Abstract
Neural control differs between position- and force-control tasks as evident from divergent effects of fatigue and pain. Unlike force-control tasks, position-control tasks focus on a postural goal to maintain a joint angle. Cortical involvement is suggested to be less during postural control, but whether this differs between position- and force-control paradigms remains unclear. Coherence estimates the functional communication between spatially distinct active regions within the cortex (cortico-cortical coherence; CCC) and between the cortex and muscles (corticomuscular coherence; CMC). We investigated whether cortical involvement differed between force-control and more posturally focused, position-control tasks. Seventeen adults performed position- and force-control knee extensor efforts at a submaximal load (10 % maximum voluntary contraction). Surface electromyography was recorded from the right knee extensor and flexor muscles and brain activity using electroencephalography (EEG). CCC and CMC in the beta (13–30 Hz) and gamma (30–45 Hz) frequency bands were calculated between combinations of intra- and inter-hemispheric pairs of electrodes, and between four EEG electrodes that approximated the left motor cortical area, and right knee extensor EMG, respectively. Differences in EEG power and muscle activity were also calculated. CCC was greater across distributed regions in the force-control task. Beta EEG power in the left hemisphere was higher for the position-control task. Although averaged CMC data differed between tasks, there was no task difference for individual CMC data. Muscle activity and force did not differ between tasks. The results demonstrate differential cortical contributions to control force- versus position-control tasks. This might contribute to differences in performance outcomes of these tasks that have been shown previously.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Motor control strategies differ between force- and position-control tasks (Maluf et al. 2005). Position-control tasks focus on maintaining joint angle in a freely moving joint while supporting an inertial load. Joint angle is fixed during force-control tasks, and the aim is to maintain an equivalent isometric force (Hunter et al. 2002). Although these tasks have similar mechanical requirements and equivalent net muscle torques and only differ in task objective, sustained position-control tasks consistently fatigue more quickly and have shorter time to task failure (Maluf et al. 2005; Hunter et al. 2008; Baudry et al. 2009; Rudroff et al. 2010, 2011). Yet, muscle activity in the position-control task is less affected by the influences of pain (Poortvliet et al. 2015). A combination of central [descending drive (Maluf and Enoka 2005)], and peripheral mechanisms [stretch reflex/muscle spindle sensitivity (Maluf and Enoka 2005)] as well as task-related differences in requirements to control position of adjacent segments (Rudroff et al. 2007; Poortvliet et al. 2013) is proposed to explain these differences.
From a functional perspective, it has been argued that a position-control task represents a more postural-type task, whereas a force-control task, a more volitional type (Hunter et al. 2002). To accommodate the difference in functional and strategic goals (Poortvliet et al. 2015), each type of control is likely to involve fundamental differences in involvement of networks of motor and/or sensory cortices. Although output from the motor cortex is necessary to instigate all voluntary motor actions (Salenius et al. 1997) and contribute to the control of both voluntary and postural-type movements (Gahéry and Nieoullon 1978), an array of subcortical and spinal mechanisms make major contributions to the latter class of motor actions (Jacobs and Horak 2007a; Maki and McIlroy 2007). Differences in cortical contributions to these two tasks could contribute to the differences in performance capacity shown by previous studies (e.g. fatigue and hold time). It is plausible that the position-control task, which is more akin to a postural-type function (Hunter et al. 2002), involves a lesser contribution of cortical input. Investigation of activation of the motor cortex and the relationship between activities of the motor cortex and agonist limb muscles performing the task provides an opportunity to probe this possibility.
Cortical involvement to a task can be estimated using coherence. Coherence estimation provides a measure of functional connectivity between spatially distinct sources of oscillatory neural activity (Andrew and Pfurtscheller 1996; Pfurtscheller and Andrew 1999) by the strength of the linear relation between activation of two sites (e.g. brain and/or muscle) in the frequency domain (Thatcher et al. 1986; Nunez 1995). Coherence between cortical (EEG) and muscle (EMG) activity (corticomuscular coherence, CMC) varies as a function of cortical involvement in a task. CMC in the beta (13–30 Hz) frequency band is more pronounced during visuomotor tasks such as maintenance of a steady state contraction with visual feedback (Baker et al. 1997; Kilner et al. 2000, 2003), but is low/absent during automatic postural functions [e.g. quiet standing tasks (Masakado et al. 2008)], whereas CMC in the gamma (30–45 Hz) band is more pronounced during dynamic (Kilner et al. 2000) and more intense contractions (Salenius et al. 1997).
Coherence between spatially distinct cortical regions (cortico-cortical coherence, CCC) in the beta and gamma frequency bands is increased with task complexity (Serrien et al. 2003); attentional focus (Gerloff et al. 1998; Manganotti et al. 1998; Serrien and Brown 2004; Serrien et al. 2004); sensory–motor processing (Classen et al. 1998; Aoki et al. 1999); cognitive performance (Weiss and Rappelsberger 2000); and learning (Miltner et al. 1999), but is lower during tasks that are performed more automatically (e.g. a highly trained activity) (Deeny et al. 2001, 2003). Investigation of CMC and CCC during force- and position-control tasks provides an opportunity to objectively quantify and compare cortical involvement between tasks. We hypothesized that the more posturally focused, position-control task would involve relatively less cortical involvement (quantified as coordinated activity between brain regions; CCC) and a weaker relationship between cortical and muscle activity (CMC). The aim of this study was to test this hypothesis using standardized position- and force-control knee extension tasks.
Methods
This study was designed to compare cortical activity between position- and force-control tasks. To this end, we used signal properties in the frequency domain to quantify the relationship between activity of cortex and muscle, and between cortex regions during steady state muscle contraction. A priori, we elected to study differences in the frequency domain characteristics of coherence and EEG spectral power during the maintenance of low intensity contractions that would be minimally affected by fatigue. As our interest was to explain differences between sustained efforts and not the preparation of other temporal events, we did not investigate the time domain or other features such as movement-related cortical potentials.
Participants
Seventeen healthy adults (33 ± 6 years, 14 men) participated in this study. Volunteers were excluded if they presented with a history of neurological disorders, history of knee pain/injury or had regularly used medication that could influence neurological function within the previous 6 months. Procedures were approved by the Institutional Medical Research Ethics Committee and conformed to the Declaration of Helsinki. Participants provided written informed consent before taking part in this study.
Electromyography
Surface EMG was recorded from three components of the right knee extensor quadriceps muscle [rectus femoris (RF), vastus lateralis (VL), and vastus medialis (VM)] and two knee flexor muscles [semitendinosus (ST) and biceps femoris (BF)] using bipolar surface electrodes (AgCl, 8-mm discs, 20 mm inter-electrode distance, Noraxon, USA). EMG data were pre-amplified 1000 times, bandpass-filtered (20 Hz to 1 kHz), and sampled at 2048 Hz using a TMS Portilab (Twente Medical Systems International B.V., the Netherlands).
Electroencephalography
High-density EEG was recorded with a sensor net consisting of 128 electrodes (Geodesic Sensor Net, Electrical Geodesics, Inc., USA) that covered the entire scalp and were referenced online to the vertex electrode. The net was connected to an AC-coupled, high-input amplifier (Netamp 300, Electrical Geodesics, Inc. USA). Sensor impedances were monitored using Netstation software (Electrical Geodesics, Inc. USA) with a threshold of 10 kΩ. Between every trial, impedances were monitored and adjusted when necessary. EEG was amplified and sampled at 1000 Hz and bandpass-filtered (3–70 Hz) using Netstation software. EEG data were re-referenced offline to the average of the mastoid electrodes (M1 and M2) and exported for analysis using MATLAB (the MathWorks, USA).
Experimental procedure
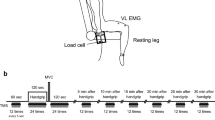
Data were collected in an electrically shielded room. Participants attended a single experimental session, during which they performed two tasks that involved a series of static contractions. All tasks were performed while lying comfortably in supine on a firm padded table. The right leg (test leg) was supported with the hip and knee flexed to ~90° by a padded bar (7 cm diameter) positioned behind the thigh (~15 cm proximal to the knee joint line). The left leg was extended at the hip and knee and rested on the table. Straps were secured around the hips and upper thigh of the test leg (Fig. 1) to minimize lateral movements during experimental tasks. Between trials, the lower leg was supported at the heel by an adjustable support sling with the knee resting at ~100° from full extension. Knee joint angle was measured with an electronic inclinometer (resolution 0.001°, Accustar, Schaevitz) attached ~10 cm distal to the lateral knee joint line (Fig. 1 circled detail). Knee extension force was measured using a strain gauge (model L2350, 300 lb, Futek, USA) attached between the lower leg (~30 cm from the lateral knee joint line) and the table via an adjustable cable (Fig. 1 main illustration).
Experimental set-up with the participant in supine and knee and hip flexed to 90°. A sensor net covered head to record brain activity, and muscle activity was recorded from bipolar electrodes placed over the knee extensor and flexor muscles. In the force-control task (main illustration), participants extended the knee to a matched target force, against a force gauge attached via an adjustable cable between the ankle and table. For the position-control task (circled detail), a load was attached to the ankle and the knee angle was unrestricted
Isometric maximal voluntary contractions (MVCs) into knee extension were performed at the start of the experimental session for quantification of MVC force and for normalization of knee extensor muscle EMG. Force was increased from rest to maximum over 3 s, maintained at maximum with strong verbal encouragement for 3 s, and return to rest. MVCs were repeated at least three times, separated by a recovery of at least 120 s, until the two highest forces differed by <5 %.
Two experimental tasks (force and position control) were performed in a mechanically identical manner, except with respect to the load type used. To minimize potential for complex variation in strategy between tasks and to maximize experimental control of all other elements of the task (e.g. body position, contraction intensity), the motor tasks in our study were isolated to a single joint. Previous studies have used target forces of up to 20 % MVC during sustained contractions until participants were unable to maintain target force/position. Optimal estimates of coherence between activity at the cortex and muscle, and between cortex regions require recordings of sufficient length to stabilize coherence values and avoid spurious coherence spectra (Mima and Hallett 1999). As coherence would be compromised by changes in muscle and/or brain activation related to fatigue, pain, or discomfort (which have all been shown to affect both tasks differently), we aimed to minimize these potential effects by selection of a submaximal resistance level equivalent to 10 % MVC in both tasks. In the position-control task [i.e. representing the control of orientation between segments, which is a critical element of postural control (Horak 2006)], participants maintained knee angle at 90° (feedback of knee joint angle) while supporting a free hanging load (10 % MVC, Fig. 1 circled detail). In the force-control task, participants generated an equivalent knee extension force (10 % MVC) against resistance of a cable attached to the table (Fig. 1 main illustration) with the knee angle fixed at 90° flexion. Task order was counter-balanced between participants. At rest, the test leg was supported at the heel with the knee at ~80° flexion using an adjustable support sling.
Participants completed six 30-s contractions for each task with similar instructions. On verbal cue from the investigator, participants gradually increased their knee angle to 90° over ~5 s, maintained the target (angle or force) for 30 s, and returned to rest over ~5 s, with 30 s rest between contractions. The adjustable cable between the ankle and the table was taut at 90° knee flexion in the force-control task, and the target of 10 % MVC was sustained isometrically at this angle. Participants were instructed to maintain the angle or force as close to the target as possible and were verbally reminded of this throughout all contractions. Feedback of target position and force was represented by a line on a standard 32.5-cm monitor fitted to the table ~1 m above the participant’s head (Fig. 1). As resolution and gain of feedback can influence task performance (Mottram et al. 2006; Hong and Newell 2008), feedback was set to 1°/cm for the position-control task and 1 % MVC/cm for the force-control task (Rudroff et al. 2010), with a 10-s time display. Participants were asked to refrain, where feasible, from eye blinks, jaw clenching, and movements of head, trunk, and arms during the contractions to minimize artefacts in the EEG recordings.
Force and angle were sampled at 2000 Hz using a Power1401 data acquisition system with Spike2 software (Cambridge Electronic Design, CED). Spike2 software generated digital trigger pulses (5 V peaks of 0.2 s length with pulse interval of 5 s) to synchronize the EMG and EEG data.
Data analysis
Signal pre-processing
Data were analysed offline using MATLAB in combination with NeuroSpec software for multivariate spectral analysis (www.neurospec.org; Halliday et al. 1995). Signal pre-processing involved several steps. EMG and force/angle data were down-sampled to 1000 Hz, and EEG and EMG signals were bandpass-filtered using a second-order Butterworth filter (cut-off frequencies: EEG and EMG 10–100 Hz) and a 50-Hz notch filter.
Strongest CMC has been shown during periods of steady-hold of muscle contractions as opposed to lower or no CMC during dynamic force productions (Baker et al. 1997; Riddle and Baker 2006; Kristeva et al. 2007). Furthermore, the changes that occur in the transition from rest to maintenance of target force or position are likely to introduce artefacts. Thus, analyses focused on 20-s epochs around the mid-region of each 30-s contraction, while the ramp phases at the start and end of each contraction were not considered for analysis. All 20-s epochs were concatenated into a continuous data set for each task (maximal length of 120 s of data from six contractions). A Tukey window (10 %) was applied to taper the start and end of each 20-s epoch such that the resulting signal had no high frequency components at the joining edges of the epochs. The continuous data were divided into successive 1024-ms windows. A discrete 4096-point Fourier transform of each successive segment allowed for a frequency resolution of 0.244 Hz in the coherence spectra.
To mitigate artefacts, EEG data were screened for high-amplitude events that exceeded a preset threshold of ±100 μV and when present, data were excluded from 0.5 s before to 1.5 s after the artefact peak. Pre-processing of the EEG and EMG signals yielded an average continuous data set length of 116 ± 14 s of artefact-free data.
Coherence analysis
Coherence analysis involved calculation of a discrete 4096-point Fourier transform for each successive segment of EEG and unrectified EMG data for the entire frequency range. A Hanning filter was used on each 1024-ms window to smooth the frequency spectrum. Power spectra were calculated as:
To limit inclusion of unrelated activity in the analysis (Serrien and Spapé 2009) and also reduce the data set to limit the chances of false positives, which increase with the number of comparisons, CCC was calculated between pairs of EEG signals recorded from 14 scalp locations evenly distributed over two hemispheres that approximated the locations of the superior parietal, sensorimotor, pre-motor and pre-frontal areas of the cortex, bilaterally [Fig. 2a; (Homan et al. 1987; Steinmetz et al. 1989)]. Analysis was performed on all possible combinations of electrode pairs (21 left, 21 right possible pairs within each hemisphere, and 49 pairs between the right and left hemispheres).
CMC was calculated between unrectified EMG signals from the three separate knee extensor muscles and EEG signals from four EEG electrodes that approximated the motor cortical area (left side) (Fig. 3). Preliminary pooled CMC analysis using all 128 electrodes averaged across participants revealed highest CMC in the area of the selected EEG electrodes (Fig. 3a, b, respectively). Although full-wave rectification of raw EMG signal has been used in some studies (Halliday et al. 1995; Myers et al. 2003; Halliday and Farmer 2010), it has been argued that rectification compromises identification of oscillatory features of EMG (Pinto Neto and Christou 2010; Bayraktaroglu et al. 2011; McClelland et al. 2012) and unrectified EMG was used here.
Contour plots of preliminary analysis of corticomuscular coherence averaged across all participants for all muscles (VM, VL, and RF) in the a force- and b position-control tasks. The four electrodes (black circles) approximate the sensorimotor cortical areas and include the region of greatest averaged coherence
The linear association between pairs of EEG recordings (CCC) and between knee extensor muscle EMG and EEG recordings (CMC) at the selected cortical electrode sites, in the range of 13 and 45 Hz (inclusive of the “beta” and “gamma” bands), was assessed as the coherence using the following equations (Halliday et al. 1995):
where
is the cross-spectrum between an EEG signal (C1) and either a second EEG signal (CCC) or an EMG signal (CMC) as C2 at frequency f. Furthermore, i is the segment number with a total of n and * denotes the complex conjugate. SP c1(f) and SP c2(f) are the power spectra for the two channels at the same frequency. This returns a real number between 0 (no coherence) and 1 (maximum coherence).
The confidence level (CL) was set at 95 %, calculated according to the recommendation of Rosenberg et al. (1989). Coherence was considered significantly different from zero if the resulting value was above the confidence level.
where α is the desired level of confidence (0.05) (Rosenberg et al. 1989). In this case, the CL was 0.01 for conditions with 120 data segments.
CCC was quantified as the area under the curve of the coherence data above the CL separately for data in the beta (13–30 Hz) and gamma (30–45 Hz) frequency bands. This was calculated for each electrode combination (left, right, and between hemispheres) (Fig. 2a).
CMC data were averaged for the four EEG electrodes, and the area under the curve of the coherence data above the CL was calculated separately for the beta (13–30 Hz) and gamma (30–45 Hz) frequency bands for each of the three knee extensor muscles. As an additional analysis, a grand ensemble average of CMC across all participants was calculated and the presence of peaks above the CL within the two frequency bands recorded. This analysis provides general information of features that were consistent across the group for each task (McClelland et al. 2012). The centres of gravity (CoG) of the peaks in the CMC spectra above the CL within each frequency band were calculated using the following equation:
where Coh i is the coherence of the ith significant bin (n) at frequency f i and CL is the 95 % confidence level.
EEG power for each of the 14 electrode sites was quantified as the area under the spectral power curve, separately for the beta and gamma frequency bands. The EEG power was transformed logarithmically to yield symmetric distributions using:
Amplitude of the myoelectric activity of the agonist (VM, VL, and RF) and antagonist muscles (ST and BF) was quantified as root-mean-square (RMS) EMG amplitude (0.1-s time constant) and normalized to MVCs. This was calculated for the 20-s segments of data used for the coherence analysis.
Statistical analysis
Statistica 9 (Statsoft, USA) was used for statistical analysis. A two-tailed, paired t test was used to compare the mean force (force-control task) and inertial weight (position-control task) between tasks. All coherence estimates were normalized using the arc hyperbolic tangent transformation to stabilize the variance for statistical analysis (Halliday et al. 1995). For cortico-cortical coherence analysis (CCC), three separate repeated measures ANOVAs were used, to compare between tasks (force vs. position), frequency bands (beta and gamma), and electrode pair combinations, separately for the analysis of the left, right, and intra-hemispheric electrode combinations. Separate repeated measures ANOVAs were used to compare CMC between tasks (force vs. position control), frequency bands (beta and gamma), and muscles (RF, VL, and VM); EEG power between tasks (force vs. position control), frequency bands (beta and gamma), and electrode (7 left and 7 right); and RMS EMG amplitudes between tasks (force vs. position control), and muscles (VM, VL, RF, ST, and BF). Statistical significance level was set at P < 0.05, and the Fisher’s least significant difference (LSD) was used for post hoc testing where required. Data are expressed as mean ± SD in text and figures.
Results
The mean force (force-control task: 46.3 ± 14.3 N) and inertial weight (position-control task: 46.2 ± 14.4 N) did not differ between tasks (t test: P = 0.99). Likewise, RMS EMG amplitude for each of the agonist and antagonist muscles did not differ between force- and position-control tasks (P = 0.84).
Corticomuscular coherence
The grand ensemble average of the coherence estimates of the cluster of four electrodes in the force- and position-control tasks for the three knee extensor muscles is shown in Fig. 4. Significant coherence during force-control task was identified in the beta (CoG at VM—18.4, VL—19.3, RF—25.3 Hz) and gamma (CoG at VM—42.0, VL—34.7, RF—35.6 Hz) bands. For the position-control task, significant coherence above the CL was only identified for the VL muscle in the gamma band (33.0 Hz).
Grand ensemble average corticomuscular coherence in the beta (13–30 Hz) and gamma (30–45 Hz) frequency bands for the force- (black line) and position-control (grey line) tasks. Data are shown for the three knee extensor muscles (VM, VL, and RF). Centres of gravity (CoG) of the significant peaks above the confidence levels (horizontal dotted lines) are highlighted by vertical lines
In contrast to the features of the ensemble-averaged CMC data, analysis of area under the curve for the peaks in CMC data above the CL for the 17 individual participants showed significant coherence for both tasks and no difference in CMC between the force- and position-control tasks (beta CMC, force: 2.36 ± 2.24, position: 2.15 ± 2.08; and gamma CMC, force: 1.66 ± 0.84, position: 1.69 ± 0.81; main effect—task; P = 0.27; interaction task × frequency band—P = 0.12).
Cortico-cortical coherence
CCC in the beta band between electrode pairs of the left hemisphere was greater for the force- (200.4 ± 119.9) than for the position-control task (190.3 ± 114.4; main effect—task; P < 0.001). There was no difference between tasks in the gamma band (force: 179.7 ± 117.4 and position: 174.1 ± 111.1; P = 0.106). Similar differences were observed in the right hemisphere (main effect—task; P < 0.001), except that greater beta band CCC for the force-control task (force: 213.4 ± 117.5 and position: 184.0 ± 110.3; P < 0.001) was now accompanied by greater gamma band CCC for the force- than for the position-control task (force: 187.7 ± 117.4 and position: 161.0 ± 108.8; P < 0.001). Likewise, CCC for inter-hemispheric electrode pairs was greater for force- than for position-control in both frequency bands (main effect—task: beta band—force: 133.6 ± 74.5 and position: 114.2 ± 68.4, P < 0.001; gamma—force: 134.1 ± 84.2 and position: 118.5 ± 73.4, P < 0.001).
EEG power spectra
Comparison of beta EEG power for the electrodes over the left hemisphere showed greater power in the position- (1.49 ± 0.08) than in the force-control task (1.48 ± 0.10; main effect—task; P < 0.05). Gamma band power was similar between tasks (force: 1.16 ± 0.12, position: 1.18 ± 0.07; main effect—task; P = 0.17). The EEG power for the electrodes over the right hemisphere showed no difference between tasks in the beta (force: 1.49 ± 0.08, position: 1.50 ± 0.08; main effect—task; P = 0.49) or the gamma (force: 1.16 ± 0.12; position: 1.17 ± 0.08; main effect—task; P = 0.15) band.
Discussion
This study aimed to test the hypothesis that cortical involvement in task performance and control would be relatively less in a position- than in a force-control task. This was tested by the investigation of the functional coupling between the cortex and the knee extensor muscles and between different regions of the cortex. Consistent with our hypothesis, the analysis of ensemble-averaged data of functional coupling between activity of the cortex and agonist muscles shows less coherence in the position-control task than in the force-control task. However, comparison of individual data showed no differences in CMC between tasks. Less functional coupling between regions of the cortex (within the left and right, and between hemispheres) was also demonstrated in the position-control than in the force-control task. Although the individual analysis of coherence between activity of the cortex and agonist muscles provided no evidence of a difference in communication between tasks, the CCC data imply differences in the underlying cortical network communication, which may contribute to the extensively shown differences in performance of the two tasks during fatigue and pain.
Comparison of corticomuscular coherence between tasks
Although analysis of the group ensemble-averaged data supported our hypothesis, analysis of CMC in the beta frequency band for individual participants did not differ between force- and position-control tasks. CMC is considered to reflect corticospinal drive to a muscle (Murthy and Fetz 1992; Baker et al. 1997; Mima and Hallett 1999; Perez et al. 2006). We hypothesized that CMC would be less in the position-control task on the premise that this task was more postural in nature. Our hypothesis was based on the argument that cortical neurons make a minor contribution to postural tasks (Slobounov et al. 2005; Jacobs and Horak 2007b; Maki and McIlroy 2007), which is reflected by an absence in CMC for leg muscles during quiet standing compared to voluntary tasks (Masakado and Nielsen 2008; Masakado et al. 2008). Greater relative contributions of other regions of the nervous system (Deliagina et al. 2007), including greater involvement of muscle spindle input to neural drive in position-control tasks (Mottram et al. 2005; Klass et al. 2008; Baudry and Enoka 2009; Rudroff et al. 2010), would be necessary to account for this difference. The contrary observation of similar CMC between position- and force-control tasks for individual analyses in the current investigation might provide support for the similarities in set-up and initial performance, as well as the equivalent physiological measures in the early phases of task performance. Differences between tasks commonly only become apparent in the later phases of the contractions when the tasks are sustained until total failure or, as we have shown previously, during experimental pain (Poortvliet et al. 2015). Thus, the similarity in CMC between tasks is reasonable, and differences in CMC might only become apparent following the onset of fatigue or when pain is induced, which requires different control strategies and adaptations in cortical involvement.
Another explanation for similarities in CMC is that coherence in the beta band is more prominent during tasks with high visuomotor demands such as tasks that follow a visual target [e.g. hold phases of static contractions with feedback (Baker et al. 1997; Kilner et al. 1999, 2000; Masakado and Nielsen 2008)]. The potential to generate identical force output in tasks with low visuomotor demand without significant CMC in the beta band (Masakado et al. 2008) implies that the primary drive to the muscles has less influence on CMC than refinement of the task using visuomotor cues. Thus, similarities in CMC between force- and position-control tasks might be explained by similar requirement to follow a visual target, despite potential differences in other sources of drive to the muscles.
Comparison of cortico-cortical coherence between tasks
In support of our first hypothesis, the results showed a task-dependent difference in CCC, which reflects communication or coupling between spatially distinct regions of the brain (Nunez 1995; Womelsdorf and Fries 2006). Although both tasks showed significant intra- and inter-hemispheric CCC in both frequency bands, the force-control task consistently involved greater CCC in the beta band than the position-control task, both within (left) and between hemispheres. A similar task effect was observed in the gamma band for most comparisons.
Beta band CCC is associated with greater cortical processing; it is increased when task-relevant information from different sources is processed simultaneously, for coordination of motor output, and is considered to reflect planning and execution of motor actions (Gerloff et al. 1998; Serrien and Brown 2002; Serrien et al. 2003). Beta band CCC between the motor and visual regions of the cortex is greater during visuomotor tasks (e.g. maintaining target force on a feedback screen) than performance of a motor task without visual feedback (Classen et al. 1998). This observation has led to the proposal that CCC may constitute an index of attentional focus. As both tasks in the present study involved attentional focus, this could arguably explain the presence of significant functional coupling in the beta band in both (albeit we did not include occipital electrodes, overlying visual cortical regions, in our analyses). Additional neural processing has been related to greater CCC in other studies (Serrien and Brown 2004; Serrien et al. 2004). The greater CCC in the force- than in the position-control task was consistent with our hypothesis of differences in the neural control of postural versus volitional motor acts, as it implies less cortical network involvement in the position-control task. This in turn implies that the contribution of subcortical (i.e. brainstem, cerebellum, spinal cord) mechanisms may be greater in this task.
Gamma band oscillations are thought to be important for cortico-cortical functional coupling (Singer 1993) and have been proposed to integrate information from individually active, widely distributed regions into a coherent cognitive process (Freeman 1975; Singer and Gray 1995; Engel et al. 2001). Compared to alpha and beta oscillatory activity, the faster gamma oscillations are involved in establishment of faster synchrony between spatially distant sources (Singer 1993). Gamma band coherence effects have been observed during tasks that involve elements of somatosensory discrimination (Sauve 1999), working memory (Tallon-Baudry et al. 1998), increased attention (Jensen et al. 2007), and sensory–motor processing (Aoki et al. 1999). CCC in the gamma band has been shown to relate to the speed of the behavioural response of monkeys to a relevant visual stimulus, indicating that the processing of information is more efficient when spatially distinct regions display greater gamma coherence (Womelsdorf and Fries 2006). In the present study, gamma band CCC in the left hemisphere was not different between tasks and this could be due to the similarities of the visuomotor aspect of both tasks. As feedback gain is known to influence task performance (Mottram et al. 2006; Hong and Newell 2008), we took great care to match feedback as closely as possible between tasks. As a result of the relationship between integrative function and gamma band coherence, this may explain the similar CCC between tasks for the left hemisphere. Unlike the left hemisphere, gamma band coherence was greater in the right hemisphere for the force- than for the position-control task. It is not uncommon to observe greater coherence in the right (ipsilateral) than in the left hemisphere among right-handed participants (Thatcher and Walker 1985; Tucker et al. 1986), as the hemispheres are asymmetrical in both anatomy and function (Stephan et al. 2007). Traditionally, the left hemisphere is considered the dominant hemisphere, associated with skilled movement and language, and the right hemisphere with visuospatial and attentional functions (Serrien et al. 2006). Anatomically, the right hemisphere consists of more diffuse neural representations that are well organized to process more global task features as well as integrate information across many different concurrent processes (Semmes 1968). A more recent view is that functional involvement of each hemisphere is flexible and dynamically driven by both task-related (type and complexity) and performer-related (skill level and attention) factors (Hellige 1990; Banich 1998; Serrien et al. 2006). More complex tasks that depend on attention and executive control and integration of different sources of information, involve a more distributed and bilateral network compared to simple tasks reliant on local neural networks. Integration over a more extensive neural network is dependent on speed of transfer and processing, which could in turn explain our observation of greater gamma band CCC. Although the exact reason remains elusive, the combined findings of greater gamma CCC in the right hemisphere for the force-control task in our study are in line with the conclusions of Serrien et al. (2006) who propose that successful task performance depends on functional hemispheric preferences that are supported by the engagement of multiple distributed neural areas.
Differences in CCC are not always accompanied by differences in EEG power (Thatcher et al. 1986). Although the present study found differences in beta and gamma band CCC in the left and right hemispheres between tasks, no difference in EEG power was found in the right hemisphere. Further, the gamma band power in the left hemisphere was also equivalent between tasks. Interestingly, beta band EEG power in the left (contralateral to movement) hemisphere was greater in the position- than in the force-control task. This task-dependent difference in EEG power could reflect a difference in active state of the cortex as EEG power is inversely correlated with the frequency of EEG activity (i.e. increased frequency results in decreased power) (Pfurtscheller and Andrew 1999). Compared to the position-control task, beta EEG power in the force-control task showed greater desynchronization of activity, which can be interpreted to indicate a more activated state of the cortex (Pfurtscheller and Lopes da Silva 1999) and supports our hypothesis that the force-control task requires greater contribution from cortical activity.
Conclusion
A plausible interpretation of the significant and similar CMC in the position- and force-control tasks implies involvement of the cortex during the postural-type position-control task and similar communication between the cortex and the muscles between contraction types. However, the greater involvement and communication between different regions of the brain in a force-control task than in a posturally focused position-control implies differences in involvement of cortex, which might contribute to the divergent adaptations of these tasks when challenged by fatigue and pain.
References
Andrew C, Pfurtscheller G (1996) Event-related coherence as a tool for studying dynamic interaction of brain regions. Electroencephalogr Clin Neurophysiol 98:144–148
Aoki F, Fetz EE, Shupe L, Lettich E, Ojemann GA (1999) Increased gamma-range activity in human sensorimotor cortex during performance of visuomotor tasks. Clin Neurophysiol 110:524–537. doi:10.1016/s1388-2457(98)00064-9
Baker SN, Olivier E, Lemon RN (1997) Coherent oscillations in monkey motor cortex and hand muscle EMG show task-dependent modulation. J Physiol (Lond) 501:225–241. doi:10.1111/j.1469-7793.1997.225bo.x
Banich MT (1998) The missing link: the role of interhemispheric interaction in attentional processing. Brain Cogn 36:128–157. doi:10.1006/brcg.1997.0950
Baudry S, Enoka RM (2009) Influence of load type on presynaptic modulation of Ia afferent input onto two synergist muscles. Exp Brain Res 199:83–88. doi:10.1007/s00221-009-1951-x
Baudry S, Rudroff T, Pierpoint LA, Enoka RM (2009) Load type influences motor unit recruitment in biceps brachii during a sustained contraction. J Neurophysiol 102:1725–1735. doi:10.1152/jn.00382.2009
Bayraktaroglu Z, von Carlowitz-Ghori K, Losch F, Nolte G, Curio G, Nikulin VV (2011) Optimal imaging of cortico-muscular coherence through a novel regression technique based on multi-channel EEG and un-rectified EMG. Neuroimage 57:1059–1067. doi:10.1016/j.neuroimage.2011.04.071
Classen J, Gerloff C, Honda M, Hallett M (1998) Integrative visuomotor behavior is associated with interregionally coherent oscillations in the human brain. J Neurophysiol 79:1567–1573
Deeny S, Hillman C, Janelle C, Hatfield B (2001) EEG coherence and psychomotor efficiency in expert and nonexpert marksmen. Psychophysiology 38:S35–S35
Deeny SP, Hillman CH, Janelle CM, Hatfield BD (2003) Cortico-cortical communication and superior performance in skilled marksmen: an EEG coherence analysis. J Sport Exerc Psychol 25:188–204
Deliagina TG, Zelenin PV, Beloozerova IN, Orlovsky GN (2007) Nervous mechanisms controlling body posture. Physiol Behav 92:148–154. doi:10.1016/j.physbeh.2007.05.023
Engel AK, Fries P, Singer W (2001) Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci 2:704–716. doi:10.1038/35094565
Freeman WJ (1975) Parallel processing of signals in neural sets as manifested in EEG. Int J Man Mach Stud 7:347–369. doi:10.1016/s0020-7373(75)80017-4
Gahéry Y, Nieoullon A (1978) Postural and kinetic coordination following cortical stimuli which induce flexion movements in the cat’s limbs. Brain Res 149:25–37. doi:10.1016/0006-8993(78)90585-1
Gerloff C, Richard J, Hadley J, Schulman AE, Honda M, Hallett M (1998) Functional coupling and regional activation of human cortical motor areas during simple, internally paced and externally paced finger movements. Brain 121:1513–1531. doi:10.1093/brain/121.8.1513
Halliday DM, Farmer SF (2010) On the need for rectification of surface EMG. J Neurophysiol 103:3547. doi:10.1152/jn.00222.2010
Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF (1995) A framework for the analysis of mixed time series/point process data—theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol 64:237–278. doi:10.1016/s0079-6107(96)00009-0
Hellige JB (1990) Hemispheric-asymmetry. Annu Rev Psychol 41:55–80. doi:10.1146/annurev.psych.41.1.55
Homan RW, Herman J, Purdy P (1987) Cerebral location of international 10–20 system electrode placement. Electroencephalogr Clin Neurophysiol 66:376–382. doi:10.1016/0013-4694(87)90206-9
Hong SL, Newell KM (2008) Visual information gain and the regulation of constant force levels. Exp Brain Res 189:61–69. doi:10.1007/s00221-008-1403-z
Horak FB (2006) Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing 35:7–11. doi:10.1093/ageing/afl077
Hunter SK, Ryan DL, Ortega JD, Enoka RM (2002) Task differences with the same load torque alter the endurance time of submaximal fatiguing contractions in humans. J Neurophysiol 88:3087–3096. doi:10.1152/jn.00232.2002
Hunter SK, Yoon TJ, Farinella J, Griffith EE, Ng AV (2008) Time to task failure and muscle activation vary with load type for a submaximal fatiguing contraction with the lower leg. J Appl Physiol 105:463–472. doi:10.1152/japplphysiol.90398.2008
Jacobs JV, Horak FB (2007a) Cortical control of postural responses. J Neural Transm 114:1339–1348. doi:10.1007/s00702-007-0657-0
Jacobs JV, Horak FB (2007b) External postural perturbations induce multiple anticipatory postural adjustments when subjects cannot pre-select their stepping foot. Exp Brain Res 179:29–42. doi:10.1007/s00221-006-0763-5
Jensen O, Kaiser J, Lachaux J-P (2007) Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci 30:317–324. doi:10.1016/j.tins.2007.05.001
Kilner JM, Baker SN, Salenius S, Jousmaki V, Hari R, Lemon RN (1999) Task-dependent modulation of 15–30 Hz coherence between rectified EMGs from human hand and forearm muscles. J Physiol (Lond) 516:559–570. doi:10.1111/j.1469-7793.1999.0559v.x
Kilner JM, Baker SN, Salenius S, Hari R, Lemon RN (2000) Human cortical muscle coherence is directly related to specific motor parameters. J Neurosci 20:8838–8845
Kilner JM, Salenius S, Baker SN, Jackson A, Hari R, Lemon RN (2003) Task-dependent modulations of cortical oscillatory activity in human subjects during a bimanual precision grip task. Neuroimage 18:67–73. doi:10.1006/nimg.2002.1322
Klass M, Levenez M, Enoka RM, Duchateau J (2008) Spinal mechanisms contribute to differences in the time to failure of submaximal fatiguing contractions performed with different loads. J Neurophysiol 99:1096–1104. doi:10.1152/jn.01252.2007
Kristeva R, Patino L, Omlor W (2007) Beta-range cortical motor spectral power and corticomuscular coherence as a mechanism for effective corticospinal interaction during steady-state motor output. Neuroimage 36:785–792. doi:10.1016/j.neuroimage.2007.03.025
Maki BE, McIlroy WE (2007) Cognitive demands and cortical control of human balance-recovery reactions. J Neural Transm 114:1279–1296. doi:10.1007/s00702-007-0764-y
Maluf KS, Enoka RM (2005) Task failure during fatiguing contractions performed by humans. J Appl Physiol 99:389–396. doi:10.1152/japplphysiol.00207.2005
Maluf KS, Shinohara M, Stephenson JL, Enoka RM (2005) Muscle activation and time to task failure differ with load type and contraction intensity for a human hand muscle. Exp Brain Res 167:165–177. doi:10.1007/s00221-005-0017-y
Manganotti P, Gerloff C, Toro C et al (1998) Task-related coherence and task-related spectral power changes during sequential finger movements. Electroencephalogr Clin Neurophysiol 109:50–62. doi:10.1016/s0924-980x(97)00074-x
Masakado Y, Nielsen JB (2008) Task-and phase-related changes in cortico-muscular coherence. Keio J Med 57:50–56
Masakado Y, Ushiba J, Tsutsumi N, Takahashi Y, Tomita Y, Kimura A, Liu M (2008) EEG-EMG coherence changes in postural tasks. Electromyogr Clin Neurophysiol 48:27–33
McClelland VM, Cvetkovic Z, Mills KR (2012) Modulation of corticomuscular coherence by peripheral stimuli. Exp Brain Res 219:275–292. doi:10.1007/s00221-012-3087-7
Miltner WHR, Braun C, Arnold M, Witte H, Taub E (1999) Coherence of gamma-band EEG activity as a basis for associative learning. Nature 397:434–436. doi:10.1038/17126
Mima T, Hallett M (1999) Electroencephalographic analysis of cortico-muscular coherence: reference effect, volume conduction and generator mechanism. Clin Neurophysiol 110:1892–1899. doi:10.1016/s1388-2457(99)00238-2
Mottram CJ, Jakobi JM, Semmler JG, Enoka RM (2005) Motor-unit activity differs with load type during a fatiguing contraction. J Neurophysiol 93:1381–1392. doi:10.1152/jn.00837.2004
Mottram CJ, Hunter SK, Rochette L, Anderson MK, Enoka RM (2006) Time to task failure varies with the gain of the feedback signal for women, but not for men. Exp Brain Res 174:575–587. doi:10.1007/s00221-006-0498-3
Murthy VN, Fetz EE (1992) Coherent 25-Hz to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci USA 89:5670–5674. doi:10.1073/pnas.89.12.5670
Myers LJ, Lowery M, O’Malley M et al (2003) Rectification and non-linear pre-processing of EMG signals for cortico-muscular analysis. J Neurosci Methods 124:157–165. doi:10.1016/s0165-0270(03)00004-9
Nunez PL (eds) (1995) Mind, brain, and electroencephalography. In: Neocortical dynamics and human EEG rhythms. Oxford University Press, pp 133–194
Perez MA, Lundbye-Jensen J, Nielsen JB (2006) Changes in corticospinal drive to spinal motoneurones following visuo-motor skill learning in humans. J Physiol (Lond) 573:843–855. doi:10.1113/jphysiol.2006.105361
Pfurtscheller G, Andrew C (1999) Event-related changes of band power and coherence: methodology and interpretation. J Clin Neurophysiol 16:512–519. doi:10.1097/00004691-199911000-00003
Pfurtscheller G, Lopes da Silva FH (eds) (1999) Functional meaning of event-related desynchronization (ERD) and synchronization (ERS). In: Handbook of electroencephalography and clinical neurophysiology, vol 6. Elsevier, Amsterdam, pp 51–65
Pinto Neto O, Christou EA (2010) Rectification of the EMG signal impairs the identification of oscillatory input to the muscle. J Neurophysiol 103:1093–1103. doi:10.1152/jn.00792.2009
Poortvliet PC, Tucker KJ, Hodges PW (2013) Changes in constraint of proximal segments effects time to task failure and activity of proximal muscles in knee position-control tasks. Clin Neurophysiol 124:732–739. doi:10.1016/j.clinph.2012.09.025
Poortvliet PC, Tucker KT, Hodges PW (2015) Experimental pain has a greater effect on single motor unit discharge during force-control than position-control tasks. Clin Neurophysiol 126:1378–1386. doi:10.1016/j.clinph.2014.10.139
Riddle CN, Baker SN (2006) Digit displacement, not object compliance, underlies task dependent modulations in human corticomuscular coherence. Neuroimage 33:618–627. doi:10.1016/j.neuroimage.2006.07.027
Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM (1989) The fourier approach to the identification of functional coupling between neuronal spike trains. Prog Biophys Mol Biol 53:1–31. doi:10.1016/0079-6107(89)90004-7
Rudroff T, Barry BK, Stone AL, Barry CJ, Enoka RM (2007) Accessory muscle activity contributes to the variation in time to task failure for different arm postures and loads. J Appl Physiol 102:1000–1006. doi:10.1152/japplphysiol.00564.2006
Rudroff T, Justice JN, Matthews S, Zuo R, Enoka RM (2010) Muscle activity differs with load compliance during fatiguing contractions with the knee extensor muscles. Exp Brain Res 203:307–316. doi:10.1007/s00221-010-2233-3
Rudroff T, Justice JN, Holmes MR, Matthews SD, Enoka RM (2011) Muscle activity and time to task failure differ with load compliance and target force for elbow flexor muscles. J Appl Physiol 110:125–136. doi:10.1152/japplphysiol.00605.2010
Salenius S, Portin K, Kajola M, Salmelin R, Hari R (1997) Cortical control of human motoneuron firing during isometric contraction. J Neurophysiol 77:3401–3405
Sauve K (1999) Gamma-band synchronous oscillations: Recent evidence regarding their functional significance. Conscious Cogn 8:213–224. doi:10.1006/ccog.1999.0383
Semmes J (1968) Hemispheric specialization: a possible clue to mechanism. Neuropsychologia 6:11–26
Serrien DJ, Brown P (2002) The functional role of interhemispheric synchronization in the control of bimanual timing tasks. Exp Brain Res 147:268–272. doi:10.1007/s00221-002-1253-z
Serrien DJ, Brown P (2004) Changes in functional coupling patterns during bimanual task performance. NeuroReport 15:1387–1390. doi:10.1097/01.wnr.0000131009.44068.51
Serrien DJ, Spapé MM (2009) The role of hand dominance and sensorimotor congruence in voluntary movement. Exp Brain Res 199:195–200. doi:10.1007/s00221-009-1998-8
Serrien DJ, Cassidy MJ, Brown P (2003) The importance of the dominant hemisphere in the organization of bimanual movements. Hum Brain Mapp 18:296–305. doi:10.1002/hbm.10086
Serrien DJ, Pogosyan AH, Brown P (2004) Cortico-cortical coupling patterns during dual task performance. Exp Brain Res 157:79–84. doi:10.1007/s00221-003-1822-9
Serrien DJ, Ivry RB, Swinnen SP (2006) Dynamics of hemispheric specialization and integration in the context of motor control. Nat Rev Neurosci 7:160–166. doi:10.1038/nrn1849
Singer W (1993) Synchronization of cortical activity and its putative role in information processing and learning. Annu Rev Physiol 55:349–374 doi:10.1146/annurev.ph.55.030193.002025
Singer W, Gray CM (1995) Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci 18:555–586. doi:10.1146/annurev.neuro.18.1.555
Slobounov S, Hallett M, Stanhope S, Shibasaki H (2005) Role of cerebral cortex in human postural control: an EEG study. Clin Neurophysiol 116:315–323. doi:10.1016/j.clinph.2004.09.007
Steinmetz H, Furst G, Meyer BU (1989) Craniocerebral topography within the international 10–20 system. Electroencephalogr Clin Neurophysiol 72:499–506. doi:10.1016/0013-4694(89)90227-7
Stephan KE, Fink GR, Marshall JC (2007) Mechanisms of hemispheric specialization: insights from analyses of connectivity. Neuropsychologia 45:209–228. doi:10.1016/j.neuropsychologia.2006.07.002
Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J (1998) Induced gamma-band activity during the delay of a visual short-term memory task in humans. J Neurosci 18:4244–4254
Thatcher RW, Walker RA (1985) EEG coherence and intelligence in children. Electroencephalogr Clin Neurophysiol 61:S161–S161. doi:10.1016/0013-4694(85)90621-2
Thatcher RW, Krause PJ, Hrybyk M (1986) Corticocortical associations and eeg coherence: a 2-compartmental model. Electroencephalogr Clin Neurophysiol 64:123–143. doi:10.1016/0013-4694(86)90107-0
Tucker DM, Roth DL, Bair TB (1986) Functional connections among cortical regions: topography of eeg coherence. Electroencephalogr Clin Neurophysiol 63:242–250. doi:10.1016/0013-4694(86)90092-1
Weiss S, Rappelsberger P (2000) Long-range EEG synchronization during word encoding correlates with successful memory performance. Cogn Brain Res 9:299–312
Womelsdorf T, Fries P (2006) Neuronal coherence during selective attentional processing and sensory-motor integration. J Physiol (Paris) 100:182–193. doi:10.1016/j.jphysparis.2007.01.005
Acknowledgments
Financial support was provided by the National Health and Medical Research Council of Australia (Research Fellowship [PH] ID401599 [KT] ID1009410; Project Grant—ID 569744). There was no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poortvliet, P.C., Tucker, K.J., Finnigan, S. et al. Cortical activity differs between position- and force-control knee extension tasks. Exp Brain Res 233, 3447–3457 (2015). https://doi.org/10.1007/s00221-015-4404-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-015-4404-8