Abstract
We investigated the effect of emotional target content on the generation of pro- and anti-saccades. Subjects had to generate saccades towards (pro-saccade) or away from (anti-saccade) peripherally presented pleasant, unpleasant or neutral pictures. Two different SOAs were used, either with simultaneous fixation offset and target onset (no gap) or with fixation offset preceding target onset by 200 ms (gap). In the pro-saccade task participants were faster to respond to emotional pictures in the left visual field. In the right visual field facilitation occurred only for pleasant pictures and saccadic reaction times towards unpleasant pictures were slowed. In the anti-saccade task more anti-saccade errors towards emotional pictures (pleasant and unpleasant) were made in the gap condition. On the whole, endogenous saccade generation appears facilitated by emotional target content, probably via increased input from extra-striate and parietal brain areas to the superior colliculus. Moderating factors such as the SOA or the visual field of presentation are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Designers of billboard advertising want their advertisements to draw the by-passers’ attention so that they will look at them. Billboards compete for people’s attention, both because people have other tasks to carry out and because often many advertisements compete. Still, billboard advertising is assumed to be fairly effective, so much so that its use along major highways is actually forbidden in some countries. But to what extent and under which circumstances do pictures as used in the media really catch people’s eyes?
A number of low-level physical stimulus properties are known to affect the deployment of spatial attention and subsequent eye movements. An object’s “salience”, i.e. its physical distinctiveness, is thought to determine whether it is selected as a new focus of attention and target location for an imminent eye movement. If multiple objects compete within a visual display, the one reaching the highest activity value is selected in a “winner takes all” manner (Fecteau and Munoz 2006).
Only recently have researchers begun to investigate whether or how the content of complex pictures affects ocular motor behaviour, providing evidence for parafoveal semantic processing. For instance, unusual, “odd”, visual configurations that violate subjects’ expectations influence eye movements: When viewing scenes that contain experimentally altered details (inverted heads, green hands), subjects fixate the altered detail earlier than when its appearance is normal (Becker et al. 2007).
Emotional relevance may be a special case of intrinsic “salience” and thus guide the deployment of attention and subsequent eye movements. However, so far only few studies directly investigated the impact of emotional stimulus content on eye movements: When a neutral picture is paired with an emotional, unpleasantly arousing picture and both are presented simultaneously, each in one hemifield, subjects’ eye movements are affected by the emotional picture: the “leftward bias”, a tendency for people to first look into the left visual field, is increased when unpleasant pictures are presented in this visual field, and attenuated or reversed when unpleasant pictures are presented in the right visual field, compared to a neutral–neutral baseline condition (LaBar et al. 2000). This pattern has by now been replicated and extended to pleasant–neutral picture pairs. In general, parafoveally presented emotional (unpleasant and pleasant) pictures are more likely to attract the initial fixation than neutral pictures when both are presented simultaneously, each in one visual field. Furthermore, emotional pictures are also fixated for a longer period of time in total (Calvo and Lang 2005). These results indicate that emotional content affects initial overt orienting as well as sustained attention.
Nummenmaa et al. (2006) further explored the issue, assessing the latency until the eye reaches first fixation and the effect of a competing instruction: Again, under free viewing instructions, participants were likely to direct first saccades towards the emotionally arousing one of two competing peripheral stimuli. Also, fixation duration was longer for emotional stimuli, with a slight advantage for pleasant pictures. The latency to reach first fixation was unaffected by emotional content. Interestingly, even under competing task instructions (fixate the neutral picture of two exemplars first) emotionally arousing pictures were looked at first more frequently and for a longer period of time than neutral pictures. Thus, the results of this report suggest that both initial orienting and subsequent attentional engagement are biased towards emotional stimuli, both during free viewing and under explicit task instructions to the opposite, with a possible advantage for pleasant contents.
Taken together, the above studies indicate first, that in situations where two stimuli compete for selection as target of an eye movement, emotionally arousing pictures are prioritized and second, that emotionally arousing pictures also counteract the experimentally imposed, top-down target selection.
Whether emotional stimulus content is capable of affecting more basic, experimentally well-studied parameters of eye movements such as saccadic reaction times or saccadic inhibition in simple single target situations has not yet been investigated, although motivational effects on saccadic reaction times have recently been demonstrated in humans: Milstein and Dorris (2007) showed that the higher the reward value of making a saccade to a peripheral target was, the shorter were saccadic reaction times. In line with this finding in humans, Ikeda and Hikosaka (2003) identified reward-dependent responses in the superior colliculus of monkeys. Thus, effects of the motivational significance of a visual target on saccadic reaction times have been demonstrated in humans and non-human primates.
Saccadic reaction times to emotionally relevant targets have not yet been studied, although saccade generation is relatively well understood and several other factors are known to affect saccadic reaction times: Stimulus brightness and contrast (Thompson and Bichot 2005), the probability of target occurrence at a certain spatial location (Dorris and Munoz 1998), and the temporal relationship between the onset of a peripheral target and the off-set of a previously fixated stimulus (Saslow 1967), have been shown to affect saccadic reaction times. Particularly the timing of fixation offset and target onset has a large effect on saccadic reaction times: If a temporal gap, i.e. a blank screen, between the offset of one target and the onset of the next target is introduced, saccadic reaction times are decreased, an effect which is mediated by disinhibtion of fixation neurons in the superior colliculus (Munoz and Wurtz 1992; Saslow 1967).
In the following, we extend previous work and test whether the emotional content of a complex target picture can affect simple saccadic reaction times in humans. Previous work has already indicated that a picture’s emotional content can affect top-down control of saccadic behaviour in a competitive manner (Nummenmaa et al. 2006), at least when two target pictures are presented simultaneously. In basic eye movement research the anti-saccade task (Hallett 1978) is often used to study the competition between exogenous, stimulus driven, and endogenous, voluntary, eye movements. In the anti-saccade task, participants are required to generate eye movements to the mirror positions of targets, i.e. upon presentation move their eyes into the opposite visual field rather than look at the presented stimulus. In anti-saccade tasks people will, in spite of the instruction not to do so, generate some erratic movements towards the peripheral target, so-called anti-saccade errors. These errors can be explained in terms of a race model. This model assumes that the stimulus driven pro-saccade program and the voluntary anti-saccade program compete for saccade initiation. If the anti-saccade program reaches a critical activation threshold fast enough, the pro-saccade program is inhibited, which is the case on the majority of trials, resulting in a correct anti-saccade. However on a minority of trials the exogenous pro-saccade will be initiated before the anti program reaches a critical threshold, leading to the generation of a pro-saccade towards the target, effectively an anti-saccade error (see Massen 2004 and Munoz and Everling 2004). On a neural level, the superior colliculus is a vital integration node, where excitatory input from parietal and occipital cortices and inhibitory signals from frontal regions and the basal ganglia converge and saccade generation is initiated. Therefore, the superior colliculus may represent a critical site for the neural implementation of this race.
Like pro-saccade performance, the performance on anti-saccade tasks is influenced by the temporal relationship between targets. If a “gap” is introduced in an anti-saccade task, saccadic reaction times are reduced and the rate of anti-saccade errors will also increase. Both of these effects are due to activity offset of fixation cells in the superior colliculus. Fixation cell activity inhibits saccadic excitability and prevents involuntary breaks of fixation.
To date, there exists only one report demonstrating an influence of semantic picture content on anti-saccade performance. Gilchrist and Proske (2006) recently showed that, when anti-saccades had to be generated away from upright faces versus faces turned by 90°, 180° and 270°, more erroneous responses were generated towards the upright than towards the turned faces. This implies that high-level visual information can affect saccade programming. The authors suggest that the effect occurs because upright faces have a stronger extra-striate representation than inverted faces and therefore lead to a stronger excitatory signal in the superior colliculus. The same may hold for emotional pictures, since these are known to lead to larger extra-striate signals than neutral ones (e.g. Junghofer et al. 2001; Keil et al. 2001, 2002).
It is currently unknown to what extent the emotional content of a visual target can influence basic pro- and/or anti-saccade parameters. However, given the literature reported above, we might expect that saccades are generated with faster reaction times when the visual target is arousing than when it is neutral. Likewise, it might be harder to suppress reflexive glances towards the visual target in an anti-saccade paradigm, when the target is affectively arousing than when it is not. Such effects may be enhanced by introducing a temporal gap between fixation cross and peripheral stimulus, since the gap renders the superior colliculus more susceptible to stimulus-driven saccade generation.
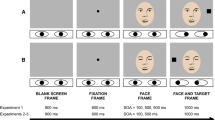
To test the effects of emotional content of pictures on pro- and anti-saccades, we conducted an experiment, where pictures varying in emotional content (pleasant, unpleasant, neutral) were presented in either visual field both under standard conditions (no gap) and under conditions that facilitate shifts of attention and eye movements (200 ms gap). Subjects were instructed to either look at the picture (pro-saccade task) or suppress the reflexive glance and look away from the picture (anti-saccade task).
Method
Participants
Twenty-four students (12 females, mean age 22.3 years, range 19–27, SD 2.1) participated in the study. All had normal or corrected-to-normal vision. They received a financial bonus of 7 € or course credit.
Materials
Twenty highly arousing unpleasant, 20 neutral and 20 highly arousing pleasant digitized pictures were selected from the International Affective Pictures System (Lang et al. 1999/2005). Pleasant and unpleasant pictures were matched for their arousal scores and differed significantly from neutral pictures on this dimension. The IAPS numbers of the pictures are given in the Appendix. Pictures were the same as previously used by Keil et al. (2001, 2002). Brightness, contrast and colour spectrum were matched across emotional categories using commercial image processing software (Adobe Photoshop).
Apparatus
Eye movements were obtained in a quiet, darkened room and measured with an infrared eye tracker (Skalar IRIS, Skalar Medical, Delft, Netherlands) with a temporal resolution of 500 Hz and a spatial resolution of 0.3°. Stimuli were peripherally presented on a 17 in. flat screen computer monitor positioned at 60 cm distance from the subjects’ eyes. Pictures subtended a visual angle of 5.8° horizontally, the eccentricity of the centre of the pictures to either side being 4.1°.
Procedure
Subjects were taken to the testing room and seated in front of the stimulus presentation monitor where the infrared measuring device was put on and adjusted to measure individual eye position. For control of head movement participants were asked to make a dental imprint on a bite bar covered with dental wax which hardens quickly. During recording the subjects were required to bite on their dental imprint to stabilize their head movements.
Prior to the actual experimental tasks, subjects were presented with calibration trials to allow for calculation of individual eye excursion at various eccentricities. Two blocks of calibration trials were presented at 15°, 12°, 10°, 8°, 6° and 3° in both visual fields. The order of tasks was counterbalanced.
Before each experimental block (pro- or anti-), the respective task was explained to the subjects. In the pro-saccade task, they were instructed to look as quickly as possible at the peripherally presented picture. In the anti-saccade task, they were instructed to not look at the peripherally presented picture, but instead look at the picture’s mirror position in the opposite visual field. Subjects were instructed to perform this task as quickly and accurately as possible. Both instructions were additionally displayed on the stimulus presentation monitor. At the beginning of each task, three practice trials were presented. These were discarded from data analysis.
Pro-saccade task
After presentation of a central fixation cross for 1.5–2.5 s, the target picture (unpleasant, neutral, or pleasant) appeared in either the left or the right visual field for 500 ms. The temporal relationship between extinction of the fixation cross and onset of the peripheral target was varied:
-
1.
0 ms gap (no gap): Target onset coincided with the extinction of the fixation cross.
-
2.
200 ms gap (gap): Extinction of the fixation cross preceded target onset by 200 ms.
For each combination of conditions (picture content: unpleasant, neutral, pleasant; visual field: left, right; stimulus onset asynchrony: no gap, gap) 20 trials were presented, resulting in a total of 240 trials presented in 2 blocks of 120. Blocks were separated by a short break. Order of pictures, target locations and fixation conditions varied pseudo-randomly.
Anti-saccade task
The stimulation in the anti-saccade task was identical to the one used in the pro-saccade task, but subjects were instructed not to look at the picture presented but instead to direct their gaze to the opposite visual field when the target appeared. The order of pro- or anti-saccade blocks was counterbalanced across subjects.
Data reduction and analysis
Ocular motor analysis
Data were analysed off-line using programs written in Matlab (The Mathworks Inc, Natick, MA, USA). Eye movement and velocity tracings were displayed on a computer monitor for visual inspection and semi-automatic saccade scoring: Saccade onset was defined as the point in time, when eye movement velocity exceeded 30°/s and marked automatically. In line with the literature, saccades that had latencies shorter than 80 ms were considered anticipatory and excluded from the analysis (e.g. Gilchrist and Proske 2006; Wenban-Smith and Findlay 1991). Manual scoring excluded trials including saccades within 200 ms prior to target offset and blinks within a time period of 200 ms before stimulus onset to 500 ms after stimulus onset. Saccadic reaction times of saccades from artefact-free trails were automatically written to computer disk and stored for statistical analysis. Directional errors in the anti-saccade task were scored manually. Scoring was carried out by a trained student research assistant who was blind to the purpose of the study.
Experimental effects on saccadic reaction times and anti-saccade error rates were statistically analysed using repeated measures analyses of variance (ANOVA). Repeated measurement factors were stimulus onset asynchrony (SOA: 200 ms gap, no gap), content (unpleasant, neutral, pleasant) and visual field (left, right). Effects of emotional content on saccadic reaction times or anti-saccade error rates were followed up using Fisher’s least significant difference tests.
Results
Pro-saccade task
There were on average 17.1 (SD 3.09) useable trials per experimental factor (2 SOAs, 3 contents, 2 visual fields), in the pro-saccade task.
Reaction times
Mean saccadic reaction times for the two SOAs, visual fields and different emotional content conditions are detailed in Table 1. Saccadic reaction times were faster in the gap than in the no gap condition [SOA, F(1, 46) = 71.2, MSE = 226.57, P < 0.0001]. Picture content tended to affect saccadic reaction times in general [content, F(2, 46) = 2.71, MSE = 109.23, P < 0.08]. There was no main effect of visual field on saccadic reaction times [F(1, 23) = 0.59, MSE = 393, P = 0.45] and the effect of picture content did not interact with the SOA [content × SOA, F(2, 46) = 0.08, MSE = 85.88, P > 0.9]. However, as shown in Fig. 1, the effect of picture content differed between the visual fields [content × visual field, F(2, 46) = 3.77, MSE = 41.2, P < 0.05). Follow up comparisons revealed that in the left visual field saccades to both pleasant and unpleasant pictures were faster than to neutral ones, while in the right visual field saccadic reaction times to unpleasant pictures were slower than both saccadic reaction times to pleasant pictures in that visual field and to unpleasant pictures presented in the left visual field (all P < 0.05, see Fig. 1). There was also a tendency for the gap effect to differ between the visual fields [SOA × visual field, F(1, 23) = 4.05, MSE = 121.19, P = 0.06). In the gap condition saccadic reaction times were somewhat faster into the left than into the right visual field. The three-way interaction had no clear impact [SOA × content × visual field, F(2, 46) = 2.4, MSE = 78.44, P = 0.09).
Anti-saccade task
There were on average 16.07 (SD 3.61) useable trials per experimental factor (2 SOAs, 3 contents, 2 visual fields) in the anti-saccade task.
Reaction times
Anti-saccade reaction times were faster in the gap than in the no gap condition [SOA, F(1, 46) = 39.28, MSE = 335.53, P < 0.001]. However, anti-saccade reaction times did not differ reliably depending on picture content, although anti-saccades away from pleasant and unpleasant pictures were minimally slower than anti-saccades away from neutral ones [content, F(2, 46) = 2.67, MSE = 321.68, P = 0.07]. Consequently, post hoc tests for the factor emotional content failed to reach statistical significance. The visual field in which targets were presented had no main effect on anti-saccade reaction times [visual field, F(1, 23) = 0.37, MSE = 1,073, P = 0.54]. There was no reliable interaction between stimulus content and SOA regarding anti-saccade reaction times [content × SOA, F(2, 46) = 1.87, MSE = 224.7, P = 0.17], but there was an interaction of the gap effect with the visual field in which targets were presented [SOA × visual field, F(2, 46) = 4.3, MSE = 308.47, P = 0.02]. Like for the pro-saccades, under gap conditions anti-saccade reaction times were faster when the target was presented in the left visual field (P < 0.05). Neither the emotional content of the pictures and the visual field in which they were presented [content × visual field, F(2, 46) = 0.37, MSE = 448.2, P = 0.69] nor the three-way interaction (SOA × content × visual field, F(2, 46) = 0.40, MSE = 350.8, P = 0.67] were significant.
Error rate
Figure 2 details the influence of picture content on anti-saccade error rates for the two stimulus onset asynchronies (gap and no gap). Overall, more anti-saccade errors were made in the gap than in the no gap condition [SOA, F(1, 46) = 4.8, MSE = 0.01, P < 0.05]. There was no main effect of picture content on anti-saccade errors [content, F(2, 46) = 1.68, MSE = 0.009, P = 0.20] and no effect of the visual field [visual field, F(1, 23) = 0.37, MSE = 0.01, P = 0.55]. However, a significant interaction between picture content and SOA was found [SOA × content, F(2, 46) = 4.6, MSE = 0.007, P < 0.05]. Individual ANOVAs revealed that emotional content affected anti-saccade errors only in the gap condition [content, F(2, 46) = 3.92, MSE = 0.012, P < 0.05] but not in the no gap condition [content, F(2,46) = 0.43, MSE = 0.004, P = 0.65]. Post hoc comparisons showed that in the gap condition significantly more anti-saccade errors were made when pleasant and unpleasant pictures were presented than when neutral pictures were shown (P < 0.05). Pleasant and unpleasant pictures did not differ. Neither the interactions between SOA and visual field [F(1, 23) = 2.57, MSE = 0.006, P = 0.12] and content and visual field [F(2, 46) = 0.97, MSE = 0.008, P = 0.39] nor the three-way interaction of SOA, content and visual field [F(2, 46) = 2.81, MSE = 0.009, P = 0.08] yielded clearly significant effects (Table 2).
Discussion
This is the first study to examine the effect of emotional picture content on pro- and anti-saccades. Emotional picture content influenced both pro- and anti-saccade parameters, but the effects interacted with experimental factors, such as stimulus onset asynchrony (gap–no gap) and the visual field in which the pictures were presented. As a whole, the present results indicate that emotional pictures act on exogenous components of the saccadic system in a facilitatory manner. Concerning simple saccadic reaction times to complex pictures, the previously reported “gap” effect was confirmed, and a numerically small, but significant effect of emotional content on saccadic reaction times was found. This effect differed between the visual fields: Saccadic reaction times of responses generated into the left visual field were faster towards both pleasant and unpleasant than towards neutral pictures, whereas responses into the right visual field were slower towards unpleasant than towards pleasant pictures. Saccadic reaction times towards unpleasant pictures in the right visual field were also slower than saccadic reaction times towards unpleasant pictures in the left visual field.
Concerning reaction times, the observed effects, although numerically small, were quite consistent. Here, saccadic reaction times may in fact be close to a floor effect as subjects were instructed to generate the saccades as quickly as possible. Consequently the reaction times were much faster than in previous studies with emotional material (e.g. Miltner et al. 2004; Nummenmaa et al. 2006). These previous studies, however, also presented multiple stimuli simultaneously which may have slowed saccadic reaction times. Nummenmaa et al. (2006), who reported the time taken to reach the first fixation on a picture, a measure related to, but not identical with, saccadic reaction time, failed to find emotional content dependent differences. Becker et al. (2007), on the other hand, do report faster fixation on an “odd” scenic detail. Likewise, Milstein and Dorris (2007) report shorter saccadic reaction times to targets with higher motivational value.
With regard to processing differences between the two visual fields the present pattern is consistent with reports of a left visual field/right hemisphere superiority in responses to emotional stimuli in general. This may be combined with a selective left hemisphere perceptual bias towards pleasant emotional stimuli and slowed processing of unpleasant stimuli in the left hemisphere. Hemispheric cooperation effects may lead to faster reaction times for pleasant stimuli and particularly slowed reaction times when unpleasant stimuli are presented in the right visual field. This pattern has been reported by several authors in emotional face perception tasks in healthy people (Hugdahl et al. 1989; Natale et al. 1983; Kimura et al. 2004). A similar suggestion has been made on the basis of lesion data: Adolphs et al. (2001), for instance, have suggested that the perception of negative affect from faces relies on the right hemisphere, whereas the processing of positive valence is supported by both hemispheres. Using functional neuroimaging in healthy subjects, Killgore and Yorgelun-Todd (2007) recently also postulated that two different systems interact during emotion perception: While a right hemispheric system is superior in emotion perception and may be specialized for the processing of emotion regardless of valence. Because of its limited capabilities with regard to emotion discrimination, a left hemispheric system may be superior in processing pleasant content, which, at least in face recognition, is less diverse than unpleasant content.
In the anti-saccade task, a higher error rate towards emotional pictures was found in the gap condition. This result is in line with previous reports of more erratic glances at an emotional picture in spite of the explicit instruction to first look at the neutral picture in a pair (Nummenmaa et al. 2006). The present results are also in line with a previous report of high level semantic visual properties on anti-saccade performance (Gilchrist and Proske 2006). In their study, more anti-saccade errors resulted when subjects were looking away from upright than from turned faces.
The question arises as to why at present the effect of stimulus content was found only in the “gap” and not in the “no gap” condition. Recall that in an anti-saccade task two processes race against each other: One associated with programming the exogenous response towards the stimulus and another one programming the endogenous response to the target’s mirror position. In the gap condition saccadic inhibition is handicapped by the fixation cells in the superior colliculus being turned off, making it easier for the reflexive error saccade to be triggered. If in this case the excitatory perceptual input from extra-striate visual and parietal areas to the superior colliculus is increased, generation of erratic pro-saccades should be facilitated. Indeed, emotional stimuli are associated with increased cortical responses in extra-striate visual and parietal areas (e.g. Cuthbert et al. 2000; Keil et al. 2001, 2002). Likewise, upright faces are associated with stronger extra-striate responses than inverted ones (Haxby et al. 1999). In the “0 gap” condition, by contrast, the increase in extra-striate visual and parietal input may not have been strong enough to break fixation. Interestingly, Gilchrist and Proske (2006) remark on the unusually high error rate in their study and attribute this to their use of a very unpredictable anti-saccade task, where the target could appear at eight different spatial locations. Thus the presence of an emotional stimulus, by itself, may not be enough to overcome the voluntary program to look away from the target. Additional factors that make the task more difficult, such as stimulus competition (Nummenmaa et al. 2006), many different target locations (Gilchrist and Proske 2006) or fixation disengagement, as in the present study, may be required.
In clinical populations, the perceptual representation of a feared stimulus may be so strong that it may, by itself, be sufficient to cause an emotional content dependent increase in anti-saccade errors. Thus the question of how emotion facilitates ocular motor behaviour may be further evaluated in experiments that combine the present paradigm with mood induction procedures or examine subjects with clinically altered processing biases. Indeed, two recent studies demonstrate effects of fear-relevant emotional content on ocular motor behaviour during visual search in spider phobic subjects (Miltner et al. 2004; Rinck and Becker 2006).
Our results, perhaps to the dismay of the advertising industry, also demonstrate limits to the power of emotions to alter behavioural responses: Under the present experimental conditions the distracting power of emotional stimuli in the anti-saccade paradigm was only significant when the visual system had not been previously engaged by some other stimulus. Still, this is the first study to demonstrate a considerable impact of emotional picture content on basic saccadic reactions. Further research will determine under which experimental conditions what types of emotion effects on ocular motor behaviour are most clearly seen.
References
Adolphs R, Jansari A, Tranel D (2001) Hemispheric perception of emotional valence from facial expressions. Neuropsychology 15:516–524
Becker MW, Pashler H, Lubin J (2007) Object-intrinsic oddities draw early saccades. J Exp Psychol Hum Percept Perform 33:20–30
Calvo MG, Lang PJ (2005) Parafoveal semantic processing of emotional visual scenes. J Exp Psychol Hum Percept Perform 31:502–519
Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ (2000) Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol Psychol 52:95–111
Dorris MC, Munoz DP (1998) Saccadic probability influences motor preparation signals and time to saccadic initiation. J Neurosci 18:7015–7026
Fecteau JH, Munoz DP (2006) Salience, relevance, and firing: a priority map for target selection. Trends Cogn Sci 10:382–390
Gilchrist ID, Proske H (2006) Anti-saccades away from faces: evidence for an influence of high-level visual processes on saccade programming. Exp Brain Res 173:708–712
Hallett PE (1978) Primary and secondary saccades to goals defined by instructions. Vision Res 18:1279–1296
Haxby JV, Ungerleider LG, Clark VP, Schouten JL, Hoffman EA, Martin A (1999) The effect of face inversion on activity in human neural systems for face and object perception. Neuron 22:189–199
Hugdahl K, Iversen PM, Ness HM, Flaten MA (1989) Hemispheric differences in recognition of facial expressions: a VHF-study of negative, positive, and neutral emotions. Int J Neurosci 45:205–213
Ikeda T, Hikosaka O (2003) Reward-dependent gain and bias of visual responses in primate superior colliculus. Neuron 39: 693–700
Junghofer M, Bradley MM, Elbert TR, Lang PJ (2001) Fleeting images: a new look at early emotion discrimination. Psychophysiology 38:175–178
Keil A, Müller MM, Gruber T, Wienbruch C, Stolarova M, Elbert T (2001) Effects of emotional arousal in the cerebral hemispheres: a study of oscillatory brain activity and event-related potentials. Clin Neurophysiol 112:2057–2068
Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert T, Lang PJ (2002) Large-scale neural correlates of affective picture processing. Psychophysiology 39:641–649
Killgore WDS, Yorgelun-Todd DA (2007) The right hemisphere and valence hypotheses: could they both be right (and sometimes left)? Soc Cogn Affect Neurosci 2:240–250
Kimura Y, Yoshino A, Takahashi Y, Nomura S (2004) Interhemispheric difference in emotional response without awareness. Physiol Behav 82:727–731
LaBar KS, Mesulam M, Gitelman DR, Weintraub S (2000) Emotional curiosity: modulation of visuospatial attention by arousal is preserved in aging and early-stage Alzheimer’s disease. Neuropsychologia 38:1734–1740
Lang PJ, Bradley MM, Cuthbert BN (1999, 2005) International affective picture system (IAPS): digitized photographs, instruction manual and affective ratings. Technical report A-6. University of Florida, Gainesville, FL
Massen C (2004) Parallel programming of exogenous and endogenous components in the antisaccade task. Q J Exp Psychol A 57:475–498
Milstein DM, Dorris MC (2007) The influence of expected value on saccadic preparation. J Neurosci 27:4810–4818
Miltner WH, Krieschel S, Hecht H, Trippe R, Weiss T (2004) Eye movements and behavioral responses to threatening and nonthreatening stimuli during visual search in phobic and nonphobic subjects. Emotion 4:323–339
Munoz DP, Wurtz RH (1992) Role of the rostral superior colliculus in active visual fixation and execution of express saccades. J Neurophysiol 67:1000–1002
Munoz DP, Everling S (2004) Look away: the anti-saccade task and the voluntary control of eye movement. Nature Rev Neurosci 5:218–228
Natale M, Gur RE, Gur RC (1983) Hemispheric asymmetries in processing emotional expressions. Neuropsychologia 21:555–565
Nummenmaa L, Hyona J, Calvo MG (2006) Eye movement assessment of selective attentional capture by emotional pictures. Emotion 6:257–268
Rinck M, Becker ES (2006) Spider fearful individuals attend to threat, then quickly avoid it: evidence from eye movements. J Abnorm Psychol 115:31–238
Saslow MG (1967) Latency for saccadic eye movement. J Opt Soc America 57:1030–1033
Thompson KG, Bichot NP (2005) A visual salience map in the primate frontal eye field. Prog Brain Res 147:251–262
Wenban-Smith MG, Findlay JM (1991) Express saccades: is there a separate population in humans? Exp Brain Res 87:218–222
Acknowledgments
We thank Andrea Feichtenschlager, Melanie Zell and Niklas Ihssen for help with data acquisition and analysis.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
IAPS numbers of stimuli used in the present study:
Pleasant pictures: 2050, 2070, 2080, 2160, 2165, 2170, 2311, 2340, 2341, 2360, 4650, 4651, 4652, 4658, 4659, 4660, 4664, 4670, 4680, 4690.
Neutral pictures: 2190, 2200, 221,0 2230, 2381, 2440, 2480, 2570, 2850, 7002, 7009, 7010, 7020, 7030, 7040, 7080, 7175, 7233, 7235, 9070.
Unpleasant pictures: 1050, 1120, 1201, 1300, 1930, 3000, 3010, 3050, 3060, 3071, 3080, 3102, 3110, 3130, 3530, 6260, 6350, 6510, 6540, 9405.
Rights and permissions
About this article
Cite this article
Kissler, J., Keil, A. Look–don’t look! How emotional pictures affect pro- and anti-saccades. Exp Brain Res 188, 215–222 (2008). https://doi.org/10.1007/s00221-008-1358-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-008-1358-0