Abstract
Chestnut shell, a by-product of chestnut processing, is rich in bioactive polyphenols, which can be divided into extractable polyphenols (EPs) and non-extractable phenols (NEPs). The EPs have arisen a lot of interests, while NEPs combined with cellulose in the residue are ignored. This work describes the development of a traditional ethanol extraction method to obtain extractable polyphenols (EPs), and the residue was treated by alkaline hydrolysis to prepare NEPs optimized by Box–Behnken experimental designs. The total phenolic contents and the scavenging effects of DPPH and ABTS+ free radicals were measured. Using UPLC–ESI-MS/MS technology, combined with principal component analysis (PCA) and orthogonal partial least square discriminant analysis (OPLS-DA), qualitative and quantitative analysis and difference analysis of EPs and NEPs were carried out. Optimal extraction conditions to extract NEPs by alkaline hydrolysis method were obtained, and the content of polyphenols obtained from NEPs was significantly higher than that of EPs. EPs have the higher scavenging effects of DPPH and ABTS+ free radicals than NEPs. A total of 466 polyphenol compounds were detected. Among them, EPs are rich in flavonoids, phenolic acids, tannins, lignans coumarins and quinones, while phenolic acids were dominant in NEPs, which provides a reference for the utilization of polyphenols from the chestnut shell. Both EPs and NEPs are abundant in phenolic composition, although there were significant differences in the compositional situation. Notably, NEPs are a widespread source of polyphenols but have not received much attention in practical applications. This study can provide a basis for the reuse of chestnut waste.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chestnut (Castanea mollissima Blume) belongs to the Fagaceae family, and it is an important food crop in many countries and regions for several centuries [1]. Chestnut fruit is the main edible part because of its rich contents of starch, amino acids, fiber, fatty acid, vitamins, minerals and other nutrients [2, 3]. At present, China has become the major chestnut producer with an annual yield of about 1.0 × 106 tons, and a large volume of useful byproducts is produced including chestnut shell [4]. Chestnut shell is accounts for about 10% of the total weight of chestnut, and is disposed as fuel, but the burning of chestnut shell will produce toxic chemicals (such as carbon monoxide, nitrogen oxide, aromatic/long-chain hydrocarbons compounds, etc.), which will cause negative impact on the environment [5, 6]. It is reported that polyphenols have antioxidant, anti-cancer, and anti-inflammatory activities, which has a positive effect on human health [7,8,9,10]. Thus, there has been a great interest in reusing chestnut waste, both because it reduces environmental pressure and represents high-value bioactive compounds, which have important physiological significance as secondary metabolites of plants [11,12,13]. It was found that chestnut shell polyphenols contained tannins, flavonoids and phenolic acids and other polyphenols, which had been proved to have a variety of biological activities. It could also be used in food anticorrosion, paper and cosmetics production and other industries [10, 14].
However, in terms of extraction process, the traditional solvent extraction method can only extract extractable polyphenols (EPs), while an important fraction of polyphenols remains retained in the extraction residue and cannot be extracted even with the advanced extraction techniques such as ultrasound assisted extraction, microwave assisted extraction, and supercritical carbon dioxide, which can give rise to greater extraction yields [15,16,17,18]. This fraction corresponds to non-extractable polyphenols (NEPs), which are high molecular weight polymeric polyphenols or individual low molecular weight phenolics cross-linked with macromolecules (e.g., protein, cellulose, hemicellulose, pectin, and lignin in plant cell walls) to form bound polyphenols [19]. For these reasons, NEPs are inaccessible to solvents during the extraction process due to their different interactions [20]. At present, the extraction and utilization of plant polyphenols mainly focus on EPs, while the utilization of NEPs is relatively less because of the difficulty to extract [21].
In terms of extraction methods, EPs in chestnut shell are usually extracted by solvent method with water, methanol, ethyl alcohol, acetone and other solvents [22,23,24]. However, the recovery process of NEPs requires hydrolysis (chemical hydrolysis or enzymatic hydrolysis) [25]. It is reported that polyphenols from chestnut shell were extracted to a greater extent by alkali solution (NaOH, Na2SO3, Na2CO3) [26, 27]. This is because alkaline hydrolysis has effective hydrolysis on both ether and ester bonds [19]. NEPs with high molecular weight are less known and they belong to proanthocyanidin group, whose monomers are flavan-3-ols, and hydrolysable tannins, derived from gallic and ellagic acid [28]. Lin et al. used lotus seed as raw material to extract bound polyphenols with alkaline hydrolysis. The results showed that the polyphenols could still be obtained after the lotus seeds were extracted by solution and hydrolyzed by alkaline hydrolysis. The polyphenol obtained after alkaline hydrolysis can inhibit obesity in mice, which may be realized by regulating AMPK signal pathway [29].
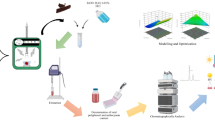
Therefore, the main purpose of this study was to study the composition and biological activity of EPs and NEPs from chestnut shell to find the suitable application direction. An efficient extraction method based on alkaline hydrolysis (NaOH) for the recovery of NEPs from chestnut shell was developed. Box–Behnken designs were used to select optimal extraction conditions including NaOH concentration (x1), liquid to material ratio (x2), and extraction time (x3). Furthermore, the antioxidant activity of EPs from chestnut shell prepared by conventional ethanol extraction method was compared with the NEPs obtained under optimal alkaline hydrolysis extraction conditions. Ultra Performance Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry (UPLC–ESI-MS/MS) was employed to estimate the phenolic compositions of the EPs and released NEPs. Raw material treatment and total experimental process are illustrated in Fig. 1.
Schematic description of raw material treatment and total experimental process. UPLC–MS ultra-performance liquid chromatography-mass spectrometry, PCA principal component analysis, OPLS-DA Orthogonal Partial Least Squares-Discriminant Analysis, EPs extractable polyphenols, NEPs non-extractable phenols
Materials and methods
Chemicals and reagents
Anhydrous methanol, anhydrous ethanol, sodium hydroxide (NaOH), hydrochloric acid solution (37%), Folin–Ciocalteu reagent, gallic acid, L-ascorbic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azinobis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS) were purchased from Merck (Darmstadt, Germany).
Plant materials
Chestnut shell was purchased at a local market (Qianxi, China) in October 2020. The mildew-free and worm-free chestnut shells were washed with clean water and dried in oven at 50 ℃. Then, they were crushed with a high-speed crusher (Model FW80, Teste Instrument, Tianjin, China) and then screened through 60-mesh sieve to remove the larger particles and fluff. The resulting chestnut shell powder was stored in a refrigerator at – 20 °C.
Preparation of EPs
Extraction of EPs from chestnut shell
The extraction of EPs was carried out according to the method of Tu [30]. Two hundred grams of chestnut shell powder were added in 3000 mL of 70% ethanol solution (V/V), and the mixture was placed in a constant temperature culture shaker with 180 rpm (THZ-100, Blue pard, Co., Ltd., Shanghai, China) at 50 ℃ for 2 h. Then, the above mixture was leached (SHZ-DIII, Yuhua Instrument Co., Ltd., Henan, China), and the filtrate was the EPs solution. Further, the residue was extracted again by the above operation for three times and all filtrate was combined together, and concentrated in a rotary evaporator (RE-52AA, Yarong Instrument Co., Ltd., Shanghai, China) at 45 ℃. Finally, the concentrated liquid was placed in the freeze dryer (LGJ-18S, Songyuanhuaxin Science and Technology Development Co., Ltd., Beijing, China) for freeze-drying to obtain the crude EPs. In addition, the residue was dried in an oven at 50 ℃ and stored in a refrigerator at – 20 ℃.
Purification of crude EPs
The crude EPs were purified according to the method described by Cheng et al., with some modifications [31]. Crude EPs freeze-dried powder was dissolved in deionized water to prepare EPs aqueous solution with a concentration of 10 mg/mL, the AB-8 resin should be soaked in anhydrous ethanol for 24 h to activation, then the activated resin was filled into a chromatographic column (chromatographic column specification: 200 mm × Ф10 mm), after that, deionized water was used to wash away the residual ethanol. After that, the above EPs aqueous solution was injected into the chromatographic column with a flow rate of 1 mL/min. When adsorption equilibrium is reached, three times resin column volume of deionized water was used for elution to remove impurities. AB-8 resin chromatography column should be eluted with 60% ethanol. The eluent was collected for concentration and freeze-drying to obtain the purified EPs powder and it was stored at – 20 ℃.
Preparation of NEPs
Optimization of alkaline hydrolysis
Referring to the method of Zheng et al., alkaline extraction was applied to release the bound phenols, with some modification [32], and 1 g of residue after extracting EPs from chestnut shell powder with ethanol was hydrolyzed with NaOH solution. The initial conditions were as follows: the concentration of NaOH was 2 mol/L, the ratio of liquid to material was 1:20, and the extraction time is 2 h. Then the optimum conditions of the three single factors were explored, that is, the concentration of NaOH was from 0.5 to 3 mol/L, the ratio of liquid to material was from 1:10 to 1:60, and the extraction time was from 1 to 5 h. The reaction was carried out at 50 ℃ in the shaker (THZ-100, Blue pard, Co., Ltd., Shanghai, China) with 180 rpm. The response surface test was designed by Box–Behnken with three factors and three levels, and the best extraction conditions were obtained. Five verification experiments were carried out using the optimal conditions, and the results were compared with the predicted values.
Preparation of crude NEPs
According to the best alkaline hydrolysis conditions obtained in the previous section, extraction residue was hydrolyzed and the pH of the mixture was quickly adjusted to 7 with an appropriate amount of hydrochloric acid. Then, the mixture was centrifuged with 5000 rpm for 10 min. The supernatant was frozen quickly (− 50 ℃), and freeze-dried in a freeze dryer to get crude NEPs, which were put in the refrigerator at – 20 ℃.
Purification of crude NEPs
The purification method of crude NEPs is similar to that of the crude EPs in 2.3.2, but the resin is changed from AB-8 resin to NKA-9 resin. The pH of crude NEP solution (5 μg/mL) was adjusted to 5 and 200 mL of the solution was put on the column with a flow rate of 1 ml/min. Then, purified NEPs were obtained after elution, concentration and lyophilization, which was the same with the method of purification of crude EPs.
Determination of total polyphenol content (TPC)
Total phenolics were quantified by the Folin–Denis spectrophotometric assay [33], and a few modifications were made using the method of Grace [34]. A portion of 0.5 mL sample solution was mixed with 4.5 mL deionized water, 0.5 mL 1 mol/L Folin–Ciocalteu reagent and 1 mL 0.5 mol/L Na2CO3 solution in a 10 mL tube. The mixture was shaken well and placed in the dark at room temperature for 1 h. After reaction, the absorbance was measured at 765 nm with UV-1800PC spectrophotometer (Aoe Instrument Co., Ltd., Shanghai, China). Standard curve with different concentrations of gallic acid (0.03 mg/mL–0.15 mg/mL) was drawn. The results were expressed as milligrams of gallic acid equivalents (GAE).
Determination of antioxidant capacity
DPPH radical scavenging assay
The DPPH free radical scavenging ability was determined by the method of Fan with a slight modification [35, 36]. A portion of polyphenol sample (2.0 mL) was mixed with 0.175 mmol/L DPPH ethanol solution (2.0 mL). After shaking well, the mixture was incubated at room temperature in the dark for 30 min and the absorbance was measured at 517 nm with deionized water as the blank control. Ascorbic acid and gallic acid were used as the positive control, and all of the experiments were repeated for three times. The free radical scavenging ability is expressed by the scavenging rate, and its formula is as follows.
where Asample is the absorbance of DPPH with the sample at 517 nm; Acontrol is the absorbance of DPPH without the sample at 517 nm.
ABTS+ radical scavenging assay
ABTS+ was determined according to Re [37]. A portion of ABTS aqueous solution (7 mmol/L) was mixed with 2.45 mmol/L potassium persulfate aqueous solution, and the mixture was placed at room temperature for 12 h under the condition of avoiding light to obtain the ABTS+ reserve solution. Then, the above reserve solution was diluted with anhydrous ethanol to the absorbance of 0.7 ± 0.0020 at 734 nm to obtain the ABTS+ working solution. The sample to be tested was dissolved in deionized water, and different concentrations of solutions were prepared, then 200 μL sample solution was added into 4 mL ABTS+ working solution. After fully mixing, the mixture was placed at room temperature for 10 min in the dark. The absorbance was determined at 734 nm, and deionized water was used as blank. Ascorbic acid and gallic acid were used as the positive control, and all of the experiments were repeated for three times. The scavenging rate of ABTS+ free radicals is calculated according to the following formula:
where Asample is the absorbance of ABTS+ with the sample at 734 nm; Acontrol is the absorbance of ABTS+ without the sample at 734 nm.
Phenolic composition analysis
Sample preparation and extraction
The extraction and preparation of samples mainly include (1) the sample to be tested is thawed and fully scrolled for 10 s to mix well; (2) place 50 mg of above sample into a 2 mL of centrifuge tube; (3) as internal standard extract, 600μL of 70% methanol was added and scrolled for 3 min; (4) centrifuge them (12,000 r/min) at 4 °C for 10 min; (5) the supernatant was filtered with a microporous filter membrane (0.22 μm) and stored in a sample flask for UPLC–ESI-MS/MS test.
Determination method of UPLC
The pretreated samples were analyzed by an UPLC–ESI-MS/MS system including UPLC (SHIMADZU Nexera X2) and MS/MS (Applied Biosystems 4500 QTRAP). The UPLC conditions mainly include: (1) Column: Agilent SB-C18 1.8 μm, 2.1 mm × 100 mm; (2) Mobile phase: phase A was ultrapure water (adding 0.1% formic acid), phase B was acetonitrile (adding 0.1% Formic acid); (3) Elution gradient: Sample measurements were performed with a gradient program that employed the starting conditions of 95% A, 5% B. Within 9 min, a linear gradient to 5% A, 95% B was programmed, and a composition of 5% A, 95% B was kept for 1 min. Subsequently, a composition of 95% A, 5.0% B was adjusted within 1.1 min and kept for 2.9 min. The flow velocity was set as 0.35 mL per minute. The injection volume was 4μL.
Determination method of ESI-MS/MS
Mass spectrometry was performed according to the method of Chen [31]. The above effluent was alternatively connected to an ESI-triple quadrupole-linear ion trap (Q TRAP)-MS/MS. Linear ion trap (LIT) and triple quadrupole (QQQ) scans were obtained on a triple quadrupole-linear ion trap (Q TRAP) mass spectrometer with AB4500 Q TRAPUPLC/MS/MS system, equipped with ESI Turbo Ion-Spray interface, which can be controlled by the Analyst 1.6.3 software (AB Sciex) to run positive and negative ion modes. The operating parameters of the ESI source were as follows: ion source, turbo spray; source temperature, 550 °C; ion spray voltage (IS), 5500 V (positive ion mode) /-4500 V (negative ion mode); ion source gas I (GSI), gas II (GSI) and curtain gas (CUR) were set to 50, 60, and 25.0 psi, respectively, and the collision induced ionization parameter was set to high. In LIT and QQQ modes, 10 and 100 μmol/L polypropylene glycol solutions were used for instrument tuning and quality calibration, respectively. The MRM mode was used for the QQQ scan and the collision gas (nitrogen) was set to medium. Through further optimization of decluttering potential (DP) and collision energy (CE), the DP and CE of each MRM transition were completed. A specific set of MRM transitions was monitored during each period.
Identification and relative intensity
Qualitative and quantitative analyses of phenolic substances were carried out according to the method of Tu [30]. Compound information of some databases was used to analyze the types and relative contents of phenolic substances in the samples. For example, the self-built database MWDB (Metware Biotechnology Co., Ltd. Wuhan, China) and the public Mass Bank (http://www.massbank.jp/), KNAPSAcK (http://kanaya.naist.jp/KNApSAcK/), HMDB (http://www.hmdb.ca/), MoTo DB (http://www.ab.wur.nl/moto/) and METLIN (http://metlin.scripps.edu/index.php).
The relative intensity of compounds was based on the MRM model to screen the precursor ions (Q1) of the target material and remove the ions corresponding to eliminate the interference. Multia Quant version 3.0.2 was used to integrate and correct chromatographic peaks, and the relative contents of the corresponding substances were calculated according to the peak area.
Statistical analysis
SPSS version 19.0 (SPSS Inc. Chicago. USA) was used to analyze the data from the three independent biological replicates and verify followed a normal distribution. All of the analyses of variance (ANOVA) followed by the least significance differences (LSD) test were performed to determine significant differences (p < 0.05). The results of response surface optimization were analyzed by the software design expert. V8.0.6.1.Composition analyses of phenolic substances were performed by R (http://www.r-project.org/) for principal component analysis (PCA) and orthogonal projections to latent structure-discriminate analysis (OPLS-DA) to study the difference between EPs and NEPs from chestnut shell.
Results and discussion
Optimization of the preparation of NEPs from chestnut shell
Alkaline hydrolysis was carried out on the residues from chestnut shell obtained after EPs extraction. NaOH concentration, liquid to material ratio and hydrolysis time are the three main factors affecting the release of NEPs from chestnut shells.
According to the results of the single factor experiment (Supplement figure S1), the Box–Behnken RSM design was utilized to optimize the influence of NaOH concentration (0.5–2.5 mol/L), liquid to material ratio (20–60 ml/g), and extraction time(1–5 h) on one response variables (TPC), and they are shown in Tables 1 and 2. Independent variables and their coded and actual values is shown in Table 1 and Table 2 showed the 17 experiments established by the experimental design. Figure 2 shows response surface diagram and isoline map of variables and their interactions. Through the analysis of variance and multiple regression fitting analysis, the quadratic multinomial regression model of the extraction content of polyphenols from chestnut shell to NaOH concentration (X1), liquid to material ratio (X2), hydrolysis time (X3) and TPC (Y) was obtained as follows:
The predicted optimum conditions were as follows: NaOH concentration, 1.68 mol/L; liquid to material ratio, 49.86 mL/g and extraction time, 4.01 h. Under these optimal conditions, TPC was predicted to be 88.44 mg GAE/g sample. Three verification experiments were carried out and compared with the predicted values, the average value obtained by the verification experiments was 87.29 ± 2.00 mg GAE /g sample, which was close to the predicted value. Moreover, the significance of the quadratic polynomial model was verified by the analysis of variance, the results are shown in Table 3. The F value of the model was 29.71, p < 0.0001, R2 was 0.9745, Radj was close to R2, CV% was 2.36%, indicating the reliability and repeatability of the model. Therefore, the model can accurately predict TPC in the hydrolyzed chestnut shell with NaOH under different alkaline hydrolysis conditions.
TPC
NEPs were prepared under the optimum extraction conditions and the TPCs of EPs and NEPs were determined. The TPCs in the chestnut shell are shown in Fig. 3A, and the results were expressed as GAE/g sample. The results showed that in each gram of chestnut shell (DW), the content of EPs was 8.23 ± 0.41 mg GAE, while the content of NEPs was 87.29 ± 2.00 mg GAE. It is obvious that the content of NEPs is more than ten times that of EPs in chestnut shell, which shows that in chestnut shell, after the EPs were extracted by ethanol solvent for several times, there was still a considerable amount of NEPs in the remaining residue, which could be released by alkaline hydrolysis. This part of NEPs exists in many plants, but there are few reports on them, and their existence was often ignored in previous experiments [38].
The TPCs of the purified EPs and purified NEPs are shown in Fig. 3B, and the results were expressed as gallic acid equivalent per milligram of purified sample. In terms of purified extracts, TPC in the purified EPs with the same mass was significantly higher than that in purified NEPs, which might be explained by the different purification conditions and polyphenol types in different samples.
Antioxidant capacity
DPPH radical scavenging activity
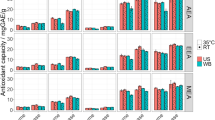
In DPPH assay, EPs showed the higher scavenging activity than NEPs (Fig. 4A). It is shown that both EPs and NEPs have good scavenging effect on DPPH free radicals; however, the scavenging ability of NEPs was slightly lower than that of EPs. A previous study about the polyphenols of black bean coat and cotyledon showed that the scavenging effect of DPPH free radicals of EPs was better than that of NEPs [39], which is consist to current research. Besides, even though the scavenging rates of EPs and NEPs were lower than ascorbic acid and gallic acid, they both exceeded 80%, which implied that EPs and NEPs possessed excellent DPPH free radical scavenging activity.
Scavenging rates of EPs and NEPs on DPPH radical A and ABTS+ radical B. The content of Eps and NEPs was converted into gallic acid equivalent; as positive control samples, contents of ascorbic acid and gallic acid were both actual mass. EPs extractable polyphenols, NEPs non-extractable phenols, AA ascorbic acid, GA gallic acid
ABTS+ radical scavenging activity
The change of the ABTS+ radical scavenging activity showed a similar tendency with that of DPPH radical scavenging activity (Fig. 4B). In the concentration range of 5 μg–70 μg, the scavenging rates of EPs were from 14.05 ± 0.47% to 99.74 ± 0.09%, and its IC50 was 22.01 ± 0.13 μg/mL, while those of NEPs were from 8.63 ± 0.39% to 89.92 ± 0.16%, and its IC50 was 29.36 ± 0.39 μg/mL. Both EPs and NEPs have good scavenging effect on ABTS+ free radicals. The ABTS+ radical scavenging activity of NEPs was also not as good as that of EPs, similar to that of DPPH. In general, results obtained demonstrated that EPs has higher antioxidant capacity than NEPs from the extraction residue of chestnut shell. However, the content of NEPs in chestnut shell is much higher than that of EPs, NEPs in chestnut shell is still valuable, even though it is currently underrated. In other words, antioxidant phenolic compounds were underestimated when the EPs were obtained.
On the other hand, according to the scavenging effects on the above two kinds of free radicals, the differences between the compositions and contents of EPs and NEPs were revealed. The antioxidant factors of polyphenols are related to the types of polyphenols, the number and relative position of phenolic hydroxyl groups, the degree of methoxylation, the ionization potential of ArOH and their intramolecular hydrogen bond interaction [40,41,42]. Therefore, the phenolic components of EP and NEP should be further analyzed to determine whether there are differences in the scavenging ability of the two free radicals due to differences in phenolic substances between them.
Individual phenolic compounds by UPLC–ESI-MS/MS
Using UPLC–ESI-MS/MS method, composition and relative content of phenolic substances of purified NEPs and EPs were studied through a widely targeted metabolism method. In this study, the experiment was divided into two groups (NEPs and EPs). Based on UPLC–ESI-MS/MS detection platform and self-built database, a total of 478 compounds were detected in positive and negative ion mode, 9 anthraquinones; including 15 coumarins, 19 lignans, 59 tannins, 175 phenolic acids, 210 flavonoids. Detailed information on 478 polyphenol compounds is shown in Supplementary material Table S1. The positive and negative total ion current diagrams of NEPs, EPs and their mixtures, as well as MRM diagrams are shown in Supplement material Fig. 2A total of 466 kinds of polyphenols in EPs were detected including flavonoids, phenolic acids, quinones and lignans and coumarins. Among them, flavonoids and phenolic acids account for a large part. The top 10 phenolic compounds in EPs included simple phenolic acids and flavonoids (Table 4). In nature, phenolic acids exist mainly in the non-extractable or bound form, while flavonoids appear in the form of glycosides, whose single or multiple glycosyl groups are connected by hydroxyl groups (o-glycosides) or carbon–carbon bonds (c-glycosides) [43]. Flavonoids form a significant proportion of antioxidants found in plants and partly account for their biological activities (Pietta, 2000). Moreover, they are usually extracted with organic solvents such as methanol, ethyl alcohol, acetone and their aqueous solution, which have better extraction effect and yield [44].
A total of 164 kinds of polyphenols in NEPs were detected, and it is mainly composed of phenolic acids, a small part of flavonoids and tannins. The composition of polyphenols of NEPs is similar to previous reports [25, 45, 46]. The top 10 phenolic compounds in NEPs were all phenolic acids (Table 4). Phenolic acids, such as hydroxycinnamic and hydroxybenzoic acids, can form ether linkages with lignin through their hydroxyl groups in the aromatic ring and ester linkages with structural carbohydrates and proteins through their carboxylic groups [19]. However, alkaline hydrolysis can destroy the ester bond between cell wall and phenolic acid, hydrolyze cell wall, decomposes lignans and proteins, and release phenolic compounds [19, 47, 48].
All detected phenolics compounds are shown in a heatmap (Fig. 5A), which showed the difference in the distribution of polyphenols in different samples. The polyphenolic profiles of EPs and NEPs had significant differences in the composition and relative content, which could lead to their different biological activities, for example, antioxidant activity. Further research is needed to figure out the specific differences between EPs and NEPs.
A Heatmap of different polyphenol components of EPs and NEPs analyzed by hierarchical clustering analysis. Each sample is displayed in a separated column, and each polyphenol component is represented by a separated row. As shown in the color legend of the heatmap, red indicates high concentration and green indicates low concentration; B PCA of differential polyphenol component analysis from EPs, NEPs and their mixed samples. EPs extractable polyphenols, NEPs non-extractable phenols, PCA principal component analysis
Comparison of phenolic composition of EPs and NEPs
PCA
Since PCA results showed the trend of separation of compound groups among groups, indicating whether there were differences among sample groups [31]. A PCA of the two sample groups (EPs and NEPs) and quality control group were carried out and the PCA score is plotted on Fig. 5B to show the difference of phenolic composition between EPs and NEPs. In the PCA score plot, a clear separation between EPs and NEPs samples was observed, and the scores of two principal components, PC1 and PC2, were 86.34% and 8.58%, respectively. These results indicate that the two samples had distinct composition characteristics and this suggests that there are differences in the composition of EPs and NEPs in chestnut shell. Quality control samples showed good repeatability of the data.
Orthogonal signal correction and partial least squares-discriminant analysis (OPLS-DA)
OPLS-DA is a multivariate statistical analysis method with supervised pattern recognition and its specific steps involve extracting the components in the independent variable X and the dependent variable Y, respectively, and calculating the correlation between the components. Compared with PCA, OPLS-DA can maximize the distinction between groups and facilitate the search for differential compounds.
According to scores OPLS-DA Plot (Supplement materials Fig. 3A), T score [1] and Orthogonal T score [1] are 97.6% and 1.29% respectively, indicating that EPs and NEPs were clearly separated. Moreover, it is proved that there were significant differences in phenolic composition between the two different forms of polyphenols in chestnut shell. In the model verification permutation test diagram (Supplement materials Fig. 3B), the parameters for the classifications were observed between EPs and NEPs (R2X = 0.989, R2Y = 1, Q2 = 1). R2X and R2Y represent the interpretation rate of the model to X and Y matrix, respectively, and Q2 indicates the prediction ability of the model. Theoretically, the closer the values of R2 and Q2 are to 1, the better the model is. The results showed that the OPLS-DA model had the good prediction performance and reliability. And the OPLS-DA model could be used to further screen differential components.
Screening of differential phenolic components
Based on the OPLS-DA results, the components of different samples can be preliminarily screened from the Variable Importance in Projection (VIP) of the multivariate analysis OPLS-DA model. At the same time, the differential components can be further screened by univariate analysis of p value or difference multiple value (fold-change). In our research, 478 kinds of polyphenols components were analyzed according to the above methods, and the polyphenols with significant differences were screened out. The detailed results are shown in Supplement materials Table S2. When fold-change ≤ 0.5 or fold-change ≥ 2, and VIP ≥ 1, it is regarded as significant difference. In Supplement materials Fig. 3C, the results showed that a total of 429 kinds of polyphenols were significantly different between NEPs and EPs, 20 kinds of polyphenols in NEPs were higher than EPs, and 409 kinds of polyphenols were lower than EPs, indicating that there were great differences in the composition and content of phenolic substances between EPs and NEPs. The detail information is shown in Supplement materials Table S2. In terms of flavonoids, the total flavonoid content in EPs was significantly higher than that in NEPs. However, epicatechin, as a flavonoid, hardly exists in EPs, and its content in NEPs is significantly higher than that in EPs. In a word, NEPs is mainly composed of phenolic acids, while flavonoids, phenolic acids, tannins and quinones, lignans and coumarins together constitute EPs, which can be used to clearly distinguish them. The species and contents of polyphenols in EPs are significantly higher than those in NEPs, which may be related to the difference in antioxidant activity of each gallic acid equivalent between EPs and NEPs.
Conclusion
In this study, EPs and NEPs were prepared to research their antioxidant activity and phenolic composition. The EPs were extracted by conventional ethanol extraction, while the NEPs were prepared by alkaline hydrolysis method, in which Box–Behnken experimental design was used to study the influence of NaOH concentration, liquid to solid ratio and extraction time on the TPC of this matrix. Optimal extraction conditions to extract NEPs by alkaline hydrolysis method were obtained as follows: the concentration of NaOH is 1.68 mol/L; the ratio of liquid to material is 49.86 mL/g; and the extraction time is 4.01 h. In the same mass of chestnut shell, the content of NEPs is much more than that of EPs. However, both EPs and NEPs have good antioxidant activity, and the scavenging effect of EPs on DPPH and ABTS+ free radicals was slightly better than that of NEPs. The composition and content of polyphenols were determined and compared. Flavonoids, phenolic acids, tannins, lignans, coumarins and quinones were detected in EPs, while phenolic acids were dominant in NEPs. There was a significant difference in the type and content of polyphenols between EPs and NEPs. In general, chestnut shell represents a rich source of polyphenols including EPs and NEPs. However, NEPs is a kind of phenolic resource that has been neglected at present and this research can provide reference for its future development as functional food components.
Data Availability
The data underlying this article are available in the article and in its online supplementary material.
References
Zhu F (2017) Properties and food uses of chestnut flour and starch. Food Bioprocess Technol 10:1173–1191
Borges OP, Carvalho JS, Correia PR, Silva AP (2007) Lipid and fatty acid profiles of Castanea sativa Mill. Chestnuts of 17 native Portuguese cultivars. J Food Compos Anal 20:80–89
Borges O, Gonçalves B, de Carvalho JLS, Correia P, Silva AP (2008) Nutritional quality of chestnut (Castanea sativa Mill.) cultivars from Portugal. Food chem 106:976–984
Cacciola NA, Cerrato A, Capriotti AL et al. (2020) Untargeted characterization of chestnut (Castanea sativa Mill.) shell polyphenol extract: A valued bioresource for prostate cancer cell growth inhibition. Molecules 25:2730
Zhang H, Ke J, Shao T et al (2014) Cytotoxic effects of procyanidins from Castanea mollissima Bl. shell on human hepatoma G2 cells in vitro. Food Chem Toxicol 64:166–176
Morana A, Squillaci G, Paixão S et al. (2017) Development of an energy biorefinery model for chestnut (Castanea sativa Mill.) Shells. Energies 10:1504
Perron NR, Brumaghim JL (2009) A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys 53:75–100
Stevens JF, Revel JS, Maier CS (2018) Mitochondria-centric review of polyphenol bioactivity in cancer models. Antioxid Redox Signaling 29:1589–1611
Yahfoufi N, Alsadi N, Jambi M, Matar C (2018) The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 10:1618
Hu M, Yang X, Chang X (2021) Bioactive phenolic components and potential health effects of chestnut shell: A review. J Food Biochem 45:e13696
Sorice A, Siano F, Capone F et al (2016) Potential anticancer effects of polyphenols from chestnut shell extracts: Modulation of cell growth, and cytokinomic and metabolomic profiles. Molecules 21:1411
Popa VI, Dumitru M, Volf I, Anghel N (2008) Lignin and polyphenols as allelochemicals. Ind Crops Prod 27(2):144–149
Tomás BFA, Ferreres F, Gil MI (2000) Antioxidant phenolic metabolites from fruit and vegetables and changes during postharvest storage and processing. Stud Nat Prod Chem 23:739–795
Ignat I, Volf I, Popa VI (2011) A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food chem 126:1821–1835
Mehmet YF, Mehmet K (2015) Optimization of extraction parameters on the isolation of phenolic compounds from sour cherry (Prunus cerasus L.) pomace. J Food Sci Technol 52:2851–2859
Kolodziejezyk K, Sojka M, Abadias M et al (2013) Polyphenol composition, antioxidant capacity, and antimicrobial activity of the extracts obtained from industrial sour cherry pomace. Ind Crops Prod 51:279–288
Simsek M, Sumnu G (2012) Microwave assisted extraction of phenolic compounds from sour cherry pomace. Sep Sci Technol 47:1248–1254
Wozniak T, Marszalek K, Skapska S (2016) Extraction of phenolic compounds from sour cherry pomace with supercritical carbon dioxide: Impact of process parameters on the composition and antioxidant properties of extracts. Sep Sci Technol 51:1472–1479
Acosta EBA, Gutiérrez UJA, Serna SSO (2014) Bound phenolics in foods, a review. Food chem 152:46–55
Pérez JJ, Torres JL (2011) Analysis of nonextractable phenolic compounds in foods: the current state of the art. J Agric Food Chem 59:12713–12724
Saura CF (2012) Concept and health-related properties of nonextractable polyphenols: The missing dietary polyphenols. J Agric Food Chem 60:11195–11200
Pinto D, Vieira EF, Peixoto AF et al (2021) Optimizing the extraction of phenolic antioxidants from chestnut shells by subcritical water extraction using response surface methodology. Food Chem 334:127521
Vella FM, Laratta B, La CF, Morana A (2018) Recovery of bioactive molecules from chestnut (Castanea sativa Mill.) by-products through extraction by different solvents. Nat Prod Res 32:1022–1032
Jing HQ, Jun W, Yi QX et al (2009) Active ingredients of phenolics and their antioxidant activities in Chinese chestnut. Acta Hortic 844:89–94
Arranz S, Saura CF, Shaha S, Kroon PA (2009) High contents of nonextractable polyphenols in fruits suggest that polyphenol contents of plant foods have been underestimated. J Agric Food Chem 57:7298–7303
Vázquez G, Fontenla E, Santos J et al (2008) Antioxidant activity and phenolic content of chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts. Ind crops and prod 28:279–285
Vázquez G, González AJ, Santos J, Freire MS, Antorrena G (2009) Evaluation of potential applications for chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts. Ind crops and prod 29:364–370
Domínguez RG, Marina ML, Plaza M (2017) Strategies for the extraction and analysis of non-extractable polyphenols from plants. J Chromatogr A 1514:1–15
Shaoling Lin,Zhenyu Wang,Yilin Lin,Shenghan Ge,Siti Sarah Hamzah,Jiamiao Hu (2019) Bound phenolics from fresh lotus seeds exert anti-obesity effects in 3T3-L1 adipocytes and high-fat diet-fed mice by activation of AMPK. Journal of Functional Foods: 58–11
Tu F, Xie CY, Li HN et al (2021) Effect of in vitro digestion on chestnut outer-skin and inner-skin bioaccessibility: The relationship between biotransformation and antioxidant activity of polyphenols by metabolomics. Food Chem 363:130277
Chen W, Gong L, Guo Z et al (2013) A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: application in the study of rice metabolomics. Mol plant 6:1769–1780
Zheng Y, Liu S, Xie J et al (2020) Antioxidant, α-amylase and α-glucosidase inhibitory activities of bound polyphenols extracted from mung bean skin dietary fiber. LWT 132:109943
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenol and other oxidation substrates and antioxidants by means of Folin-Ciocalteau reagent. Methods Enzymol 299:152–178
Grace MH, Yousef GG, Gustafson SJ et al (2014) Phytochemical changes in phenolics, anthocyanins, ascorbic acid, and carotenoids associated with sweetpotato storage and impacts on bioactive properties. Food chem 145:717–724
Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of a free radical methodto evaluate antioxidant activity. LWT – Food Science and Technology, 28(1),25–30.
Fan R, Xiang J, Li N, Jiang X, Gao Y (2016) Impact of extraction parameters on chemical composition and antioxidant activity of bioactive compounds from Chinese licorice (Glycyrrhiza uralensis Fisch.) by subcritical water. Sep Sci Technol 51:609–621
Re RPN, Proteggente APA, Yang M, Rice EC (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med 26:1231–1237
Pérez JJ, Saura CF (2018) Fruit peels as sources of non-extractable polyphenols or macromolecular antioxidants: Analysis and nutritional implications. Food Res Int 111:148–152
Peng H, Li W, Li H, Deng Z, Zhang B (2017) Extractable and non-extractable bound phenolic compositions and their antioxidant properties in seed coat and cotyledon of black soybean (Glycinemax (L.) merr). J Funct Foods 32:296–312
Yordi EG, Pérez EM, Matos MJ, Villares EU (2012) Antioxidant and pro-oxidant effects of polyphenolic compounds and structure-activity relationship evidence. Nutrition, well-being and health 2:23–48
de Heer MI, Korth HG, Mulder P (1999) Poly methoxy phenols in solution: O-H bond dissociation enthalpies, structures, and hydrogen bonding. J org chem 64:6969–6975
Quideau S, Deffieux D, Douat-Casassus C, Pouységu L (2011) Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed 50:586–621
Nardini M, Ghiselli A (2004) Determination of free and bound phenolic acids in beer. Food Chem 84:137–143
Chávez GML, Sepúlveda L, Verma DK et al (2020) Conventional and emerging extraction processes of flavonoids. Processes 8:434
Aires A, Carvalho R, Saavedra MJ (2016) Valorization of solid wastes from chestnut industry processing: extraction and optimization of polyphenols, tannins and ellagitannins and its potential for adhesives, cosmetic and pharmaceutical industry. Waste Manage 48:457–464
Jara P-J, Elena D-R, Fulgencio S-C et al (2014) Non-Extractable Polyphenols in Plant Foods: Nature. Isolation, and Analysis 10:203–218
Gonzales GB, Raes K, Vanhoutte H et al (2015) Liquid chromatography–mass spectrometry coupled with multivariate analysis for the characterization and discrimination of extractable and nonextractable polyphenols and glucosinolates from red cabbage and Brussels sprout waste streams. J Chromatogr A 1402:60–70
Deshpande SS, Sathe SK and Salunkhe DK (1984) Chemistry and safety of plant polyphenols. Nutritional and toxicological aspects of food safety 457–495
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 31701604), Engineering Research Center of Phosphorus Resources Development and Utilization of Ministry of Education (Grant No. LCX2021002), Open Project of Key Laboratory of Green Chemical Engineering Process of Ministry of Education (Grant No. GCP202103), and Supported by Graduate Innovative Fund of Wuhan Institute of Technology (No. CX2022562).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human and animal studies
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, F., Wei, D., Li, J. et al. Chestnut shell represents a rich source of polyphenols: preparation methods, antioxidant activity and composition analysis of extractable and non-extractable polyphenols. Eur Food Res Technol 249, 1273–1285 (2023). https://doi.org/10.1007/s00217-023-04212-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-023-04212-1