Abstract
Saccadic movements are well known to involve specific top-down or bottom-up processes depending on the task and paradigm characteristics. For example, after the Gap bottom-up effect, it has been shown that an Instruction effect, i.e., asking to identify a peripheral target instead of simply look toward it, reduces latencies in prosaccade (PS) but not in antisaccade (AS) tasks. Nevertheless, in a mixed task comprising AS, PS and nosaccade trials, such differences vanished. Thus, it has been suggested that a top-down effect could be dependent on tonic or phasic neuronal activation and that only the tonic frontal activation could enable interferences with other cortical regions involved. In this study, we tested the interference of emotional information with saccadic performance depending on cognitive cost of the task. We used emotional facial expression cues in block and mixed paradigms. Using a generalized linear mixed model for the analysis, we found a main effect of the paradigm, with task and emotional effects only in mixed saccadic task that could suggest a top-down effect of emotional information processing over the regions involved in saccadic performances. Moreover, we demonstrated that prosaccades latencies are significantly reduced by emotion, while antisaccades are significantly increased, suggesting a disinhibition of reflexive saccades.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Saccades are rapid eye movements daily used to place an object of interest in the fovea in order to analyze its visual components precisely. Saccadic movements are one of the best-known processes in its neural networks involvements (Hutton and Ettinger 2006; Munoz and Everling 2004; Pierrot-Deseilligny et al. 2003; Schall et al. 2002). In this context, prosaccade (PS) and antisaccade (AS) are the more frequently used tasks in neurophysiological studies. In PS tasks, participants have to look as fast as possible toward a peripheral cue, whereas in AS they have to inhibit the reflexive movement toward this cue (in this case, a distractor) and instead produce a saccade in the mirror position.
AS and PS tasks are generally used with block paradigms, in which participants perform a full set of the same kind of trials. As seen with the Gap effect (Saslow 1967) or more recently with the Instruction effect (Guyader et al. 2010; Malsert et al. 2012a; Trottier and Pratt 2005), some modifications in tasks are accompanied by modification of performances. The Gap effect is observed when the fixation point disappeared before the onset of the peripheral cue. It has been showed that the gap reduces latency in PS as well as in AS, due to a disinhibition of superior colliculus (SC) neurons, therefore via a “bottom-up” process. The Instruction effect, associated with the decrease of PS latencies when participants have to identify a target rather than to simply look toward it, would be due to a top-down effect, from frontal cortical areas involved in task programing. Nevertheless, even if the Instruction effect is systematically observed in reflexive PS tasks, it is less important in voluntary PS and absent in AS blocks (Guyader et al. 2010). The interference of the Instruction effect could be dependent on the available resources managed by frontal areas and then opposite to the cognitive cost of the task.
In the following study (Malsert et al. 2012a), this effect has been tested in block tasks (PS and AS) parallel to a mixed paradigm combining PS, AS and nosaccade (NS) trials (SPAN task), which increases the executive control cost, in order to switch between the different instructions. Surprisingly, the Instruction effect was observed in the SPAN paradigm with a decrease of saccadic reaction times (SRTs) in PS and even in AS trials. The identification instruction resulted also in an increased error rate in AS.
In a neuroimaging study, Dyckman et al. (2007) showed increased PFC activity during AS blocks compared to PS blocks, but did not find any difference in PFC activity between saccade types when AS and PS were mixed. Thus, strong activations of the PFC were observed during all types of saccades and not only during AS as in blocked experiments. Based on these data, the observation of an Instruction effect on latencies for both AS and PS in SPAN paradigm could be explained by different types of activations of the PFC between blocked AS and SPAN. The main difference may be due to “tonic” versus “phasic” activations of the PFC (Malsert et al. 2012a). In the mixed saccadic task, tonic activations of the PFC have been shown, whereas during blocked AS phasic activations have been demonstrated (Dyckman et al. 2007).
Indeed, at a neural cellular level, cell discharges can be either tonic or phasic (Farrant and Nusser 2005; Mody 2001). When phasic discharges generate brief spikes periods at the onset of stimulation, tonic discharges generate spikes continuously throughout the stimulation period. This phasic intensive generation of spikes for a brief period can be followed by a refractory period during which cells cannot discharge. On the other hand, tonic activation necessary in SPAN paradigm for maintaining task instruction in working memory may allow a top-down control by sharing resources (Malsert et al. 2012a). In this view, the instructions used could change the functioning of cerebral areas involved in these saccades depending on the experimental design (blocked vs. mixed).
This study aimed at testing another kind of interference in saccadic performances, with emotional components in order to observe if their frontal processing applies a top-down influence, and if yes, how it impacts. Actually, emotional information in one hand is thought to be process differentially by left and right prefrontal areas (Davidson 2004; Davidson et al. 1979), while on the other hand, the dominance hypothesis proposed that the right hemisphere would be more involved in emotional processing in general (Ley and Bryden 1979).
The link between emotion and frontal lateralization is still not well understood. One of the oldest theories about emotional processing is that the right hemisphere would be dominant whatever the valence of the emotional stimuli. This hypothesis is based, for example, on studies which have shown a more important expression on the left side of the face in healthy subjects (Sackeim et al. 1978), or an easier discrimination of emotion presented in the left ear (Erhan et al. 1998) or visual field (Levine and Levy 1986). On the other hand, a more recent hypothesis extends the concept of the lateralization of emotion with a theory in which each hemisphere could integrate emotional stimuli depending on their valence or approach/avoidance behavioral action tendencies. In this view, the authors suggest that left hemisphere is dominant for processing positive emotions or approach tendencies and right hemisphere negative ones or withdrawal (Davidson 1992; Gur et al. 1994; Robinson and Starkstein 1989; Sackeim et al. 1978, 1982). These observations were done in patients with frontal lobe damage who became depressed (Sackeim et al. 1982) or with behavioral and neuroimaging studies (Davidson and Fox 1982; Tucker et al. 1981). The most recent observations are not able to select one of the theories formulated previously. Some studies have found a right hemisphere predominance for negative emotion (Kumar and Srinivasan 2011; Onal-Hartmann et al. 2012; Sedda et al. 2013); a right dominance for all emotional processing (Alves et al. 2009; Bourne 2010; Cheng et al. 2012; Irish et al. 2014; Killgore and Yurgelun-Todd 2007; Najt et al. 2013; Yuvaraj et al. 2013); or no asymmetries for positives emotion (Kumar and Srinivasan 2011; Najt et al. 2013; Onal-Hartmann et al. 2012; Sedda et al. 2013; Zhang et al. 2011).
Few studies have used emotional stimuli in saccadic paradigm in order to test the role of emotion in attentional processes. In their study, Kissler and Keil (2008) tested the effect of peripheral pleasant or unpleasant cues in PS and AS tasks. They showed that emotional target facilitates endogenous saccade generation. Moreover, this facilitation was modulated by visual field lateralization and by the Gap effect. In another study, West et al. (2011) presented an emotional facial expression in the center of the screen before a simultaneous distractor and target onset. They expected an effect in SRTs linked to a bottom-up process from the superior colliculus. They observed a decrease in SRTs in the Gap condition with fear trials compared to neutral ones. The use of a lateralized saccadic task could help the understanding of emotional processing in case of interference. Indeed, saccadic movement can be analyzed by visual hemifield and could be dependent on the contralateral prefrontal cortex (Johnston and Everling 2006).
On the other hand, previous studies have shown that a top-down effect requires available resources from prefrontal cortex, allowed by the use of a SPAN paradigm compared to blocked one (Malsert et al. 2012a).
We therefore hypothesize that if tonic activation is necessary to observe top-down interference and if the emotional components involve frontal areas, we should observe an interaction between emotional valence of facial expression distractor and performance in the SPAN paradigm but not in the blocked ones. Indeed, the emotional component will be able to interfere in SPAN on frontal activations and then should reduce saccadic latencies as observed in previous studies (Kissler and Keil 2008; West et al. 2011).
Furthermore, theories of emotional lateralization provide distinct hypotheses: hemispheric lateralization depending on emotional valence or approach/avoidance action tendencies (Davidson 1992; Davidson and Irwin 1999; Gur et al. 1994; Robinson and Starkstein 1989) or right hemisphere dominance (Mandal et al. 1996; Sackeim et al. 1978; Weddell 1994). Overall, the use of lateralized saccades could allow us to observe performance changes depending on visual hemifield and thus on cerebral hemisphere. An inversion of lateralized effect depending on emotion would base the valence hypothesis, whereas a unilateral decrease toward left visual hemifield would emphasize the right hemisphere one.
Methods
Participants
Forty students (19 Females, mean age 25.7, ±5.1) of the University of Geneva took part in the study. All were naive to the purpose of the study, had normal or corrected to normal vision and no history of psychiatric disorder or medication.
Participants gave their informed consent to the study approved by the local ethical committee and received 10 CHF as a compensation.
Apparatus and stimuli
A camera-based eye tracker (Eyelink 1000 from SR Research, Kanata, ON, Canada) with a temporal resolution of 1000 Hz and an accuracy of 0.25° was used in the pupil tracking. Prior to the experiment, participants’ gaze location was calibrated using a 3 × 3 points grid. Fixations and saccades were detected automatically by the eye tracker. Saccades were automatically detected in the data file as eye movements with a velocity of over 30°/s and an acceleration of over 9500°/s2.
All stimuli were displayed on a mid-gray level screen (10 cd/m2 CIE luminance). They were displayed on a 21-in. screen located 57 cm from participants (1 cm on the screen = 1°) with a resolution of 1024 × 768 pixels and a refresh rate of 85 Hz. The experiment took place in a darkened room.
Hundred and twenty pictures from the Karolinska Directed Emotional Faces database (Lundqvist et al. 1998) were used for the standardized presentation of the emotional expressions. Twenty male and twenty female actors expressing fear, happiness and displaying a neutral expression were selected and standardized in black and white pictures of 5° × 6.8° of visual angle.
Procedure
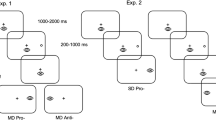
After a mood assessment by a Beck Depression Inventory (BDI) to control potential mood disorder influencing saccadic performances (Malsert et al. 2012b, c), participants completed three sessions of saccadic tasks: a blocked PS, a blocked AS and a SPAN (PS, AS and NS trials) paradigm. The order was counterbalanced between participants. In the SPAN paradigm, they were instructed to make a PS when fixation point was green—i.e., to look as quickly as possible toward a peripheral cue when it appears. After the red fixation point, participants had to make an AS—i.e., to look directly and as quickly as possible in the mirror position of the cue. When the fixation point was blue, participants were required to do a NS, i.e., to maintain their gaze in a central position and to refrain from making any eye movements.
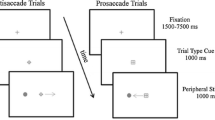
The procedure for the three tasks was nearly identical, trials began with the participant gazing at the fixation dot (SPAN) or cross (Block) for 1000, 1500, or 2000 ms. In SPAN, the white fixation dot was followed by a color fixation dot for 2 s which indicate the instruction for the coming trial.
If the participant maintained his gaze in the central position (±0.5° on the x-axis and ±2° on the y-axis), an emotional facial expression appeared in the center of the screen for 50 ms and was followed by a 200 ms gap. Afterward, a cue was flashed for 50 ms, 10° on the left or right side of the screen, and the target appeared as soon as participants looked at the correct location (±2° on the x-axis and ±4° on the y-axis) or after a delay of 2 s and remained for 1 s (Fig. 1).
Block and SPAN paradigms. In prosaccade (PS) and antisaccade (AS) block tasks, instructions to look toward or away of the cue, respectively, were given before a training session. In the SPAN paradigm, which mixed PS, AS and nosaccade (NS) trials, instruction for the kind of saccades is given by a colored fixation point. Blue: NS, Green: PS, Red: AS. For NS trials, participants have to fixate the center of the screen despite the onset of the peripheral distractor (color figure online)
All tasks started with a learning phase of six trials for PS and 12 for AS and SPAN paradigm followed by 90 testing trials for the blocked tasks and 120 for the SPAN. SPAN testing phase had 48 PS and 48 AS, but just 24 NS to be more difficult to inhibit, then loading the cognitive cost.
Results
We recorded the eye positions of participant during the experiment, and we extracted the latencies of the first saccades after the appearance of the cues and the saccade errors (saccades toward the wrong direction).
Data from the forty participants were analyzed. Eight participants having a score higher than 11 at BDI questionnaire were not considered in the analyses, this score being considered as mild mood disturbance (Jindal 2013).
For each trial, the accuracy of the eye movements was measured. We lost some saccades (around 2 %) because the eye tracker lost the pupil image (blinks). From the recordings of all eye movements, we extracted the saccadic reaction time (SRT) of the first correct saccade after the cue onset.
A saccade was considered as a correct saccade if it was the first eye movement after the cue onset and if the eye reached an area of 4° of visual angle around the target (2° on each side of the target position). We removed all the anticipated saccades (i.e., saccades with latencies below 70 ms, less than 0.5 %). Moreover, very long saccades (i.e., saccades with latencies >500 ms for PS and >700 ms for AS) were rare and excluded from the analysis (less than 1 %).
We choose to use the generalized linear mixed model (GLMM) analysis which estimates the variability of fixed and random effects and shows to have higher statistical power (Moscatelli et al. 2012). Using a linear mixed model with subjects and items specified as crossed random factors, this analysis allows us to compare the tasks effect as well as conditions (Kliegl et al. 2010).
Saccadic reaction times
A GLMM analysis on task (block/SPAN) × saccade type (PS/AS) × cue lateralization (left/right) × emotion (facial expressions: happy/neutral/fearful) on correct SRTs for the 32 subjects was performed using the lme4 package (Bates et al. 2015) in R Development Core Team (2008). Results are presented in Fig. 2.
A main effect of task was observed (χ 2 = 154.31, p < .001) with shorter SRTs in block versus SPAN tasks (183 vs. 203 ms) as well as the main effect of saccade type (χ 2 = 2460.7, p < .001) with a strong increase in RT for AS compared to PS (240 vs. 151 ms). Nevertheless, there is no main effect of emotion (χ 2 = 1.29, n.s.) but a trend level for interaction between emotion and cue lateralization (χ 2 = 4.75, p < .09; Fig. 3a).
We found an interaction between task and saccade type, and SRTs are more influenced by task in PS (Block 142 vs. SPAN 168 ms) than in AS (Block 235 vs. SPAN 249 ms) trials (χ 2 = 36.72, p < .001).
The interaction between task, saccade type, emotion and cue lateralization is significant (χ 2 = 76.46, p < .001).
We analyzed contrasts and observed a trend level of significance for SRTs between neutral and positive emotional distractors (χ 2 = 3.28, p = .07), with SRTs shorter in PS and higher in AS for emotional ones. The decrease of SRTs in PS with emotional distractors versus Neutral ones is significant (χ 2 = 9.08, p < .005). Nevertheless, this effect of emotional versus neutral distractors is significant only in SPAN task (χ 2 = 6.48, p < .005), but not in block task (χ 2 = 2.64, n.s.).
Inhibition error rates
A GLMM analysis on task (Block/SPAN) × cue lateralization (left/right) × emotion (facial expressions: happy/neutral/fearful) was performed on AS inhibition error rate, i.e., when the first saccade was in direction of the cue.
We observed a main effect of task, with higher error rate in SPAN (30.8 %) than in block (18.9 %) AS (χ 2 = 93.503, p < .001), and a trend level for interaction between emotion and cue lateralization (χ 2 = 5.6454, p < .06; Fig. 3b).
NS inhibition error rates are not included in the analysis because not used in block paradigm. SPAN NS inhibition error rates are comprised between 6 and 8 %.
Discussion
In the previous parts, we discussed how saccadic performances could be influenced by different top-down or bottom processes and how those interferences, for some specific components, can help the understanding of the neural network involvement. This study aimed at testing whether an emotional interference effect could be observed in saccadic tasks and, if applicable, to compare interference in a mixed and a blocked saccadic condition. An emotional facial expression was presented centrally and implicitly in foveal area before cue onset in PS and AS block tasks or in a mixed AS, PS and NS paradigm (SPAN).
Previous studies have shown different effects interfering on SRTs and/or error rates, either from subcortical areas by bottom-up processes (e.g., Gap effect, Saslow, 1967), or from cortical areas by top-down processes (e.g., Instruction effect, Trottier and Pratt, 2005; Malsert et al. 2012a).
It has been suggested that the Instruction effect, known to be robust in PS tasks (Madelain et al. 2007; Montagnini and Chelazzi 2005; Trottier and Pratt 2005) is dependent on the cognitive cost of the task (Guyader et al. 2010). Its observation in the SPAN task with high cognitive cost suggests that tonic activation of the PFC in mixed paradigms allows this top-down effect irrespective of saccade type, contrary to phasic activation in block tasks which causes a refractory period.
Thus, top-down effect from frontal cortical areas involved in the task programing could be dependent on available resources and then on the cognitive complexity of the task instruction. Nevertheless, available resources are not only dependent on how much the task costs impact on a specific cognitive level, but also how the different sub-frontal brain regions are involved in the different demands.
In this study, we tested the hypothesis of how emotional stimuli could interfere on saccadic performance, and in this case, whether such interference would also depend on cognitive cost as previously observed with Instruction effect. Actually, we suggested that emotional interference could be driven by top-down effects from frontal cortex like Instruction effect, but it could also be a bottom-up process from subcortical areas. Then, testing an interference process with emotional distractors could help its understanding if it confirms the available resources dependence of a top-down effect.
We observed a main effect of task type, for SRTs as well as for inhibition error rates with an increase of both in SPAN paradigm compared to block one. These observations showed that the cognitive cost of SPAN paradigm cannot be offset by strategies such as slowing down saccadic movements in order to reduce inhibition errors.
However, SPAN paradigm showed different significant effects not observed in block paradigms. We observed that emotional stimuli decrease latencies in PS trials but increase them in AS trials compared to neutral stimuli. The emotional facial expression in the central visual field helped to produce a reflexive PS toward peripheral visual field independently of its valence.
Such as for Instruction effect (Malsert et al. 2012a), Emotional effect is released by SPAN paradigm. This mixed effect was explained in the previous study by the neuronal kind of activation in SPAN. Indeed, the main difference between mixed and blocked saccadic tasks may be the result of, respectively, tonic versus phasic activations of the prefrontal cortex (Dyckman et al. 2007).
By looking more closely at the emotional interference observed in this study, we note that emotional stimuli decreased latencies in PS whereas they increased them in AS. The emotional component seems to not interfere with inhibition performance but with the reflexive programing of saccadic movement as observed previously by Kissler and Keil (2008). The disinhibition of reflexive saccades could thus facilitate the production of PS but be more costly for AS programing. The emotional effect seems to be equivalent for both valences, suggesting that cortical or/and subcortical network in emotional process might be similar for positive and negative valences in this context.
The statistical trend for interaction between cue lateralization and emotion is really interesting in relation to the current theoretical debate about lateralization of emotional processes. Indeed, even if the right hemisphere dominance for emotional process seems to dominate in some studies (Dimberg and Petterson 2000; Sackeim et al. 1978), the lateralization depending on the emotional valence has also been proposed by several authors (Davidson 2004; Davidson et al. 1979; Silberman and Weingartner 1986). Here, we found that with emotions, latencies were shorter in left visual field than in right visual field, contrary to neutral facial expressions. We could suggest that if the right hemisphere dominates in emotional processing, its activation by emotional cues might preferentially facilitate for saccades controlled by the right hemisphere, i.e., toward the left visual field. The increase in error rates in the same visual field supports also this hypothesis.
Our results are therefore consistent with the previous study (Malsert et al. 2012a) about the releasing of top-down effect by tonic activation suggesting that mixed SPAN paradigm induces interference contrary to block paradigm.
Moreover, the direct statistical comparison between SPAN and block paradigms was not evaluated in previous studies and confirm the task effect in performances. This study shows that the emotional interaction would be dependent on a cortical top-down and not a bottom-up subcortical process. Furthermore, emotion could interfere particularly with the reflexive component of saccadic programing. The interhemispheric asymmetry observed could be in favor of the right hemisphere dominance hypothesis, at least for positive emotional stimuli. Presently, more researches are needed with brain imaging in order to precise the involvement of different frontal subregions for the different components manipulated in such saccadic paradigms. Thus, an accurate understanding of these networks would allow us evaluating emotional saccadic performances in patients suffering of mood disorders and objectivize them.
References
Alves NT, Aznar-Casanova JA, Fukusima SS (2009) Patterns of brain asymmetry in the perception of positive and negative facial expressions. Laterality 14:256–272. doi:10.1080/13576500802362927
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Statistical Softw 67:1–48
Bourne VJ (2010) How are emotions lateralised in the brain? Contrasting existing hypotheses using the chimeric faces test. Cognit Emot 24:903–911
Cheng Y, Lee SY, Chen HY, Wang PY, Decety J (2012) Voice and emotion processing in the human neonatal brain. J Cognit Neurosci 24:1411–1419. doi:10.1162/jocn_a_00214
Davidson RJ (1992) Anterior cerebral asymmetry and the nature of emotion. Brain Cognit 20:125–151
Davidson RJ (2004) What does the prefrontal cortex “do” in affect: perspectives on frontal EEG asymmetry research. Biol Psychol 67:219–233. doi:10.1016/j.biopsycho.2004.03.008
Davidson RJ, Fox NA (1982) Asymmetrical brain activity discriminates between positive and negative affective stimuli in human infants. Science 218:1235–1237
Davidson RJ, Irwin W (1999) The functional neuroanatomy of emotion and affective style. Trends Cognit Sci 3:11–21
Davidson RJ, Schwartz GE, Saron C, Bennett J, Goleman DJ (1979) Frontal versus parietal EEG asymmetry during positive and negative affect. Psychophysiology 16:202–203
Dimberg U, Petterson M (2000) Facial reactions to happy and angry facial expressions: evidence for right hemisphere dominance. Psychophysiology 37:693–696
Dyckman KA, Camchong J, Clementz BA, McDowell JE (2007) An effect of context on saccade-related behavior and brain activity. NeuroImage 36:774–784. doi:10.1016/j.neuroimage.2007.03.023
Erhan H, Borod JC, Tenke CE, Bruder GE (1998) Identification of emotion in a dichotic listening task: event-related brain potential and behavioral findings. Brain Cognit 37:286–307. doi:10.1006/brcg.1998.0984
Farrant M, Nusser Z (2005) Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci 6:215–229. doi:10.1038/nrn1625
Gur RC, Skolnick BE, Gur RE (1994) Effects of emotional discrimination tasks on cerebral blood flow: regional activation and its relation to performance. Brain Cognit 25:271–286. doi:10.1006/brcg.1994.1036
Guyader N, Malsert J, Marendaz C (2010) Having to identify a target reduces latencies in prosaccades but not in antisaccades. Psychol Res 74:12–20. doi:10.1007/s00426-008-0218-7
Hutton SB, Ettinger U (2006) The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiology 43:302–313. doi:10.1111/j.1469-8986.2006.00403.x
Irish M, Hodges JR, Piguet O (2014) Right anterior temporal lobe dysfunction underlies theory of mind impairments in semantic dementia brain: A J Neurol 137(4):1241–1253
Jindal SK (2013) World clinics: pulmonary & critical care medicine: chronic obstructive pulmonary disease, vol 1. Jaypee Bros Medical Publishers, New Delhi
Johnston K, Everling S (2006) Neural activity in monkey prefrontal cortex is modulated by task context and behavioral instruction during delayed-match-to-sample and conditional prosaccade-antisaccade tasks. J Cognit Neurosci 18:749–765. doi:10.1162/jocn.2006.18.5.749
Killgore WD, Yurgelun-Todd DA (2007) The right-hemisphere and valence hypotheses: could they both be right (and sometimes left)? Soc Cognit Affect Neurosci 2:240–250. doi:10.1093/scan/nsm020
Kissler J, Keil A (2008) Look-don’t look! How emotional pictures affect pro- and anti-saccades. Exp Brain Res 188:215–222. doi:10.1007/s00221-008-1358-0
Kliegl R, Masson MEJ, Richter EM (2010) A linear mixed model analysis of masked repetition priming. Vis Cognit 18:655–681. doi:10.1080/13506280902986058
Kumar D, Srinivasan N (2011) Emotion perception is mediated by spatial frequency content. Emotion 11:1144–1151. doi:10.1037/a0025453
Levine SC, Levy J (1986) Perceptual asymmetry for chimeric faces across the life span. Brain Cognit 5:291–306
Ley RG, Bryden MP (1979) Hemispheric differences in processing emotions and faces. Brain Lang 7:127–138
Lundqvist D, Flykt A, Ohman A (1998) The Karolinska directed emotional faces (KDEF). Department of Neurosciences Karolinska Hopital, Stockholm
Madelain L, Champrenaut L, Chauvin A (2007) Control of sensorimotor variability by consequences. J Neurophysiol 98:2255–2265. doi:10.1152/jn.01286.2006
Malsert J, Guyader N, Chauvin A, Marendaz C (2012a) Having to identify a target reduces antisaccade latencies in mixed saccadic paradigms: a top-down effect released by tonic prefrontal activation? Cognit Neurosci 3:105–111. doi:10.1080/17588928.2012.666965
Malsert J et al (2012b) Antisaccades as a follow-up tool in major depressive disorder therapies: a pilot study. Psychiatry Res 200:1051–1053. doi:10.1016/j.psychres.2012.05.007
Malsert J, Guyader N, Chauvin A, Polosan M, Szekely D, Bougerol T, Marendaz C (2012c) Saccadic performance and cortical excitability as trait-markers and state-markers in rapid cycling bipolar disorder: a two-case follow-up study. Front Psychiatry 3:112. doi:10.3389/fpsyt.2012.00112
Mandal MK, Mohanty A, Pandey R, Mohanty S (1996) Emotion-specific processing deficit in focal brain-damaged patients. Int J Neurosci 84:87–95
Mody I (2001) Distinguishing between GABA(A) receptors responsible for tonic and phasic conductances. Neurochem Res 26:907–913
Montagnini A, Chelazzi L (2005) The urgency to look: prompt saccades to the benefit of perception. Vis Res 45:3391–3401. doi:10.1016/j.visres.2005.07.013
Moscatelli A, Mezzetti M, Lacquaniti F (2012) Modeling psychophysical data at the population-level: the generalized linear mixed model. J Vis 12:26. doi:10.1167/12.11.26
Munoz DP, Everling S (2004) Look away: the anti-saccade task and the voluntary control of eye movement. Nat Reviews Neurosci 5:218–228. doi:10.1038/nrn1345
Najt P, Bayer U, Hausmann M (2013) Models of hemispheric specialization in facial emotion perception—a reevaluation. Emotion 13:159–167. doi:10.1037/a0029723
Onal-Hartmann C, Pauli P, Ocklenburg S, Gunturkun O (2012) The motor side of emotions: investigating the relationship between hemispheres, motor reactions and emotional stimuli. Psychol Res 76:311–316. doi:10.1007/s00426-011-0337-4
Pierrot-Deseilligny C, Muri RM, Ploner CJ, Gaymard B, Demeret S, Rivaud-Pechoux S (2003) Decisional role of the dorsolateral prefrontal cortex in ocular motor behaviour. Brain J Neurol 126:1460–1473
R Development Core Team (2008) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Robinson RG, Starkstein SE (1989) Mood disorders following stroke: new findings and future directions. J Geriatr Psychiatry 22:1–15
Sackeim HA, Gur RC, Saucy MC (1978) Emotions are expressed more intensely on the left side of the face. Science 202:434–436
Sackeim HA, Greenberg MS, Weiman AL, Gur RC, Hungerbuhler JP, Geschwind N (1982) Hemispheric asymmetry in the expression of positive and negative emotions: neurologic evidence. Arch Neurol 39:210–218
Saslow MG (1967) Latency for saccadic eye movement. J Opt Soc Am 57:1030–1033
Schall JD, Stuphorn V, Brown JW (2002) Monitoring and control of action by the frontal lobes. Neuron 36:309–322
Sedda A et al (2013) Ambiguous emotion recognition in temporal lobe epilepsy: the role of expression intensity. Cognit Affect Behav Neurosci 13:452–463. doi:10.3758/s13415-013-0153-y
Silberman EK, Weingartner H (1986) Hemispheric Lateralization of Functions Related to Emotion. Brain Cognit 5:322–353. doi:10.1016/0278-2626(86)90035-7
Trottier L, Pratt J (2005) Visual processing of targets can reduce saccadic latencies. Vis Res 45:1349–1354. doi:10.1016/j.visres.2004.12.007
Tucker DM, Stenslie CE, Roth RS, Shearer SL (1981) Right frontal lobe activation and right hemisphere performance: decrement during a depressed mood. Arch Gen Psychiatry 38:169–174
Weddell RA (1994) Effects of subcortical lesion site on human emotional behavior. Brain Cognit 25:161–193. doi:10.1006/brcg.1994.1029
West GL, Al-Aidroos N, Susskind J, Pratt J (2011) Emotion and action: the effect of fear on saccadic performance. Exp Brain Res 209:153–158. doi:10.1007/s00221-010-2508-8
Yuvaraj R, Murugappan M, Norlinah MI, Sundaraj K, Khairiyah M (2013) Review of emotion recognition in stroke patients. Dement Geriatr Cognit Disord 36:179–196. doi:10.1159/000353440
Zhang J, Zhou R, Oei TP (2011) The effects of valence and arousal on hemispheric asymmetry of emotion. J Psychophysiol 25(2):95–103
Acknowledgments
We thank Ernest Boninchi foundation which supported this research by a Grant at Geneva University and the Swiss Center for Affective Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malsert, J., Grandjean, D. Mixed saccadic paradigm releases top-down emotional interference in antisaccade and prosaccade trials. Exp Brain Res 234, 2915–2922 (2016). https://doi.org/10.1007/s00221-016-4693-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-016-4693-6