Abstract
Despite several pieces of evidence, which suggest that the human brain employs internal models for motor control and learning, the location of these models in the brain is not yet clear. In this study, we used transcranial direct current stimulation (tDCS) to manipulate right cerebellar function, while subjects adapt to a visuomotor task. We investigated the effect of this manipulation on the internal forward and inverse models by measuring two kinds of behavior: generalization of training in one direction to neighboring directions (as a proxy for inverse models) and localization of the hand position after movement without visual feedback (as a proxy for forward model). The experimental results showed no effect of cerebellar tDCS on generalization, but significant effect on localization. These observations support the idea that the cerebellum is a possible brain region for internal forward, but not inverse model formation. We also used a realistic human head model to calculate current density distribution in the brain. The result of this model confirmed the passage of current through the cerebellum. Moreover, to further explain some observed experimental results, we modeled the visuomotor adaptation process with the help of a biologically inspired method known as population coding. The effect of tDCS was also incorporated in the model. The results of this modeling study closely match our experimental data and provide further evidence in line with the idea that tDCS manipulates FM’s function in the cerebellum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is believed that the CNS uses some kind of internal models for motor learning, planning and execution (Flanagan and Wing 1997; Kawato 1999; Wolpert and Kawato 1998; Wolpert et al. 1998). In general, internal models can be categorized into two main groups: forward models (FMs) and inverse models (IMs). FMs try to model the input–output function of body segments involved in the movements, and therefore they can be used as predictors (Flanagan et al. 2003; Flanagan and Wing 1997; Jordan and Rumelhart 1992; Shadmehr et al. 2010; Wolpert et al. 1995). On the other hand, inverse models mimic the inverse of these transfer functions, and they can be used as controllers (Katayama and Kawato 1993; Kawato 1999; Kawato and Gomi 1992b; Sabes 2000; Shidara et al. 1993; Wolpert et al. 1998). Model-based theories of human motor control take root in control theory (Miall et al. 1993; Towhidkhah et al. 1997). In recent years, scientists have tried to find physiological evidence and anatomical basis for these theories (Blakemore et al. 2001; Cerminara et al. 2009; Gomi and Kawato 1992; Higuchi et al. 2007; Imamizu et al. 2000, 2003; Kawato and Gomi 1992b; Kawato et al. 2003; Lisberger 2009). Several studies point to the cerebellum as the candidate region for the formation and storage of internal models (Cullen et al. 2011; Kawato 1999; Kawato et al. 1987; Kawato and Gomi 1992a; Lisberger 2009; Miall et al. 2007; Miall et al. 1993; Nowak et al. 2007; Pasalar et al. 2006; Wolpert et al. 1995; Wolpert and Kawato 1998). However, primary motor cortex is also considered as a candidate region for the formation of internal inverse models (Li et al. 2001; Richardson et al. 2006; Shadmehr 2004; Shadmehr and Krakauer 2008; Yavari et al. 2013).
In order to investigate the possible formation/adaptation of FM and/or IM in the cerebellum, we applied tDCS over subjects’ right cerebellum to manipulate its function, while subjects learn a new visuomotor task. To the best of our knowledge, there are only two behavioral studies that investigated the formation of internal models in the brain (Izawa et al. 2012; Miall et al. 2007). Miall et al. (2007) used transcranial magnetic stimulation (TMS) to disrupt cerebellar function, while subjects reached toward a visual target. They found that cerebellum disruption resulted in movements that were based on out-of-date estimations of hand position (Miall et al. 2007). This shows cerebellum’s role in state estimation, one of the characteristics of the FM. In another study, Izawa et al. (2012) designed an experiment to examine whether the cerebellum can be seen as FM, IM or both. They compared performance of healthy subjects and cerebellar patients and concluded that the cerebellum has a critical role in formation of FMs but not IMs.
The research protocol in the current study is a modified version of the one presented by Izawa et al. (2012). We used tDCS to manipulate cerebellum function, while subjects adapted to a visuomotor task. There were three groups of subjects who received anodal (facilitatory), cathodal (inhibitory) and sham stimulation. We investigated the effect of tDCS on FM and IM formation. IM can act as a controller and produces the necessary motor commands for movement. If motor learning results in formation/modification of IM, then we should expect that generalization from trained to untrained workspace should follow the IM commands. In our experiment, subjects learned a new visuomotor transformation while reaching to a single target. Then, they were asked to reach to new sets of targets without visual feedback to estimate the motor generalization. If cerebellum has a role in IMs formation, then one expects to observe differences in the subjects’ performance under different tDCS electrode montages; i.e., it is expected to observe some differences in the way in which they alter their motor commands in the presence of perturbation and also the way in which they generalize this learning to the other directions. This helped us to investigate and compare IM formation in our three experimental groups. On the other hand, if the brain forms a FM to predict the sensory consequences of motor commands, then we expect that the FM changes one’s perception of hand position after learning a new visuomotor transformation. We developed a new method to measure a subject’s perception of his hand’s position in space. Subjects were asked to report their unseen hand’s position after a shooting movement. These measures were obtained in different places in the workspace and were used to study and compare FMs in different groups. In summary, if internal inverse/forward models were formed in the cerebellum, it is expected that tDCS application changes the observed generalization/estimation pattern and produces significant difference between these three experimental groups. Our experimental results showed that tDCS changes subject’s position estimation and does not change generalization pattern. Therefore, our findings are in agreement with FM formation in the cerebellum.
We used a realistic human head model to simulate electrical field and current density distribution over the stimulated area of the brain. These modeling results showed that the applied current would pass through the cerebellum.
Inspired by previous biological and experimental observations (Bindman et al. 1962; Gartside 1968; Purpura and McMurtry 1965), the process of visuomotor adaptation in the presence of tDCS was also modeled with a computational framework known as population coding. Our experimental and simulation results show that the cerebellum can be seen as a candidate region for internal forward (and not inverse) model.
Materials and methods
Subjects
Twenty-nine volunteers participated in this study (mean age 24 ± 5 years; 12 men). All the subjects were right-handed and healthy, with no history of neurological or psychiatric illness. They all had normal or corrected-to-normal vision and were naive to the experimental manipulations. All subjects signed a consent form approved by Amirkabir University of Technology Ethics Committee and were compensated for their time.
Subjects were randomly assigned to one of the three experimental groups in which they received either cerebellar anodal, cathodal or sham stimulation (10, 10 and 9 subjects, respectively). They were blind to the type of stimulation.
We performed a neurological assessment test before and after the experimental manipulation, which involves paper-and-pencil tests of motor graphic (Archimed spiral, horizontal lines and broken lines test) (Ferrucci et al. 2008, 2012, 2013). We used visual analog scale (VAS) before and after tDCS to assess subjects’ attention, mental and physical fatigue, and perceived pain as a result of tDCS in which 0 and 100 represented minimum and maximum levels of attention, fatigue or pain, respectively (Galea et al. 2009, 2011). At the end of each session, participants completed an ad hoc questionnaire to test for possible adverse effects (including headache, dizziness and nausea).
Transcranial cerebellar direct current stimulation (tcDCS)
tcDCS studies have focused on two aspects of the cerebellum’s role: (1) nonmotor cerebellar functions: for example, working memory (WM) (Boehringer et al. 2013; Ferrucci et al. 2008), processing facial emotions (Ferrucci et al. 2012) and cognitive tasks (Pope and Miall 2012); and (2) motor cerebellar functions (Block and Celnik 2013; Ferrucci et al. 2013; Galea et al. 2011; Jayaram et al. 2012; Morton and Bastian 2006; Sadnicka et al. 2013; Shah et al. 2013).
In this study, we aimed to modulate cerebellar function during a visuomotor adaptation task to investigate its involvement in the internal model formation. Stimulation parameters were chosen based on several previous studies as follows: (1) electrodes: regular rectangular sponge electrodes with dimensions 5 × 5 cm2 soaked in saline (Boehringer et al. 2013; Galea et al. 2009, 2011; Jayaram et al. 2012; Pope and Miall 2012); (2) current amplitude: 2 mA (Boehringer et al. 2013; Ferrucci et al. 2008, 2012, 2013; Galea et al. 2009, 2011; Jayaram et al. 2012; Pope and Miall 2012); (3) stimulation duration: 15 min (Ferrucci et al. 2008; Galea et al. 2011; Jayaram et al. 2012; Shah et al. 2013); (4) stimulation type: anodal, cathodal and sham (Ferrucci et al. 2008, 2012; Galea et al. 2009; Jayaram et al. 2012; Pope and Miall 2012; Shah et al. 2013); (5) active electrode: over right cerebellum 3 cm lateral to the inion (Galea et al. 2009, 2011; Jayaram et al. 2012; Shah et al. 2013); and (6) passive electrode: over the right buccinator muscle (Boehringer et al. 2013; Galea et al. 2009, 2011; Jayaram et al. 2012; Shah et al. 2013).
A modeling study by Ferrucci et al. (2013) found that extra-cephalic reference electrodes produce maximum stimulation effect in the posterior cerebellar cortex. However, cell recording in animal studies (Cerminara et al. 2009; Laurens et al. 2013; Liu et al. 2003) and also imaging studies (Blakemore et al. 2001; Higuchi et al. 2007; Imamizu and Kawato 2012; Imamizu et al. 2000, 2003; Kawato et al. 2003; Milner et al. 2007) suggested that lateral and anterior cerebellum are the probable sites of formation or storage of internal models (e.g., see Fig. 3 in Imamizu et al. 2003). Since we studied the effect of cerebellar stimulation on internal models, the passive electrode was placed over the buccinator instead of deltoid muscle. We expected that with this montage, current would pass through the lateral and anterior parts of the cerebellum. Our simulation of electrical field and current density distribution using a realistic human head model showed that these regions are affected. Moreover, this montage does not lead to stimulation of brainstem or occipital cortex (Galea et al. 2009, 2011).
At the beginning of each tDCS stimulation, electrical current was introduced in a ramp-and hold manner similar to previous studies (Hummel et al. 2005; Nitsche et al. 2003a, b). Current density was 0.08 mA/cm2 which is safe and far below the threshold for tissue damage (Boggio et al. 2006; Iyer et al. 2005; Liebetanz et al. 2009). After stimulation start, subjects felt a transient and mild itching sensation under electrodes, which is normal. For sham stimulation, current was applied only for 30 s to produce the initial sensation and turned off afterward.
Experimental procedure and protocol
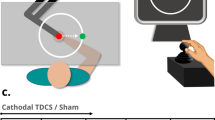
The experimental setup was almost identical to the one used in some previous studies (Gentili et al. 2013; Saidi et al. 2012). Subjects sat in a chair in front of a horizontal LCD monitor in which the visual information was provided to the subject. They performed shooting hand movements (Block and Celnik 2013; Galea et al. 2011; Izawa et al. 2012) while holding a digitizing tablet’s (9 × 12 Inches, Intuos3) pen with their right hand. Direct view of subjects’ right hand was occluded by the table. There was a one-to-one mapping between movements on the tablet screen and the visual feedback on the monitor. Start and target’s positions were displayed as black circles with 5 mm diameter. Subject hand movement was also projected in the monitor.
Our experiment consisted of three phases: preadaptation, adaptation and post-adaptation. Figure 1a shows number of blocks and trials in each phase. An eye schematic in Fig. 1a shows the presence of visual feedback in that block. In the experimental blocks without visual feedback, the cursor disappeared from the display as soon as the subject’s hand moved out of the start position. Each block’s description is explained bellow.
a Time course and different blocks of the experiment. There were three phases on this experiment: preadaptation (seven blocks), adaptation (one block) and post-adaptation (three blocks). tcDCS was applied during visuomotor adaptation phase (gray box). Eye schematic inside the blocks shows the presence of visual feedback. b Each trial in the localization blocks consisted of four consecutive steps: 1 Start position was displayed on the monitor; 2 subject should move his/her hand to the start point after which a 5-mm-length line was shown next to the start position; 3 subjects are asked to execute a shooting movement in that specific direction. Afterward, they were asked to move their hand to the bottom right corner of the tablet screen. 4 A graded arc was displayed, and subjects were asked to report the remembered location of their right hand as it crossed the arc. c Close-up of the graded arc
Familiarization
In this block, after the first touch of pen with the tablet, a cursor appeared on the monitor and subjects were asked to move the cursor to the start position. When subjects reached the start point, then target appears 10 cm from the start point and in 45° angle. A countdown next to the start point gives the subject the go cue, and at the same time, target color changes to red. Subjects were asked to start their shooting movement as soon as they see the go cue. They were instructed to make straight movements with no corrections. The visual feedback of the hand was removed as soon as the subject’s hand crossed the 10-cm mark from the start point, and feedback was provided to the subject. After nonsuccessful movements, a green 5-mm circle was displayed at the point in which subjects passed the 10-cm distance from the start point. After successful movements, the target circle was changed to a smiley face. Subjects could use this feedback to modify their future movements. A text message (“too fast!”, “too slow!”, and “good”) next to the end point was used to provide the velocity feedback to the subject. A movement was considered successful if it both crosses the target and its duration from start to target point was within 275–375 ms. Subjects were told that spatial accuracy should be their first priority.
At the end of each trial, subjects had to return to the start point. The cursor reappears only when the distance to the start point is less than 2 cm.
Subjects performed forty trials in this block. For the first 15 trials, subjects were not provided with the velocity feedback to make the movements easier. Number of successful trials was shown next to the start point.
Baseline with visual feedback
This block was identical to the familiarization phase with the exception that the velocity criterion was imposed for all 50 trials.
Generalization and localization
We employed generalization and localization blocks to examine the inverse and forward models, respectively. Our generalization blocks were similar to Izawa et al.’s study (2012). In each trial, subjects reach to one of the seven targets 15°, 25°, 35°, 45°, 55°, 65° and 75° relative to the tablet’s coordinate system. Visual feedback was only provided for the 45° target. In the preadaptation block, 20 out of 50 trials were toward the 45° target. In the post-adaptation block, 107 out of 137 trials were toward the 45° target. Each one of the peripheral angles was displayed 5 times (Izawa et al. 2012). The goal of these blocks was to evaluate the generalization of learning from 45° target to the other targets. The other aspects of these trials were similar to that of familiarization block. Based on its definition, internal inverse model associates desired sensory states with the required motor command for achieving these states (Katayama and Kawato 1993; Kawato 1999; Kawato and Gomi 1992b; Sabes 2000; Shidara et al. 1993; Wolpert et al. 1998). In our experiment, subjects trained movements toward a target at 45° with and without perturbation, and then in generalization blocks, motor commands which subjects generated for movement toward other targets were measured.
On the other hand, if the brain uses FM to predict the sensory consequences of hand motions, then localization task can help us to investigate this phenomenon. In a study by Izawa et al. (2012), subjects performed shooting movements without any visual target for the localization task. They were then asked to report with their left hand the remembered final location of their right hand. However, it has been shown that training with the right hand can transfer to the left hand (Balitsky Thompson and Henriques 2010; Birbaumer 2007; Parlow and Kinsbourne 1989; Sainburg and Wang 2002; Taylor et al. 2011; Wang 2008; Wang and Sainburg 2006). Therefore, the left hand’s pointing task could be affected by the training performed with the right hand. To solve this problem, we developed a new method to estimate subjects’ position perception of their unseen hand. At the beginning of each trial, start position was displayed on the monitor (block 1 in Fig. 1b). Subjects were asked to bring the pen tip to this point and click once. Then a 5-mm-length line was shown next to the start point for 0.7 s (block 2 in Fig. 1b). The direction of this line was randomly chosen from 5° to 85° with step size 5°. Subjects were asked to perform a shooting movement from the start point in the specified direction. Then they asked to move their hand to the bottom right corner of the tablet screen (block 3 in Fig. 1b). Visual feedback of the hand trajectory was absent during the movement. When subject’s hand crossed the 10-cm-radius circle centered on the start point, an arc appeared on the monitor. This 10-cm-radius arc was from −18° to 107° and graded from 1 to 26 with step size of 5° (block 4 in Fig. 1b, c). Subjects were asked to report the number in which they crossed the arc. Each subject repeated this movement 30 times and in different randomly selected directions. The reported numbers were recorded and used to compute the errors in the subjects’ estimation of the positions.
Since there was no target in the localization blocks, we color-coded the arc to provide the subjects with velocity feedback.
Working memory (WM) test
Subjects should be able to remember where they crossed the arc in the localization task. In this part, we wanted to test the subjects’ ability to maintain a sensory stimulus in their WM for about 1 s. After subjects clicked once at the start point, a “Go!” signal was displayed next to the start point. They were asked to do a shooting movement without any visual feedback in an arbitrary direction within the first quadrant (0°–90°). When they passed the 10-cm mark from the start point, a 5-mm-diameter red circle was temporarily shown at a random angle, from 5° to 85° with step size of 5°. After one second, this circle faded and a graded arc similar to the one in the localization block was displayed. Subjects reported where in the arc the red circle appeared. The shooting movement was added to this block to make it more similar to the localization block.
Adaptation phase
In this part, a 30° CCW rotation was imposed between hand motion and the cursor on the screen that represents the hand (Block and Celnik 2013; Galea et al. 2011; Izawa et al. 2012). Subjects did not know in advance about the rotation. All other aspects of shooting movements were similar to that of the familiarization block. All participants learned to successfully compensate for the cursor rotation after a short practice. Target direction was kept at 45°. Subjects completed VAS forms before and after this phase, and tDCS was applied during this phase.
Visuomotor rotation was kept constant through all the blocks of the post-adaptation phase.
Retrain block
Subjects performed 60 additional movements toward 45° in the presence of 30° visuomotor rotation.
Movement without visual feedback
In the baseline generalization block, Izawa et al. (2012) observed that movements without visual feedback tend to be attracted to a 45° target in which subject had visual feedback. It was not clear whether this observation was due to movement repetition toward 45° or other factors. In this initial block, subjects were asked to reach toward different targets [15°, 25°, 35°, 45°, 55°, 65°, 75°] without visual feedback to examine whether this directional preference happens without repetition.
Modeling the current pathway
Although behavioral studies of tDCS have become more frequent, the mechanism by which tDCS affects the brain and the localization of its effected regions are not totally clear. To better understand these effects, computational models and specifically finite element models can help us to estimate the affected area. We built a realistic human head model to visualize electric field (E) or current density distribution in the brain. High-resolution anatomical scans of a healthy subject (24 years old, male) were used to build masks of scalp, skull, cerebrospinal flow, gray matter and white matter of the brain. We assumed isotropic electrical conductivity for each of these tissues. Table 1 shows electrical conductivity of different tissues in low frequency ranges (Shahid et al. 2014). The electrodes size and montage were similar to our experimental procedure (Fig. 2a).
Simulation of current density distribution using two square pad electrodes. The colorbar scale is from Min = 0 to Max = 0.17 A/m2, for b, c and d plots. a Electrodes’ montage. b Back view of current density distribution on the surface of gray matter. c An arbitrary slice in axial plane corresponding to the dashed line in b (y = 171) shows the induced current density across lateral and anterior parts of the cerebellum. d Sagittal view of cortical surface shows induced current density across lateral part of the cerebellum. Selected region by red box is expanded for better view of the obtained result. e Induced current density vectors across a sagittal plane are visualized with red cones. Close-up of the region in the dashed square is shown in inset (color figure online)
We used quasi-static approximation of Maxwell’s equation to determine the induced electric potential by tDCS (Plonsey and Heppner 1967). Under this assumption, electric potential on volume conductor model can be described by Laplace equation:
where σ and V are the conductivity of biological tissue and the electric potential, respectively. Estimation of induced E was obtained by gradient of V. Moreover, Ohm’s law predicts the current density:
J, the normal component (perpendicular component) of current density corresponding to 2 mA current intensity, was applied to an active electrode, and ground boundary condition was assigned to the reference electrode. We assume all external boundaries to be isolated and continuity of normal current density to be preserved for all inner layers.
To quantify the maximum value of current density (electric field), we calculated the average of values greater than 0.75 of maximum value.
Computational model of visuomotor adaptation
We used population-coding framework, which was developed to simulate information encoding in the brain, to model the adaptation process in the presence of tDCS. In this method, if the stimuli are in a neuron’s response field, then the neuron will fire. A large population of neurons can cover variety of stimuli (Pouget et al. 2000). Each neuron’s response to a stimulus is defined as the number of spikes per second (firing rate). Average responses of each cell are assumed to be Gaussian (Pouget et al. 2000). An average over all cells’ responses specifies the full response to a stimulus (Maunsell and Van Essen 1983). Therefore, amplitude of these Gaussian functions corresponds to the firing rates of the neurons. On the other hand, anodal (cathodal) tDCS has been shown to increase (decrease) spontaneous firing rate of neurons (Bindman et al. 1964; Nitsche et al. 2008; Nitsche and Paulus 2000). This has been reported in early animal studies (Bindman et al. 1962; Gartside 1968; Purpura and McMurtry 1965). By changing the amplitude of these Gaussian functions, we can incorporate the effect of tDCS to our population-coding model. Amplitudes of Gaussian functions were set to 0.5, 0.8 and 1.1 for cathodal, sham and anodal stimulation, respectively.
Experimental studies have shown that the population-coding approach is a good model of activity in the sensory and motor regions of brain. Some of the well-known examples of this strategy are the responses of “place cells” within the hippocampus, neurons in the vision’s cortical areas (Maunsell and Van Essen 1983; Usrey and Reid 1999) and neurons in the motor cortex (Georgopoulos et al. 1986; Pouget et al. 2000). This property has also been observed in cerebellar neurons (Coltz et al. 2000; Krauzlis 2000; Taguchi et al. 2004).
It has been suggested that in visuomotor adaptation, the brain uses prediction error in each trial to update the FM such that its prediction would be closer to the actual value for the next trial. We implement an FM with a set of Gaussian basis functions (\(g(u)\)), i.e., population-coding structure:
\(\hat{p}\), \(u\) and \(w_{i}\) are FM output, motor command and weights of FM, respectively. The standard deviation and distance of two neighboring Gaussian functions were assumed to be 20° and 5°. The predicted sensory consequence (\({\hat{\text{c}}}\)) of motor command u is computed as follows:
For each trial, u (motor command) is determined by minimizing the difference between the target position (\(c^{*}\)) and the predicted sensory consequence as follows:
The produced motor command \(u(n)\) will result in cursor motion \(c(n)\). Prediction error is computed as follows:
Weights of FM are modified to minimize the prediction error (\(e(n)\)) based on a gradient descent algorithm (Izawa et al. 2012):
The learning parameters were set to α = 0.99 and η = 0.1. The pseudocode for the adaptation algorithm is explained in (Yavari and Towhidkhah 2014). Simulations were performed using a perturbation value similar to the experimental setup, i.e., 30°. Learning rates for three simulated groups can be compared with the data in adaptation phase of the experiment. After training of the model, we used Eq. 3 to compute FM predictions for different motor commands (movement directions). These results were compared with obtained experimental results in the localization blocks. Similarly, Eq. 6 was used for quantifying the required motor commands for movement toward targets at various angles which can be compared with the results of generalization blocks.
Results
Experiment results
Performance during the preadaptation phase
In the first block of the experiment, subjects performed shooting movements toward different targets with no visual feedback. Figure 3 shows the movement errors for all subjects. As it can be seen, 60° angle worked like an attractor direction which means performance error is zero in this direction and positive and negative for smaller and greater angles. One-way ANOVA revealed a significant effect of angle on error (P < 0.001). Various factors can contribute to this effect, e.g., nonlinear dynamics of arm, direction-dependent changes in the arm stiffness (Shadmehr 2004) and direction dependency of proprioceptive sensory (Rossetti et al. 1994; van Beers et al. 1998, 2002; Wilson et al. 2010). This effect has also been observed in some previous modeling studies (Bhushan and Shadmehr 1999; Shadmehr and Mussa-Ivaldi 1994).
Mean and SEM of reach movement error in different angles in baseline generalization block (first block in the experiment) and preadaptation generalization block. Error bars show SEMs. As it can be seen, performance error is zero in 60°/45° direction and positive and negative for smaller and greater angles in baseline/preadaptation generalization block
Interestingly, in the baseline generalization block, the movements were attracted toward the 45° target (similar to Izawa et al. 2012). The observed shift of attractor from 60° to 45° may come as a result of movement repetition toward the 45° direction in the previous blocks (Diedrichsen et al. 2010) and/or the presence of visual feedback for movement toward this direction.
One-way ANOVA revealed that performance of three groups was not significantly different in familiarization and baseline blocks (P > 0.05). Moreover, WM test results showed that subjects are able to correctly recall the stimuli after one second. The mean (±SD) error of the remembered position was −0.35 (±1.2)°, −0.48 (±1.26)° and −0.22 (±1.3)° for subjects in anodal, cathodal and sham groups, respectively. There was no significant difference between three groups in WM performance (ANOVA and post hoc Dunnett’s t test; P > 0.05).
Performance during the adaptation phase
All subjects reported their perceived level of attention, mental and physical fatigue, and pain before and after tDCS. One-way ANOVA revealed no significant differences between groups in these parameters (P > 0.05; Table 2).
In the adaptation phase, all subjects finished 200 trials toward the 45° target while receiving tcDCS. All subjects successfully adapted to the visuomotor rotation. The average reach direction for all groups as a function of trial during the adaptation phase is shown in Fig. 4a. In order to explain the effect of tcDCS on adaptation rate, learning curves were normalized and smoothed with the help of a 7-sample-length moving average window. We then used an exponential function to fit the learning curves for each group. The parameters of fitted functions for anodal and cathodal curves are given in Table 3. The adaptation rate was significantly greater when anodal stimulation has been used (P < 0.05). The fitted exponential functions to anodal and cathodal groups are shown in Fig. 4b. This has also been reported in previous studies (Block and Celnik 2013; Galea et al. 2011; Jayaram et al. 2012). Moreover, Table 3 also shows the larger final asymptotic value for cathodal stimulation (P < 0.05). Simulation results suggest that this observation might also be resulted from tcDCS effect on FMs.
Performance change in localization and generalization blocks
As it was expected, in both generalization blocks the presence of visual feedback for the movements toward 45° led to the elimination of error in this direction. This means that after adaptation, subjects moved their hand toward 15° so that the rotated visual feedback passed through the 45° target. This adaptation also generalized to the neighboring angles. The magnitude of generalization decreased as the deviation from 45° target increased.
To partially account for inter-individual differences, we calculated the difference in the performance from pre- to post-adaptation generalization block individually for each subject. Changes in the subjects’ behavior for these blocks are shown in Fig. 5. Our three experimental groups did not show any significant difference in any of the angles, i.e., 15°, 25°, 35°, 45°, 55°, 65° and 75° (one-way ANOVA and post hoc Dunnett’s t test, P > 0.05). Generalization blocks were added to evaluate IM. The absence of any significant difference between groups may demonstrate that tcDCS did not influence IM.
An interesting observation, shown in Fig. 5, is the asymmetry of generalization function, i.e., learning in 45° direction produced more generalization for larger angles compared to smaller ones. This observation may be the result of mechanical properties of arm and/or dependency of sensors precision on joints’ angles.
In the localization phase, subjects’ estimation of their unseen hand’s position was measured. Subjects can use at least three sources of information for positional sense: vision, proprioception and FM’s output (Yavari et al. 2014). However, in localization blocks, subjects did not have the visual feedback. Moreover, subjects were asked to replace their hand to the bottom of the screen before their answer. This helped us minimize the effect of proprioception. Therefore, in this phase, subjects’ answers were based on the sensory consequences of their own action.
Before visuomotor adaptation, subjects’ error in their perception of hand position was small. There is some evidence that visuomotor adaptation changes subjects’ perception of their hand’s position (Cressman and Henriques 2009; Synofzik et al. 2008). Similar to the generalization block, for each subject we calculated differences in the position perception from before to after adaptation to minimize the individual differences. Since maximum changes in perception for our three groups happened around 30°, we used a bin around this direction and compared the results. Figure 6 shows changes in perception for the first five trials of post-adaptation localization block. One-way ANOVA found a significant effect of electrode montage (P < 0.05). We followed our ANOVA with Bonferroni-corrected post hoc and found a significant difference between our anodal and cathodal groups (P < 0.05). We only used the first five trials of post-adaptation localization task in Fig. 6 as movement repetition without visual feedback is shown to washout the learning effects (Kitago et al. 2013; Smeets et al. 2006). Interesting observations in the localization phase were as follows: (1) Perception change for anodal group is greater than cathodal; (2) for all three groups, the maximum perceptual change happens at an angle smaller than 45°. This is consistent with the observed data for healthy subjects in Izawa’s study (Izawa et al. 2012).
As we explained before, FM provides subjects with another source of information for perception of their hand’s position. Having this as well as the facilitatory/inhibitory effect of anodal/cathodal tDCS stimulation in mind, our first observation is consistent with the hypothesis that tDCS changes FMs. However, our experimental result cannot explain the other observation. To explain this observation, we decided to run a simulation study, which is described in the next section. Our simulation results suggest that this observation might also be a result of changes to FMs by tDCS.
Since localization task evaluates forward model (Izawa et al. 2012), significant difference between groups in this task may demonstrate tcDCS effect on forward model. This can be considered as evidence for the existence of internal FMs in cerebellum.
Simulation results
Obtained results from simulations are described in this section.
Current density distribution
We simulated the electrical current flow in subjects’ heads for 2 mA current intensity and electrode configuration that is shown in Fig. 2a. Surface maps of current density demonstrate that current predominantly flows laterally and passes through anterior parts of cerebellum (Fig. 2b–d).
In most tDCS studies with targets other than the cerebellum [e.g., primary motor cortex (Nitsche et al. 2003a, b) and dorsolateral prefrontal cortex (Fregni et al. 2005)], current amplitude is set to 1 mA. In targeting the cerebellum, however, 2 mA amplitude has been used (Boehringer et al. 2013; Ferrucci et al. 2008, 2012, 2013; Galea et al. 2009, 2011; Jayaram et al. 2012; Pope and Miall 2012). Because scalp and skull are much thicker in the occipital lobe, it is expected that these local variations have an effect on current intensity in the cerebellar cortex. Our simulation results showed peak cortical current density of 0.21 A/m2. Moreover, maximum cortical electric field was estimated to be 0.67 V/m. A similar value has been reported for 1 mA primary motor cortex stimulation (Datta et al. 2009). This confirms a need for larger amplitude current in cerebellar stimulation.
Induced current density vectors across a sagittal plane are shown in Fig. 2e. As it can be seen, current density reduces as electrode distance from cortex increased. Moreover, due to high impedance of the skull, much of the current is shunted along the skin; i.e., current tends to flow through the scalp rather than passing through the skull.
Population coding
All three models were trained to learn 30° CCW rotation in movement toward 45°. Error reduction curves are shown in Fig. 7a. In this figure, two features are similar to the experimental results (Table 3): (1) higher error reduction rate in anodal compared to that in cathodal group and (2) greater asymptotic value in cathodal compared to that in anodal group.
Perception changes for three groups were obtained from models (Fig. 7b). Two features in this figure are also similar to the experimental results: (1) Maximum change in perception happens in angles smaller than 45° for all three groups; (2) maximum amplitude of curves decreases from anodal to sham and cathodal groups.
Generalization profiles obtained from models are shown in Fig. 7c. We found better generalization for angles above 45° for all three groups. This asymmetry has also been observed in experimental results. It should be noted that generalization pattern is also largely affected by mechanical properties of elbow and arm or upper-level control signals. This may explain the differences between experimental and model results.
Interestingly, simulation results demonstrated that the model could account for some key observations in our experiment. Therefore, these observations may be, at least partly, explained by a forward model in cerebellum that is affected by tDCS.
Discussion
In this paper, we used tDCS in a visuomotor adaptation paradigm to investigate the formation of an internal model in the cerebellum. Current density and electric field distribution were simulated using a realistic human head model. Visuomotor adaptation in the presence of tcDCS was simulated using a population-coding framework.
Experimentally, there are some improvements to the experiments in Izawa et al. (2012):
-
1.
In their position perception task to evaluate FM’s function, subjects pointed to their right hand’s location with their left hand. It has been shown that training with right hand changes the left hand’s movements (Balitsky Thompson and Henriques 2010; Birbaumer 2007; Parlow and Kinsbourne 1989; Sainburg and Wang 2002; Taylor et al. 2011; Wang 2008; Wang and Sainburg 2006). Therefore, the final pointed location could be affected by the subject’s right hand training. To solve this problem, we modified this phase of the experiment with a novel method to evaluate subjects’ perceptions of their hand’s positions. In our method, we used a graded arc such that subjects’ report where they believed their hand crossed the arc. Other methods have been proposed to examine perception of position for the unseen hand. In (Haggard et al. 2000; Jola et al. 2011; Jones et al. 2010; van Beers et al. 1998; von Hofsten and Rosblad 1988), subjects were asked to move their unseen hand to the position of a visual target or to align their unseen hand with their other hand, while their eyes were either open or closed. In (Darainy et al. 2013; Jones et al. 2010), subjects reported whether their unseen hand’s position is at the right or left of their body midlines. All these methods can estimate the subjects’ perceptions for only a single point (visual target, seen hand position or body midline) and as such are not effective to study the role of FM in perception. We think that our method overcomes these limitations.
-
2.
Brain lesion studies have some fundamental limitations: (a) It is usually very difficult to find patients with lesions located exactly in the area of interest; (b) most cerebellar degeneration occurs by chronic disease often accompanied by compensatory neural mechanisms affecting other brain regions; and (c) each patient’s individual lesion has its own characteristics, making it difficult to compare different patients’ data. An alternative method to avoid these problems is to use brain stimulation techniques, e.g., r/TMS and tDCS. These techniques can temporarily manipulate the function of our region of interest. In this study, we applied anodal, cathodal and sham tDCS over the right cerebellum, while subjects adapted to a visuomotor task.
-
3.
In their study, Izawa et al. (2012) observed that reaching movements without visual feedback were attracted to 45° target. However, it was not clear whether this observation comes as a result of movement repetition toward this direction or other factors. In the first phase of our experiment, we added a block of reaching movements toward different targets in the first quarter without visual feedback to investigate whether this attraction occurs without repetition. Our result showed an attractor in the 60° direction which might be a result of mechanical properties of the arm (mechanical impedance, arm configuration, etc.) or pattern of proprioceptive precision and acuity. Interestingly, this attractor angle was shifted to the 45° direction in baseline generalization block in which movement errors generally decreased compared to the first phase. This could be as a result of visual feedback in this angle and/or movement repetition toward this direction.
We observed that subjects’ perceptions of their hand position changed after visuomotor adaptation. This is in agreement with previous experimental results (Cressman and Henriques 2009; Synofzik et al. 2008). Maximum perceptual change for all groups occurred at an angle smaller than 45°. This is consistent with the healthy subjects’ results in Izawa et al. (2012) paper. In summary, lack of generalization of motor learning and changes that we have observed in the subject’s perception of hand position as a result of different tDCS manipulations indicate that the cerebellum can be seen as internal FM but not IM. We also modeled visuomotor adaptation in the presence of tcDCS using a biologically inspired structure. Interesting common features between simulation and experimental results confirmed that our experimental outcomes could be caused by manipulation of FM by tcDCS. In simulations, adaptation of FM to 30° perturbation gradually changes reach direction so that the cursor hit the target at 45° (Fig. 7a). During the adaptation, FM weights are tuned as a function of both previous motor commands and sensory prediction errors (Eq. 7) in a way that the peak of the forward model gradually shifts from 45° angle to smaller angles, i.e., the most repeated sensory states. This is consistent with the results of previous studies (Gonzalez Castro et al. 2011; Izawa et al. 2012). The simulation results show difference in the peak angle and the magnitude of the subjects’ “illusion” about their hand position depending on the amplitude of Gaussian functions. However, the values are different in the measured data and simulations, but similar trends are consistent with the hypothesis that these features might be resulted from FM manipulation by tcDCS.
To check whether the applied current would pass through the cerebellum, we also performed a simulation study using a realistic human head model. The obtained results confirmed that the current intensity and electrode montage used in our experiments would result in the passage of current from the intended regions of the cerebellum. Also in this study, we tried to partly compensate for the individual differences between subjects by comparing each subject’s performance before and after adaptation instead of comparing mean behavior of all subjects before and after adaptation.
tDCS is a relatively new technique in neuroscience studies. Its capability to modify spontaneous neuronal excitability by de- or hyperpolarization of resting membrane potential turns it to a valuable research tool. In general, cathodal stimulation hyperpolarizes neurons and diminishes cerebral excitability, while anodal stimulation can result in neuronal depolarization and increase in excitability (Bindman et al. 1962; Purpura and McMurtry 1965). Published papers suggest that tDCS is an effective technique to manipulate desired brain regions and to study the effect of this manipulation.
However, the current pathways in the head are not exactly known. Modeling studies (Bikson et al. 2012) can be used to estimate the electrical current pathways in brain and map out the exact affected brain regions. On the other hand, HD electrodes (Datta et al. 2009) can be used to sharpen the focal point of stimulation and therefore increase the precision of this technique. These could be addressed in future works.
References
Balitsky Thompson AK, Henriques DY (2010) Visuomotor adaptation and intermanual transfer under different viewing conditions. Exp Brain Res 202(3):543–552. doi:10.1007/s00221-010-2155-0
Bhushan N, Shadmehr R (1999) Computational nature of human adaptive control during learning of reaching movements in force fields. Biol Cybern 81(1):39–60
Bikson M, Rahman A, Datta A, Fregni F, Merabet L (2012) High-resolution modeling assisted design of customized and individualized transcranial direct current stimulation protocols. Neuromodulation 15(4):306–315. doi:10.1111/j.1525-1403.2012.00481.x
Bindman LJ, Lippold OC, Redfearn JW (1962) Long-lasting changes in the level of the electrical activity of the cerebral cortex produced by polarizing currents. Nature 196:584–585
Bindman LJ, Lippold OC, Redfearn JW (1964) The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol 172:369–382
Birbaumer N (2007) Motor learning: passing a skill from one hand to the other. Curr Biol 17(23):R1024–R1026
Blakemore S-J, Frith CD, Wolpert DM (2001) The cerebellum is involved in predicting the sensory consequences of action. NeuroReport 12(9):1879–1884
Block H, Celnik P (2013) Stimulating the cerebellum affects visuomotor adaptation but not intermanual transfer of learning. Cerebellum 12(6):781–793. doi:10.1007/s12311-013-0486-7
Boehringer A, Macher K, Dukart J, Villringer A, Pleger B (2013) Cerebellar transcranial direct current stimulation modulates verbal working memory. Brain Stimul 6(4):649–653. doi:10.1016/j.brs.2012.10.001
Boggio PS, Ferrucci R, Rigonatti SP, Covre P, Nitsche M, Pascual-Leone A, Fregni F (2006) Effects of transcranial direct current stimulation on working memory in patients with Parkinson’s disease. J Neurol Sci 249(1):31–38
Cerminara NL, Apps R, Marple-Horvat DE (2009) An internal model of a moving visual target in the lateral cerebellum. J Physiol 587(Pt 2):429–442. doi:10.1113/jphysiol.2008.163337
Coltz JD, Johnson MT, Ebner TJ (2000) Population code for tracking velocity based on cerebellar Purkinje cell simple spike firing in monkeys. Neurosci Lett 296(1):1–4
Cressman EK, Henriques DY (2009) Sensory recalibration of hand position following visuomotor adaptation. J Neurophysiol 102(6):3505–3518. doi:10.1152/jn.00514.2009
Cullen KE, Brooks JX, Jamali M, Carriot J, Massot C (2011) Internal models of self-motion: computations that suppress vestibular reafference in early vestibular processing. Exp Brain Res 210(3–4):377–388
Darainy M, Vahdat S, Ostry DJ (2013) Perceptual learning in sensorimotor adaptation. J Neurophysiol 110(9):2152–2162. doi:10.1152/jn.00439.2013
Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M (2009) Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul 2(4):201.e201–207.e201. doi:10.1016/j.brs.2009.03.005
Diedrichsen J, White O, Newman D, Lally N (2010) Use-dependent and error-based learning of motor behaviors. J Neurosci 30(15):5159–5166. doi:10.1523/JNEUROSCI.5406-09.2010
Ferrucci R, Marceglia S, Vergari M, Cogiamanian F, Mrakic-Sposta S, Mameli F, Priori A (2008) Cerebellar transcranial direct current stimulation impairs the practice-dependent proficiency increase in working memory. J Cogn Neurosci 20(9):1687–1697. doi:10.1162/jocn.2008.20112
Ferrucci R, Giannicola G, Rosa M, Fumagalli M, Boggio PS, Hallett M, Priori A (2012) Cerebellum and processing of negative facial emotions: cerebellar transcranial DC stimulation specifically enhances the emotional recognition of facial anger and sadness. Cogn Emot 26(5):786–799. doi:10.1080/02699931.2011.619520
Ferrucci R, Brunoni AR, Parazzini M, Vergari M, Rossi E, Fumagalli M, Priori A (2013) Modulating human procedural learning by cerebellar transcranial direct current stimulation. Cerebellum 12(4):485–492. doi:10.1007/s12311-012-0436-9
Flanagan JR, Wing AM (1997) The role of internal models in motion planning and control: evidence from grip force adjustments during movements of hand-held loads. J Neurosci 17(4):1519–1528
Flanagan JR, Vetter P, Johansson RS, Wolpert DM (2003) Prediction precedes control in motor learning. Curr Biol 13(2):146–150
Fregni F, Boggio PS, Nitsche M, Bermpohl F, Antal A, Feredoes E, Pascual-Leone A (2005) Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res 166(1):23–30. doi:10.1007/s00221-005-2334-6
Galea JM, Jayaram G, Ajagbe L, Celnik P (2009) Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci 29(28):9115–9122. doi:10.1523/JNEUROSCI.2184-09.2009
Galea JM, Vazquez A, Pasricha N, de Xivry JJ, Celnik P (2011) Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb Cortex 21(8):1761–1770. doi:10.1093/cercor/bhq246
Gartside IB (1968) Mechanisms of sustained increases of firing rate of neurons in the rat cerebral cortex after polarization: reverberating circuits or modification of synaptic conductance? Nature 220(5165):382–383
Gentili RJ, Shewokis PA, Ayaz H, Contreras-Vidal JL (2013) Functional near-infrared spectroscopy-based correlates of prefrontal cortical dynamics during a cognitive-motor executive adaptation task. Front Hum Neurosci 7:277. doi:10.3389/fnhum.2013.00277
Georgopoulos AP, Schwartz AB, Kettner RE (1986) Neuronal population coding of movement direction. Science 233(4771):1416–1419
Gomi H, Kawato M (1992) Adaptive feedback control models of the vestibulocerebellum and spinocerebellum. Biol Cybern 68(2):105–114
Gonzalez Castro LN, Monsen CB, Smith MA (2011) The binding of learning to action in motor adaptation. PLoS Comput Biol 7(6):e1002052. doi:10.1371/journal.pcbi.1002052
Haggard P, Newman C, Blundell J, Andrew H (2000) The perceived position of the hand in space. Percept Psychophys 62(2):363–377
Higuchi S, Imamizu H, Kawato M (2007) Cerebellar activity evoked by common tool-use execution and imagery tasks: an fMRI study. Cortex 43(3):350–358
Hummel F, Celnik P, Giraux P, Floel A, Wu W-H, Gerloff C, Cohen LG (2005) Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain 128(3):490–499
Imamizu H, Kawato M (2012) Cerebellar internal models: implications for the dexterous use of tools. The Cerebellum 11(2):325–335
Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B, Kawato M (2000) Human cerebellar activity reflecting an acquired internal model of a new tool. Nature 403(6766):192–195. doi:10.1038/35003194
Imamizu H, Kuroda T, Miyauchi S, Yoshioka T, Kawato M (2003) Modular organization of internal models of tools in the human cerebellum. Proc Natl Acad Sci U S A 100(9):5461–5466. doi:10.1073/pnas.0835746100
Iyer M, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann E (2005) Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology 64(5):872–875
Izawa J, Criscimagna-Hemminger SE, Shadmehr R (2012) Cerebellar contributions to reach adaptation and learning sensory consequences of action. J Neurosci 32(12):4230–4239. doi:10.1523/JNEUROSCI.6353-11.2012
Jayaram G, Tang B, Pallegadda R, Vasudevan EV, Celnik P, Bastian A (2012) Modulating locomotor adaptation with cerebellar stimulation. J Neurophysiol 107(11):2950–2957. doi:10.1152/jn.00645.2011
Jola C, Davis A, Haggard P (2011) Proprioceptive integration and body representation: insights into dancers’ expertise. Exp Brain Res 213(2–3):257–265
Jones SA, Cressman EK, Henriques DY (2010) Proprioceptive localization of the left and right hands. Exp Brain Res 204(3):373–383. doi:10.1007/s00221-009-2079-8
Jordan MI, Rumelhart DE (1992) Forward models: supervised learning with a distal teacher. Cognitive science 16(3):307–354
Katayama M, Kawato M (1993) Virtual trajectory and stiffness ellipse during multijoint arm movement predicted by neural inverse models. Biol Cybern 69(5–6):353–362
Kawato M (1999) Internal models for motor control and trajectory planning. Curr Opin Neurobiol 9(6):718–727
Kawato M, Gomi H (1992a) The cerebellum and VOR/OKR learning models. Trends Neurosci 15(11):445–453
Kawato M, Gomi H (1992b) A computational model of four regions of the cerebellum based on feedback-error learning. Biol Cybern 68(2):95–103
Kawato M, Furukawa K, Suzuki R (1987) A hierarchical neural-network model for control and learning of voluntary movement. Biol Cybern 57(3):169–185
Kawato M, Kuroda T, Imamizu H, Nakano E, Miyauchi S, Yoshioka T (2003) Internal forward models in the cerebellum: fMRI study on grip force and load force coupling. Prog Brain Res 142:171–188. doi:10.1016/S0079-6123(03)42013-X
Kitago T, Ryan SL, Mazzoni P, Krakauer JW, Haith AM (2013) Unlearning versus savings in visuomotor adaptation: comparing effects of washout, passage of time, and removal of errors on motor memory. Front Hum Neurosci 7:307. doi:10.3389/fnhum.2013.00307
Krauzlis RJ (2000) Population coding of movement dynamics by cerebellar Purkinje cells. NeuroReport 11(5):1045–1050
Laurens, J., Meng, H., & Angelaki, D. E. (2013). Computation of linear acceleration through an internal model in the macaque cerebellum. Nature neuroscience
Li CS, Padoa-Schioppa C, Bizzi E (2001) Neuronal correlates of motor performance and motor learning in the primary motor cortex of monkeys adapting to an external force field. Neuron 30(2):593–607
Liebetanz D, Koch R, Mayenfels S, König F, Paulus W, Nitsche MA (2009) Safety limits of cathodal transcranial direct current stimulation in rats. Clin Neurophysiol 120(6):1161–1167
Lisberger SG (2009) Internal models of eye movement in the floccular complex of the monkey cerebellum. Neuroscience 162(3):763–776. doi:10.1016/j.neuroscience.2009.03.059
Liu X, Robertson E, Miall RC (2003) Neuronal activity related to the visual representation of arm movements in the lateral cerebellar cortex. J Neurophysiol 89(3):1223–1237
Maunsell JH, Van Essen DC (1983) Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. J Neurophysiol 49(5):1127–1147
Miall RC, Weir DJ, Wolpert DM, Stein JF (1993) Is the cerebellum a smith predictor? J Mot Behav 25(3):203–216. doi:10.1080/00222895.1993.9942050
Miall RC, Christensen LO, Cain O, Stanley J (2007) Disruption of state estimation in the human lateral cerebellum. PLoS Biol 5(11):e316. doi:10.1371/journal.pbio.0050316
Milner TE, Franklin DW, Imamizu H, Kawato M (2007) Central control of grasp: manipulation of objects with complex and simple dynamics. Neuroimage 36(2):388–395. doi:10.1016/j.neuroimage.2007.01.057
Morton SM, Bastian AJ (2006) Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci 26(36):9107–9116. doi:10.1523/JNEUROSCI.2622-06.2006
Nitsche MA, Paulus W (2000) Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527(Pt 3):633–639
Nitsche MA, Liebetanz D, Antal A, Lang N, Tergau F, Paulus W (2003a) Modulation of cortical excitability by weak direct current stimulation–technical, safety and functional aspects. Suppl Clin Neurophysiol 56(3):255–276
Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, Tergau F (2003b) Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci 15(4):619–626. doi:10.1162/089892903321662994
Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Pascual-Leone A (2008) Transcranial direct current stimulation: state of the art 2008. Brain Stimul 1(3):206–223. doi:10.1016/j.brs.2008.06.004
Nowak DA, Topka H, Timmann D, Boecker H, Hermsdorfer J (2007) The role of the cerebellum for predictive control of grasping. Cerebellum 6(1):7–17. doi:10.1080/14734220600776379
Parlow SE, Kinsbourne M (1989) Asymmetrical transfer of training between hands: implications for interhemispheric communication in normal brain. Brain Cogn 11(1):98–113
Pasalar S, Roitman A, Durfee W, Ebner T (2006) Force field effects on cerebellar Purkinje cell discharge with implications for internal models. Nat Neurosci 9(11):1404–1411
Plonsey R, Heppner DB (1967) Considerations of quasi-stationarity in electrophysiological systems. Bull Math Biophys 29(4):657–664
Pope PA, Miall RC (2012) Task-specific facilitation of cognition by cathodal transcranial direct current stimulation of the cerebellum. Brain Stimul 5(2):84–94. doi:10.1016/j.brs.2012.03.006
Pouget A, Dayan P, Zemel R (2000) Information processing with population codes. Nat Rev Neurosci 1(2):125–132. doi:10.1038/35039062
Purpura DP, McMurtry JG (1965) Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol 28:166–185
Richardson AG, Overduin SA, Valero-Cabré A, Padoa-Schioppa C, Pascual-Leone A, Bizzi E, Press DZ (2006) Disruption of primary motor cortex before learning impairs memory of movement dynamics. J Neurosci 26(48):12466–12470
Rossetti Y, Meckler C, Prablanc C (1994) Is there an optimal arm posture? Deterioration of finger localization precision and comfort sensation in extreme arm-joint postures. Exp Brain Res 99(1):131–136
Sabes PN (2000) The planning and control of reaching movements. Curr Opin Neurobiol 10(6):740–746
Sadnicka A, Kassavetis P, Saifee TA, Parees I, Rothwell JC, Edwards MJ (2013) Cerebellar transcranial direct current stimulation does not alter motor surround inhibition. Int J Neurosci 123(6):425–432. doi:10.3109/00207454.2012.763165
Saidi M, Towhidkhah F, Lagzi F, Gharibzadeh S (2012) The effect of proprioceptive training on multisensory perception under visual uncertainty. J Integr Neurosci 11(4):401–415. doi:10.1142/S0219635212500276
Sainburg RL, Wang J (2002) Interlimb transfer of visuomotor rotations: independence of direction and final position information. Exp Brain Res 145(4):437–447. doi:10.1007/s00221-002-1140-7
Shadmehr R (2004) Generalization as a behavioral window to the neural mechanisms of learning internal models. Hum Mov Sci 23(5):543–568
Shadmehr R, Krakauer JW (2008) A computational neuroanatomy for motor control. Exp Brain Res 185(3):359–381. doi:10.1007/s00221-008-1280-5
Shadmehr R, Mussa-Ivaldi FA (1994) Adaptive representation of dynamics during learning of a motor task. J Neurosci 14(5 Pt 2):3208–3224
Shadmehr R, Smith MA, Krakauer JW (2010) Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci 33:89–108. doi:10.1146/annurev-neuro-060909-153135
Shah B, Nguyen TT, Madhavan S (2013) Polarity independent effects of cerebellar tDCS on short term ankle visuomotor learning. Brain Stimul. doi:10.1016/j.brs.2013.04.008
Shahid SS, Bikson M, Salman H, Wen P, Ahfock T (2014) The value and cost of complexity in predictive modelling: role of tissue anisotropic conductivity and fibre tracts in neuromodulation. J Neural Eng 11(3):036002. doi:10.1088/1741-2560/11/3/036002
Shidara M, Kawano K, Gomi H, Kawato M (1993) Inverse-dynamics model eye movement control by Purkinje cells in the cerebellum. Nature 365(6441):50–52. doi:10.1038/365050a0
Smeets JB, van den Dobbelsteen JJ, de Grave DD, van Beers RJ, Brenner E (2006) Sensory integration does not lead to sensory calibration. Proc Natl Acad Sci 103(49):18781–18786
Synofzik M, Lindner A, Thier P (2008) The cerebellum updates predictions about the visual consequences of one’s behavior. Curr Biol 18(11):814–818
Taguchi S, Tabata H, Shibata T, Kawato M (2004) Transformation from population codes to firing rate codes by learning: neural representation of smooth pursuit eye movements. Syst Comput Jpn 35(6):79–88
Taylor JA, Wojaczynski GJ, Ivry RB (2011) Trial-by-trial analysis of intermanual transfer during visuomotor adaptation. J Neurophysiol 106(6):3157–3172. doi:10.1152/jn.01008.2010
Towhidkhah F, Gander RE, Wood HC (1997) Model predictive impedance control: a model for joint movement. J Mot Behav 29(3):209–222. doi:10.1080/00222899709600836
Usrey WM, Reid RC (1999) Synchronous activity in the visual system. Annu Rev Physiol 61:435–456. doi:10.1146/annurev.physiol.61.1.435
van Beers RJ, Sittig AC, Denier van der Gon JJ (1998) The precision of proprioceptive position sense. Exp Brain Res 122(4):367–377
van Beers RJ, Wolpert DM, Haggard P (2002) When feeling is more important than seeing in sensorimotor adaptation. Curr Biol 12(10):834–837
von Hofsten C, Rosblad B (1988) The integration of sensory information in the development of precise manual pointing. Neuropsychologia 26(6):805–821
Wang J (2008) A dissociation between visual and motor workspace inhibits generalization of visuomotor adaptation across the limbs. Exp Brain Res 187(3):483–490. doi:10.1007/s00221-008-1393-x
Wang J, Sainburg RL (2006) The symmetry of interlimb transfer depends on workspace locations. Exp Brain Res 170(4):464–471. doi:10.1007/s00221-005-0230-8
Wilson ET, Wong J, Gribble PL (2010) Mapping proprioception across a 2D horizontal workspace. PLoS ONE 5(7):e11851. doi:10.1371/journal.pone.0011851
Wolpert DM, Kawato M (1998) Multiple paired forward and inverse models for motor control. Neural Netw 11(7):1317–1329
Wolpert DM, Ghahramani Z, Jordan MI (1995) An internal model for sensorimotor integration. Science-New York then Washington, 1880–1880
Wolpert DM, Miall RC, Kawato M (1998) Internal models in the cerebellum. Trends Cogn Sci 2(9):338–347
Yavari F, Towhidkhah F (2014) Modeling the effect of explicit information in visuomotor adaptation. Paper presented at the 22nd Iranian Conference on electrical engineering (ICEE)
Yavari F, Towhidkhah F, Ahmadi-Pajouh MA (2013) Are fast/slow process in motor adaptation and forward/inverse internal model two sides of the same coin? Med Hypotheses 81(4):592–600. doi:10.1016/j.mehy.2013.07.009
Yavari F, Towhidkhah F, Darainy M (2014) A hypothesis on the role of perturbation size on the human sensorimotor adaptation. Front Comput Neurosci 8:1–3
Acknowledgments
The authors would like to thank Prof. Jun Izawa for his valuable comments throughout this research and also Dimitrios Palidis for his great comments in editing the manuscript grammatically.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
I hereby confirm that there is no conflict of interest in this research.
Rights and permissions
About this article
Cite this article
Yavari, F., Mahdavi, S., Towhidkhah, F. et al. Cerebellum as a forward but not inverse model in visuomotor adaptation task: a tDCS-based and modeling study. Exp Brain Res 234, 997–1012 (2016). https://doi.org/10.1007/s00221-015-4523-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-015-4523-2