Abstract
The possible effects of hot water treatments (HWT) on the main quality parameters and bioactive compounds of two sweet cherry cultivars were examined over two years. Cherries were dipped in hot water (48 °C, 2 min), stored 3 weeks at 1 °C (CS) and for 2 additional days at 20 °C for simulated shelf-life (SL). Except for a slight decrease in firmness generally observed in treated samples (5–6%), no difference was observed between HWT and control fruits, neither for the main quality parameters nor the bioactive compounds during CS and/or SL. On average, the percentage of rotten fruit was more than 50% lower in HWT samples compared to the controls. These results show that the HWT conditions examined in this study effectively reduce the incidence of sweet cherry decay without impairing, after CS and/or SL, most of the main quality parameters or the content of the main bioactive nutrients. Because HWT are residue-free, easy to apply and effective in reducing post-harvest losses while preserving the organoleptic and nutritional quality of cherries, HWT are a good strategy for both cherry professionals and consumers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With a global production increase of about 50% between 2001 and 2021, 1.8 and 2.7 million tons, respectively [1], sweet cherry (Prunus avium L.) is a very popular fruit worldwide with a high market value. Highly appreciated by consumers for its attractive color and sweetness, sweet cherry is also known to be a good source of health beneficial compounds, such as vitamins and phenolics [2,3,4,5]. In particular, sweet cherries have been reported to exhibit potential cancer chemopreventive properties, or to reduce the risk of developing diabetes and Alzheimer’s disease [6,7,8]. In recent years, consumers have become increasingly aware of the impact of food on health, and many of them are willing to pay higher prices for fresher, tastier, and healthier products [9]. As a non-climacteric fruit, sweet cherry must be picked when fully ripe to guarantee good quality [10], but due to its short postharvest shelf-life, cold storage is commonly used to extend its market window. Nevertheless, as the storage time increases fruit quality generally significantly decreases (water loss, softening, …) and postharvest rots, mainly caused by fungal pathogens (Monilinia spp., Botrytis cinerea, Penicillium expansum, …) often occur [11, 12]. Although these fungal pathogens can be controlled using synthetic fungicides, there is a growing demand from consumers and regulatory authorities to decrease, or even prohibit, their use [13]. Pre-storage hot water treatments (HWT) have previously been reported to be effective and natural alternatives to synthetic chemical treatments against postharvest rots in many fruits including cherries [14,15,16,17].
Nevertheless, information on the influence of HWT on nutritional and bioactive compounds is scarce, and apart from some work [18,19,20,21,22], little is known about their potential impacts on polyphenolic compounds. Therefore, the objective of this study was to determine over two years the effects of HWT on the levels of vitamin C and polyphenols in two sweet cherry cultivars after 3 weeks of cold storage at 1 °C and after an additional shelf-life period of 2 d at 20 °C. Because firmness, sweetness and acidity are decisive characteristics for consumer acceptance of sweet cherries [23], fruit firmness, soluble solids content, titratable acidity as well as levels of individual sugars and organic acids were determined. The efficiency of HWT on fruit rot was also evaluated.
Materials and methods
Chemicals and reagents
All chemicals and reagents were as previously described [24] unless otherwise indicated.
Materials
In 2020 and 2021, two sweet cherry (Prunus avium L.) cultivars, Balrine and Regina, were manually harvested at commercial ripening stage in the experimental orchard of the Interprofessional Technical Center for Fruit and Vegetables (CTIFL) of Balandran (Gard, France), and then transferred to the laboratory within two hours. For each year and cultivar, about 6–8 kg of homogeneous fruits in color and size and without any visual defects were selected. Fruits were then randomly packed into commercial punnets of 250 g. Three punnets of 25 fruits were then randomly selected and directly analyzed at harvest as described below. Remaining punnets were then randomly divided into two groups: one group was the untreated control, whereas the other was dipped into hot water at 48 °C for 2 min (HWT). These conditions were chosen based on results obtained in preliminary experiments carried out at the CTIFL (data not shown). Punnets were left for 2 h at room temperature until the water had evaporated, and then punnets of control and HWT fruits were stored for 3 weeks at 1 °C (90% RH). At the end of cold storage (CS), an additional shelf-life period of 2 d at 20 °C (SL) was applied. Three punnets of control and HWT fruits were analyzed at the end of CS, and at the end of SL. Each punnet was considered as a biological replicate.
Fruit decay evaluation
Fruit rot was visually evaluated at the end of CS and SL. Fruit that showed any sign of surface mycelia development were considered as decayed. Rot ratio was expressed as a percentage of infected sweet cherry fruits.
Firmness
Firmness was performed on both sides of each fruit from each replicate using an electronic Durofel (licensed by CTIFL-Copa Technologie, Saint Etienne du Grès, France) fitted with a 0.25 mm tip. The measurements, ranging from 0 (no resistance) to 100 (maximum resistance), are expressed in ID25. Fruits were then hand-pitted, cut into small cubes (< 1 cm3) and frozen under liquid nitrogen. The cubes were then immediately reduced to powder using an IKA A11 analytical mill for 30 s (IKA, Staufen, Germany) with liquid nitrogen, and total vitamin C was immediately determined as described below (see “Vitamin C” section). The frozen powders were then stored at − 80 °C until analysis.
Soluble solids content (SSC) and titratable acidity (TA)
About 20 g of frozen powder (− 80 °C) were then thawed for 30 min at room temperature and then centrifuged (14,000g, 5 min, 4 °C) (Sigma 4K15, Sigma Laborzentrifugen GmbH, Osterode am Harz, Germany). SSC was determined from the supernatant with an Atago PR-32 digital refractometer (Atago Co., Ltd., Tokyo, Japan). TA was determined by diluting 5 mL of supernatant with 30 mL of deionized water, and titrating to pH 8.1 with 0.1 N NaOH using an automatic titrator with autosampler (Titroline 7000, Schott SI Analytics, Mainz, Germany).
Individual sugars and organic acids
The levels of sugars and organic acids were simultaneously determined by HPLC as previously described [24]. Briefly, 1 mL of supernatant previously obtained, diluted 20-fold with deionized water, was filtered (RC 0.2 µm Phenex; Phenomenex, Le Pecq, France) and directly injected into the HPLC system. Identifications were performed by comparing retention times (tR) with those of standards and by spiking samples with pure compounds.
Vitamin C
Total vitamin C was determined by HPLC as previously described [24]. Briefly, 1 g of frozen powder added to 10 mL of metaphospheric acid (MPA) (2%) was homogenized for 60 s at 20,000 rpm with an Ultra-Turrax (IKA T25-Digital). After centrifugation (Sigma 4K15, 14,000g, 5 min, 4 °C), the supernatant was filtered (RC 0.2 µm Phenex; Phenomenex). Total vitamin C was determined by HPLC after 3 h of reduction under agitation at room temperature of 500 µL of supernatant added to 500 µL of tris-(2-carboxy-ethyl)-phosphine TCEP (0.01 mol/L) in MPA (2%). Chromatographic conditions were as previously described. Identifications were performed by comparing retention times (tR) with that of the standard of ascorbic acid and by spiking samples with a pure compound.
Polyphenols
The levels of polyphenols were determined by UPLC-MS as previously described [24]. Briefly, four grams of frozen powder added with 20 mL of a mixture of MeOH/H2O/formic acid (60/38/2 v/v/v) and 100 µL of methyl 4-hydroxybenzoate (3515 mg/L in MeOH/formic acid; 95/5 v/v) (internal standard) were homogenized for 60 s at 20,000 rpm with an Ultra-Turrax (IKA T25-Digital) and centrifuged (Sigma 4K15, 10,000g, 5 min, 4 °C). 10 mL of supernatant was concentrated to dryness under a stream of nitrogen using an XcelVap automated evaporation system (Horizon technology, New Hampshire, USA). The residue was dissolved in 1 mL of mixture of MeOH/formic acid (95/5 v/v), filtered on Phenex RC 0.2 µm (Phenomenex) and then injected in UPLC-MS. The UPLC-MS analyses were performed on an ACQUITY UPLC system equipped with a photo-diode array (PDA) detector (Waters, Milford, MA, USA) coupled to a mass single-quadrupole detector (QDa, Waters), which is a compact detector with an electrospray ionization (ESI) interface. Empower 3 software was used for data acquisition and instrument control. Separation was carried out at 35 °C using a Waters Acquity HSS T3 column (100 mm × 2.1 mm i.d., 1.8 μm) protected by a precolumn filter (Waters). A binary solvent system was used at a 0.4 mL/min flow rate with solvent A (water/formic acid; 98.5:1.5 v/v) and solvent B (methanol). The elution gradient was as follows: 0–1 min, isocratic 0% B; 1–18 min, linear 0–74.5% B; 18–22 min, isocratic 95% B; 22–27 min, isocratic 0% B. The volume of injection was 1 μL. With the PDA detector, the flavonols were detected at 255 nm, flavanols at 280 nm, hydroxycinnamic acids at 320 nm, and anthocyanins at 505 nm. Compound identification was achieved by comparing the retention times (tR) and their UV–Vis spectra from 240 to 600 nm with those of standards, and by spiking samples with pure compounds whenever possible. The QDa detector was operated in negative and positive ion modes for polyphenol and anthocyanin characterization. ESI capillary voltage and cone voltage were set at 0.8 kV and 15V, respectively. Probe temperature was set at 600 °C. A full mass spectrum between m/z 100 and 1200 was acquired at a sampling rate of 8.0 points/sec. Flavonols were quantified by comparisons with an external standard of quercetin-3-glucoside, flavan-3-ols as (+)-catechin, hydroxycinnamic acid derivatives as 5-caffeyolquinic acid, and anthocyanins as cyanidin-3-glucoside. Levels of phenolic compounds were expressed as milligrams per 100 g of FW.
Statistical analysis
Data are given as mean ± standard deviation (n = 3). Significant differences between the five treatments (Harvest, Control CS, HWT CS, Control SL, and HWT SL) were evaluated year by year for each cultivar through one-way analysis of variance (ANOVA). Homogeneity of variance was tested using Levene’s median test. Welch-ANOVA was applied when data were not homoscedastic. Multiple comparisons of means were carried out using Duncan's test (p < 0.05). Data were also analyzed by three-way ANOVA using a completely randomized design (2 years × 2 cultivars × 5 treatments × 3 replicates). The effect size of the different factors and their interaction was evaluated using omega squared value (ω2) calculated as follow: ω2 = (SSFactor − dfFactorxMSError)/(SSTotal + MSError), where SSFactor = sum of squares of each factor, dfFactor = degrees of freedom of each factor, MSError = mean square error and SSTotal = sum of squares total [25]. The obtained value, multiplied by 100, thus represents the variance in the population as explained by each factor. As compared to eta squared and partial eta squared, ω2 was preferred because this estimation resulted in less bias when dealing with small samples. Statistical analyses of data were performed using XLSTAT 2019.1.1 (Addinsoft, Paris, France).
Results and discussion
Fruit decay and firmness
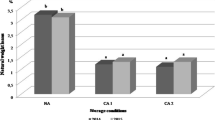
Three-way ANOVA results (Fig. 1A) showed that the percentage of rotten fruit was highly affected (p < 0.001) by the postharvest treatment and the cultivar. According to the omega-squared value (ω2), which explains the contribution of each factor to the observed variability, the postharvest treatment was the most significant factor (ω2 = 66%), followed by cultivar (ω2 = 6%). Year (p < 0.05) and the interaction cultivar × treatment (p < 0.01) also affected the percentage of rotten fruit, but the ω2 values were below 5%. No significant interaction year × cultivar or treatment, or year × cultivar × treatment was observed. As shown in Fig. 1A, HWT notably reduced the percentage of rotten fruit as compared to untreated samples, particularly after the two additional days of shelf-life at 20 °C. Over the two years and for the two cultivars, the percentage of rotten fruit in HWT samples (8%) was on average more than half that in the controls (18%). Lastly, as indicated Fig. 1B, flesh firmness was mainly affected (p < 0.001) by the year (ω2 = 56%), followed by the postharvest treatment (ω2 = 15%) and the interaction year × cultivar (ω2 = 15%). The interaction year × treatment (p < 0.01) also affected the firmness but the ω2 values was below 5%. Flesh firmness was not influenced by cultivar. No significant interaction cultivar × treatment or cultivar × year × treatment was observed. As observed, at the end of CS, flesh firmness of fruit was generally similar between samples, except for Regina in 2021, where the flesh firmness of HWT fruit was about 3% lower than in controls. As previously reported [26], firmness of heated fruits were after SL less firm (5–6%) compared to control ones, excepted for Balrine in 2021 where no difference was observed.
Percentage of rotten fruits (A) and firmness (B) in control and HWT sweet cherries after 21 d of cold storage at 1 °C (CS) and additional 2 d of shelf-life at 20 °C (SL) in 2020 and 2021. Values are the mean ± standard deviation (n = 3). For a given year and cultivar, values with different letters are significantly different (p < 0.05, Duncan’s test). For three-way ANOVA results, only ω2 > 5% are indicated in parenthesis. ns (not significant); * (p < 0.05); ** (p < 0.01); *** (p < 0.001)
Soluble solids content (SSC) and titratable acidity (TA)
As observed in Table 1, SSC ranged from 16.4 to 19.9°Brix in the different sweet cherry samples and TA from 8.5 to 13.5 meq/100g. Three-way ANOVA results showed that SSC was mainly affected (p < 0.001) by the year (ω2 = 42%), the cultivar (ω2 = 24%) and the interaction year × cultivar (ω2 = 18%). As shown, neither the treatment factor nor its interaction with year and/or cultivar significantly affected SSC. In contrast, TA was mainly affected (p < 0.001) by the postharvest treatment (ω2 = 45%), followed by the year (ω2 = 24%), the interaction year × cultivar (ω2 = 17%) and the cultivar (ω2 = 5%). No interaction treatment with year and/or cultivar was observed for TA. For both years and both cultivars (Table 1), SSC remained constant in control fruits during the 21 d of cold storage at 1 °C (CS) and/or after the two additional days of shelf-life at 20 °C (SL), whereas TA decreased significantly by about 16–23% during CS and then remained constant during SL. These results agree with those previously reported in other sweet cherry cultivars, and confirm that SSC is generally not affected by cold storage whereas a loss of acidity is observed [27,28,29]. As shown in Table 1, no significant difference was observed between HWT and control fruits for the SSC and TA values, either after CS and/or 2 d of SL.
Sugars and organic acids
In this study, the quantified sugars were glucose, fructose, sorbitol and sucrose, on average 46%, 37%, 16% and 1% respectively of the total sugars, that ranged between 14.9 and 19.2 g/100g FW in the different samples (Table 1). Three-way ANOVA results showed that total sugars were highly affected by the year (ω2 = 60%), the cultivar (ω2 = 15%), and the interaction year × cultivar (ω2 = 11%). Similar trends were observed for glucose and fructose, while sorbitol and sucrose were mainly affected by the year (ω2 = 69–74%). With levels ranging from 0.9 to 1.2 g/100g FW (Table 1), malic acid was, as previously reported in other sweet cherry cultivars [5, 30], the main organic acid detected in Balrine and Regina cherries. Citric and shikimic acids were also detected in this study, but at trace levels, and therefore not quantified. Three-way ANOVA results showed malic acid, was mainly affected (p < 0.001) by the postharvest treatment (ω2 = 46%), followed by the year (ω2 = 31%), the interaction year × cultivar (ω2 = 12%) and the cultivar (ω2 = 2%). No interaction treatment with year and/or cultivar was observed. For both years and both cultivars (Table 1), the levels of the three main sugars remained constant in control fruit during CS and/or after SL, whereas those of malic acid decreased significantly by about 12–18% during CS and then remained constant during SL. Consistent with results of SSC and TA, these results are also in agreement with those previously reported in other sweet cherry cultivars [31, 32].
As shown in Table 1, no significant difference was observed between HWT and control fruits for the levels of glucose, fructose, sorbitol and malic acid, either after CS and/or SL.
Vitamin C
In this study, vitamin C contents in the different sweet cherry samples ranged between 6.5 and 15.8 mg/100g FW (Table 1). Three-way ANOVA results showed vitamin C was mainly affected (p < 0.001) by the postharvest treatment (ω2 = 58%), followed by the cultivar (ω2 = 21%), and the year (ω2 = 10%). No interaction year × cultivar or year × treatment was observed. Significant interaction cultivar × treatment (p < 0.001) and year × cultivar × treatment (p < 0.05) were observed but the ω2 values were lower than 5%. For both years and both cultivars, levels of vitamin C in control fruits significantly decreased from between 16 and 33% after CS, and then decreased again from between 8 and 22% after SL. These results are in agreement with those previously reported in other sweet cherry cultivars, and confirm that vitamin C drastically decreases during cold storage and/or shelf-life [29, 33, 34]. As shown in Table 1, a similar trend was observed in HWT fruits. Whether after CS and/or SL, levels of vitamin C in treated and untreated fruits were generally not statistically different, except for Balrine where the decrease observed in HWT fruits after CS in 2020 was half of that observed in control fruits (12% and 24%, respectively).
Polyphenols
In this study, six hydroxycinnamic acid derivatives (HA1–HA6), four flavan-3-ols (FA1–FA4), three anthocyanins (AN1–AN3) and three flavonols (FO1–FO3) were identified and quantified using UPLC-PDA-QDa (Table 2). To our knowledge, this is the first time that a detailed quantitative analysis of individual phenolic compounds is reported in the Balrine sweet cherry cultivar. As previously reported in other cultivars [2, 35,36,37], hydroxycinnamic acids were predominant (32–50% of the total polyphenol content), followed by anthocyanins (21–42%), flavan-3-ols (20–29%), and flavonols (3–4%) (Table 3). Among hydroxycinnamic acids, neochlorogenic acid (HA1; 17.0–27.4mg/100g FW), 3-p-coumaroylquinic acid (HA2; 10.5–17.9mg/100g FW), and chlorogenic acid (HA3; 3.2–5.3 mg/100g FW) were predominant and together accounted for 93–96% of total hydroxycinnamic acids. Three-way ANOVA results indicated that the levels of the three main hydroxycinnamic acids were significantly (p < 0.001) affected by year and cultivar. According to ω2 value, the year explained 65–73% of the total variability of neo and chlorogenic acid, while the cultivar explained 65% of the total variance of 3-p-coumaroylquinic acid. As indicated in Table 3, the levels of the three main hydroxycinnamic acids were not affected by the treatment factor. No interaction was observed for neochlorogenic acid. A significant year × cultivar interaction (p < 0.001) was observed for 3-p-coumaroylquinic acid and chlorogenic acid but the ω2 values were below 5%. Remaining interactions, year × treatment for 3-p-coumaroylquinic acid (p < 0.05) or cultivar × treatment for chlorogenic acid (p < 0.05), were also below 5%.
Previous studies reported that cold storage has variable effects on phenolic acids in sweet cherries, depending mainly on cultivar and storage conditions [38]. While a decrease of neochlorogenic acid and 3-p-coumaroylquinic acid was observed after a month at 4 °C in the sweet cherries of Bing cultivar [39], their levels were found to remain relatively constant in the cultivars Van and Burlat after 30 d of storage but were found to decrease in the cultivars Saco and Summit during the same period of time [2]. Similarly, while the levels of these two compounds were not affected in the Lambert Compact cultivar after 12 d of storage at 2–4 °C [40], their levels sharply increased in the Sweetheart cultivar after 27 d at 0 °C [41]. In our study, for both years and both cultivars (Table 3), no significant change was observed in control fruits for the levels of the three main hydroxycinnamic acids after CS and/or after SL. Similar results were observed in HWT fruits. No significant difference was observed between HWT and control fruits for the levels of the three main hydroxycinnamic acids, either after CS and/or SL.
Cyanidin-3-rutinoside (AN2; 16.9–51.7 mg/100g FW) was the major anthocyanin detected in this study and accounted for 88–94% of total anthocyanins (Table 3). Three-way ANOVA results showed cyanidin-3-rutinoside was mainly affected (p < 0.001) by the cultivar (ω2 = 77%) and the postharvest treatment (ω2 = 10%). Cyanidin-3-rutinoside was also affected (p < 0.001) by the year and the interaction year × cultivar but the ω2 values were below 5%. No significant interaction between treatment with year and/or cultivar was observed. As shown in Table 3, for both years and both cultivars, levels of cyanidin-3-rutinoside significantly increased in control fruits by about 3–18% during CS, and then increased again by about 19–31% after subsequent SL. These results agree with those previously reported in other cherry cultivars [2, 40, 42, 43]. The accumulation of anthocyanins during storage and/or subsequent SL is attributed to normal sweet cherry ripening, as has been found in other fruits [24, 44, 45]. A similar trend was observed in HWT fruits, and, whether after CS and/or SL, no significant difference was observed between treated and untreated samples.
With ω2 values ranging from 54 to 67%, three-way ANOVA results showed that the cultivar was the most relevant factor (p < 0.001) in explaining the variability of the four flavan-3-ols detected in this study (Table 3). Except for (+)-catechin (FA2), the year factor also significantly (p < 0.001) accounted for a proportion of variance for three of the four flavan-3-ols, ranging from 25% for procyanidin dimer B2 (FA3) to 34% for procyanidin dimer B1 (FA1). As indicated, levels of flavan-3-ols were not affected by the treatment factor. A significant (p < 0.01) year × cultivar interaction was observed for (+)-catechin but the ω2 value was less than 5%. Remaining interactions, year × cultivar for procyanidin dimer B1 (p < 0.05) and (−)-epicatechin (p < 0.05), cultivar × treatment for procyanidin dimer B2 (p < 0.05) and (−)-epicatechin (p < 0.05) were all less than 5%.
For a given year and cultivar (Table 3), no significant difference was observed for the levels of the four flavan-3-ols between the control and HWT samples, whether after CS and/or SL.
Among the three flavonols detected in this study, quercetin 3-O-rutinoside (FO2) was predominant (62–71% of total flavonols) (Table 3). Three-way ANOVA results showed that quercetin 3-O-rutinoside and quercetin triglycoside (FO1) were mainly affected (p < 0.001) by the cultivar (ω2 = 62–81%). The year factor significantly influenced (p < 0.001) the variability of quercetin triglycoside (ω2 = 14%) and to a lesser extent (p < 0.05) that of kaempferol 3-O-rutinoside (FO3; ω2 = 8%) and quercetin 3-O-rutinoside (ω2 < 5%). The treatment factor only affected quercetin 3-O-rutinoside (p < 0.001, ω2 = 10%). A significant interaction year × cultivar was also observed for kaempferol 3-O-rutinoside (p < 0.01; ω2 = 9%) and quercetin triglycoside (p < 0.05; ω2 < 5%). As shown in Table 3, for both years and both cultivars, levels of quercetin triglycoside and kaempferol 3-O-rutinoside remained constant in control and HWT fruits, whether after CS and/or SL. As regards quercetin 3-O-rutinoside, despite some significant differences, no clear trend could be observed.
Conclusion
In conclusion, our results showed that the HWT conditions examined in this study (48 °C, 2 min) significantly reduced the percentage of rotten fruit after storage and/or shelf-life, without having any adverse effects, either on the level of bioactive compounds (vitamin C, polyphenols), or on the main cherry quality parameters (SSC, TA, sugars, organic acids). This study not only confirms that hot water treatment is an effective method against post-harvest rot in cherries, but also that it's a good strategy both for sweet cherry professionals and for consumers. For the former, because it's a simple method to apply to significantly reduce post-harvest losses, and for the latter, because it's a natural method that preserves the organoleptic and nutritional quality of cherries without using synthetic chemicals.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
(2021) FAOSTAT. In: Food Agric. Data Food Agric. Organ. U. N. Retrieved from https://www.fao.org/faostat/en/#home. Accessed 12 Sept 12 2023. Accessed 10 Jan 2021
Gonçalves B, Landbo A-K, Knudsen D et al (2004) Effect of ripeness and postharvest storage on the phenolic profiles of cherries (Prunus avium L.). J Agric Food Chem 52:523–530
Jakobek L, Šeruga M, Novak I, Medvidovic̀-Kosanović M (2007) Flavonols, phenolic acids and antioxidant activity of some red fruits. Dtsch Lebensm Rundsch 103:369–377
McCune LM, Kubota C, Stendell-Hollis NR, Thomson CA (2010) Cherries and health: a review. Crit Rev Food Sci Nutr 51:1–12
Usenik V, Fabčič J, Štampar F (2008) Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.). Food Chem 107:185–192
Kang S-Y, Seeram NP, Nair MG, Bourquin LD (2003) Tart cherry anthocyanins inhibit tumor development in ApcMin mice and reduce proliferation of human colon cancer cells. Cancer Lett 194:13–19
Kim D-O, Heo HJ, Kim YJ et al (2005) Sweet and sour cherry phenolics and their protective effects on neuronal cells. J Agric Food Chem 53:9921–9927
Lachin T (2014) Effect of antioxidant extract from cherries on diabetes. Recent Pat Endocr Metab Immune Drug Discov 8:67–74
Alsubhi M, Blake M, Nguyen T et al (2023) Consumer willingness to pay for healthier food products: a systematic review. Obes Rev 24:e13525
Kader AA (1999) Fruit maturity, ripening, and quality relationships. In: International symposium effect of pre-& postharvest factors in fruit storage, vol 485, pp 203–208
Alonso J, Alique R (2006) Sweet cherries. In: Hui YH, Barta J, Cano MP, Gusek TW, Sidhu JS, Sinha NK (eds) Handbook of fruits and fruit processing. Blackwell Publishing, Oxford, pp 359–367
Romanazzi G, Nigro F, Ippolito A, Salerno M (2001) Effect of short hypobaric treatments on postharvest rots of sweet cherries, strawberries and table grapes. Postharvest Biol Technol 22:1–6
Liu X, Cao A, Yan D et al (2021) Overview of mechanisms and uses of biopesticides. Int J Pest Manag 67:65–72
Chailoo MJ, Asghari MR (2011) Hot water and chitosan treatment for the control of postharvest decay in sweet cherry (Prunus avium L.) cv. Napoleon (Napolyon). J Stored Prod Postharvest Res 2:135–138
Fallik E, Ilić Z (2017) Hot water treatments. In: Pareek S, Pareek S (eds) Novel postharvest treatments of fresh produce. CRC Press, Boca Raton, pp 241–258
Feng X, Hansen JD, Biasi B et al (2004) Use of hot water treatment to control codling moths in harvested California ‘Bing’sweet cherries. Postharvest Biol Technol 31:41–49
Usall J, Ippolito A, Sisquella M, Neri F (2016) Physical treatments to control postharvest diseases of fresh fruits and vegetables. Postharvest Biol Technol 122:30–40
Aguayo E, Requejo-Jackman C, Stanley R, Woolf A (2015) Hot water treatment in combination with calcium ascorbate dips increases bioactive compounds and helps to maintain fresh-cut apple quality. Postharvest Biol Technol 110:158–165
Kim Y, Brecht JK, Talcott ST (2007) Antioxidant phytochemical and fruit quality changes in mango (Mangifera indica L.) following hot water immersion and controlled atmosphere storage. Food Chem 105:1327–1334
Kim Y, Lounds-Singleton AJ, Talcott ST (2009) Antioxidant phytochemical and quality changes associated with hot water immersion treatment of mangoes (Mangifera indica L.). Food Chem 115:989–993
Michailidis M, Karagiannis E, Tanou G et al (2019) An integrated metabolomic and gene expression analysis identifies heat and calcium metabolic networks underlying postharvest sweet cherry fruit senescence. Planta 250:2009–2022
Schirra M, Palma A, D’Aquino S et al (2008) Influence of postharvest hot water treatment on nutritional and functional properties of kumquat (Fortunella japonica Lour. Swingle Cv. Ovale) fruit. J Agric Food Chem 56:455–460
Ricardo-Rodrigues S, Laranjo M, Agulheiro-Santos AC (2023) Methods for quality evaluation of sweet cherry. J Sci Food Agric 103:463–478
Aubert C, Bruaut M, Chalot G, Cottet V (2021) Impact of maturity stage at harvest on the main physicochemical characteristics, the levels of vitamin C, polyphenols and volatiles and the sensory quality of Gariguette strawberry. Eur Food Res Technol 247:37–49
Plasquy E, Florido MC, Sola-Guirado RR, García JM (2021) Effects of a harvesting and conservation method for small producers on the quality of the produced olive oil. Agriculture 11:417
Vangdal E, Nordbø R, Flatland S (2004) Postharvest calcium and heat treatments of sweet cherries (Prunus avium L.). In: V international postharvest symposium, vol 682, pp 1133–1136
Agulheiro Santos AC, Palma V, Rato AE et al (2009) Quality of ‘Sweetheart’ cherry under different storage conditions. In: VI international cherry symposium, vol 1020, pp 101–110
Drake SR, Elfving DC (2002) Indicators of maturity and storage quality of ‘Lapins’ Sweet Cherry. HortTechnology 12:687–690
Tian S-P, Jiang A-L, Xu Y, Wang Y-S (2004) Responses of physiology and quality of sweet cherry fruit to different atmospheres in storage. Food Chem 87:43–49
Serrano M, Guillén F, Martínez-Romero D et al (2005) Chemical constituents and antioxidant activity of sweet cherry at different ripening stages. J Agric Food Chem 53:2741–2745
Esti M, Cinquanta L, Sinesio F et al (2002) Physicochemical and sensory fruit characteristics of two sweet cherry cultivars after cool storage. Food Chem 76:399–405
Goliáš J, Němcová A, Šuderlová L (2003) Effect of low oxygen and anaerobic conditions as post-harvest treatment on the quality of sweet cherry fruit. Mitt Klosterneubg 53:123–131
Aglar E, Ozturk B, Guler SK et al (2017) Effect of modified atmosphere packaging and ‘Parka’ treatments on fruit quality characteristics of sweet cherry fruits (Prunus avium L. ‘0900 Ziraat’) during cold storage and shelf life. Sci Hortic 222:162–168
Zhao H, Wang B, Cui K et al (2019) Improving postharvest quality and antioxidant capacity of sweet cherry fruit by storage at near-freezing temperature. Sci Hortic 246:68–78
Kosińska A, Diering S, Prim D et al (2013) Phenolic compounds profile of strawberry fruits of Charlotte cultivar. J Berry Res 3:15–23
Nowicka A, Kucharska AZ, Sokół-Łętowska A, Fecka I (2019) Comparison of polyphenol content and antioxidant capacity of strawberry fruit from 90 cultivars of Fragaria× ananassa Duch. Food Chem 270:32–46
Seeram NP, Lee R, Scheuller HS, Heber D (2006) Identification of phenolic compounds in strawberries by liquid chromatography electrospray ionization mass spectroscopy. Food Chem 97:1–11
Habib M, Bhat M, Dar BN, Wani AA (2017) Sweet cherries from farm to table: a review. Crit Rev Food Sci Nutr 57:1638–1649
Sharma M, Jacob JK, Subramanian J, Paliyath G (2010) Hexanal and 1-MCP treatments for enhancing the shelf life and quality of sweet cherry (Prunus avium L.). Sci Hortic 125:239–247
Mozetič B, Simčič M, Trebše P (2006) Anthocyanins and hydroxycinnamic acids of Lambert Compact cherries (Prunus avium L.) after cold storage and 1-methylcyclopropene treatment. Food Chem 97:302–309
González-Gómez D, Bernalte MJ, Ayuso MC et al (2013) Evaluation of different postharvest conditions to preserve the amount of bioactive compounds, physicochemical quality parameters and sensory attributes of ‘Sweetheart’ cherries. In: VII international cherry symposium, vol 1161 pp 581–586
Serrano M, Diaz-Mula HM, Zapata PJ et al (2009) Maturity stage at harvest determines the fruit quality and antioxidant potential after storage of sweet cherry cultivars. J Agric Food Chem 57:3240–3246
Valero D, Diaz-Mula HM, Zapata PJ et al (2011) Postharvest treatments with salicylic acid, acetylsalicylic acid or oxalic acid delayed ripening and enhanced bioactive compounds and antioxidant capacity in sweet cherry. J Agric Food Chem 59:5483–5489
Díaz-Mula HM, Zapata PJ, Guillen F et al (2008) Changes in physicochemical and nutritive parameters and bioactive compounds during development and on-tree ripening of eight plum cultivars: a comparative study. J Sci Food Agric 88:2499–2507
Zheng Y, Yang Z, Chen X (2008) Effect of high oxygen atmospheres on fruit decay and quality in Chinese bayberries, strawberries and blueberries. Food Control 19:470–474
Acknowledgements
Thanks to Janet Zaragoza for proofreading the article.
Author information
Authors and Affiliations
Contributions
All authors conceived and designed the study. FB and GC performed the experiments and acquired data. CA performed statistical analysis and wrote the paper. All authors contributed to the discussion of the data and critically revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aubert, C., Bulver, F., Chalot, G. et al. Effects of hot water treatments on the main physicochemical characteristics and the levels of vitamin C and polyphenols of two sweet cherry cultivars (Prunus avium L.) during cold storage and shelf-life. Eur Food Res Technol 250, 1641–1651 (2024). https://doi.org/10.1007/s00217-024-04495-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-024-04495-y