Abstract
Purpose
Repetitive or sustained simple muscle contractions have been shown to alter corticomotor excitability. The present study investigated the effects of a sustained handgrip contraction with the right hand on motor-evoked potentials (MEPs) in task-unrelated knee extensor muscles and determined whether the effects are influenced by intensity of the handgrip contraction.
Methods
Subjects performed a 120-s sustained handgrip contraction at 10% or 50% maximal voluntary contraction (MVC) using the right hand. MEPs in vastus lateral (VL) muscles elicited by transcranial magnetic stimulation were measured before, during, and after the handgrip contraction.
Results
Both the handgrip contractions at 10 and 50% MVC induced significant greater MEPs in the left VL muscle (121.5 ± 25.7%) than in the right VL muscle (97.9 ± 17.4%) from 10 min after the handgrip contraction (P < 0.05). MEPs in both the right and left VL muscles were significantly increased by the handgrip contractions at 10% MVC (124.8 ± 45.2%, P < 0.05), but were not increased by the handgrip contractions at 50% MVC.
Conclusion
The results of the present study indicate that a unilateral sustained handgrip contraction can differentially alter corticomotor excitability in knee extensor muscles ipsilateral and contralateral to the exercised hand after the handgrip and that the intensity of the handgrip contraction influences corticomotor excitability in both knee extensor muscles after the handgrip.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In our daily lives and during sport activities, human movements gradually adapt to varying situations, environments, and task requirements. These adaptations are associated with plastic changes in neural circuits for motor control. These circuits include descending pathways from the primary motor cortex (M1) to muscle fibres (i.e. corticomotor pathways). Indeed, skill learning of rapid finger movements (Rosenkranz et al. 2007), locomotor training in people with spinal cord injury (Knikou 2012), and motor skill training involving ankle muscles (Perez et al. 2004) have altered the resting corticomotor excitability measured in muscles involved in the task or training. In many studies, corticomotor excitability has been assessed by motor-evoked potentials (MEPs) using transcranial magnetic stimulation (TMS) to the M1 (Di Lazzaro et al. 2004).
Not only skill training or learning of motor tasks but also repetitive or sustained simple muscle contractions can alter corticomotor excitability following the contractions (i.e. post-contraction MEPs). For example, sustained or repetitive muscle contractions have been demonstrated to alter post-contraction resting MEPs measured in task-related muscles (Brasil-Neto et al. 1993; Liepert et al. 1996; Maruyama et al. 2006; Zanette et al. 1995), and sustained or intermittent unilateral contractions of upper limb muscles influence post-contraction resting or active MEPs in the contralateral homologous muscle (Aboodarda et al. 2016; Bonato et al. 1996; Takahashi et al. 2009). Furthermore, sustained or intermittent unilateral or bilateral lower limb muscle contractions have been shown to alter post-contraction resting MEPs measured in the upper limb muscles not involved in the contractions (i.e. task-unrelated muscles) (Aboodarda et al. 2017; Matsuura and Ogata 2015; Šambaher et al. 2016; Takahashi et al. 2011). Thus, extensive evidence suggests that voluntary muscle contractions influence post-contraction resting corticomotor excitability of not only task-related muscles but also task-unrelated muscles. Nevertheless, the neural mechanisms responsible for the alterations of resting MEPs after voluntary muscle contractions (i.e. the changes in post-contraction resting MEPs) remain unclear for task-related and task-unrelated muscles.
For task-related muscles, it is thought that the degree of change in post-contraction resting MEPs is contingent on force levels and the type of muscle contraction. Miyaguchi et al. (2016) found that non-fatiguing high-intensity or isotonic contractions strongly depressed post-contraction resting MEPs in the task-related muscles compared to low-intensity or isometric contractions, respectively. They speculated that greater M1 activities during high-intensity or isotonic muscle contractions were responsible for the greater degree of decrease in post-contraction resting MEPs, since the levels of activity in the sensorimotor cortex area have been shown to scale with the intensity of finger flexion (Thickbroom et al. 1998) or index finger abduction (van Duinen et al. 2008) in functional magnetic resonance imaging studies. Further, isotonic contractions have been reported to enhance corticomotor excitability measured in the task-related muscle compared to isometric contractions in TMS studies (Saito et al. 2014; Yahagi et al. 2003). Although further studies are required to elucidate whether the depression of post-contraction resting MEPs in task-related muscles varies in function of activities only of the M1 during muscle contractions, it is likely that the degree of facilitations of MEPs in task-related muscles during muscle contractions determines the changes in post-contraction resting MEPs in task-related muscles.
For task-unrelated muscles, relationships between post-contraction resting MEPs and facilitation of MEPs during muscle contractions have never been determined. When upper or lower limb muscles are contracted, the MEPs of lower or upper limb muscles have been demonstrated to increase, respectively (Borroni et al. 2004; Péréon et al. 1995; Shironouchi et al. 2019; Tazoe et al. 2009). This suggests that the muscle contractions of the upper or lower limb facilitate the corticomotor excitability of lower or upper limb muscles alongside the contractions, respectively. If changes in post-contraction resting MEPs in task-unrelated muscles are associated with the degree of facilitation of corticomotor excitability in task-unrelated muscles alongside the muscle contractions, upper or lower limb muscle contractions can alter post-contraction resting MEPs in task-unrelated lower or upper limb muscles, respectively. However, although lower limb muscle contractions have been shown to change post-contraction resting MEPs of task-unrelated upper limb muscles (Aboodarda et al. 2017; Matsuura and Ogata 2015; Šambaher et al. 2016; Takahashi et al. 2011), the effects of upper limb muscle contractions on post-contraction resting MEPs in task-unrelated lower limb muscles have not been addressed. We hypothesised that upper limb muscle contractions alter post-contraction resting MEPs in task-unrelated lower limb muscles. Additionally, since it has been reported that the degree of facilitation of the MEPs in task-unrelated muscles alongside the muscle contractions was contingent on the intensity of the contractions (Tazoe et al. 2009), the degree of changes in post-contraction resting MEPs in task-unrelated lower limb muscles may be influenced by the intensity of upper limb muscle contractions as reported in the available literature for exercised muscles (Miyaguchi et al. 2016).
The aims of the present study were to investigate the effects of unilateral sustained handgrip contraction on MEPs in task-unrelated knee extensor muscles and to determine whether the effects may be influenced by the intensity of the handgrip contraction.
Methods
Subjects
Nine healthy subjects [male 8; female 1; mean ± standard deviation (SD): age 26.3 ± 5.6 years; height 174.6 ± 10.1 cm; weight 71.0 ± 15.8 kg] were recruited for the present study. We conducted a power analysis using PANGEA version 0.2 (Westfall 2016). As we were mostly interested if post-contraction resting, MEPs are affected by intensity of handgrip and lower limb sides, we calculated the sample size required for a three-way analysis of variance (ANOVA) with repeated measures for intensity (two levels; low intensity vs. high intensity), side (two levels; right vs. left), and time (seven levels; before the hand contraction, 5, 10, 15, 20, 25, and 30 min after the hand contractions) as fixed factors and the participants as the random factor. Taking discomfort by TMS over lower limb M1 area into account, we determined the minimal sample size needed to detect three-way interactions with a large effect size (0.4; Cohen 1992) and power of 0.8 (n = 9; statistical power = 0.817).
All participants were right handed and right footed, based on self-reports. All subjects gave written informed consent to participate in the study, and the experimental procedures were carried out in accordance with the Declaration of Helsinki. The Ethics Committee of the Joetsu University of Education approved the study (approval number 26-41). The subjects were requested to abstain from strenuous physical activity, drinking alcohol, and taking caffeine for 24 h prior to each experimental session. The subjects were informed of the experimental procedures but were kept unaware of the precise experimental hypotheses.
Experimental setup
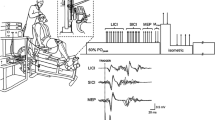
Subjects were comfortably seated in a custom-built chair with a backrest board equipped with two padded stoppers to maintain the position of the upper body and head with the knee and hip angles positioned at 90° and 110°, respectively. The seat height was set such that the subject’s feet were in the air to prevent the knee extensors from exerting any force on the floor during the handgrip contraction. The subject’s elbow was fully extended. The custom-built handgrip device was grasped by the right hand (Fig. 1a). The width of the handgrip was adjusted to each subject’s hand size so that the proximal interphalangeal joints of the four fingers rested on one side of the handgrip and that of the thumb rested on the other side. The force of a handgrip was measured by a load cell (LC1205-K100; A&D, Tokyo, Japan) attached to the device on the side where the four fingers rested. Signals from the load cell were amplified, digitised at a sampling rate of 2 kHz, low-pass filtered (40 Hz), and then displayed on a PC monitor facing the subjects. A red target line corresponding to the required force was created and displayed on the PC monitor so that subjects could produce the required handgrip force.
Experimental setup and protocol. a Schematic illustration of the experimental setup. Subjects were instructed to perform a sustained unilateral isometric handgrip contraction as an intervention. The custom-built handgrip device was placed in the right hand. Subjects were asked to keep the non-exercising leg at rest. Transcranial magnetic stimulation (TMS) was applied to the vertex to elicit motor-evoked potentials (MEPs) in the right or left vastus lateralis (VL) muscles. b Protocol for assessing MEPs in the VL muscles depending on the level of intensity of the handgrip contractions. The subject was asked to perform 120-s sustained handgrip contractions at 10% (LOW sessions) or 50% (HIGH sessions) of the maximal voluntary contraction (MVC). TMS to elicit MEPs in the right (IPSI sessions) or left (CONTRA sessions) VL muscles was applied before (PRE trial, 12 times), during (HAND trial, 24 times), and after (RECOVERY trial, 24 times; each POST trial, 12 times) the handgrip contractions
Electromyography recordings
Electromyographic (EMG) activity was recorded bilaterally from the vastus lateralis (VL) through surface electrodes (Ag/AgCl; 10-mm diameter) secured to the skin using the belly-tendon montage. The ground electrode was placed at the right malleolus lateralis. EMG signals were amplified (gain × 1000), filtered (bandwidth 15–1000 Hz) with a bioamplifier (AB-611J; Nihon Kohden, Tokyo, Japan), and then stored on a computer with a sampling rate of 2 kHz using an analog-to-digital converter (PowerLab 8/30; AD Instruments, Bella Vista, NSW, Australia) for later off-line analysis (LabChart version 8.1.3 for Windows; AD Instruments). The raw signals were displayed on the PC monitor so that subjects could maintain EMG activities in the VL silent throughout the experiments.
Experimental design
Subjects reported to the laboratory on four separate sessions at the same time of the day. In each session, subjects received TMS before, during, and after a 120-s sustained isometric handgrip contraction with the right hand. The four separate sessions consisted of two sessions in which MEPs were measured in the right VL (IPSI sessions) and two sessions in which MEPs were measured in the left VL (CONTRA sessions). In the IPSI and CONTRA sessions, the subjects performed a 120-s sustained handgrip contraction with either 10% or 50% (LOW and HIGH sessions, respectively) of the handgrip maximal voluntary contraction (MVC). Thus, each subject underwent the following four sessions: LOW-IPSI, LOW-CONTRA, HIGH-IPSI, and HIGH-CONTRA (Fig. 1b). The order of sessions was randomly assigned. Each session was separated by at least 1 week.
TMS
TMS was delivered from a magnetic stimulator (Magstim 2002; Magstim Co., Whitland, UK) with a monophasic current waveform. A double cone coil (each 110 mm in diameter) was centred over the vertex. The TMS-induced current in the cortex was set to flow from posterior to anterior and was assumed to produce D and I wave activations of corticospinal neurons (Di Lazzaro et al. 2004). The coil position was adjusted to elicit the largest MEPs in the right or left VL at rest for each subject using a suprathreshold intensity (50–80% of maximum stimulator output), and the adjusted position was defined as the optimal position (i.e. hot spot). The optimal position was marked on a tight-fitting swimming cap that covered the subject’s head to ensure constant positioning of the coil throughout the experiment. The experimenter held the TMS coil to the subject’s head. The TMS measurements included the resting motor threshold (RMT, only before the sustained handgrip) and MEPs in the resting right or left VL. RMT was defined as the minimal stimulus intensity required to induce MEPs > 50 µV peak-to-peak amplitude in 5 of 10 consecutive trials in the resting right or left VL.
Before the sustained unilateral handgrip contractions (PRE trial), TMS was delivered 12 times with stimulus intervals of approximately 5 s. The stimulus intensity (SI) was determined as the peak-to-peak amplitude of 0.3–0.5 mV and the SI was maintained constant throughout the experiment. During the sustained unilateral handgrip contractions (HAND trial), each subject received 24 stimulations with stimulus intervals of approximately 5 s. Immediately after the end of the hand contraction (< 10 s), TMS was delivered 24 times with stimulus intervals of approximately 5 s for 120 s (RECOVERY trial). Thereafter, 12 MEPs with intervals of approximately 5 s were measured 5, 10, 15, 20, 25, and 30 min after the hand contractions (POST trials). Although repetitive TMS has been shown to change resting MEPs, resting MEPs were not changed by the 0.2 or 0.3 Hz TMS used in the present study (Cincotta et al. 2003; Murase et al. 2005). Additionally, the number of stimuli (12 or 24 times) used in the present study was much less than that (100–900 times) used by repetitive TMS (Fitzgerald et al. 2006).
The size of the MEPs was defined as the peak-to-peak amplitude. For analysis of data obtained during the PRE trial and each POST trial, MEPs were averaged over 12 times, and for data obtained during the HAND and RECOVERY trials, the MEP averaged every 6 stimulations (i.e. for 30 s) was used to analyse the MEP profile (30, 60, 90, and 120 s). All MEPs were normalised to values of the PRE trial. Background EMG activity was calculated as the root mean square of EMG activity for a period of 100 ms before TMS. In all trials, background EMG activity in both VL muscles did not exceed 20 µV (Tazoe et al. 2009).
Handgrip contractions
To determine MVC in the right handgrip, the subjects carried out three unilateral maximal isometric handgrip tests for 5 s separated by 1 min of rest on the first visit. The warm-up was performed with unilateral right isometric handgrip contractions for 5 s and consisted of two contractions performed at 50% and one each at 70%, and 100% of the participants perceived maximal handgrip contraction. A 1 min rest was provided between contractions. During the maximal tests, subjects were verbally encouraged to perform maximally and visual feedback was provided. The mean force for each maximal test was calculated from a 1 s window defined as 0.5 s before and after the peak force of each test. The highest mean force was defined as the MVC for handgrip contractions. Based on this MVC, subjects were asked to sustain 10 (LOW-IPSI and LOW-CONTRA) or 50% of the handgrip MVC (HIGH-IPSI and HIGH-CONTRA) with visual feedback of the exerted force from the PC monitor for 120 s. Subjects were repeatedly reminded to relax their lower limbs. In the HIGH sessions, subjects were asked to maintain the handgrip force at the highest level possible if the required force could not be maintained. During the sustained handgrip contraction (HAND trial), the handgrip force was calculated as the mean handgrip force for a period of 100 ms before each TMS and the force averaged every six intervals (i.e. for 30 s) was used to analyse the profile of handgrip force (30, 60, 90, and 120 s). Subjects also performed the maximal handgrip test (POST trial) immediately after (< 2 s) the sustained handgrip contraction in each session. Handgrip force for each trial was normalised to the PRE trial values (i.e. MVC).
Sense of effort
Using the Borg rating of perceived exertion (RPE) scale (15 points; range 6–20), we asked each subject to assess the sense of effort at 30, 60, 90, and 120 s during the sustained handgrip contractions (HAND-30, HAND-60, HAND-90, and HAND-120 trials, respectively).
Statistical analysis
Group data are presented as mean ± SD. For MEPs, a three-way ANOVA with repeated measures was performed separately with intensity, side, and time as factors in each trial (i.e. HAND, RECOVERY, and POST trials). A three-way repeated ANOVA was performed to compare intensity, side, and time as factors for handgrip force and RPE during the HAND trial. A two-way ANOVA was performed to compare intensity and side as factors for the absolute amplitudes of MEPs during the PRE trial, the SI of TMS, and handgrip force during the POST trial. All variables were examined using Mendoza’s multisample sphericity test. Whenever the data violated the assumption of sphericity, P values based on the Greenhouse–Geisser correction were reported. After ANOVA, Shaffer’s modified sequentially rejective Bonferroni procedure was performed for multiple comparisons. Partial η2 (η2p ) and Cohen’s d values are presented as measures of effect size. A P value of < 0.05 was considered statistically significant.
Results
Handgrip contraction
The MVC in handgrip contraction was 471.5 ± 124.1 N (range 245–615 N). Figure 2a illustrates the changes in handgrip force during the HAND trial in the four sessions. Three-way ANOVA (Table 1) showed no significant three-way interaction among intensity, side, and time (P = 0.22), but there was significant two-way interaction between intensity and side (P = 0.047). Handgrip force during the HAND trial was significantly higher in the HIGH sessions than in the LOW sessions during both the IPSI (LOW-IPSI: 9.6 ± 1.0% of MVC vs. HIGH-IPSI: 38.0 ± 7.9% of MVC; P < 0.01) and CONTRA sessions (LOW-CONTRA: 9.5 ± 0.6% of MVC vs. HIGH-CONTRA: 41.4 ± 6.1% of MVC; P < 0.01) (Fig. 2b). The comparison of effects of sides in each intensity session showed no significant differences in the LOW (LOW-IPSI: 9.6 ± 1.0% of MVC vs. LOW-CONTRA: 9.5 ± 0.6% of MVC; P = 0.70) and HIGH sessions (HIGH-IPSI: 38.0 ± 7.9% of MVC vs. HIGH-CONTRA: 41.4 ± 6.1% of MVC; P = 0.07). Other two-way interactions were not significant (intensity × time, ε = 0.36, P = 0.08; side × time, ε = 0.55, P = 0.24). No significant main effect due to time was found (ε = 0.36, P = 0.06). These results showed that handgrip force was maintained at a higher level during the HAND trial in the HIGH sessions than in the LOW sessions.
Handgrip force during the 120-s sustained handgrip contraction. A Circle symbols show group data during the handgrip contraction at 10% of maximal voluntary contraction (MVC) in two conditions in which motor-evoked potentials (MEPs) by transcranial magnetic stimulation (TMS) were elicited in the right (open circles, LOW-IPSI) and left (filled circles, LOW-CONTRA) vastus lateralis (VL) muscles. Square symbols show group data during the handgrip contraction at 50% of MVC in two conditions in which MEPs by TMS were elicited in the right (open squares, HIGH-IPSI) and left (filled squares, HIGH-CONTRA) VL muscles. B Mean values for force over the 120-s handgrip contraction in the LOW-IPSI, LOW-CONTRA, HIGH-IPSI, and HIGH-CONTRA sessions. Error bars indicate standard deviation (SD). ††Significant intensity effect in each side session (P < 0.01)
In the POST trial, two-way ANOVA (Table 1) revealed no significant intensity × side interactions (P = 0.15). Handgrip force in the maximal handgrip test immediately after the sustained handgrip (LOW-IPSI: 86.2 ± 9.1%, LOW-CONTRA: 81.9 ± 14.6%, HIGH-IPSI: 47.5 ± 10.5%, HIGH-CONTRA: 48.8 ± 14.7%) was significantly lower in the HIGH sessions than in the LOW sessions (P < 0.01) and was not significantly different between the IPSI and CONTRA sessions (P = 0.63).
MEPs
The SI was determined as the peak-to-peak amplitude of 0.3–0.5 mV. For the SI (Table 2), two-way ANOVA revealed no significant intensity × side interaction (P = 0.58) or significant main effects due to intensity or side (intensity, P = 0.97; side, P = 0.54). For the amplitude value of MEPs in the PRE trial (Table 2), two-way ANOVA revealed no significant intensity × side interaction (P = 0.83) or significant main effects due to side (P = 0.55), but the amplitude was significantly greater in the HIGH sessions than in the LOW sessions (P < 0.01). For all sessions, the SI corresponded to approximately 130% of the RMT. For the SI relative to RMT (Table 2), no significant intensity × side interaction (P = 0.67) or significant main effects that can be attributed to intensity or side (intensity, P = 0.82; side, P = 0.07) were identified.
In the HAND trial, one subject was excluded from the analysis because data of the subject exceeded the mean ± 2SD of group data at all time points in the HIGH sessions. Figure 3a (n = 8) shows the changes in MEPs during the HAND trial in the four sessions. Three-way ANOVA (Table 3) showed no significant three-way interaction among intensity, side, and time (P = 0.75), but there was a significant two-way interaction between intensity and time (P = 0.03). In the HIGH and LOW sessions (Fig. 3b), the amplitude of MEPs significantly increased over time (HIGH, P < 0.01; LOW, P < 0.01), and post hoc analysis showed significant difference in the amplitude of MEPs between 30 and 90 s only in the HIGH sessions (P < 0.01, d = 0.95). Across all time points, the amplitude of MEPs was significantly higher in the HIGH sessions than in the LOW sessions (30 s, P < 0.01; 60 s, P < 0.01; 90 s, P < 0.01; 120 s, P < 0.01) (Fig. 3b). Other two-way interactions were not significant (intensity × side, P = 0.95; side × time, P = 0.59). No significant main effect due to side was observed (P = 0.47). These results showed that the degree of facilitation of MEPs throughout the handgrip contraction and the rate of increase in the amplitude of MEPs over time were greater in the HIGH sessions than in the LOW sessions and that the changes in MEPs were not different between the IPSI and CONTRA sessions.
Motor-evoked potentials (MEPs) recorded from the resting right (open symbols, IPSI) and left (filled symbols, CONTRA) vastus lateralis (VL) muscles during the 120-s handgrip contraction (HAND trial). A, Group data during the handgrip contraction at 10% (circle symbols, LOW-IPSI and LOW-CONTRA) and 50% (square symbols, HIHG-IPSI and HIGH-CONTRA) of the maximal voluntary contraction (MVC). B, Mean values for amplitudes of MEPs across the IPSI and CONTRA sessions in each intensity session (grey circles, LOW sessions; grey squares, HIGH sessions). Error bars indicate standard deviation (SD). ##Significant time effect in the HIGH session (P < 0.01). **Significant difference vs. 30 s in the HIGH session (P < 0.01). §Significant time effect in the LOW session (P < 0.05). ††Significant intensity effect (P < 0.01)
In the RECOVERY trial (Fig. 4, n = 9), three-way ANOVA (Table 3) revealed no significant three-way interaction among intensity, side, and time (P = 0.88) or two-way interactions (intensity × side, P = 0.92; intensity × time, ε = 0.55, P = 0.18; side × time, ε = 0.54, P = 0.89). No significant main effects of the intensity or side were found (intensity, P = 0.75; side, P = 0.18). The amplitude of MEPs significantly decreased for 120 s after the handgrip contraction in all sessions (P < 0.01), and post hoc analysis revealed a significant smaller amplitude of MEPs at 60, 90, and 120 s than at 30 s (60 s, P < 0.01, d = 0.68; 90 s, P < 0.01, d = 0.73; 120 s, P < 0.01, d = 0.89). These results indicated that the amplitude of MEPs decreased for 120 s after the handgrip contraction and that the changes in MEPs were similar across all sessions.
Motor-evoked potentials (MEPs) recorded from the resting right (open symbols, IPSI) and left (filled symbols, CONTRA) vastus lateralis (VL) muscles for 120 s after the end of the 120-s handgrip contraction (RECOVERY trial) at 10% (circle symbols, LOW-IPSI and LOW-CONTRA) and 50% (square symbols, HIHG-IPSI and HIGH-CONTRA) of the maximal voluntary contraction (MVC). Error bars indicate standard deviation (SD). ##Significant difference vs. 30 s in all sessions (P < 0.01)
In the POST trials (Fig. 5a), three-way ANOVA (Table 4) revealed no significant three-way interaction among intensity, side, and time interaction (P = 0.23), but there were significant intensity × time (P < 0.01) and side × time (P = 0.01) interactions. For the intensity × time interaction (Fig. 5b), the amplitude of MEPs significantly increased over time in the LOW sessions (ε = 0.29, P = 0.03). Post hoc analysis showed a significantly greater amplitude of MEPs in the POST-30 trial than in the PRE (P < 0.01, d = 1.30) and POST-10 trials (P = 0.02, d = 0.89) and significantly increased amplitude of MEPs at the POST-25 trial compared to that at the PRE trial (P < 0.01, d = 1.20). In the HIGH sessions, MEPs did not significantly change (P = 0.47). At the POST-15, POST-25, and POST-30 trials, the amplitude of MEPs was significantly greater in the LOW sessions than in the HIGH sessions (POST-15, P = 0.02; POST-25, P = 0.02; POST-30, P = 0.04). For the side × time interaction (Fig. 5c), in the CONTRA sessions, the amplitude of MEPs significantly increased over time (P < 0.01), but post hoc analysis showed no significant differences in the amplitude of MEPs (PRE vs. POST-10, P = 0.06, d = 1.18; PRE vs. POST-25, P = 0.06, d = 1.43). In the IPSI sessions, MEPs did not change significantly (P = 0.33). At the POST-10, POST-15, POST-20, POST-25, and POST-30 trials, the amplitude of MEPs was significantly greater in the CONTRA sessions than in the IPSI sessions (POST-10, P = 0.01; POST-15, P = 0.02; POST-20, P = 0.03; POST-25, P = 0.04; POST-30, P = 0.04). These results indicated that the high-intensity handgrip contraction resulted in smaller amplitude of MEPs than the low-intensity handgrip contraction and that the handgrip contraction with the right hand induced a greater amplitude of MEPs in the left VL than in the right VL. However, the differential effect of the handgrip contraction on MEPs in the right and left VL muscles did not depend on the intensity of the handgrip contraction.
Motor-evoked potentials (MEPs) recorded from the resting right (open symbols, IPSI) and left (filled symbols, CONTRA) vastus lateralis (VL) muscles at 5, 10, 15, 20, 25, and 30 min (POST-5, POST-10, POST-15, POST-20, POST-25, and POST-30, respectively) after the handgrip contractions. A Group data after the handgrip contraction at 10% (circle symbols, LOW-IPSI and LOW-CONTRA) and 50% (square symbols, HIHG-IPSI and HIGH-CONTRA) of maximal voluntary contraction (MVC). B Mean values for amplitudes of MEPs across the IPSI and CONTRA sessions in each intensity session (grey circles, LOW sessions; grey squares, HIGH sessions). C Mean values for amplitudes of MEPs across the LOW and HIGH sessions in each side session (open diamonds, IPSI sessions; filled diamonds, CONTRA sessions). Error bars indicate standard deviation (SD). §Significant time effect in the LOW session (P < 0.05). **Significant difference vs. PRE in the LOW session (P < 0.01). †Significant intensity effect (P < 0.05). ¶¶Significant time effect in the CONTRA sessions (P < 0.01). ‡Significant side effect (P < 0.05)
Sense of effort
Figure 6a shows the changes in RPE across all sessions. Three-way ANOVA (Table 5) indicated no significant three-way interaction among intensity, side, and time interaction (P = 0.95) or significant two-way interactions (intensity × side, P = 0.84; side × time, ε = 0.39, P = 0.68), except for intensity × time interaction (P < 0.01). In the LOW and HIGH sessions (Fig. 6b), the RPE significantly increased with time (ε = 0.48, P < 0.01; HIGH, P < 0.01). Post hoc analysis showed significantly higher RPEs at the HAND-60, HAND-90, and HAND-120 trials than at the HAND-30 trial in the LOW (HAND-60, P < 0.01, d = 0.59; HAND-90, P < 0.01, d = 1.36; HAND-120, P < 0.01, d = 1.69) and HIGH sessions (HAND-60, P < 0.01, d = 1.69; HAND-90, P < 0.01, d = 2.65; HAND-120, P < 0.01, d = 3.67). At each time point, the RPE was significantly higher in the HIGH sessions than in the LOW sessions (HAND-30, P < 0.01; HAND-60, P < 0.01; HAND-90, P < 0.01; HAND-120, P < 0.01). No significant main effect due to side was found (P = 0.87). Inspection of the data indicated that the rate of increase in RPE was greater during the high-intensity handgrip contraction than during the low-intensity handgrip contraction.
Rating of perceived exertion (RPE) reported at 30, 60, 90, and 120 s during the handgrip contraction (HAND-30, HAND-60, HAND-90, and HAND-120, respectively). A Circle symbols show group data during the handgrip contraction at 10% of maximal voluntary contraction (MVC) in two conditions in which motor-evoked potentials (MEPs) by transcranial magnetic stimulation (TMS) were elicited in the right (open circles, LOW-IPSI) and left (filled circles, LOW-CONTRA) vastus lateralis (VL) muscles. Square symbols show group data during the handgrip contraction at 50% of MVC in two conditions in which MEPs by TMS were elicited in the right (open squares, HIGH-IPSI) and left (filled squares, HIGH-CONTRA) VL muscles. B Mean values for RPE values across the IPSI and CONTRA sessions in each intensity session (grey circles, LOW sessions; grey squares, HIGH sessions). Error bars indicate standard deviation (SD). ##Significant time effect in the HIGH session (P < 0.01). **Significant difference vs. HAND-30 in the HIGH session (P < 0.01). §§Significant time effect in the LOW session (P < 0.01). &&Significant difference vs. HAND-30 in the LOW session (P < 0.01). ††Significant intensity effect (P < 0.01)
Discussion
The present study revealed that a unilateral sustained handgrip contraction differentially influenced post-contraction resting MEPs in task-unrelated knee extensor muscles ipsilateral and contralateral to the hand and that the intensity of the handgrip contraction significantly influenced post-contraction resting MEPs regardless of the ipsilateral or contralateral leg.
According to reports, resting MEPs were facilitated when a muscle in a different segment was contracted (Furubayashi et al. 2003; Sugawara et al. 2005; Tazoe et al. 2009). This facilitation has been shown to be proportional to the intensity of the muscle contractions and gradually increased during sustained muscle contractions regardless of actual muscle force output (Tazoe et al. 2009). The present results are consistent with these previous results for resting MEPs in task-unrelated muscles alongside the muscle contractions. This is the first study to demonstrate gradual facilitations of resting MEPs in the task-unrelated right and left knee extensor muscles when a unilateral upper limb muscle contraction is isometrically sustained. According to Tazoe et al. (2009), gradual facilitations of resting MEPs in task-unrelated muscles alongside the sustained muscle contractions are considered to depend on the level of effort driving the contractions. However, the authors did not measure the sense of effort during the muscle contractions with different intensities or durations. In the present study, the rates of increase in RPE and the amplitude of resting MEPs during the handgrip contraction showed a similar trend and were greater in the HIGH sessions than in the LOW sessions. These results may support the possibility that sense of effort during upper (or lower) limb muscle contractions is closely related to facilitation of resting MEPs in task-unrelated lower (or upper) limb muscles. Although the present study cannot determine the neural processes involved in the gradual facilitation of resting MEPs of the knee extensor muscles, supraspinal factors may contribute to the gradual facilitation. Indeed, a unilateral sustained isometric wrist flexion at 10% MVC did not facilitate spinal reflex excitability of the proximal thigh muscles assessed by transcutaneous spinal cord stimulation (Kato et al. 2019). Nevertheless, future studies should simultaneously measure the intracortical and spinal excitability of the lower limb muscles during upper limb muscle contractions.
For post-contraction resting MEPs, the amplitude of the contralateral MEPs was greater than that of the ipsilateral MEPs beginning at 10 min after the cessation of the handgrip contraction independent of the intensity of the contraction. Matsuura and Ogata (2015) found that unilateral lower limb muscle contractions differentially influenced post-contraction resting MEPs in the hand muscles ipsilateral and contralateral to the lower limb muscles. More specifically, the amplitude of MEPs in the contralateral hand muscles was greater than that in the ipsilateral hand muscles beginning at 10 min after the lower limb muscle contractions (Matsuura and Ogata 2015). The present results are in line with these previous findings. This suggests that neural processes that mediate the differential effects of muscle contractions with one limb on post-contraction corticomotor excitability innervating the homolateral and diagonal limb muscles may be shared between upper and lower limb muscle contractions. In the present study, the exact mechanisms involved in the increased amplitude of post-contraction resting MEPs in the left VL muscle are unclear. Since the intensity of the sustained handgrip contraction did not influence the differential effect on post-contraction resting MEPs in the right and left VL muscles and there was no significant difference in facilitations of resting MEPs between the right and left VL muscles alongside the handgrip contraction, it is likely that the facilitations of resting MEPs alongside the contractions did not mediate the differential changes in post-contraction resting MEPs in the ipsilateral and contralateral legs. A potential mechanism responsible for the differential changes in post-contraction resting MEPs in the ipsilateral and contralateral legs may involve the interhemispheric interactions between the left and right M1s innervating the VL muscles. However, Matsuura and Ogata (2015) found that fatiguing unilateral plantar flexions did not depress interhemispheric inhibition from the hand M1 contralateral to the ankle to that ipsilateral to the ankle when the plantar flexions increased post-contraction corticomotor excitability of the contralateral hand muscle (i.e. innervated by the ipsilateral M1 hand area). Nevertheless, there is currently no available evidence describing the effects of unilateral upper limb muscle contractions on interhemispheric interactions between the leg M1s during or after the contractions. Therefore, further studies are needed to clarify neural mechanism for the differential effects of unilateral upper limb muscle contractions on the post-contraction excitability of corticomotor pathways innervating the proximal thigh muscles.
Consistent with our expectations, post-contraction resting MEPs in the VL muscles were influenced by the intensity of the unilateral sustained handgrip contraction. Specifically, the amplitude of the post-contraction resting MEPs decreased from 15 min after the high-intensity handgrip contraction compared to the low-intensity contraction. A previous study showed that high-intensity or isotonic muscle contractions greatly depressed post-contraction resting MEPs measured in the task-related muscle compared to low-intensity or isometric contractions (Miyaguchi et al. 2016). The study speculated that the depressed post-contraction resting MEPs in the task-related muscle was contingent on the activation of the M1 during the muscle contractions (Miyaguchi et al. 2016), since greater M1 activities or corticomotor excitability have been reported to occur during high-intensity or isotonic muscle contractions compared to low-intensity or isometric contractions (Saito et al. 2014; Thickbroom et al. 1998; van Duinen et al. 2008; Yahagi et al. 2003). In the present study, resting MEPs measured in the VL muscles were more strongly facilitated alongside the high-intensity handgrip contraction than the low-intensity contraction. This difference in the degree of facilitation during the handgrip contraction might result in intensity-dependent effects on post-contraction resting MEPs in the VL muscles. We found no evidence of the neural mechanisms that mediate the intensity-dependent effects on post-contraction resting MEPs in the task-unrelated muscles. Nevertheless, based on previous findings on spinal excitability involving corticospinal–motoneuronal synapses, which was measured by cervicomedullary MEPs (CMEPs), we speculate that the relative contributions of cortical and spinal excitability to corticomotor excitability can explain the intensity-dependent effects on post-contraction resting MEPs in the task-unrelated muscles.
For post-contraction resting MEPs and resting CMEPs measured in task-related muscles, sustained isometric MVC, non-fatiguing intermittent isometric contractions at 75% MVC, or brief intermittent contractions at 50% MVC induced a similar time course of resting CMEPs with a late prolonged facilitation from 5 to 6 min after the contraction in the context of varying changes in MEPs (Aboodarda et al. 2015; Gandevia et al. 1999; Nuzzo et al. 2016). A recent study has reported that contraction intensity in a single session of resistance training with elbow flexors affected only post-contraction resting MEPs but not post-contraction resting CMEPs measured in the task-related muscle (Colomer-Poveda et al. 2019). These results suggest that the changes in spinal excitability after muscle contractions are less affected by intensity, duration, and type of the contraction and that differences in resting corticomotor excitability (i.e. resting MEPs) after different protocols of muscle contractions may mainly reflect changes in cortical excitability. Thus, the post-contraction resting corticomotor excitability in task-related muscles would be depressed if depression of cortical excitability exceeded spinal facilitation, and vice versa. Furthermore, in task-unrelated elbow flexor muscles, post-contraction resting CMEPs have been shown to be facilitated after fatiguing dynamic bilateral knee extensor contractions (Šambaher et al. 2016) or after two sets of 100-s unilateral isometric dominant knee extensions (Aboodarda et al. 2017). Therefore, it is speculated that the high-intensity handgrip contraction strongly depressed the post-contraction resting cortical excitability of the task-unrelated knee extensor muscles compared to the low-intensity contraction. In sessions accompanied by a late facilitation of resting MEPs except for the HIGH-IPSI session, spinal facilitation might exceed cortical depression over time.
An alternative explanation for the intensity-dependent effects on post-contraction resting MEPs in the task-unrelated muscles is activation of the group III/IV muscle afferents derived from the task-related muscle, which transfer excitatory and inhibitory inputs to the cortical and spinal circuitries innervating the task-unrelated muscle (Sidhu et al. 2014). Since unilateral handgrip contractions at 30% MVC induce greater intramuscular metabolic perturbations compared to those at 10% MVC (Boushel et al. 1998), the high-intensity handgrip contraction could induce greater intramuscular metabolic perturbations and then lead to greater activation of group III/IV muscle afferents compared to the low-intensity handgrip contraction in the present study. Although it cannot be totally excluded that the greater activation of group III/IV muscle afferents led to the lower amplitude of post-contraction resting MEPs in the task-unrelated VL muscles in the high-intensity sessions compared to the low-intensity sessions, no study has investigated whether activation of group III/IV muscle afferents due to fatiguing upper limb muscle contractions affects post-contraction resting MEPs measured in task-unrelated muscles. Further studies should assess whether post-contraction resting MEPs measured in task-unrelated muscles adjust to group III/IV muscle afferent activities derived from task-related muscles.
Our findings can be applied in the improvement of the quality of walking training by hemiparetic patients. Wang et al. (2012) have reported that 30-min walking training for 10 sessions over 2 weeks combined with preceding low-frequency repetitive TMS over the leg area of the M1 of the unaffected hemisphere enhanced the effect of the training in those with chronic stroke by increasing gait spatial symmetry and corticomotor excitability symmetry. This intervention combined low-frequency repetitive TMS and walking training resulted in decreased corticomotor excitability of unaffected hemisphere and increased corticomotor excitability of affected hemisphere (Wang et al. 2012). Therefore, unilateral handgrip by non-hemiparetic hand may be a useful intervention for improving corticomotor excitability asymmetry in the leg M1 area between unaffected and affected hemispheres before walking training.
Limitations
Some limitations should be considered in the present study. First, supraspinal and spinal motoneuronal excitability could not be investigated separately. Consequently, there was no direct evidence that shows whether the origin of resting MEP changes in the task-unrelated VL muscles was supraspinal or spinal during and after the handgrip contractions. Hence, conclusions from the present study are limited to corticomotor excitability. Second, central fatigue elicited by the handgrip contractions was not assessed. Central fatigue of the first dorsal interosseous muscle was different between during low- and high-force sustained submaximal contractions (Eichelberger and Bilodeau 2007). Thus, it is possible that there was a significant difference in central fatigue due to handgrip contraction between the HIGH and LOW sessions and that the difference in central fatigue between the sessions was responsible for the intensity-dependent effect on post-contraction resting MEPs. For task-related muscle, it has been shown that the time course of post-contraction resting MEPs did not parallel that of central fatigue during the recovery period after fatiguing muscle contractions (Gandevia et al. 1999), suggesting that the differences in central fatigue between the LOW and HIGH sessions were not related to the changes in post-contraction resting MEPs in the VL muscles. Even though changes in post-contraction resting MEPs were related to central fatigue, differences in central fatigue between the LOW and HIGH sessions would be diminished quickly because central fatigue has been shown to recover quickly (2–3 s) after termination of voluntary muscle contractions (Mira et al. 2017). Therefore, it is likely that the effects of the handgrip contraction on post-contraction resting MEPs in the VL muscles are primarily explained by factors other than central fatigue. Third, each experimental session was performed on a separate day in the present study. As a result, there were significant differences in the amplitude of MEPs at the PRE trial between the LOW and HIGH sessions. The smaller amplitudes of MEPs at the PRE trial in the LOW sessions compared to the HIGH sessions might lead to overestimation of facilitation of MEPs during and after handgrip contraction. However, the SI relative to RMT was approximately 130% in all sessions and was not different between sessions. The MEPs of the quadriceps muscles have been shown not to produce floor and ceiling effects when the SI was at 120–140% of RMT (Chiou et al. 2013). Therefore, the differences in the amplitudes of MEPs at the PRE trial between the LOW and HIGH sessions did not alter the conclusions of the present study. Another limitation of the present study was that the sample size was relatively small. Consequently, our sample size may have been insufficient to detect some subtle differences in the HAND and RECOVERY trials, and future studies might want to confirm the results with larger samples. However, given the fact that effects of handgrip contraction were found within the sample suggests that the effect sizes may be substantial.
Conclusions
This study indicates that a unilateral sustained upper limb contraction can differentially alter corticomotor excitability in lower limb muscles ipsilateral and contralateral to the upper limb after muscle contraction and that the intensity of the upper limb contraction influences corticomotor excitability in both the lower limb muscles after the contraction. It is likely that mechanisms responsible for the modulation of corticomotor excitability after muscle contractions are partly shared between task-related and task-unrelated muscles. Corticomotor excitability has been suggested to be associated with neural processes involving motor skill acquisition and improvements of motor function. Thus, since it is possible that the changes in MEPs after muscle contractions exert a potentially positive or negative effect on subsequent motor learning or rehabilitation, the present findings may contribute to the development of new motor learning or rehabilitation protocols.
Abbreviations
- ANOVA:
-
Analysis of variance
- CMEPs:
-
Cervicomedullary motor-evoked potentials
- CONTRA:
-
Side contralateral to the exercised hand
- EMG:
-
Electromyography
- HAND:
-
Time point during the sustained handgrip contraction
- HAND-30:
-
Time point at 30 s during the sustained handgrip contraction
- HAND-60:
-
Time point at 60 s during the sustained handgrip contraction
- HAND-90:
-
Time point at 90 s during the sustained handgrip contraction
- HAND-120:
-
Time point at 120 s during the sustained handgrip contraction
- HIGH:
-
Intensity corresponding to 50% of maximal voluntary contraction
- IPSI:
-
Side ipsilateral to the exercised hand
- LOW:
-
Intensity corresponding to 10% of maximal voluntary contraction
- MEPs:
-
Motor-evoked potentials
- M1:
-
Primary motor cortex
- MVC:
-
Maximal voluntary contraction
- POST:
-
Time point after the sustained handgrip contraction
- POST-5:
-
Time point at 5 min after the sustained handgrip contraction
- POST-10:
-
Time point at 10 min after the sustained handgrip contraction
- POST-15:
-
Time point at 15 min after the sustained handgrip contraction
- POST-20:
-
Time point at 20 min after the sustained handgrip contraction
- POST-25:
-
Time point at 25 min after the sustained handgrip contraction
- POST-30:
-
Time point at 30 min after the sustained handgrip contraction
- PRE:
-
Time point before the sustained handgrip contraction
- RECOVERY:
-
Time point for 120 s after the end of the sustained handgrip contraction
- RMT:
-
Resting motor threshold
- RPE:
-
Rating of perceived exertion
- SD:
-
Standard deviation
- SI:
-
Stimulus intensity
- TMS:
-
Transcranial magnetic stimulation
- VL:
-
Vastus lateralis
References
Aboodarda SJ, Copithorne DB, Pearcey GEP, Button DC, Power KE (2015) Changes in supraspinal and spinal excitability of the biceps brachii following brief, non-fatiguing submaximal contractions of the elbow flexors in resistance-trained males. Neurosci Lett 607:66–71. https://doi.org/10.1016/j.neulet.2015.09.028
Aboodarda SJ, Šambaher N, Behm DG (2016) Unilateral elbow flexion fatigue modulates corticospinal responsiveness in non-fatigued contralateral biceps brachii. Scand J Med Sci Sports 26:1301–1312. https://doi.org/10.1111/sms.12596
Aboodarda SJ, Šambaher N, Millet GY, Behm DG (2017) Knee extensors neuromuscular fatigue changes the corticospinal pathway excitability in biceps brachii muscle. Neuroscience 340:477–486. https://doi.org/10.1016/j.neuroscience.2016.10.065
Bonato C, Zanette G, Manganotti P, Tinazzi M, Bongiovanni G, Polo A, Fiaschi A (1996) ‘Direct’ and ‘crossed’ modulation of human motor cortex excitability following exercise. Neurosci Lett 216:97–100. https://doi.org/10.1016/0304-3940(96)13010-X
Borroni P, Cerri G, Baldissera F (2004) Excitability changes in resting forearm muscles during voluntary foot movements depend on hand position: a neural substrate for hand–foot isodirectional coupling. Brain Res 1022:117–125. https://doi.org/10.1016/j.brainres.2004.07.003
Boushel R, Madsen P, Nielsen HB, Quistorff B, Secher NH (1998) Contribution of pH, diprotonated phosphate and potassium for the reflex increase in blood pressure during handgrip. Acta Physiol Scand 164:269–275. https://doi.org/10.1046/j.1365-201X.1998.00429.x
Brasil-Neto JP, Pascual-Leone A, Valls-Sole J, Cammarota A, Cohen LG, Hallett M (1993) Postexercise depression of motor evoked potentials: a measure of central nervous system fatigue. Exp Brain Res 93:181–184. https://doi.org/10.1007/BF00227794
Chiou SY, Wang RY, Liao KK, Yang YR (2013) Homologous muscle contraction during unilateral movement does not show a dominant effect on leg representation of the ipsilateral primary motor cortex. PLoS One 8:e72231. https://doi.org/10.1371/journal.pone.0072231
Cincotta M, Borgheresi A, Gambetti C, Balestrieri F, Rossi L, Zaccara G, Ulivelli M, Rossi S, Civardi C, Cantello R (2003) Suprathreshold 0.3 Hz repetitive TMS prolongs the cortical silent period: potential implications for therapeutic trials in epilepsy. Clin Neurophysiol 114:1827–1833. https://doi.org/10.1016/S1388-2457(03)00181-0
Cohen J (1992) A power primer. Psychol Bull 112:155–159. https://doi.org/10.1037/0033-2909.112.1.155
Colomer-Poveda D, Romero-Arenas S, Lundbye-Jensen J, Hortobágyi T, Márquez G (2019) Contraction intensity-dependent variations in the responses to brain and corticospinal tract stimulation after a single session of resistance training in men. J Appl Physiol 127:1128–1139. https://doi.org/10.1152/japplphysiol.01106.2018
Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC (2004) The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol 115:255–266. https://doi.org/10.1016/j.clinph.2003.10.009
Eichelberger TD, Bilodeau M (2007) Central fatigue of the first dorsal interosseous muscle during low–force and high–force sustained submaximal contractions. Clin Physiol Funct Imaging 27:298–304. https://doi.org/10.1111/j.1475-097X.2007.00751.x
Fitzgerald PB, Fountain S, Daskalakis JZ (2006) A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol 117:2584–2596. https://doi.org/10.1016/j.clinph.2006.06.712
Furubayashi T, Sugawara K, Kasai T, Hayashi A, Hanajima R, Shiio Y, Iwata NK, Ugawa Y (2003) Remote effects of self-paced teeth clenching on the excitability of hand motor area. Exp Brain Res 148:261–265. https://doi.org/10.1007/s00221-002-1299-y
Gandevia SC, Petersen N, Butler JE, Taylor JL (1999) Impaired response of human motoneurones to corticospinal stimulation after voluntary exercise. J Physiol 521:749–759. https://doi.org/10.1111/j.1469-7793.1999.00749.x
Kato T, Sasaki A, Yokoyama H, Milosevic M, Nakazawa K (2019) Effects of neuromuscular electrical stimulation and voluntary commands on the spinal reflex excitability of remote limb muscles. Exp Brain Res. https://doi.org/10.1007/s00221-019-05660-6
Knikou M (2012) Plasticity of corticospinal neural control after locomotor training in human spinal cord injury. Neural Plast 2012:254948. https://doi.org/10.1155/2012/254948
Liepert J, Kotterba S, Tegenthoff M, Malin JP (1996) Central fatigue assessed by transcranial magnetic stimulation. Muscle Nerve 19:1429–1434. https://doi.org/10.1002/(SICI)1097-4598(199611)19:11%3c1429:AID-MUS7%3e3.0.CO;2-E
Maruyama A, Matsunaga K, Tanaka N, Rothwell JC (2006) Muscle fatigue decreases short-interval intracortical inhibition after exhaustive intermittent tasks. Clin Neurophysiol 117:864–870. https://doi.org/10.1016/j.clinph.2005.12.019
Matsuura R, Ogata T (2015) Effects of fatiguing unilateral plantar flexions on corticospinal and transcallosal inhibition in the primary motor hand area. J Physiol Anthropol 34:4. https://doi.org/10.1186/s40101-015-0042-x
Mira J, Lapole T, Souron R, Messonnier L, Millet GY, Rupp T (2017) Cortical voluntary activation testing methodology impacts central fatigue. Eur J Appl Physiol 117:1845–1857. https://doi.org/10.1007/s00421-017-3678-x
Miyaguchi S, Kojima S, Kirimoto H, Tamaki H, Onishi H (2016) Do differences in levels, types, and duration of muscle contraction have an effect on the degree of post-exercise depression? Front Hum Neurosci 10:159. https://doi.org/10.3389/fnhum.2016.00159
Murase N, Rothwell JC, Kaji R, Urushihara R, Nakamura K, Murayama N, Igasaki T, Sakata-Igasaki M, Mima T, Ikeda A, Shibasaki H (2005) Subthreshold low-frequency repetitive transcranial magnetic stimulation over the premotor cortex modulates writer’s cramp. Brain 128:104–115. https://doi.org/10.1093/brain/awh315
Nuzzo JL, Barry BK, Gandevia SC, Taylor JL (2016) Acute strength training increases responses to stimulation of corticospinal axons. Med Sci Sports Exerc 48:139–150. https://doi.org/10.1249/MSS.0000000000000733
Péréon Y, Genet R, Guihéneuc P (1995) Facilitation of motor evoked potentials: timing of Jendrassik maneuver effects. Muscle Nerve 18:1427–1432. https://doi.org/10.1002/mus.880181213
Perez MA, Lungholt BKS, Nyborg K, Nyborg K, Nielsen JB (2004) Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res 159:197–205. https://doi.org/10.1007/s00221-004-1947-5
Rosenkranz K, Kacar A, Rothwell JC (2007) Differential modulation of motor cortical plasticity and excitability in early and late phases of human motor learning. J Neurosci 27:12058–12066. https://doi.org/10.1523/JNEUROSCI.2663-07.2007
Saito K, Sugawara K, Miyaguchi S, Matsumoto T, Kirimoto H, Tamaki H, Onishi H (2014) The modulatory effect of electrical stimulation on the excitability of the corticospinal tract varies according to the type of muscle contraction being performed. Front Hum Neurosci 8:835. https://doi.org/10.3389/fnhum.2014.00835
Šambaher N, Aboodarda SJ, Behm DG (2016) Bilateral knee extensor fatigue modulates force and responsiveness of the corticospinal pathway in the non-fatigued, dominant elbow flexors. Front Hum Neurosci 10:18. https://doi.org/10.3389/fnhum.2016.00018
Shironouchi F, Ohtaka C, Mizuguchi N, Kato K, Kakigi R, Nakata H (2019) Remote effects on corticospinal excitability during motor execution and motor imagery. Neurosci Lett 707:134284. https://doi.org/10.1016/j.neulet.2019.134284
Sidhu SK, Weavil JC, Venturelli M, Garten RS, Rossman MJ, Richardson RS, Gmelch BS, Morgan DE, Amann M (2014) Spinal µ-opioid receptor-sensitive lower limb muscle afferents determine corticospinal responsiveness and promote central fatigue in upper limb muscle. J Physiol 592:5011–5024. https://doi.org/10.1113/jphysiol.2014.275438
Sugawara K, Furubayashi T, Takahashi M, Ni Z, Ugawa Y, Kasai T (2005) Remote effects of voluntary teeth clenching on excitability changes of the human hand motor area. Neurosci Lett 377:25–30. https://doi.org/10.1016/j.neulet.2004.11.059
Takahashi K, Maruyama A, Maeda M, Etoh S, Hirakoba K, Kawahira K, Rothwell JC (2009) Unilateral grip fatigue reduces short interval intracortical inhibition in ipsilateral primary motor cortex. Clin Neurophysiol 120:198–203. https://doi.org/10.1016/j.clinph.2008.10.003
Takahashi K, Maruyama A, Hirakoba K, Maeda M, Etoh S, Kawahira K, Rothwell JC (2011) Fatiguing intermittent lower limb exercise influences corticospinal and corticocortical excitability in the nonexercised upper limb. Brain Stimul 4:90–96. https://doi.org/10.1016/j.brs.2010.07.001
Tazoe T, Sakamoto M, Nakajima T, Endoh T, Shiozawa S, Komiyama T (2009) Remote facilitation of supraspinal motor excitability depends on the level of effort. Eur J Neurosci 30:1297–1305. https://doi.org/10.1111/j.1460-9568.2009.06895.x
Thickbroom GW, Phillips BA, Morris I, Byrnes ML, Mastaglia FL (1998) Isometric force-related activity in sensorimotor cortex measured with functional MRI. Exp Brain Res 121:59–64. https://doi.org/10.1007/s002210050437
van Duinen H, Renken R, Maurits NM, Zijdewind I (2008) Relation between muscle and brain activity during isometric contractions of the first dorsal interosseus muscle. Hum Brain Mapp 29:281–299. https://doi.org/10.1002/hbm.20388
Wang RY, Tseng HY, Liao KK, Wang CJ, Lai KL, Yang YR (2012) rTMS combined with task-oriented training to improve symmetry of interhemispheric corticomotor excitability and gait performance after stroke: a randomized trial. Neurorehabil Neural Repair 26(3):222–230. https://doi.org/10.1177/1545968311423265
Westfall J (2016) Power ANalysis for GEneral Anova designs. https://jakewestfall.shinyapps.io/pangea/
Yahagi S, Ni Z, Takahashi M, Takeda Y, Tsuji T, Kasai T (2003) Excitability changes of motor evoked potentials dependent on muscle properties and contraction modes. Mot Control 7:328–345. https://doi.org/10.1123/mcj.7.4.329
Zanette G, Bonato C, Polo A, Tinazzi M, Manganotti P, Fiaschi A (1995) Long-lasting depression of motor-evoked potentials to transcranial magnetic stimulation following exercise. Exp Brain Res 107:80–86. https://doi.org/10.1007/BF00228019
Acknowledgements
The authors are grateful to Dr. Tokuo Yano for helpful discussions. This study was supported by JSPS KAKENHI (Grant number 25871146).
Author information
Authors and Affiliations
Contributions
RM contributed to the conception and design of the experiment. RM, TY, and KS conducted the experiments. YO contributed to the execution of the experiments (management of risks of transcranial magnetic stimulation). RM analysed the data. RM and TY contributed to the interpretation of the data. RM drafted the article. YO critically reviewed the manuscript for important intellectual content. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest, financial or otherwise to report, regarding this manuscript.
Research involving human participants and/or animals
The experimental procedures were carried out in accordance with the Declaration of Helsinki. The Ethics Committee of the Joetsu University of Education approved the study (approval number 26-41).
Informed consent
All subjects gave written informed consent to participate in the study.
Additional information
Communicated by Toshio Moritani.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Matsuura, R., Yunoki, T., Shirakawa, K. et al. Effects of sustained unilateral handgrip on corticomotor excitability in both knee extensor muscles. Eur J Appl Physiol 120, 1865–1879 (2020). https://doi.org/10.1007/s00421-020-04414-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-020-04414-5