steps executed following a backward perturbation were larger in size and duration.
For forward perturbations, peak trunk extension displayed no main effect of task [F(1, 8) = 2.843, p = 0.130, ηp2 = 0.262] despite trunk extension being significantly larger following the large perturbations [F(1, 8) = 11.725, p = 0.009, ηp2 = 0.594; Fig. 4a]. For backward perturbations, there was a significant magnitude by task interaction for peak trunk flexion [F(1, 8) = 8.046, p = 0.022, ηp2 = 0.501; Fig. 4b)]. Trunk flexion was increased with (baseline + perturbation) only if a small perturbation was used (p = 0.039) as opposed to a large perturbation (p = 0.161).
a Peak trunk extension (mean ± SE) following forward perturbations of a small (dashed line) or large (continuous line) magnitude under the different experimental conditions. Only an effect of magnitude was observed (double asterisk); the large perturbation resulted in significantly greater degree of trunk extension (p < 0.05). Figure <link b Peak trunk flexion (mean ± SE) following backward perturbations of a small (dashed line) or large (continuous line) magnitude under ST and DT conditions. A significant magnitude by cognitive-task interaction was observed (***p < 0.05). Analyses of simple effects revealed a significant effect when also performing a cognitive task, but for only the small perturbations (i.e. fixed base of support response; *p < 0.05); no effect was observed for the large perturbations (recovery step, change in support; p > 0.05). Trunk angular position less than 90° was indicative of flexion; greater than 90° was extension
Discussion
The current study investigated the effects of performing the auditory Stroop task concurrently with a fBoS or ∆BoS (i.e. recovery step) postural response in terms of cognitive task performance and postural stability. Specifically, we investigated whether altering the complexity of the postural response by manipulating both direction and magnitude of the perturbation applied reflects changes associated with cognitive-task interference. In brief, small perturbations evoking a fBoS response combined with a cognitive task resulted in changes in postural stability and altered corrections of CoM motion without any corresponding changes in Stroop response times (RTCs). Large perturbations evoking a step (∆BoS) revealed greater cognitive-task interference for the cognitive task (increased response time RTCs) though no changes in the motor responses were identified (i.e. no change in MoS). We also observed some additional perturbation direction main effects. For example, while we saw no changes in Stroop response times we did find that steps generated following a backward perturbation were larger in size and duration. Measures of trunk control did display consistent effects for each perturbation direction; changes in cognitive-task interference for backward perturbations manifested only following those of a small magnitude.
Stroop task performance
Participants had increased RTCs only when performed simultaneously with a ∆BoS (e.g. a large perturbation). Surprisingly, differences were observed only with respect to performance during fBoS responses as opposed to the baseline trials that included a Stroop task performed during quiet standing. This finding suggests an increased requirement for cognitive resources when responding to a perturbation that requires executing a step. Similar findings have been previously reported in young adults (Brauer et al. 2002) and older adults (Brown et al. 1999). Unanswered previously in the literature is the observed lack of difference between forward and backward steps. Despite the lack of visual input and altered mechanics associated with a backward protective step, which arguably made the task more cognitively challenging, no changes in cognitive input were present in the cognitive task performance. This may be due to fact that the backward steps were initiated earlier (i.e. MoSoff was larger for forward perturbations).
We expected to observe reduced Stroop task performance for the large perturbation trials compared to quiet standing alone, though this was not the case. A possible explanation for this finding may be related to the experimental design. Baseline, single-task trials were randomized amongst cognitive-task conditions. Inherently, anticipation of an impending perturbation may have resulted in greater variability of baseline response times potentially causing the negative mean RTCs observed for fBoS response. If true, it could reflect participants adopting a “posture first” strategy in which the postural task is prioritized in terms of cognitive resource allocation (Redfern et al. 2002). Prior work using EEG methodology has demonstrated that even in randomized experimental protocols, anticipatory cortical activity precedes an ‘expected’ perturbation; this may help explain our observations for the baseline, single task performance trials (Jacobs et al. 2008).
Perturbation task: postural control and stability
An unexpected finding was how healthy young adults changed MoSpeak and MoSmin during cognitive-task scenarios. Researchers such as Norrie et al. (2002) have observed factors such as increased center of pressure excursions (indicative of reduced postural stability) associated with dual-task scenarios. The contrasting increase in MoS we observed suggests a change in control strategy associated with limiting the degrees of freedom akin to previous findings reported by Etemadi et al. (2016). However, increased trunk flexion was observed following backward perturbations combined with the cognitive task (baseline) suggesting the opposite. Brown et al. (1999) observed no effect of adding a simultaneous cognitive task on the selection of postural strategy. Conversely, our observations suggest a drive towards use of a hip strategy following a backward perturbation when also performing the cognitive task; recall that this strategy maintains balance via counter-rotations of the trunk with respect to the CoM (Horak and Nashner 1986; Hof et al. 2007). Reflecting on whether cognitive-task interference is direction-dependent when considering balance recovery using a fBoS, values of MoS suggest dependency does not exist and did not confirm our hypotheses. If considering how this interference manifests, then an effect of direction becomes apparent. While the sagittal MoSpeak is higher following a forward perturbation, the simultaneous performance of the cognitive task seemed to induce a “stiffening” strategy, perhaps to reduce the degrees of freedom to be controlled (Maki et al. 1996; Etemadi et al. 2016). The same cognitive-task scenario but with a backward perturbation that induced a lower, often negative MoS theoretically shifted the postural strategy towards one with less reliance on center of pressure manipulation (Hof et al. 2005; Hof 2007).
Initial responses to a slip perturbation (Maki and McIlroy 2007), obstacle avoidance (Bernard-Demanze et al. 2014) and tripping responses (Bohm et al. 2012; Bolton 2015) appear to be similar to a reflex or stored-triggered response (Potocanac et al. 2015); thus many authors have suggested that it is the later phases of the postural response execution that are most impacted with the presence of a concurrent cognitive task (Redfern et al. 2002; Norrie et al. 2002). The observed reductions in MoScorr support this notion; it appears that when the baseline + perturbation tasks occurred simultaneously, young adults overcorrected their return to an upright posture following the early phase of an fBoS response (> 250–300 ms). This overcorrection happened regardless of the initial perturbation direction and, in addition to the increased MoSpeak, could additionally reflect an immediate prioritization of correcting the initial disturbance (as suggested by Redfern et al. [2002]); available resources can then be shifted back to the cognitive task during later phases of the combined task. The result is a rapid, correct response to the auditory Stroop task, but this unfortunately results in reduced postural control at the later stages of the movement. This initial prioritization of the postural task is further indicated by the improvements observed in MoSpeak values (reported above).
When analyzing the ∆BoS postural responses evoked by large perturbations, postural stability was not influenced by the presence of a concurrent cognitive task; neither MoS at foot-off, MoS at foot contact, nor any step parameters displayed cognitive-task interference. It is interesting that MoS magnitude and step size/duration were affected by the direction of perturbation, but simultaneous performance of a cognitive task did not further influence this relationship. Thus, the hypothesis that backward, reactive stepping requires additional allocation of cognitive resources relative to forward was not confirmed. Despite the increased difficulty related to the execution of a backward step, when a perturbation is sufficient enough to require a ∆BoS (i.e. a step to prevent falling) cognitive input is prioritized towards maintaining balance in a “posture-first” manner. This raises the question as to whether it is possible to train these young adults into performing both tasks competently; the degree to which young adults’ strategy may be shifted is quite interesting. Future work should address these ideas in addition to investigating how this may change for populations with reduced cognitive resources, e.g. older adults.
Limitations
The current study is not without its limitations. Foremost, individual experimental conditions were limited to a total of four trials within our experimental design; this provided an opportunity to collect a variety of experimental conditions while limiting potential physical and/or cognitive fatigue effects that would be induced in a longer more extensive testing protocol. Additionally, we randomized experimental trials within presentation blocks which essentially created a situation where perturbations may, or may not, have been experienced while performing the Stroop task. We acknowledge that our experimental design may have resulted in the allocation of attention to the motor task (i.e. direction and magnitude of the perturbation). The RTC effects we report here are, therefore, related to responses observed when no perturbation was present. Although we did not observe differences between incongruent/congruent Stroop conditions (“Stroop effect”) we believe that our high accuracy rates (> 97%) are indicative of a high cognitive input during the experimental trials.
Conclusion
In conclusion, our results demonstrate that direction of perturbation influences the complex fBoS postural control strategies evoked when responding to a sudden perturbation when also performing a cognitive task. Interestingly, despite differences in the characteristics of the postural responses following forward and backward perturbations, the CNS was able to maintain a consistent outcome: improving postural stability although cognitive task performance was altered. Cognitive-task interference was not influenced by the direction of a reactive step (∆BoS) used to recover balance. Rather, young adults prioritized their motor responses to the postural disturbance regardless of perturbation direction, perhaps by re-allocating cognitive resources towards centers responsible for step execution, which in turn resulted in delayed response to the Stroop task. Thus, the “posture first” strategy employed by young adults in response to complex control scenarios reinforces the important role of cognition in the maintenance of upright balance.
References
Bernard-Demanze L, Léonard J, Dumitrescu M et al (2014) Static and dynamic posture control in postlingual cochlear implanted patients: effects of dual-tasking, visual and auditory inputs suppression. Front Integr Neurosci. https://doi.org/10.3389/fnint.2013.00111
Bohm S, Mersmann F, Bierbaum S et al (2012) Cognitive demand and predictive adaptational responses in dynamic stability control. J Biomech 45:2330–2336
Bolton DAE (2015) The role of the cerebral cortex in postural responses to externally induced perturbations. Neurosci Biobehav Rev 57:142–155
Brauer SG, Woollacott M, Shumway-Cook A (2002) The influence of a concurrent cognitive task on the compensatory stepping response to a perturbation in balance-impaired and healthy elders. Gait Posture 15:83–93
Brown LA, Shumway-Cook A, Woollacott MH (1999) Attentional demands and postural recovery: the effects of aging. J Gerontol A Biol Sci Med Sci 54:M165-171
Chen HC, Schultz AB, Ashton-Miller JA et al (1996) Stepping over obstacles: dividing attention impairs performance of old more than young adults. J Gerontol A Biol Sci Med Sci 51:M116-122
Curtze C, Hof AL, Otten B, Postema K (2010) Balance recovery after an evoked forward fall in unilateral transtibial amputees. Gait Posture 32:336–341
Dijkstra TM, Schöner G, Gielen CC (1994) Temporal stability of the action-perception cycle for postural control in a moving visual environment. Exp Brain Res 97:477–486
Ebersbach G, Dimitrijevic MR, Poewe W (1995) Influence of concurrent tasks on gait: a dual-task approach. Percept Mot Skills 81:107–113
Etemadi Y, Salavati M, Arab AM, Ghanavati T (2016) Balance recovery reactions in individuals with recurrent nonspecific low back pain: Effect of attention. Gait Posture 44:123–127
Hasson CJ, Van Emmerik REA, Caldwell GE (2008) Predicting dynamic postural instability using center of mass time-to-contact information. J Biomech 41:2121–2129
Hof AL (2007) The equations of motion for a standing human reveal three mechanisms for balance. J Biomech 40:451–457
Hof AL, Gazendam MGJ, Sinke WE (2005) The condition for dynamic stability. J Biomech 38:1–8
Horak FB, Nashner LM (1986) Central programming of postural movements: adaptation to altered support-surface configurations. J Neurophysiol 55:1369–1381
Jacobs JV, Fujiwara K, Tomita H et al (2008) Changes in the activity of the cerebral cortex relate to postural response modification when warned of a perturbation. Clin Neurophysiol 119:1431–1442
Joseph Jilk D, Safavynia SA, Ting LH (2014) Contribution of vision to postural behaviors during continuous support-surface translations. Exp Brain Res 232:169–180
Kahneman D, Chajczyk D (1983) Tests of the automaticity of reading: dilution of Stroop effects by color-irrelevant stimuli. J Exp Psychol Hum Percept Perform 9:497–509
Kelly VE, Janke AA, Shumway-Cook A (2010) Effects of instructed focus and task difficulty on concurrent walking and cognitive task performance in healthy young adults. Exp Brain Res 207:65–73
Kerr B, Condon SM, McDonald LA (1985) Cognitive spatial processing and the regulation of posture. J Exp Psychol Hum Percept Perform 11:617–622
Lajoie Y, Teasdale N, Bard C, Fleury M (1993) Attentional demands for static and dynamic equilibrium. Exp Brain Res 97:139–144
Lamoth CJ, van Deudekom FJ, van Campen JP et al (2011) Gait stability and variability measures show effects of impaired cognition and dual tasking in frail people. J Neuroeng Rehabil 8:2
Little CE, Woollacott M (2015) EEG measures reveal dual-task interference in postural performance in young adults. Exp Brain Res 233:27–37
Maki BE, McIlroy WE (1997) The role of limb movements in maintaining upright stance: the “change-in-support” strategy. Phys Ther 77:488–507
Maki BE, McIlroy WE (2007) Cognitive demands and cortical control of human balance-recovery reactions. J Neural Transm (Vienna) 114:1279–1296
Maki BE, McIlroy WE, Perry SD (1996) Influence of lateral destabilization on compensatory stepping responses. J Biomech 29:343–353
Melzer I, Benjuya N, Kaplanski J (2001) Age-related changes of postural control: effect of cognitive tasks. Gerontology 47:189–194. doi: 52797
Morgan AL, Brandt JF (1989) An auditory Stroop effect for pitch, loudness, and time. Brain Lang 36:592–603
Nashner LM, Shupert CL, Horak FB, Black FO (1989) Organization of posture controls: an analysis of sensory and mechanical constraints. Prog Brain Res 80:411-418-397
Norrie RG, Maki BE, Staines WR, McIlroy WE (2002) The time course of attention shifts following perturbation of upright stance. Exp Brain Res 146:315–321
O’Connor CM, Thorpe SK, O’Malley MJ, Vaughan CL (2007) Automatic detection of gait events using kinematic data. Gait Posture 25:469–474
Patel PJ, Bhatt T (2015) Attentional demands of perturbation evoked compensatory stepping responses: examining cognitive-motor interference to large magnitude forward perturbations. J Mot Behav 47:201–210
Potocanac Z, Smulders E, Pijnappels M et al (2015) Response inhibition and avoidance of virtual obstacles during gait in healthy young and older adults. Hum Mov Sci 39:27–40
Redfern MS, Müller MLTM., Jennings JR, Furman JM (2002) Attentional dynamics in postural control during perturbations in young and older adults. J Gerontol A Biol Sci Med Sci 57:B298–B303
Redfern MS, Chambers AJ, Jennings JR, Furman JM (2017) Sensory and motoric influences on attention dynamics during standing balance recovery in young and older adults. Exp Brain Res 235:2523–2531. https://doi.org/10.1007/s00221-017-4985-5
Schaefer S, Krampe RT, Lindenberger U, Baltes PB (2008) Age differences between children and young adults in the dynamics of dual-task prioritization: body (balance) versus mind (memory). Dev Psychol 44:747–757
Siu K-C, Catena RD, Chou L-S et al (2008) Effects of a secondary task on obstacle avoidance in healthy young adults. Exp Brain Res 184:115–120
Weerdesteyn V, Schillings AM, van Galen GP, Duysens J (2003) Distraction affects the performance of obstacle avoidance during walking. J Mot Behav 35:53–63
Wellmon R, Barr-Gillespie AE, Newton R et al (2013) The effects of aging on the attentional demands of walking toward and stepping up onto a curb. Gait Posture 38:198–202
Winter DA, Patla AE, Prince F et al (1998) Stiffness control of balance in quiet standing. J Neurophysiol 80:1211–1221
Worden TA, Vallis LA (2014) Concurrent performance of a cognitive and dynamic obstacle avoidance task: influence of dual-task training. J Mot Behav 46:357–368
Worden TA, Vallis LA (2016) Stability control during the performance of a simultaneous obstacle avoidance and auditory Stroop task. Exp Brain Res 234:387–396
Worden TA, Mendes M, Singh P, Vallis LA (2016) Measuring the effects of a visual or auditory Stroop task on dual-task costs during obstacle crossing. Gait Posture 50:159–163
Acknowledgements
The authors would like to acknowledge funding provided by a NSERC Discovery Grant (awarded to LAV), Ontario Graduate Student scholarship and NSERC summer student fellowship (awarded to KAI) and Canadian Foundation for Innovation and Ontario Research Fund Research Infrastructure Grants for equipment. The authors would also like to thank Tim Worden and Rhianna Malcolm for assistance with experimental design and data collection and Dr. John Zettel for use of laboratory equipment.
Funding
This study was funded by Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant awarded to LAV (Grant number #261854).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Inkol, K.A., Huntley, A.H. & Vallis, L.A. Do perturbation-evoked responses result in higher reaction time costs depending on the direction and magnitude of perturbation?. Exp Brain Res 236, 1689–1698 (2018). https://doi.org/10.1007/s00221-018-5249-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-018-5249-8