Abstract
Does the brain use a separate internal model for cursor mechanics during visuomotor adaptation? We compared the amount of adaptation and transfer to the opposite arm when subjects reached the targets under different viewing conditions of the arm during reaching. If the brain forms separate models, we predict a difference in the amount of adaptation and transfer for each viewing condition. If the brain forms one model, we predict equivalent amounts of adaptation and transfer between the two hands for each viewing condition. Separate groups of subjects performed a reaching task with either a rotated view of cursor motion representing their unseen hand or a rotated view of their actual hand. The two groups were further divided so that the magnitude of the rotation was either 45° or 75° counter-clockwise. After adapting to the rotation with one hand, subjects reached the same targets under the same viewing condition but with the opposite hand. Similar amounts of adaptation and intermanual transfer were found across the different magnitudes of rotation and across patterns of hand-order. Our results suggest that the brain may not be learning a distinct model for cursor mechanics, or if it is, it must be equivalent or overlapping with the arm model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Visually guided reaching is fundamental to our daily activities. An example of a visually guided action is the use of a computer mouse to move a cursor to a desired icon on a computer screen. We perform such reaches so often in our day that it is easy to take for granted the complexity of this dynamic action. In order to reach to a visual object, the brain needs to integrate multiple sensory modalities, such as vision and proprioception, based on prior experience and current context. Vision plays the dominant role in developing our motor programmes for the planning and control of reaching tasks (Held and Bauer 1974). When vision and proprioception are incongruous, the brain must change its motor commands to accurately move the hand so that the visual representation of the hand reaches the desired location. So, when visual feedback of the hand is altered (i.e., movements no longer produce the expected visual representation), one must adapt to the new context. This learning requires the control system to compensate for changes in the sensory and motor relationship (Wolpert et al. 1995). The neural representations of this new sensorimotor motor relationship result in a new internal model, or new predictions of the motor command outcomes (Shadmehr and Moussavi 2000).
Learning a new internal model and generalization of adaptation to other contexts could depend on where the brain attributes errors in performance (i.e., whether the error signal is associated with the hand itself or to an external effector such as the cursor). Perhaps the source of error in reaching is an injured right arm. Adjustments made to the right arm would not be transferred to the healthy left arm. If the source of error is the cursor, we would expect both arms to make adjustments in their reach. Studies that examined visuomotor learning found that subjects show better performance with vision of a cursor compared to vision of their finger (Clower and Boussaoud 2000) or compared to a picture of an arm (Sober and Sabes 2005) suggesting that we use different mechanisms for each view. In agreement, other studies suggest that we have specific internal models for external information that are distinct from the internal model of the arm (Cothros et al. 2006; Berniker and Kording 2008; Kluzik et al. 2008). Cothros et al. 2006 showed that subjects associated the adaptation of the task with the tool used in the task, which could not be generalized to performance of the same task without the tool (i.e., a free hand). Where does incoming error originate, the internal dynamics of the arm or the external end-effector represented by a cursor? To explore where the brain assigns the error signal, we compared performance when subjects see a cursor representation of their hand compared to vision of their actual hand.
Intermanual transfer—where adapting to a new task on one hand influences subsequent performance with the opposite, untrained hand—occurs following adaptation of reaches to force perturbations (Dizio and Lackner 1995; Wang and Sainburg 2003; Galea et al. 2007), to rotated visual feedback of the hand represented by a cursor (Sainburg and Wang 2002; Criscimagna-Hemminger et al. 2003), and to a reversed image of the hand (Dionne and Henriques 2008). Adaptation to an external cursor is not arm-specific, so it is not surprising that generalization of the task occurs across arms. Intermanual transfer does not always follow adaptation to prisms (Taub and Goldberg 1973; Choe and Welch 1974) or gradual exposure to a rotation where error is more associated with the arm (Malfait and Ostry 2004). In the case where exposure to a rotation is gradual, the results suggested that intrinsic arm co-ordinates were used. The intrinsic coordinates were arm-specific; as a result, the authors did not observe transfer across arms. Dionne and Henriques (2008) found that intermanual transfer occurred when feedback of the actual hand, that is, a video image of the hand and forearm, was mirror-reversed. So, when subjects reached with their right hand, the left–right mirror-reverse rotation of the hand produced feedback of the hand that resembled the opposite, (i.e., left) hand. In this case, generalization to the opposite arm was possible because the mirror-reversed left hand looked like the already adapted right hand (Dionne and Henriques 2008). These findings suggest that the type of visual feedback individuals receive affects transfer of adaptation to their unexposed arm. Intermanual transfer can help determine the extent of learning and evaluate the differences between viewing conditions as it depends on how the brain attributes errors (i.e., whether learning involves forming an internal model of the arm or an external effector).
Intermanual transfer seems to be asymmetrical from dominant hand training compared with non-dominant hand training (Sainburg and Kalakanis 2000; Sainburg 2002; Criscimagna-Hemminger et al. 2003; Wang and Sainburg 2007). Whether the direction of generalization is from the dominant to non-dominant hand or non-dominant to dominant hand is still under debate.
To test the effect of the type of visual feedback of the effector on learning, we exposed subjects to different viewing conditions (cursor or hand view), with different misalignments (45° and 75°) while they reached the visual targets. In the cursor view condition, participants saw a cursor representation of their hand movement. In the hand view condition, participants saw their actual hand on the screen captured by video in real time. Different misalignments were used to test whether a larger angle would be more difficult to learn and perhaps less transferable to the opposite hand. After subjects learned to accurately reach under one of these conditions with the right hand, they performed the same task with the left, untrained hand. To assess the asymmetry and direction of intermanual transfer, we had a second group of subjects learn the task with their left hand and test with their right hand for all viewing conditions.
Our first objective was to evaluate differences in performance between viewing conditions (i.e., cursor view and hand view). The visual feedback of the cursor in the cursor view condition is external to the body such that it appears the same on the screen whether the right or left hand is used. In contrast, the visual feedback of the hand in the hand view condition is related to the body such that the right and left hands will appear differently on the screen. Since the cursor is dissociated from the body and appears to be the same across hands, we might expect more generalization of the adaptation to the task across arms. Transfer differences between the cursor and hand view conditions may suggest that the brain uses separate internal models to represent external cursor mechanics versus the internal information of the hand.
Our second objective was to evaluate differences in performance between hand-order, (dominant to non-dominant and non-dominant to dominant). In support of the asymmetrical transfer literature, we expect to find differences in transfer across arms for direction of hand-order. The direction of asymmetrical transfer could suggest that one arm has access to more bilateral recruitment of the motor areas compared to the other arm.
Methods
Participants
One hundred and forty-two right-handed healthy individuals with normal, or corrected to normal, vision (108 female, 34 male, mean age = 21.9, ±4.2) participated in this study. All subjects gave institutionally approved informed consent and received credit towards an undergraduate psychology course. Data from ten subjects were not included because they did not show normal visuomotor adaptation (i.e., they did not learn the task).
Apparatus
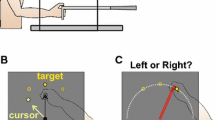
Figure 1a shows a subject seated in front of a digitizing tablet (Wacom Intuos3, 12″ × 12″ digitizing surface, resolution of 5,080 lines per inch) positioned at waist level facing a vertical screen at a distance of 60 cm (Dionne and Henriques 2008). The subjects’ hand and the digitizing tablet were hidden by an occluding plaque positioned 25 cm above the digitizing tablet and tilted 45° away from the subject. Hand position and movement speed were recorded continuously (sampled every 20 ± 7 ms) as subjects moved a stylus pen across the digitizing tablet.
A projector (Optiplex GX620) rear-projected targets (1.5 cm dots) and the cursor (Fig. 1b) or an image of the hand and arm (captured by a Logitech webcam—Quickcam; Fig. 1c) in real time onto the vertical screen. The inset in Fig 1a shows ten cyan dots surrounding a green centre dot forming a circle with a 10-cm radius. The dots were positioned at angles of 0° (directly to the right of start position), 22.5°, 45°, 135°, 157.5°, 180°, 202.5°, 225°, 315° and 337.5°. Subjects used the stylus pen to make movements on the tablet that were represented on the vertical screen by a cursor (white circle of 0.5 cm diameter) or an image of the actual hand and arm (so that all subjects could see their hand and about one-third of their forearm on the screen). Movements on the tablet corresponded to movements on the vertical screen in a 1:1 ratio (i.e., a movement of 1 cm with the stylus pen produced a 1 cm movement of the cursor).
Procedure
For all conditions, subjects performed a centre-out reaching task to one of ten targets while either seeing an image of their hand as it moved to the target, or only a cursor representing their hand. Subjects began each trial by moving the stylus pen on the tablet to position the cursor or image of the hand on the vertical screen to the start target at the centre of the radial targets. After the subject reached the starting position, one of the peripheral targets appeared, and subjects were instructed to move smoothly and accurately to that target. Once the reaching movement was completed (indicated by the start target re-appearing), subjects returned to the start position to begin the next trial. To mimic natural movements, subjects were given no specific instructions about where to look. This was the general procedure for all paradigms with minor differences detailed for each paradigm below. Subjects performed four paradigms: a baseline for each hand; adaptation with one hand; and transfer with the opposite hand.
In the baseline paradigm, subjects made reaches with veridical feedback of hand motion while the target remained visible. Veridical feedback meant that the movement of the cursor or the image of the hand moved in the same direction as the actual hand relative to the targets. Two baselines were performed—one for the right, and one for the left hand. Each peripheral target appeared three times, in random order, for a total of 30 trials for each hand.
The objective of the adaptation paradigm was to evaluate how subjects learned to accurately reach under altered visual feedback of the hand. In this paradigm, the movement path of the cursor or hand image was rotated 45° or 75° counter-clockwise such that to achieve rightward movement on the vertical screen, subjects had to produce a movement down (at either 45° or 75° respectively). One set of subjects performed the task with their dominant (right) hand and the transfer task with their non-dominant (left) hand for both viewing conditions and all rotations. Another set of subjects performed the task with their non-dominant (left) hand and the transfer task with their dominant (right) for both view conditions. We had a total of eight groups of subjects for the viewing, rotation magnitude, and hand-order conditions, as listed in the first column in Table 1, along with the sample size of each group. Once subjects reached the central target, one of the peripheral targets flashed for 500 ms. After the peripheral target disappeared, the subject had 6 s to reach the remembered target. Upon reaching their final position, subjects were instructed to hold the position until the same target reappeared, providing subjects with knowledge of results. Each peripheral target appeared 20 times, in pseudo-random order, for a total of 200 trials.
The objective of the transfer paradigm was to see how adaptation to altered feedback transferred to an untrained hand. In this paradigm, subjects reached with their opposite, untrained, hand under the same rotated visual feedback experienced in the adaptation paradigm. The target did not reappear at the end of the trial. Each peripheral target appeared 3 times, in pseudo-random order, for a total of 30 trials.
The entire experiment took less than 1.5 h to complete. After the experimental session, subjects reported that they noticed something in the adaptation paradigm, but were not aware of the nature of the manipulation.
Data analysis
The hand path trajectory and velocity profile of each outward reach for every trial for every subject were screened using custom software developed for Matlab 7.1 (The MathWorks, Natick MA) to verify that only outward reaches were included in analysis. Movement times that were more than two standard deviations from an individual’s mean for each block of ten trials were removed. The control paradigms provided a baseline measure for hand path deviation for each hand. We calculated deviations from a straight line as absolute angle at peak velocity (Dionne and Henriques 2008). The calculated deviations in hand paths were analysed in SPSS (SPSS, Chicago IL).
The calculated deviations in hand paths were analysed using analyses of variance (ANOVA) in SPSS (SPSS, Chicago IL). To evaluate adaptation, we used a 2 (view: cursor, hand) × 2 (hand-order: RL, LR) × 20 (training blocks: 1–20) mixed ANOVA for each angle of rotation. To evaluate transfer across arms we used a 2 (view: cursor, hand) × 2 (hand-order: RL, LR) × 2 (blocks: initial training, initial transfer) mixed ANOVA for each angle of rotation.
To control for differences in initial deviation, we looked at normalized percentages of adaptation and transfer. All values were compared to baseline. For adaptation percentage, we took the difference between initial and final performance and divided this difference by initial performance. For transfer percentage, we took the difference between initial performance in the adaptation condition and initial performance in the transfer condition and divided this difference by initial adaptation performance. To evaluate adaptation and transfer, we used 2 (view: cursor, hand) × 2 (hand-order: RL, LR) × 2 (rotation magnitude: 45°, 75°) independent ANOVAs.
Results
Path trajectories
Trials were averaged into blocks of ten trials. Figure 2 shows path trajectories of all subjects reaching under the eight viewing conditions for both dominant to non-dominant (2a) and non-dominant to dominant (2b) hand transfer. Trajectories for each of the ten targets are normalized to the target at zero degrees and averaged across ten trials for each subject. Black traces are hand paths produced when subjects were shown a cursor representing their unseen hand while grey traces are those when they were shown a rotated image of their hand. The columns represent the initial block of adaptation, the last block of adaptation and the first block of transfer.
Hand trajectories normalized to the target at zero degrees which is directly right of the start position. Each thin line represents a mean of ten reaches for each subject. Thick lines represent group means averaged across subjects. Black lines represent cursor view and grey lines represent hand view. Trajectories for participants who adapted with their right hand (a) and trajectories for participants who adapted with their left hand first (b)
Hand path deviations across trials
Figure 3 shows angular deviations averaged into blocks of up to ten trials for each subject and then averaged across subjects for each group. Black symbols indicate deviations in hand path when the cursor represented hand position, while grey symbols are those when the image of the hand was shown. Circles represent subjects who adapted with their right hand first and squares represent subjects who adapted with their left hand first.
Adaptation (a, b) and transfer (c–f). Angular deviations averaged into blocks of ten trials and then averaged across subjects for all feedback conditions. Black lines represent cursor view and grey lines represent hand view. Circles represent adaptation with the right hand and squares represent adaptation with the left hand. a and b show reach deviations across blocks of trials for the 45° and 75° rotation, respectively, during the adaptation condition. c–f represent angular deviations in the first three blocks of trials in the adaptation and transfer conditions for right–left hand order at 45° rotation (c), left–right at 45° (d), right–left at 75° (e), and left–right at 75° (f). Solid lines represent hand deviations during the adaptation condition (performance with one hand) and dashed lines represent those during the transfer conditions (performance with the opposite hand). Error bars represent SEM
Adaptation
Figure 3a, b shows performance in the adaptation paradigm for the 45° and 75° rotations, respectively. All eight feedback conditions showed significant decreases across the 20 blocks (200 trials) of adaptation for the 45° rotation (F(19, 1235) = 84.32, p < 0.01) and 75° rotation (F(19, 1235) = 57.00, p < 0.01). Consistent with other studies, decreases in angular deviation did not always reach baseline (Ghilardi et al. 1995; Abeele and Bock 2003; Klassen et al. 2005).
Adaptation across blocks was similar whether subjects saw a cursor representation of their movement or a view of their hand for the 45° rotation group (a) (F(19, 1235) = 1.29, p = 0.18), but not for the 75° rotation group (b) (F(19, 1235) = 3.48, p < 0.01). Pair-wise comparisons revealed higher initial errors for the cursor view in blocks one to seven (asterisks; p < 0.05), but no difference between views in the final blocks of adaptation (p > 0.05). Adaptation across blocks was similar whether participants adapted with the right or left arm for the 45° rotation (F(19, 1235) = 1.44, p = 0.10) and the 75° rotation (F(19, 1235) = 1.44, p = 0.10).
Intermanual transfer
Figure 3c–f shows transfer from the arm used in adaptation (solid lines) to the opposite untrained arm (dashed lines) for the 45° rotation (c, d) and the 75° rotation (e, f). We found significant transfer, indicated by a significant decrease in angular deviation from the adapted to the non-adapted arm for the 45° rotation (F(1, 63) = 26.46, p < 0.01) and the 75° rotation (F(1, 63) = 26.46, p < 0.01). However, transfer for the 45° rotation depended on hand-order (F(1, 65) = 16.95, p < 0.01), in that transfer only occurred from right to left hand (c, p < 0.05 as indicated by the asterisks) and not from left to right hand (d, p > 0.05). For the 75° rotation group (e, f), transfer was similar from right to left hand compared to left to right hand (F(1, 63) = 2.11, p = 0.15).
In general, in Fig. 3e, f deviations in the first blocks of the adaptation and transfer condition are larger when subjects see a cursor compared to a hand view (F(1, 63) = 11.09, p < 0.01). But, more important, transfer, the decrease in angular deviation across the first block of adaptation to the first block of transfer, was similar whether subjects could see a cursor representing their movements or a view of their hand for the 45° (F(1, 65) = 0.12, p = 0.72) and the 75° rotation (F(1, 63) = 0.18, p = 0.67).
Normalized adaptation and transfer percentages
Since initial deviations differed depending on the degree of rotation introduced, we calculated and evaluated a normalized percentage of adaptation and transfer. Figure 4 shows adaptation and transfer percentages where 100 would represent complete adaptation or transfer and zero would indicate none.
Adaptation (a) and transfer percentages (b) for all groups. Black bars represent cursor view and grey bars represent hand view. Solid bars represent adaptation with the right hand and checkered bars represent adaptation with the left hand. A value of 100 represents complete adaptation or transfer. Positive percentages indicate an improved performance with the untrained arm and negative percentages indicate a poorer initial performance with the untrained arm. Error bars represent SEM
Adaptation
Figure 4a shows that subjects adapted, or improved performance; i.e., one-sample t-tests were all significantly different from zero (p < 0.01) for all cases (Table 1). There was no difference in the adaptation percentage when subjects viewed a cursor compared to an image of their hand (F(1, 125) = 1.66, p = 0.20). But the percentage of adaptation did vary across hand-order as a function of viewing condition. F(1, 125) = 5.60, p < 0.05. Groups who adapted with their non-dominant (left) hand (checkers bars), showed larger adaptation percentages than groups who adapted with their dominant (right) hand (solid bars; F(1, 125) = 5.55, p < 0.05), but only for the cursor view (black bars) and not the hand view (grey bars; F(1, 125) = 5.60, p < 0.05). This is not surprising since we found larger initial deviations in the 75° groups for the cursor view.
Intermanual transfer
Figure 4b shows that in all cases where subjects adapted with their right arm, there was significant transfer to their left arm; one-sample t-tests were all significantly greater than zero (p > 0.01); Interestingly, when subjects adapted with their left arm, they showed no transfer to their right arm (checkered bars); one-sample t-tests were not significantly greater than zero (p < 0.01, Table 1). Transfer percentages were significantly larger for groups who adapted the dominant arm (solid bars) compared to groups who adapted with the non-dominant hand (checkered bars; F (1, 125) = 19.25, p < 0.05), but this does not vary with viewing condition (F (1, 125) = 0.01, p < 0.95).
Discussion
Our first objective was to evaluate differences in performance between viewing conditions, cursor view and hand view. We speculated that intermanual transfer may be greater in the case where errors can be attributed to an external cursor rather than to the arm. Yet, when subjects learned to reach with a 45° or 75° rotation, the rate of learning and its transfer to the opposite arm was equivalent across viewing conditions. Normalized percentages of adaptation and transfer consistently showed no difference between viewing conditions across either the viewing conditions or the two angles, suggesting that our brain treats external information of the cursor mechanics the same as internal information about the arm. If the brain is learning a separate internal model for the cursor, it is not evident from the amount of adaptation in the two viewing conditions.
Adaptation and generalization depend partly on how to the brain attributes the sources of errors; whether specifically to changes in the world or changes in the body (Berniker and Kording 2008). Here we wanted to test whether the brain attributes the source of error differently when people adapt to a misaligned cursor, which is similar across arms (more dependent on properties of the environment), or to an image of their arm which is distinct to each arm (more dependent on properties of the body). In addition, we wanted to know whether the brain forms distinct internal models. In a preliminary study that compared different viewing conditions, Clower and Boussaoud (2000) compared reaching with a view of the fingertip to a view of a cursor representation of fingertip position. When subjects reached the targets while wearing displacing prisms, they found no difference in errors between the two viewing conditions during the adaptation trials, but observed greater aftereffects (an indication of the formation of an internal model) in the condition where subjects saw their fingertip compared with subjects who saw a cursor representing finger position. They suggest that the brain must use separate mechanisms, such as different internal models, for different viewing conditions. Perhaps it is not where the brain attributes the error—the internal dynamics of the arm or an external effector, but how the brain deals with where incoming error originates. Since prisms displace the entire visual field including the target, it is not clear whether these effects were due to adaptation to the shift of the seen hand, or the shift of the seen target or movement goals.
In a study by Sober and Sabes (2005) where they use virtual reality to displace the view of the end-effector, they found subjects were less accurate in reaching the targets when they could see a displaced (horizontal shift) drawn outline of hand position compared to a displaced cursor representing hand location. They suggest that different mechanisms for each view may be at work here. However, reaching with a drawn outline of the hand could compromise the accuracy in reaching to the target. The difference in accuracy could be a result of reaching with a large end-effector compared with a small cursor. Here, the outline of the hand did not resemble a real hand. Since Graziano et al. (2000) showed that monkeys respond to reaching with realistic false arms, but not to unrealistic false arms, it is difficult to generalize the results of Sober and Sabes to reaching with an actual view of the hand.
It has been suggested that we have internal models specific to external information, whether it is a robot or a cursor, and these models are distinct from the internal model of the arm (Cothros et al. 2006; Kluzik et al. 2008). In the study by Cothros et al. (2006), subjects first learned to reach the targets within a curl force field and were then required to reach to the same targets without the imposed forces (null field) while either gripping the same robot handle or moving the hand freely. In the case where subjects continued to reach while gripping the robot handle in the null field, they produced typical large aftereffects—an indication of the formation of a new internal model. But for subjects who reached the same targets with a free hand, the aftereffects were smaller and decayed faster than the aftereffects for subjects who continued to grip the handle. Furthermore, subjects who moved the robot in the null field showed better retention of the first task when asked to reach in the force field again. This suggests that learning was associated or paired with the gripping of the robot and we form separate models for the external robot and the internal dynamics of the arm (Cothros et al. 2006). In a similar study, Kluzik et al. (2008) also showed that the internal models for the robot and arm are distinct, although they possibly partially overlap. In this study, when the one group of subjects reached in free space following adaptation to the force field, they were grasping a detached robot handle. They also found greater generalization to reaching in a null field, but generalization to reaching in free-space while gripping the detached handle was greater than that found in the study by Cothros and colleagues (whose subjects did not grasp anything). When the experiment was repeated, but with a gradual exposure to the force field during the adaptation phase, subjects reaching in free space showed an increase in generalization (from 40 to 60%), suggesting that with gradual exposure, we tend to assign error to the arm. These studies suggest that our internal model associated with the robot differs from the model of our arm.
Our results seem to suggest otherwise. Following the results of the aforementioned studies, we expected to find less transfer when we view an image of the hand because the error associated with the right arm should be specific to the right arm and not generalize to the left arm. Since we found equivalent learning and transfer across view conditions, it may indicate that our brain is treating the cursor and the hand image in the same manner.
Perhaps the brain creates a combined internal model of the cursor and hand so that we see no difference in performance between the two view conditions. The brain may be coding the cursor as an extension of the hand. Obayashi et al. (2001) showed that when monkeys used a rake to reach for objects, the body schema was modified—neurons expected to code for the schema of the hand in space were activated when using the rake. The visual receptive field, which codes peripersonal space, was altered when the monkey used the rake such that the monkey’s image of the hand was expanded to include the tip of the rake. In our study, the brain may extend its hand representation to the cursor, creating a combined internal model of the cursor and hand.
Hand-order and direction of transfer
Our second objective was to evaluate differences in performance between hand-order (dominant to non-dominant and non-dominant to dominant). After finding no difference in learning between the left and right hand, with the exception of a larger transfer percentage driven by larger initial errors in the 75° rotation for the cursor view, we found that transfer was asymmetrical. For the 45° and 75° rotation, we found similar amounts of transfer from the right to the left arm, but not always from the left to the right arm. The hand path trajectories showed that transfer occurred in both directions of hand-order for the 75° rotation, but this was not the case when we normalized the data to account for differences in initial errors for the two magnitudes of rotation, where we found transfer to be consistently asymmetrical. Since the 75° rotation had larger initial deviation overall, the amount of learning transferred to the right hand was significant, but not a large proportion of what was learned.
Generalization across arms does not always show symmetry across hand-order (from dominant to non-dominant and non-dominant to dominant). This asymmetry has been referred to the dynamic-dominance hypothesis (Sainberg and Wang 2002), where different aspects of a task are transferred across arms. But there is disparity in the literature regarding the direction of transfer. When subjects learn a visuomotor adaptation with their dominant hand, the non-dominant hand (untrained hand) shows improved performance in initial endpoint accuracy, but not in direction accuracy. The opposite occurred when the non-dominant hand learned the task first; only initial direction accuracy was transferred (Sainburg and Kalakanis 2000; Sainburg and Wang 2002; Wang and Sainburg 2007). Others have found the opposite pattern (Parlow and Kinsbourne 1989; Criscimagna-Hemminger et al. 2003). Crisciamagna-Hemminger et al. (2003) found that when subjects made reaching movements with a robot-manipulandum, the dynamics of the reach path were generalized from the dominant to the non-dominant hand, but not from the non-dominant to the dominant hand (Criscimagna-Hemminger et al. 2003). Our results support the latter direction of transfer where the initial error of the reach decreases from the dominant to the non-dominant hand, but not for the reverse hand-order. Criscimagna-Hemminger et al. (2003) also found that in the absence of the corpus callosum, intermanual transfer was still achieved. This could suggest that the dominant hemisphere has intrahemispheric connections that allow for some ipsilateral control. When the dominant hemisphere of right-handers (left hemisphere) is learning a task with the contralateral arm (right arm), the dominant hemisphere’s ipsilateral control results in transfer of the task to the ipsilateral arm (left arm).
Learning by observation
Across viewing conditions (cursor representation and hand view) we found consistent amounts of adaptation and transfer. For the hand view, some may argue that subjects are generally learning the task by simply watching themselves move their hand to targets. Learning by observation studies show that motor learning can be achieved without directly performing a task (Mattar and Gribble 2005; Nyberg et al. 2006; Mercier et al. 2008). Mattar and Gribble (2005) showed that when subjects watched a video of an individual learning how to accurately reach with a robot perturbing the hand, the subjects performed better than naïve subjects. Subjects were able to learn the task by simply observing others. Nyberg et al. (2006) showed that mental rehearsal of a task improves subsequent performance, with similar changes in brain activity that accompany direct motor learning. These studies show that a motor task can be learned, complete with changes in the brain, without physically doing the task. Both of these studies, however, show that learning by observing or rehearsing occurs when the same effector is used to perform the task. Similarly, Dionne and Henriques (2008) showed that seeing a reversed image of the hand (i.e., the right hand appears to be the left hand), increased transfer to the opposite arm compared with viewing a rotation of the arm. Seeing what looks like the left hand seemed to facilitate generalization to that same hand.
Our results of partial transfer cannot be explained by learning through observation of one’s hand performing the task on the screen. If this were the case, we would have observed transfer in both directions across arms. This was not the case; therefore learning by observation cannot explain intermanual transfer in our hand view condition.
Conclusions
When learning a visuomotor task, the brain seems to treat a view of cursor mechanics and a view of the actual hand in the same manner. This is true across different magnitudes of rotation and across patterns in hand-order. Transfer of adaptation to the opposite arm occurred in one direction, from the dominant to the non-dominant arm, supporting an asymmetrical pattern of generalization and suggesting that the dominant hemisphere has partial ipsilateral control of the arm.
References
Abeele S, Bock O (2003) Transfer of sensorimotor adaptation between different movement categories. Exp Brain Res 148:128–132
Berniker M, Kording K (2008) Estimating the sources of motor errors for adaptation and generalization. Nat Neurosci 11:1454–1461
Choe CS, Welch RB (1974) Variables affecting the intermanual transfer and decay of prism adaptation. J Exp Psychol 102:1076–1084
Clower DM, Boussaoud D (2000) Selective use of perceptual recalibration versus visuomotor skill acquisition. J Neurophysiol 84:2703–2708
Cothros N, Wong JD, Gribble PL (2006) Are there distinct neural representations of object and limb dynamics? Exp Brain Res 173:689–697
Criscimagna-Hemminger SE, Donchin O, Gazzaniga MS, Shadmehr R (2003) Learned dynamics of reaching movements generalize from dominant to nondominant arm. J Neurophysiol 89:168–176
Dionne JK, Henriques DYP (2008) Interpreting ambiguous visual information in motor learning. J Vis 8:1–10
Dizio P, Lackner JR (1995) Motor adaptation to Coriolis force perturbations of reaching movements: endpoint but not trajectory adaptation transfers to the nonexposed arm. J Neurophysiol 74:1787–1792
Galea JM, Miall RC, Woolley DG (2007) Asymmetric interlimb transfer of concurrent adaptation to opposing dynamic forces. Exp Brain Res 182:267–273
Ghilardi MF, Gordon J, Ghez C (1995) Learning a visuomotor transformation in a local area of work space produces directional biases in other areas. J Neurophysiol 73:2535–2539
Graziano SA, Cooke DF, Taylor CSR (2000) Coding the location of the arm by sight. Science 290:1782–1786
Held R, Bauer JA Jr (1974) Development of sensorially-guided reaching in infant monkeys. Brain Res 71:265–271
Klassen J, Tong C, Flanagan JR (2005) Learning and recall of incremental kinematic and dynamic sensorimotor transformations. Exp Brain Res 164:250–259
Kluzik J, Diedrichsen J, Shadmehr R, Bastian AJ (2008) Reach adaptation: what determines whether we learn an internal model of the tool or adapt the model of our arm? J Neurophysiol 100:1455–1464
Malfait N, Ostry DJ (2004) Is interlimb transfer of force-field adaptation a cognitive response to the sudden introduction of load? J Neurosci 24:8084–8089
Mattar AA, Gribble PL (2005) Motor learning by observing. Neuron 46:153–160
Mercier C, Aballea A, Vargas CD, Paillard J, Sirigu A (2008) Vision without proprioception modulates cortico-spinal excitability during hand motor imagery. Cereb Cortex 18:272–277
Nyberg L, Eriksson J, Larsson A, Marklund P (2006) Learning by doing versus learning by thinking: an fMRI study of motor and mental training. Neuropsychologia 44:711–717
Obayashi S, Suhara T, Kawabe K, Okauchi T, Maeda J, Akine Y, Onoe H, Iriki A (2001) Functional brain mapping of monkey tool use. Neuroimage 14(4):853–861
Parlow SE, Kinsbourne M (1989) Asymmetrical transfer of training between hands: implications for interhemispheric communication in normal brain. Brain Cogn 11:98–113
Sainburg RL (2002) Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res 142:241–258
Sainburg RL, Kalakanis D (2000) Differences in control of limb dynamics during dominant and nondominant arm reaching. J Neurophysiol 83:2661–2675
Sainburg RL, Wang J (2002) Interlimb transfer of visuomotor rotations: independence of direction and final position information. Exp Brain Res 145:437–447
Shadmehr R, Moussavi ZM (2000) Spatial generalization from learning dynamics of reaching movements. J Neurosci 20:7807–7815
Sober SJ, Sabes PN (2005) Flexible strategies for sensory integration during motor planning. Nat Neurosci 8:490–497
Taub E, Goldberg LA (1973) Prism adaptation: control of intermanual transfer by distribution of practice. Science 180:755–757
Wang J, Sainburg RL (2003) Mechanisms underlying interlimb transfer of visuomotor rotations. Exp Brain Res 149:520–526
Wang J, Sainburg RL (2007) The dominant and nondominant arms are specialized for stabilizing different features of task performance. Exp Brain Res 178:565–570
Wolpert DM, Ghahramani Z, Jordan MI (1995) An internal model for sensorimotor integration. Science 269:1880–1882
Acknowledgments
The authors gratefully acknowledge Dr. Erin Cressman and Aidan Thompson for their helpful suggestions on the manuscript and John Stemberger and Kemar Trenchfield for their programming expertise. This work was funded by grants from the Canadian Institute of Health Research (CIHR), Institute of Musculoskeletal Health and Arthritis (IMHA) and the Banting Research Foundation. D.Y.P. Henriques is an Alfred P Sloan fellow.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balitsky Thompson, A.K., Henriques, D.Y.P. Visuomotor adaptation and intermanual transfer under different viewing conditions. Exp Brain Res 202, 543–552 (2010). https://doi.org/10.1007/s00221-010-2155-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-010-2155-0