Abstract
In everyday life, vestibular sensors are activated by both self-generated and externally applied head movements. The ability to distinguish inputs that are a consequence of our own actions (i.e., active motion) from those that result from changes in the external world (i.e., passive or unexpected motion) is essential for perceptual stability and accurate motor control. Recent work has made progress toward understanding how the brain distinguishes between these two kinds of sensory inputs. We have performed a series of experiments in which single-unit recordings were made from vestibular afferents and central neurons in alert macaque monkeys during rotation and translation. Vestibular afferents showed no differences in firing variability or sensitivity during active movements when compared to passive movements. In contrast, the analyses of neuronal firing rates revealed that neurons at the first central stage of vestibular processing (i.e., in the vestibular nuclei) were effectively less sensitive to active motion. Notably, however, this ability to distinguish between active and passive motion was not a general feature of early central processing, but rather was a characteristic of a distinct group of neurons known to contribute to postural control and spatial orientation. Our most recent studies have addressed how vestibular and proprioceptive inputs are integrated in the vestibular cerebellum, a region likely to be involved in generating an internal model of self-motion. We propose that this multimodal integration within the vestibular cerebellum is required for eliminating self-generated vestibular information from the subsequent computation of orientation and posture control at the first central stage of processing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction and summary
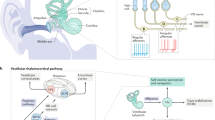
In this review, we consider the results of recent investigations that have focused on the information encoded by single neurons during voluntary (i.e., active) head movements. Early investigations involving in vitro, reduced, and anesthetized preparations provided important insights into the functional circuitry, intrinsic electrophysiology, and neuropharmacology of the vestibular nuclei. These studies revealed a unique feature of the vestibular system, namely that the neurons at the first central stage of processing (i.e., in the vestibular nuclei) are both sensory and premotor neurons. Notably, neurons in the vestibular nuclei constitute the middle link of the three-neuron vestibulo-ocular reflex (VOR) pathway, which produces eye movements to stabilize gaze during self-motion (Fig. 1a; VOR pathway). Likewise, the vestibular nuclei provide the middle link of the pathway linking the labyrinth and the spinal cord, which mediates the vestibulo-spinal reflexes required for balance and posture (Fig. 1a; vestibulo-spinal pathway). As a result, subsequent in vivo studies focused principally on understanding the responses of neurons within the vestibular nuclei in relation to the sensorimotor transformations that are required for reflex generation, by applying passive vestibular stimulation (i.e., head motion input) and recording the resulting neuronal and behavioral responses.
Methods: a Schematic of the central vestibular pathway. The vestibular sensors located in the inner ear send information via the 8th nerve to neurons in the vestibular nuclei. Specifically, vestibular-only (VO) neurons project to higher-order centers as well as the spinal cord, whereas position-vestibular-pause (PVP) and floccular target neurons (FTN) project to ocular motoneurons. b During experiments, monkeys were seated on a turntable, which could be translated or rotated. c–d Neurons were recorded during active and passive head-on-body rotations (c) and translations (d), as well as passive whole-body rotations (c) and translations (d). Examples of velocity and acceleration profiles during rotation and translation, respectively, are shown in the bottom panel. Blue lines represent passive motion and red lines represent active motion
However, a second essential feature of the vestibular system when compared to other sensory systems is that information processing is strongly multisensory and multimodal at the first stage of central processing (Fig. 1a; inputs; reviewed in Cullen and Roy 2004). The vestibular nuclei receive inputs from a wide range of cortical, cerebellar, and other brainstem structures in addition to direct inputs from the vestibular afferents. The integration of vestibular information and extra-vestibular cues is essential for higher-order vestibular functions, such as self-motion perception and spatial orientation (Fig. 1a; ascending vestibular pathways). Moreover, recent studies in alert animals have emphasized the importance of extra-vestibular signals in shaping the ‘simple’ sensory-motor transformations that mediate vestibulo-ocular and vestibulo-spinal reflexes.
In contrast to the long history of characterizing vestibular processing in conditions where the head is restrained and vestibular stimuli are passively applied, more recent investigations have now begun to examine the responses of single neurons during active head movements. Here, we review the results of studies examining the differential processing of active versus passive head motion, with a focus on the role of extra-vestibular signals and underlying mechanisms. First, we review the literature showing that neuronal responses to self-generated motion are selectively suppressed for a distinct class of neurons in the vestibular nuclei: vestibular-only (VO) neurons, which have descending projections to vestibulo-spinal pathways and ascending projections to the thalamus, such that they contribute to vestibulo-spinal reflexes and self-motion perception. In contrast, the responses of primary vestibular afferents and other classes of vestibular nuclei neurons are similarly robust during active and passive motion. Notably, we discuss the implications of our finding that the VOR interneurons (i.e., position-vestibular-pause (PVP) neurons) of the vestibular nuclei do not discriminate between passive and self-generated motion at the first central stage of processing. We then further show that the suppression of “vestibular-only” neurons responses to self-produced vestibular stimulation occurs only if the actual movement matches the intended one. Notably, the brain only generates a cancellation signal when the activation of proprioceptors matches the motor-generated expectation, consistent with an internal model of the sensory consequences of active motion. Finally, we examine the mechanisms that underlie this comparison. The results of recent experiments, focused on the integration of vestibular and extra-vestibular information in the vestibular cerebellum, yield insights into the origins of the suppression of self-generated vestibular stimulation. Our recent findings provide new evidence that the multimodal integration within the vestibular cerebellum provides the inputs required to update an internal model of active self-motion, which in turn is used to eliminate self-movements from the subsequent computation of orientation and posture control.
General approach
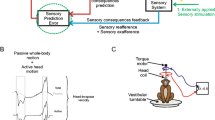
Macaque monkeys were prepared for chronic extracellular recording in the vestibular nerve and nuclei using aseptic surgical techniques similar to those previously described by Roy and Cullen (2001, 2004). All experimental protocols were approved by the McGill University Animal Care Committee and were in compliance with the guidelines of the Canadian Council on Animal Care. Monkeys were trained to follow a target light (HeNe laser) to generate pursuit and gaze shift movements. During the experiments, the monkey sat comfortably in a primate chair, placed on a servo-controlled vestibular turntable. Neuronal activity was initially recorded in the head-restrained condition during voluntary eye movements and passive whole-body rotation or translation (Fig. 1b). After a neuron was fully characterized in the head-restrained condition, the monkey’s head was slowly and carefully released so that the neuron’s activity could be characterized during active as well as passive movements of the head relative to the body (Fig. 1c and d).
Extracellular single-unit activity from afferent and vestibular-only neurons, horizontal gaze, and head position, target position, and vestibular turntable velocity were recorded and stored on DAT tape for playback. Action potentials were first discriminated during playback using a windowing circuit (BAK), and then spike density was calculated by convolving a Gaussian function with the spike train (SD of 10 ms). Subsequent analysis was performed using custom algorithms.
The differential processing of active and passive motion: conceptual frameworks
The ability to distinguish sensory inputs that are a consequence of our own actions from those that result from changes in the external world is essential for perceptual stability and accurate motor control. This distinction is particularly important in the vestibular system because of the short latency vestibular reflexes needed to stabilize gaze and head in space. For example, while vestibulo-spinal reflexes are essential for providing robust postural responses to unexpected vestibular stimuli, they can be counter-productive when the goal is to make active movements that result in head motion. It is thus theoretically advantageous to distinguish between vestibular inputs that arise as a result of active self-motion and those that are the result of unexpected events in external world.
Vestibular canal and otolith afferents do not differentially encode active and passive head motion
Evidence for the proposal that the efferent vestibular system has an important role in the differential processing of active and passive movements came from several converging lines of research. First, the afferents of the vestibular periphery receive bilateral efferent projections (Gacek and Lyon 1974; Dickman and Correia 1993; Myers et al. 1997; Plotnik et al. 2002), and studies in model systems (goldfish, Hartmann and Klinke 1980; frog, Caston and Bricout-Berthout 1984; toadfish, Boyle and Highstein 1990a) had suggested that vestibular efferent fibers carry extra-vestibular signals (e.g., somatosensory, proprioceptive or efference copy signals), which could serve to modulate afferent responses during voluntary movements (Klinke 1970; Goldberg and Fernandez 1980). Consistent with this idea, activation of the efferent vestibular system had been further shown to produce an increase in afferent resting discharge rate (Goldberg and Fernandez 1980; Boyle and Highstein 1990b; Plotnik et al. 2002, 2005; Marlinski et al. 2004; Sadeghi et al. 2009) and a reduction in afferent response sensitivity (Goldberg and Fernandez 1980). Finally, studies of the toadfish periphery had reported a comparable increase in the background discharge rate and a decrease in the sensitivity of afferents preceding the generation of an escape response (Highstein and Baker 1985; Boyle and Highstein 1990b; Highstein 1991, 1992). Thus, taken together, these results have been used to support the proposal that during active head movements, activation of the efferent system is used to decrease the probability of inhibitory cutoff or excitatory saturation of afferents, thereby effectively increasing the dynamic range available for unexpected vestibular input (Goldberg and Fernandez 1980).
However, more recent studies by our laboratory have shown that the vestibular afferents in alert behaving monkeys do not differentially encode active and passive head motion. Neuronal responses were compared during passive and active head-on-body translations or rotations in order to assess the relative influence of neck proprioceptive and motor-related signals on head motion coding in vestibular afferents. Figure 2 shows the activity of example regular and irregular semicircular canal (A) and otolith (B) afferents in response to head rotation and translation, respectively. The vestibular sensitivities of both regular and irregular afferents during active motion were comparable to those recorded during passive movements for both types of motion (Cullen and Minor 2002; Sadeghi et al. 2007a; Jamali et al. 2009). Furthermore, while previous studies have reported that the effects of activation of the efferents is greatest for irregularly discharging afferents in primates (Goldberg and Fernandez 1980; Sadeghi et al. 2009), we found that a given afferent’s response was comparable in active and passive conditions regardless of the regularity of its discharge or resting rate. Thus, the results of these more recent experiments do not support the proposal that the efferent vestibular system plays an important role in the differential processing of active and passive movements; both regular and irregular afferent responses were comparable during self-generated and passive head-on-body movements. Accordingly, in primates, vestibular inputs that are self-generated versus those that are the result of passive motion are similarly encoded by the firing rates of primary sensory afferents.
Vestibular afferents do not distinguish between active and passive motion. a–b Responses of an example regular and irregular semicircular canal (a) and otolith (b) afferent during passive and active head movements. Superimposed on the actual firing rate (gray) in each condition is an estimate of the firing rate (passive: blue trace, active: red trace) based on the vestibular sensitivities and bias discharge. In the active condition, the firing rate could be predicted (dashed blue traces) by a model based on the bias and the sensitivity values calculated in the passive condition
The response of vestibular-only neurons of the vestibular nuclei are cancelled during active head motion
The processing of vestibular information at the first central synapse had been studied by recording from neurons in the vestibular nuclei of head-restrained animals, during passive stimulation protocols that resulted in movement of the head and body together relative to space. This approach has been useful for categorizing neurons into different subgroups including position-vestibular-pause neurons, which mediate the VOR, and vestibular-only (or non-eye movement neurons), which contribute to vestibulo-spinal pathways. In addition to their vestibular inputs, however, neurons in the vestibular nuclei also receive convergent information from the areas carrying proprioceptive (Boyle and Pompeiano 1981; Anastasopoulos and Mergner 1982; Wilson et al. 1990; Wilson 1991) and efference copy signals, as well as projections from higher-order cortical areas (for review, see Fukushima 1997). These extra-vestibular inputs could be used to discriminate between self-motion that is the result of passive stimulation and that is the result of active motion. Accordingly, more recently, investigators have quantified the information encoded by these different neuron subgroups, comprising the first central stage of vestibular processing, to examine whether each subclass differentially encodes vestibular inputs arising from self-generated versus externally applied head motion.
Indeed, the results of studies in our laboratory as well as others have shown that the responses of a particular group of neurons in the vestibular nuclei—the vestibular-only neurons—are dramatically attenuated during active movements (compare panels 3a, b) (McCrea et al. 1999; Roy and Cullen 2001). The attenuation of vestibular modulation during active motion is specific to this class of neurons, which project to the spinal cord and are thought to mediate, at least in part, vestibulo-spinal reflexes, but do not contribute to the direct VOR pathways (Wilson et al. 1990; Boyle et al. 1996; Gdowski and McCrea 1999). Notably, vestibular-only neurons also continue to respond selectively to passively applied head motion when a monkey generates active head-on-body movements (Fig. 3c, see also Roy and Cullen 2001). The ability of these neurons to selectively respond to passive vestibular stimuli during concurrent active and passive motion is likely to underlie our ability to reflexively respond to changes in vestibular input that the brain does not expect. For example, in order to recover from tripping over an obstacle while walking or running, it is vital that postural reflexes generate robust responses to the unexpected component of head motion.
Vestibular-only neurons are attenuated during active head movements. Activity of an example vestibular-only neuron is shown during active and passive paradigms. a Neurons were identified based on their response to horizontal head movements during whole-body rotations. b Example of neuronal responses during active head-on-body rotations. The firing rate could not be predicted based on the vestibular sensitivity calculated during passive rotations (red trace overlaying firing rate). c Example response of the same neuron during a paradigm where the monkey generated voluntary head-on-body movements while being passively rotated. Head-in-space movement is the sum of the passive rotation generated by the turntable and voluntary head-on-body movements. The modulation of the neuron was well correlated with the passive prediction (blue trace) but was poorly related to the vestibular prediction (red trace) based on total head movement. The black traces overlaying the firing rates represent the estimation. d Histogram comparing sensitivity to active head movements of vestibular afferents (canal and otolith, N = 24 and 48) and vestibular nuclei neurons (vestibular only (VO), and position-vestibular-pause (PVP) during gaze stabilization, N = 52 and 24)) normalized to sensitivity estimated during passive head motion
The VOR interneurons (i.e., PVP neurons) of the vestibular nuclei do not differentially encode active and passive head motion
In contrast to VO neurons, we have demonstrated that the position-vestibular-pause (PVP) neurons of the vestibular nuclei, which mediate the vestibulo-ocular reflex, do not differentially encode active and passive motion (Roy and Cullen 1998, 2002). These neurons continue to reliably encode head velocity during either active or passive head motion, when stable gaze is required, again consistent with their role in generating the VOR. Furthermore, by characterizing neuronal responses during a variety of experimental conditions, we were able to specifically characterize the multimodal processing that occurs in PVP neurons. We found that neither neck proprioceptive inputs, an efference copy of neck motor commands, nor the monkey’s knowledge of its self-motion influence the activity of PVP neurons per se. Instead, an efference copy of the saccadic system’s motor command to move the eye (i.e., redirect gaze) is responsible for the behaviorally dependent modulation of position-vestibular-pause neurons (and by extension for modulation of the status of the VOR) reported during gaze redirection (see for example, Cullen and Roy 2004). As a result, the efficacy of the VOR pathway is suppressed whenever the animal’s current behavioral goal is to redirect gaze. For example, the VOR is counterproductive during rapid eye-head gaze shifts, since it would generate an eye movement command in the direction opposite to the intended change in gaze. Accordingly, the inhibitory input from the saccadic pathways (i.e., a gaze efference copy) produces a pause in the responses of PVP neurons resulting in the suppression of the VOR’s efficacy during rapid gaze redirection.
Summary of primary afferent and central neuron response sensitivity during active vs. passive motion
Thus, in summary unlike primary canal or otolith afferents, central neurons can preferentially encode passive versus active head motion. Importantly, however, this was true only for a subclass class of neurons at the first central stage of processing, namely VO neurons, which have descending projections to vestibulo-spinal pathways and ascending projections to the thalamus, and contribute to vestibulo-spinal reflexes and the self-motion perception. In contrast, when the goal was to stabilize gaze, VOR interneurons, like primary afferents similarly encode motion in both conditions. Results from the population analysis of the different classes of primary afferents and central neurons are plotted in Fig. 3d. On average, the vestibular-only neuron population response was unique in that it was profoundly (70%) reduced during active when compared to passive head movements. In contrast, population responses of VOR interneurons (i.e., position-vestibular-pause neurons (PVPs)), primary canal, and otolith afferents were unchanged. Notably, this difference in the coding strategy of the two central neuron groups is consistent with their different functional roles; the responses of neurons that comprise vestibulo-spinal pathways are suppressed when the goal is to generate an active head movements, whereas the responses of VOR interneurons remain robust when the goal is to stabilize gaze (i.e., regardless of whether the head movement is active or passive). Instead, these neurons process vestibular information in a behaviorally dependent manner.
The spike train regularity of vestibular afferent and vestibular nuclei neurons is unchanged during active head motion
As summarized above (Figs. 2, 3d), our previous analyses of neuronal firing rates did not successfully provide any compelling evidence for the existence of selective efferent-mediated affects on afferent responses during active versus passive movements in alert primates (Cullen and Minor 2002; Jamali et al. 2009). A recent study, however, has proposed that activation of the efferent system could potentially change the firing regularity, as well as excitability, of afferent fibers (Kalluri et al. 2010). In particular, since differences in channels across afferents are linked to differences in the regularity of spike timing, it follows that the effect of efferent synapse acetylcholine release (Goldberg and Fernandez 1980; Perachio and Kevetter 1989) could modulate channels present in the calyceal afferents to alter discharge regularity. In turn, a substantial effect on regularity could alter the information carried by the afferent, since more regular afferents encode information in a spike timing as well as rate-based code (Sadeghi et al. 2007b). Thus, to explore this possibility further, we compared the firing rate variability of both primary afferent and vestibular-only neurons in the vestibular nuclei before, during, and after active head movements.
The results of this analysis are shown for an example afferent and central neuron in Fig. 4 (left panel and right panel, respectively). Notably, we found that discharge regularity was not significantly altered during active head movements at either of these sequential stages of processing. First, we estimated each afferent’s variability by computing the residual of its modulation relative to a simple model of its response to head motion:
Response regularity of afferent and central vestibular neurons does not change during active head movements. a Activity of an example irregular afferent (A1) and vestibular-only (VO) (A2) neuron during active movements. Top traces show superimposed head velocity and bottom traces show the corresponding firing rates. b Raster plots for each head movement (in a) for the same afferent (B1) and vestibular-only neuron (B2). c Residuals of the regression analysis (i.e., difference between actual and estimated firing rate) during active head movements shown above. d Standard deviation of residuals for the population of afferents (D1, n = 10) and vestibular-only neurons (D2, n = 5) during each of the five epochs of the active movement (denoted by the five gray boxes). This gives us an estimation of the variability in the spontaneous discharge of the neuron. Inset shows CV of the interspike interval for vestibular-only neurons before, during, and after the active head movement
Where \( \widehat{fr} \) is the estimated firing rate, Sv-vest and Sa-vest are coefficients representing sensitivities to head velocity and acceleration, and b is a bias term. Specifically, the residual was computed by taking the difference of the actual firing rate (fr), and the estimate (\( \widehat{fr} \)) provided by the best fit to Eq. 1. Analysis of the central neuron, which was more straightforward since the neuron was not responsive to active head movements, similarly showed that their response variability was not altered during active head movements. The histograms shown in Fig. 4 D1, D2 summarize the average standard deviations of the residuals before, during (3 epochs corresponding to early, middle, and late stages), and after active head motion. In addition, we also compared the average coefficient of variation (CV) at each interval for a population of central neurons that were unresponsive to active head motion. Overall, we found that there was no significant change in either the variability of the residual firing rate or CV when compared before, during, and active head movement, (paired t test, p > 0.62). Thus, our analysis of both primary afferents and central neurons in the vestibular nuclei suggests that the efferent system does not play a role in the differential processing of active and passive motion; we observed neither a change in average sensitivities (i.e., neuronal rate coding, Fig. 2) nor differences in spike-time variability (i.e., temporal coding, Fig. 4).
An internal model of the sensory consequences of active motion is used for suppression
The differential encoding of active and passive movements observed in the firing rates of second-order vestibular nuclei neurons is consistent with Von Holst and Mittelstaedt’s (1950) original proposal that the brain compares an “efference copy” of the motor command generated during voluntary movements with an incoming sensory input to distinguish between exafference and reafference. As detailed above, the suppression of vestibular reafference is specific to a class of second-order neurons, which had been classically termed vestibular-only (or alternatively non-eye movement) neurons on the basis of their lack of eye movement–related responses in head-restrained animals (e.g., Fuchs and Kimm 1975; Keller and Daniels 1975; Lisberger and Miles 1980; Chubb et al. 1984; Tomlinson and Robinson 1984; Scudder and Fuchs 1992; Cullen and McCrea 1993). Thus, the results of head unrestrained studies have shown that this nomenclature is misleading given that these neurons reliably encode passively applied head velocity (i.e., vestibular exafference) but not active head velocity. Accordingly, we propose that nomenclature such as ‘vestibular exafference selective’ would provide a more accurate description of the responses of this group of neurons.
How does the brain distinguish between active and passive head movements at the first stage of central processing in the vestibular system? Theoretically, the existence of extensive multimodal convergence of other sensory and motor signals with vestibular information in the vestibular nuclei provides several possible solutions. In particular, it is important to note that neuronal responses were compared in two very different conditions: (a) during self-generated head movements that were produced by activation of the neck musculature (i.e., voluntary head-on-body movements) and (b) during passive movements that were generated by whole-body rotations (i.e., the traditional stimulus for quantifying vestibular responses). Accordingly, our more recent studies in alert rhesus monkeys have focused on the implications of the difference between the extra-vestibular cues that were present in these two conditions. First, because neck proprioceptors as well as vestibular receptors are stimulated during active head-on-body movements, we began by examining whether this additional information might alter neuronal responses during active head-on-body movements. However, while the neck-related inputs conveyed to the vestibular nuclei via disynaptic pathways (Sato et al. 1997) influence the vestibular nuclei neuron activity in decerebrate animals (Boyle and Pompeiano 1981; Anastasopoulos and Mergner 1982; Wilson et al. 1990), vestibular nuclei neurons are unresponsive to passive activation of neck proprioceptors in alert rhesus monkeys (Roy and Cullen 2003, 2004). Similarly, this same series of studies demonstrated that neurons do not respond to the generation of a neck motor command to move the head when the head is experimentally restrained such that it cannot move (as verified by the measuring neck torque in head-restrained monkeys). Thus, neither neck motor efference copy nor proprioception cues alone are sufficient to account for the elimination of neuronal sensitivity to active head rotation.
The results described in the paragraph above appear to suggest that signals related to neck motor commands do not play an important role in mediating the reduction in vestibular-only neuron responses during active motion. However, although the monkey generated neck motor commands during the experiments, the movement of the head relative to the neck was experimentally constrained to prevent the actual realization of the intended head movement. Accordingly, the question arises whether an inhibitory neck proprioceptive signal might be gated in only when the actual activation of neck proprioceptors matches an internal model of the sensory consequence of head motion. By experimentally controlling the correspondence between intended and actual head movement, it was demonstrated that indeed a cancellation signal is generated when the activation of neck proprioceptors matches the motor-generated expectation (Roy and Cullen 2004). Accordingly, we have proposed that an internal model of the sensory consequences of active head motion is used to selectively suppress reafference at the vestibular nuclei level. The schematic in Fig. 5 shows our current working model of the neural mechanisms in the vestibular nuclei that underlie the ability to distinguish self-generated from passively applied head motion.
Proposed mechanisms for the attenuation of vestibular reafference. During the active head movements, an efference copy is processed by an internal model, which computes the expected sensory consequence of the motor command. Neck proprioceptive inputs are compared with this estimate of reafference in a putative matching center in the cerebellum. If these signals match, a cancellation signal is sent to vestibular-only neurons in the vestibular nuclei
Multimodal integration in the vestibular cerebellum and the origins of the suppression of self-generated vestibular stimulation
The cerebellum is thought to integrate convergent information encoding goals, motor commands, and movement feedback signals to compute the correspondence between the predicted and actual sensory outcome of a motor command. Consistent with this proposal, prior studies in the electric fish have shown that its cerebellum-like electrosensory lobes play a key role in the attenuation of sensory responses to self-generated electrical stimulation (Bell et al. 1999; Mohr et al. 2003; Sawtell et al. 2007). Furthermore, fMRI studies in humans have similarly provided evidence that the cerebellum plays a similar role in the suppression of tactile stimulation during self-produced ‘tickle’ (Blakemore et al. 1998; 1999a; b).
To date, however, the site where multimodal signals combine in order to cancel vestibular reafference has not yet been identified. Our previous results suggest that the site responsible for predicting the sensory consequences of active motion should logically be characterized by three elements, namely, neurons should receive (1) proprioceptive information, (2) a copy of the motor command, or alternatively an estimate of the expected sensory feedback that results from self-generated movement, as well as (3) vestibular input. For this reason we predicted that the rostral fastigial nucleus (FN), the most medial of the deep cerebellar nuclei, should be an excellent candidate site for the generation of a cancellation signal during active head-on-body movements. This nucleus receives descending projections from the anterior vermis (Batton et al. 1977; Yamada and Noda 1987), a region of the cerebellum that receives direct projections from cortical structures involved in producing head and neck movement (Alstermark et al. 1992a, b). In addition, it receives ascending neck proprioceptive input via the central cervical nucleus and the external cuneate nucleus and vestibular input from the vestibular nuclei (Voogd et al. 1996). In turn, the rostral FN projects to the vestibular nuclei and spinal cord, and lesion studies have confirmed its important contribution to the control of voluntary head movements as well as posture (Thach et al. 1992; Kurzan et al. 1993; Pélisson et al. 1998).
To explicitly test whether neurons in the rostral FN encode and integrate multimodal information in manner consistent with that required for the cancellation of vestibular reafference, we recently completed a series of single-unit recording studies. Notably, we found, for the first time, that single neurons dynamically encode both proprioceptive and vestibular information during passive stimulation conditions (Brooks and Cullen 2009). In particular, we have established that the convergence of vestibular and proprioceptive inputs provides the required foundation for computing an internal estimate of body motion in space. Notably, neurons encoding body motion are characterized by the convergence of vestibular and proprioceptive signals. In contrast, neurons only sensitive to vestibular inputs encode head motion like those in the vestibular nerve and nuclei (see Roy and Cullen 2001, 2004; Cullen and Minor 2002; Jamali et al. 2009). Overall, we found that approximately half of the neurons in the rostral FN selectively encode passive body motion when the head and body are moved separately, while the other half encodes head motion (Brooks and Cullen 2009). This convergence of vestibular and proprioceptive inputs most likely underlies the transformation of vestibular signals from a head to a body reference frame in the rostral FN (Kleine et al. 2004; Shaikh et al. 2004). Indeed, our recent studies have further revealed similar tuning of proprioceptive and vestibular responses in relation to changes in head-on-body position and thus provide insight into the specific computation that accomplishes this transformation (Fig. 6d–f, Brooks and Cullen 2009). Importantly, to date, our experiments in the rostral FN have exclusively tested neuronal responses to passively applied movements. Our current experiments are now focused on understanding how these same neurons respond when proprioceptive and vestibular inputs are stimulated as a result of active self-motion. In particular, these studies will allow us to test whether this multimodal integration within this region of the vestibular cerebellum underlies the brain’s computation of exafference during self-motion.
Rostral FN neuron responses to vestibular and neck proprioceptive stimulation. a–c Activity of example unimodal (top gray filled trace) and bimodal (bottom gray filled trace) neurons are shown during whole-body (a), body-under-head (b) and head-on-body rotations (c). Thick lines overlaying the firing rate represent a model based on estimated resting discharge and vestibular (a), or neck proprioception sensitivity (b); in C thick lines represents a prediction based on the vestibular sensitivity (blue) or a linear summation of vestibular and neck sensitivities (red). d–e Sensitivity to vestibular (d) and neck proprioceptive (e) stimulation of the example bimodal but not unimodal neuron varies as a function of head-on-body position. (f) Average tuning curves for bimodal and unimodal neurons for different neck positions to vestibular and neck proprioceptive stimulation
Conclusions
Neurons at the first central stage of vestibular processing in the vestibular nuclei can distinguish between self-generated and passive movements. Notably, during active movements, a cancellation signal is sent to a distinct group of neurons termed vestibular-only neurons when the activation of proprioceptors matches the motor-generated expectation. The cancellation signal predicts the vestibular activation which is the consequence of a motor command to effectively eliminate self-generated movements from subsequent computation of orientation and postural control. However, the ability to distinguish actively generated and passive stimuli is not a general feature of all early central vestibular processing; central vestibular neurons process vestibular information in a manner that is consistent with their functional role. In particular, central neurons controlling gaze, rather than posture and spatial orientation (i.e., PVP neurons), do not differentially encode active and passive head motion. Instead, these neurons process vestibular information in a manner that depends on current gaze strategy: their responses are only suppressed when the animal’s goal is to redirected gaze. This is logical since these neurons should continue to generate a VOR when the goal is to stabilize gaze—regardless of whether head motion is active or passive. Thus, for example, while the activity of PVP neurons is suppressed during the initial phase of eye-head gaze shifts (i.e., when gaze is redirected), it (and thus the VOR) is again robust toward the end of the gaze shift when the eye is on target but the head is still catching up (Roy and Cullen 1998, 2002). Our most recent studies have furthered this line of research by identifying a site of where multimodal signals are likely combined in order to cancel vestibular reafference. Neurons in the cerebellar nuclei integrate extra-vestibular and vestibular inputs, and as a result encode information that could potentially contribute to the ability of human subjects to accurately perceive rotation of the body independently of rotation of the head during everyday activities (Mergner et al. 1981, 1983, 1991). To date, however, it is currently unknown whether single neurons in the vestibular cerebellum differentially encode body and head motion when movements are the result of voluntary behavior (i.e., reafference) versus passively applied stimulation (i.e., exafference) in a manner consistent with the cancellation of vestibular reafference. Further experiments will be needed in order to establish whether this region plays a role in reafference cancellation.
References
Alstermark B, Pinter MJ, Sasaki S (1992a) Descending pathways mediating disynaptic excitation of dorsal neck motoneurones in the cat: facilitatory interactions. Neurosci Res 15:32–41
Alstermark B, Pinter MJ, Sasaki S (1992b) Descending pathways mediating disynaptic excitation of dorsal neck motoneurones in the cat: brain stem relay. Neurosci Res 15:42–57
Anastasopoulos D, Mergner T (1982) Canal-neck interaction in vestibular nuclear neurons of the cat. Exp Brain Res 46:269–280
Batton RR III, Jayaraman A, Ruggiero D, Carpenter MB (1977) Fastigial efferent projections in the monkey: an autoradiographic study. J Comp Neurol 174:281–305
Bell CC, Han VZ, Sugawara Y, Grant K (1999) Synaptic plasticity in the mormyrid electrosensory lobe. J Exp Biol 202:1339–1347
Blakemore SJ, Wolpert DM, Frith CD (1998) Central cancellation of self-produced tickle sensation. Nat Neurosci 1:635–640
Blakemore SJ, Wolpert DM, Frith CD (1999a) The cerebellum contributes to somatosensory cortical activity during self-produced tactile stimulation. Neuroimage 10:448–459
Blakemore SJ, Frith CD, Wolpert DM (1999b) Spatio-temporal prediction modulates the perception of self-produced stimuli. J Cogn Neurosci 11:551–559
Boyle R, Highstein SM (1990a) Efferent vestibular system in the toadfish: action upon horizontal semicircular canal afferents. J Neurosci 10:1570–1582
Boyle R, Highstein SM (1990b) Resting discharge and response dynamics of horizontal semicircular canal afferents of the toadfish, Opsanus tau. J Neurosci 10:1557–1569
Boyle R, Pompeiano O (1981) Convergence and interaction of neck and macular vestibular inputs on vestibulospinal neurons. J Neurophysiol 45:852–868
Boyle R, Belton T, McCrea RA (1996) Responses of identified vestibulospinal neurons to voluntary eye and head movements in the squirrel monkey. Ann N Y Acad Sci 781:244–263
Brooks JX, Cullen KE (2009) Multimodal integration in rostral fastigial nucleus provides an estimate of body movement. J Neurosci 29:10499–10511
Caston J, Bricout-Berthout A (1984) Responses to somatosensory input by afferent and efferent neurons in the vestibular nerve of the frog. Brain Behav Evol 24:135–143
Chubb MC, Fuchs AF, Scudder CA (1984) Neuron activity in monkey vestibular nuclei during vertical vestibular stimulation and eye movements. J Neurophysiol 52:724–742
Cullen KE, McCrea RA (1993) Firing behavior of brain stem neurons during voluntary cancellation of the horizontal vestibuloocular reflex. I. Secondary vestibular neurons. J Neurophysiol 70:828–843
Cullen KE, Minor LB (2002) Semicircular canal afferents similarly encode active and passive head-on-body rotations: implications for the role of vestibular efference. J Neurosci 22:RC226
Cullen KE, Roy JE (2004) Signal processing in the vestibular system during active versus passive head movements. J Neurophysiol 91:1919–1933
Dickman JD, Correia MJ (1993) Bilateral communication between vestibular labyrinths in pigeons. Neuroscience 57:1097–1108
Fuchs AF, Kimm J (1975) Unit activity in vestibular nucleus of the alert monkey during horizontal angular acceleration and eye movement. J Neurophysiol 38:1140–1161
Fukushima K (1997) Corticovestibular interactions: anatomy, electrophysiology, and functional considerations. Exp Brain Res 117:1–16
Gacek RR, Lyon M (1974) The localization of vestibular efferent neurons in the kitten with horseradish peroxidase. Acta Otolaryngol 77:92–101
Gdowski GT, McCrea RA (1999) Integration of vestibular and head movement signals in the vestibular nuclei during whole-body rotation. J Neurophysiol 82:436–449
Goldberg JM, Fernandez C (1980) Efferent vestibular system in the squirrel monkey: anatomical location and influence on afferent activity. J Neurophysiol 43:986–1025
Hartmann R, Klinke R (1980) Efferent activity in the goldfish vestibular nerve and its influence on afferent activity. Pflugers Arch 388:123–128
Highstein SM (1991) The central nervous system efferent control of the organs of balance and equilibrium. Neurosci Res 12:13–30
Highstein SM (1992) The efferent control of the organs of balance and equilibrium in the toadfish, Opsanus tau. Ann N Y Acad Sci 656:108–123
Highstein SM, Baker R (1985) Action of the efferent vestibular system on primary afferents in the toadfish, Opsanus tau. J Neurophysiol 54:370–384
Jamali M, Sadeghi SG, Cullen KE (2009) Response of vestibular nerve afferents innervating utricle and saccule during passive and active translations. J Neurophysiol 101:141–149
Kalluri R, Xue J, Eatock RA (2010) Ion channels set spike timing regularity of Mammalian vestibular afferent neurons. J Neurophysiol 104:2034–2051
Keller EL, Daniels PD (1975) Oculomotor related interaction of vestibular and visual stimulation in vestibular nucleus cells in alert monkey. Exp Neurol 46:187–198
Kleine JF, Guan Y, Kipiani E, Glonti L, Hoshi M, Buttner U (2004) Trunk position influences vestibular responses of fastigial nucleus neurons in the alert monkey. J Neurophysiol 91:2090–2100
Klinke R (1970) Efferent influence on the vestibular organ during active movements of the body. Pflugers Arch 318:325–332
Kurzan R, Straube A, Buttner U (1993) The effect of muscimol micro-injections into the fastigial nucleus on the optokinetic response and the vestibulo-ocular reflex in the alert monkey. Exp Brain Res 94:252–260
Lisberger SG, Miles FA (1980) Role of primate medial vestibular nucleus in long-term adaptive plasticity of vestibuloocular reflex. J Neurophysiol 43:1725–1745
Marlinski V, Plotnik M, Goldberg JM (2004) Efferent actions in the chinchilla vestibular labyrinth. J Assoc Res Otolaryngol 5:126–143
McCrea RA, Gdowski GT, Boyle R, Belton T (1999) Firing behavior of vestibular neurons during active and passive head movements: vestibulo-spinal and other non-eye-movement related neurons. J Neurophysiol 82:416–428
Mergner T, Anastasopoulos D, Becker W, Deecke L (1981) Discrimination between trunk and head rotation; a study comparing neuronal data from the cat with human psychophysics. Acta Psychol (Amst) 48:291–301
Mergner T, Nardi GL, Becker W, Deecke L (1983) The role of canal-neck interaction for the perception of horizontal trunk and head rotation. Exp Brain Res 49:198–208
Mergner T, Siebold C, Schweigart G, Becker W (1991) Human perception of horizontal trunk and head rotation in space during vestibular and neck stimulation. Exp Brain Res 85:389–404
Mohr C, Roberts PD, Bell CC (2003) The mormyromast region of the mormyrid electrosensory lobe. I. Responses to corollary discharge and electrosensory stimuli. J Neurophysiol 90:1193–1210
Myers SF, Salem HH, Kaltenbach JA (1997) Efferent neurons and vestibular cross talk in the frog. J Neurophysiol 77:2061–2070
Pélisson D, Goffart L, Guillaume A (1998) Contribution of the rostral fastigial nucleus to the control of orienting gaze shifts in the head-unrestrained cat. J Neurophysiol 80:1180–1196
Perachio AA, Kevetter GA (1989) Identification of vestibular efferent neurons in the gerbil: histochemical and retrograde labelling. Exp Brain Res 78:315–326
Plotnik M, Marlinski V, Goldberg JM (2002) Reflections of efferent activity in rotational responses of chinchilla vestibular afferents. J Neurophysiol 88:1234–1244
Plotnik M, Marlinski V, Goldberg JM (2005) Efferent-mediated fluctuations in vestibular nerve discharge: a novel, positive-feedback mechanism of efferent control. J Assoc Res Otolaryngol 6:311–323
Roy JE, Cullen KE (1998) A neural correlate for vestibulo-ocular reflex suppression during voluntary eye-head gaze shifts. Nat Neurosci 1:404–410
Roy JE, Cullen KE (2001) Selective processing of vestibular reafference during self-generated head motion. J Neurosci 21:2131–2142
Roy JE, Cullen KE (2002) Vestibuloocular reflex signal modulation during voluntary and passive head movements. J Neurophysiol 87:2337–2357
Roy JE, Cullen KE (2003) Brain stem pursuit pathways: dissociating visual, vestibular, and proprioceptive inputs during combined eye-head gaze tracking. J Neurophysiol 90:271–290
Roy JE, Cullen KE (2004) Dissociating self-generated from passively applied head motion: neural mechanisms in the vestibular nuclei. J Neurosci 24:2102–2111
Sadeghi SG, Minor LB, Cullen KE (2007a) Response of vestibular-nerve afferents to active and passive rotations under normal conditions and after unilateral labyrinthectomy. J Neurophysiol 97:1503–1514
Sadeghi SG, Chacron MJ, Taylor MC, Cullen KE (2007b) Neural variability, detection thresholds, and information transmission in the vestibular system. J Neurosci 27:771–781
Sadeghi SG, Goldberg JM, Minor LB, Cullen KE (2009) Efferent-mediated responses in vestibular nerve afferents of the alert macaque. J Neurophysiol 101:988–1001
Sato H, Ohkawa T, Uchino Y, Wilson VJ (1997) Excitatory connections between neurons of the central cervical nucleus and vestibular neurons in the cat. Exp Brain Res 115:381–386
Sawtell NB, Williams A, Bell CC (2007) Central control of dendritic spikes shapes the responses of Purkinje-like cells through spike timing-dependent synaptic plasticity. J Neurosci 27:1552–1565
Scudder CA, Fuchs AF (1992) Physiological and behavioral identification of vestibular nucleus neurons mediating the horizontal vestibuloocular reflex in trained rhesus monkeys. J Neurophysiol 68:244–264
Shaikh AG, Meng H, Angelaki DE (2004) Multiple reference frames for motion in the primate cerebellum. J Neurosci 24:4491–4497
Thach WT, Goodkin HP, Keating JG (1992) The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci 15:403–442
Tomlinson RD, Robinson DA (1984) Signals in vestibular nucleus mediating vertical eye movements in the monkey. J Neurophysiol 51:1121–1136
von Holst E, Mittelstaedt H (1950) Das reafferenzprinzip. Naturwissenschaften 37:464–476
Voogd J, Gerrits NM, Ruigrok TJ (1996) Organization of the vestibulocerebellum. Ann N Y Acad Sci 781:553–579
Wilson VJ (1991) Vestibulospinal and neck reflexes: interaction in the vestibular nuclei. Arch Ital Biol 129:43–52
Wilson VJ, Yamagata Y, Yates BJ, Schor RH, Nonaka S (1990) Response of vestibular neurons to head rotations in vertical planes. III. Response of vestibulocollic neurons to vestibular and neck stimulation. J Neurophysiol 64:1695–1703
Yamada J, Noda H (1987) Afferent and efferent connections of the oculomotor cerebellar vermis in the macaque monkey. J Comp Neurol 265:224–241
Acknowledgments
We thank Steve Nuara for assistance with animal care, and Walter Kucharski for excellent technical assistance, and D.E. Mitchell for critically reading this manuscript. This work was funded by Canadian Institutes of Health Research, Le Fonds québécois de la recherche sur la nature et les technologies, and the National Institute of Health (R01DC2390).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cullen, K.E., Brooks, J.X., Jamali, M. et al. Internal models of self-motion: computations that suppress vestibular reafference in early vestibular processing. Exp Brain Res 210, 377–388 (2011). https://doi.org/10.1007/s00221-011-2555-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-011-2555-9