Abstract
The polyphenol extract from olive stone was optimized by the simultaneous maximization of the yield in total phenolic content (TPC), total flavonoids content (TFC), 2,2-diphenyl-1-picrylhydrazyl free radical-scavenging activity (DPPH), ferric reducing/antioxidant power (FRAP), and three individual polyphenols (hydroxytyrosol, syringic acid, and oleuropein) using response surface methodology (RSM). Extraction temperature (40, 50, and 60 °C), extraction time (30, 60, and 90 min), and solvent type (methanol, ethanol, and acetone) were identified as the main variables influencing the extraction efficiency. Face-centered central composite design (FCCD), which consists of 39 factorial experiments including 5 replicates at the central point, generated satisfactory models for the optimization of independent variables. The results showed that the extraction temperature, extraction time, and solvent type had significant effects on all responses except DPPH and FRAP. The optimum conditions for extraction temperature, extraction time, and solvent type were determined as 40 °C, 89.49 min, and methanol, respectively. These values verified with confirmation experiments, and thus, the suitability of the developed models was proven. This methodology could be preferred to ensure that polyphenol extracts were obtained efficiently from the by-products, such as the olive stone in the food industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, there has been a growing interest in natural antioxidants. This is not only due to the possible carcinogenic effects of synthetic antioxidants in foods but also from the antioxidant capacities of various phytochemicals [1]. In this context, phenolic compounds are the most promising molecule group because of their high antioxidant activities [2]. There are many studies on polyphenols due to their beneficial effects on human health such as having protective effects against diseases like chronic degenerative diseases (cataracts, neurodegenerative diseases, macular degeneration, and diabetes mellitus), cancer, and cardiovascular diseases [3]. It has been proved that they have a mechanism to scavenge free radicals and non-radical reactive oxygen species which can lead to oxidative stress [1].
Olive tree (Olea europaea L.), one of the oldest known cultivated plants, is generally native to Mediterranean countries [4]. The cultivation of the olive tree has spread globally over the past two decades because of the health benefits attributed to the consumption of table olive and olive oil [5]. The most important wastes and by-products of table olive and olive oil industries are olive leaves, cake, pomace, paste, and olive mill wastewater [6]. Apart from these, the olive stone is a by-product separated from the pulp in the pitted olive industry and olive oil extraction and used for many applications such as the productions of activated carbon, plastic fillers, liquid and gas fuel produced by pyrolysis, cosmetics, abrasives, and furfural production [7]. On the other hand, the olive stone is a very rich source of nutritionally valuable compounds such as polyphenols (nüzhenide, verbascoside, tyrosol, hydroxytyrosol, oleuropein, etc.) and can be also evaluated as a source of added-value products [6, 8].
Considering the diversity of polyphenols in the composition of their natural sources, their structure, and physicochemical properties, a universal extraction protocol is not conceivable, and specific processes for each phenolic source must be designed and optimized [2]. Conventional solid–liquid extraction is a widely used technique for antioxidants like phenolic compounds, plus is cheap, safe, and easy to scale up [9]. A solid–liquid extraction process involves the separation of the target component from the solid matrix. As soon as the solvent reaches the solid phase that contains a target component, the sorption process begins. The solvent goes through pores and penetrates the dense solid matrix. The target component, trapped in solid matrix, releases and transfers into the solvent [10]. Many factors such as the solvent type, matrix composition, extraction temperature, extraction time, solvent-to-solid ratio, and sample particle size are influential on the efficacy of solid–liquid extraction [11]. Extraction conditions must be optimized to increase the efficiency of extraction.
In the optimization of the extraction procedure, the use of a traditional one-factor-at-a-time approach, where one factor varies at a time while all others are kept constant, is less efficient because of being time-consuming and costly [1]. On the other hand, statistical optimization methods show the interactions between the variables when generating the response. In this context, the most commonly used technique for the optimization of the entire system is the response surface methodology (RSM). In RSM, a model equation that fits the experimental data is derived and the optimal response of the system is calculated by a limited number of experiments [3]. It is one of the most popular optimization techniques in the field of food science [1]. So far, it has been used for the optimization of polyphenol extraction of the different dietary sources [11,12,13,14,15,16,17,18,19,20].
The objective of the present study is to optimize the conventional solid–liquid extraction conditions, i.e., solvent type, extraction temperature, and time, of olive stone to maximize simultaneously the yields of total phenolic content (TPC), total flavonoids content (TFC), hydroxytyrosol content, syringic acid content, and oleuropein content with two in vitro methodologies of measuring antioxidant activities (2,2-diphenyl-1-picrylhydrazyl free radical-scavenging activity (DPPH) and ferric reducing/antioxidant power (FRAP)) by applying the RSM approach.
Materials and methods
Materials
Olive stones belonging to Domat type olives were purchased from a local producer in Manisa, Turkey and dried for a week in a dark place at room temperature. Before the analysis, they were converted to powder (< 500 µm) using a hammer mill (Brook Crompton series 2000, UK). The chemicals used in this research were purchased from Sigma-Aldrich (Darmstadt, Germany) and were of analytical grade except for the standards of phenolic compounds.
Extraction procedure
The process of solid–liquid extraction from olive stone powder was carried out using a water bath (Memmert WNB 10, Germany). Olive stone powder (10 g) was mixed with 20 mL of the solvent (methanol, ethanol, and acetone) heated to the desired temperature and kept in the water bath for different durations (30, 60, and 90 min) at required temperatures (40, 50, and 60 °C). Then, the olive stone extract was centrifuged at 10,000×g-forces at 4 °C for 10 min. Extractions were performed in duplicate.
Total phenolic contents, total flavonoid contents, and DPPH and FRAP assays
A modified Folin–Ciocalteu method was used for determining TPC of olive stone and results were expressed as mg gallic acid equivalent (GAE)/kg DW [21, 22]. The TFC of olive stone was determined by the aluminum chloride colorimetric method described by Heimler et al. [23] and results were expressed as (−)-epicatechin equivalents ((−)-EC)/kg DW. The scavenging activity of DPPH radical of the olive stone extract was measured according to the modified methods reported by Cheung et al. [24] and Chu et al. [25] and the percentage of inhibition of the DPPH radical was calculated as follows:
The ferric reducing antioxidant potential of the olive stone extract was estimated by the modified methods of Guo et al. [26] and Xu et al. [27] and the results were reported as mg of reduced iron equivalents (FeSO4) per kg DW. All spectroscopic analyzes were performed at least three times for each extract and only average values were reported.
Hydroxytyrosol, syringic acid, and oleuropein contents
Hydroxytyrosol, syringic acid, and oleuropein contents of the olive stone were determined simultaneously by using an Agilent 1200 LC system (Agilent, Santa Clara, CA) equipped with a quadratic pump, an autosampler, a diode array detector ((DAD)), and a Chemstation software system. First, 20 µL of extract was injected into the C18 (5 µm, 4.6 mm × 250 mm) Nucleosil C18 (MACHEREY–NAGEL GmbH & Co, Germany) column at 40 °C, and the flow rate was 0.7 mL/min. DAD was set to 280 nm, and methanol (A) and 2.5% acetic acid in water (v/v) (B) were used as mobile phases. The mobile phase gradient elution was 5% A at first 2 min, 25% A at 5 min, 40% A at 15 min, 50% A at 23 min, and 100% A at 37 min. Each phenolic compound was identified by matching UV spectra and retention times of samples with standards and standard addition method was utilized. It was quantified using a respective calibration curve. Results were expressed as mg/kg DW. Chromatographic separations of phenolic compounds were carried out at least three times for each extract and only mean values were reported.
Experimental design
The extraction parameters were optimized by applying RSM. The relationship between the three independent factors and the responses (dependent variables) were identified using a face-centered central composite design (FCCD). Extraction temperature (X1), extraction time (X2), and solvent type (X3) were chosen as independent variables. The range and central point values of independent variables were given in Table 1. TPC (YTPC), TFC (YTFC), DPPH (YDPPH), FRAP (YFRAP), hydroxytyrosol content (YHydroxytyrosol), syringic acid content (YSyringic acid), and oleuropein content (YOleuropein) were selected as the responses for the combination of the independent variables and presented in Table 2. The experimental design consists of 39 factorial experiments including 5 replicates at the central point. Analysis of the experimental design data and calculation of predicted responses were performed using Design Expert software (Version 11, Stat-Ease, Inc., Minneapolis, MN, USA). The general form of the polynomial model equation used for each response is given below in Eq. (1):
where Xi and Xj are the independent variables, Y is predicted response, β0, βi,βii, and βij are the regression coefficients of intercept, linear, quadratic, and interaction terms, respectively, and ε is the error.
Experimental data were fitted to the above model equation and regression coefficients for each response were obtained. Analysis of variance (ANOVA) with a 95% confidence level was used to determine the significance and adequacy of the developed models. After the statistically non-significant terms (p > 0.05) had been removed from the initial models, the experimental data were refitted to produce the final models. It was proved that the final models had high F values and p values less than 0.05 and insignificance of lack of fit for each response. The model adequacies were also checked by examining R2 and adjusted-R2. The interactions among the independent variables and their effects on the responses were indicated by analyzing the response surface 3D plots. Then, the desirability function was generated to optimize multiple responses, simultaneously. According to this method, a desirability function for each response should be determined that reflects the desirable interval between 0 and 1. For each response, an importance term (1–5) can be assigned for their significance in the optimization [28]. In this study, it was aimed to determine the solid–liquid extraction parameters in which the olive stone extract with the highest polyphenol composition and antioxidant activity can be obtained. For this reason, the same importance for all responses (r = 3) was appointed and the desirability functions were developed so that all responses would be maximum. In addition, one sample t-test was applied using SPSS v.20.0 statistical package for the verification of optimization.
Results and discussion
In the current research aimed at determining the optimum extraction parameter, the effects of the three process variables (extraction temperature (X1: 40, 50, 60 °C), extraction time (X2: 30, 60, 90 min), and solvent type (X3: methanol, ethanol, acetone)) were investigated. As shown in Table 3, the values of TPC and TFC varied between 139.92 and 211.63 mg GAE/kg DW and 91.06–145.06 mg (−)-EC/kg DW, respectively. Antioxidant activity values changed from 7.53 to 19.01%, 219.84–289.02 mg FeSO4/kg DW for the DPPH and FRAP assays, respectively. The contents of hydroxytyrosol and oleuropein were determined as 5.55–26.85 mg/kg DW and 3.06–36.99 mg/kg DW, respectively while the range of syringic acid content was between 0.29 and 0.69 mg/kg DW. The highest TPC, TFC, and oleuropein content were obtained under the experimental conditions of X1 = 40 °C, X2 = 90 min, and X3 = methanol. The highest hydroxytyrosol content was seen under conditions of X1 = 40 °C, X2 = 60 min, and X3 = methanol. The highest syringic acid content was determined under conditions of both X1 = 40 °C, X2 = 90 min, and X3 = methanol and X1 = 50 °C, X2 = 60 min, and X3 = methanol. The highest antioxidant activity for DPPH assay was found under conditions of X1 = 50 °C, X2 = 30 min, and X3 = methanol, whereas for FRAP assay under conditions of X1 = 40 °C, X2 = 30 min, and X3 = methanol. Consequently, it is proven that it was necessary to optimize the process conditions of solid–liquid extraction for obtaining the extract which maximized both the polyphenolic content and the antioxidant activity of olive stone.
Model fitting
A quadratic model was selected for the RSM analysis and the results of fitting models to the data were given in Table 2. According to ANOVA results, the contributions of fitting models were significant (p < 0.05). According to the best solvent type (methanol) specified in Verification of predictive models section, the fitted modified quadratic models for TPC, DPPH, FRAP, hydroxytyrosol and oleuropein, modified 2FI model for TFC, and linear model for syringic acid in coded variables were obtained as follows:
The significance of each coefficient was found by the F-test and p-value. Also, the absence of any lack of fit (p > 0.05) strengthened the reliability of all models (Table 2).
Total phenolic contents, total flavonoid contents, and antioxidant activities (DPPH and FRAP)
It could be seen that the effects of extraction temperature (X1), extraction time (X2), and solvent type (X3) on TFC were significant (p < 0.05) in first-order linear effects (X1, X2, X3), second-order quadratic effects effects related to the squares of extraction temperature and time (X12, X22) and interaction effects related to extraction temperature and solvent type (X1X3), extraction time and solvent type (X2X3), extraction temperature, time and solvent type (X1X2X3), the square of extraction temperature and extraction time (X12X2), the square of extraction temperature and solvent type (X12X3) and extraction temperature and the square of the extraction time (X1X22).. Although the effect of extraction temperature and time (X1X2) on TFC was insignificant (p = 0.0675), it was added to the model for supporting hierarchy.
It was determined that the linear terms of extraction temperature (X1) and solvent type (X3) for TFC showed the largest effects (p < 0.05), followed by the linear term of extraction time (X2). The interaction term of extraction temperature and solvent type (X1X3) had the lowest effect on the TFC yield.
The results given in Table 3 demonstrate that the change of extraction temperature, extraction time, and solvent type significantly influence the yield of both TPC and TFC (p < 0.05).
For DPPH, the extraction temperature (X1) and solvent type (X3) were significant (p < 0.05) in two linear effects (X1, X3), and one quadratic effect related to the square of extraction temperature (X12). The quadratic term of extraction temperature (X12) showed the lowest effect on the DPPH yield.
For FRAP, the extraction temperature (X1) and solvent type (X3) were also significant (p < 0.05) in two linear effects (X1, X3), one quadratic effect related to the extraction temperature (X12), and one interaction effect related to the square of the extraction temperature and solvent type (X12X3). The interaction term of extraction temperature and solvent type (X1X3), which had an insignificant effect on FRAP (p = 0.0543), was added to the model for supporting hierarchy.
As shown in Table 3, the change of extraction temperature and solvent type significantly affects the antioxidant activity (p < 0.05).
The R2 of the predicted models for TPC, TFC, DPPH, and FRAP were between 0.8490 and 0.9975 and p-values for lack of fit ranged from 0.1216 to 0.9969 (Table 3). These values express that the responses (TPC, TFC, DPPH, and FRAP) can be sufficiently explained by the models in Eq. 2–5.
Hydroxytyrosol, syringic acid, and oleuropein contents
For hydroxytyrosol contents, it was observed that all linear terms (X1, X2, X3), quadratic term of extraction temperature (X12), interaction term of extraction temperature and time (X1X2) and interaction term of extraction temperature and solvent type (X1X3) generated a significant effect. All linear terms (X1, X2, X3), the quadratic term of extraction time (X22), interaction term of extraction temperature and solvent type (X1X3), and interaction term of extraction time and solvent type (X2X3) for oleuropein content were significant. Yet, only linear terms (X1, X2, X3) were significant for syringic acid (p < 0.05). It was determined that all linear terms (X1, X2, X3) of independent variables for hydroxytyrosol, syringic acid, and oleuropein contents demonstrated the largest effects (p < 0.05). The interaction term of extraction temperature and extraction time (X1X2) had the lowest effect on hydroxytyrosol yield while the interaction term of the extraction time and solvent type (X2X3) had the lowest effect on oleuropein yield.
According to Table 4, it is deduced that the extraction temperature, extraction time, and solvent type have a significant effect on the yield of each of the three phenolic compounds.
The models for phenolic compounds gave the R2 values in the range of 0.9503 to 0.9803. The p-values of the lack of fit test ranged from 0.1728 to 0.4040 (Table 4), indicating that the fitting of these models is adequate to describe the experimental data.
Interpretation of response surface models
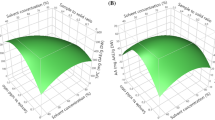
3D response surface plots of TPC, TFC, DPPH, FRAP, hydroxytyrosol, syringic acid, and oleuropein as a function of extraction time and temperature are shown in Figs. 1a–d and 2a–c. From the results given in Verification of predictive models section, it is understood that the solvent type which maximizes all responses is methanol and it significantly affects the responses. Therefore, 3D plots showed the patterns of the effects of extraction time and temperature in each individual response for methanol.
Total phenolic contents, total flavonoid contents, and antioxidant activities (DPPH and FRAP)
3D response surface plots given in Fig. 1a-d show the changes in TPC, TFC, DPPH, and FRAP values as a function of extraction time and temperature. The results indicated that the decrease in extraction temperature and the increase in extraction time had a positive effect on both TPC and TFC, and thus, a higher yield of TPC and TFC could be obtained (Fig. 1a, b). For example, when extraction temperature was decreased from 60 to 40 °C and extraction time was increased from 30 to 90 min by using methanol as an extraction solvent, an increase in TPC from 174.64 to 211.63 mgGAE/kg DW and in TFC from 102.20 to 145.06 mg (−)-EC/kg DW were observed. Figure 1c, d displayed that the decrease in extraction temperature gave a higher value of DPPH and FRAP, irrespective of the extraction time. It was also observed that the extraction time did not significantly affect the change of antioxidant activity. The higher values of DPPH and FRAP recorded in the range between 40 and 50 °C by using methanol as an extraction solvent (Fig. 1c, d). These results are in agreement with the studies of Assefa et al. [29] and Saha et al. [1], where they found that the yield of TPC and TFC were higher at extraction temperatures between 30 and 50 °C and prolonged the extraction time. Furthermore, Assefa et al. [29] reported that antioxidant activity was higher when 45 °C was used as the extraction temperature, and prolonged extraction time had an insignificant effect on the yield of antioxidant compounds.
Hydroxytyrosol, syringic acid, and oleuropein contents
The changes in hydroxytyrosol, syringic acid, and oleuropein contents as a function of extraction time and the temperature is demonstrated by 3D response surface plots in Fig. 2a–c. As a result of the use of methanol as the extraction solvent; hydroxytyrosol, syringic acid, and oleuropein yields increased by decreasing the extraction temperature and prolonging the extraction time. The maximum amount of syringic acid (0.69 mg/kg DW) and oleuropein (36.99 mg/kg DW) was determined at the extraction temperature of 40 °C and the extraction time of 90 min. Besides, when the extraction time was increased from 30 to 60 min at the extraction temperature of 40 °C, the content of hydroxytyrosol increased by 9.80% (Table 2). Similar results were also observed by Assefa et al. [29], who proved that the yield of individual phenolic compounds such as hesperidin, naringin, and phloretin was increased by using moderate extraction temperature in the range of 30 and 60 °C and increasing extraction time.
Extraction temperature, extraction time, and type of solvent are the most important parameters affecting the efficacy of conventional solid–liquid extraction. A slight increase in the temperature can enhance the extracted phenolic content by increasing phenolic solubility and diffusion rate, and decreasing solvent viscosity and surface tension. However, a further increase in temperature leads to a reduction in phenolic content resulting in thermal degradation [11]. The antioxidant can be lost [1]. This behavior of the polyphenols as a function of the temperature proves that the solid–liquid extract contained heat-sensitive phenolic compounds [11]. It was determined that the phenolic compounds contained in the solid–liquid extract of the olive stone in this study were also highly sensitive to heat, and as a result, the increase in temperature adversely affected the phenolic content. It was also confirmed that the extraction rate of antioxidants in the olive stone at lower temperatures was higher than their decomposition rate. Similar to the results of the present study, Gan and Latiff [30] obtained the highest values of TPC, DPPH, and FRAP from stink bean pod, when a temperature of 35 °C was used. They determined the highest TFC value from the stink bean pod at 48.5 °C.
The longer extraction time prolongs the interaction between extraction solvent and olive stone. It facilitates extraction. However, if the extraction time is too long, the risk of oxidation of polyphenols may increase [31]. It can also increase the solvent loss by vaporization, which directly affects the loss of mass transfer throughout extraction. Therefore, the use of moderate extraction time in the extraction process is generally preferred for better protection of polyphenols and antioxidants. In this study, the yield of polyphenols increased until extraction time reaches 90 min, but it was found that the extraction time applied up to 90 min had no effect on antioxidant activity. Similar studies were reported in the literature, which optimum extraction times of 100 min (105.9 for TFC) [30] and 119.6 min [29] were determined for maximum yield of polyphenols and antioxidants.
The difference in polarities of the extraction solvents can affect the solubility of polyphenols and antioxidants in olive stone and their extraction yield. Hence, the selection of an appropriate solvent system is very important for optimizing the recovery of TPC, TFC, and other antioxidant compounds from olive stone. As a result of appropriate solvent selection, it is possible to obtain the extract which has a higher content of polyphenol and antioxidants at a shorter extraction time [32]. Among the extraction solvents used in the current research, methanol (partition coefficient = − 0.77) was the most polar solvent, followed by ethanol (partition coefficient = − 0.31), and acetone (partition coefficient = − 0.24), respectively [33]. In this study, extraction yields of polyphenols and antioxidants in olive seeds were found to be higher in methanol, indicating that these compounds in the structure could be more polar than ethanol and acetone. Moreover, the use of aqueous acetone gives better results in the extraction of higher molecular weight flavonols while methanol is more effective in the extraction of low molecular weight polyphenols [34]. Among the pure solvents, methanol is the most efficient solvent in the extraction of antioxidants, followed by water, ethanol, and acetone. This is due to the better dissolution of antioxidants in the fruits and vegetables as a consequence of interactions (hydrogen bonds) between the polar sites of the antioxidants and methanol. Ethanol is less efficient than methanol in the extraction of antioxidants. The ethyl radical in ethanol is longer than the methyl radical in methanol and this leads that antioxidants have a lower solubility in ethanol. Antioxidants such as polyphenols have the lower solubility ratio in acetone compared to methanol and ethanol. The reason is that acetone molecules are proton acceptors while the other solvents (methanol, ethanol, and water) are proton donors [35]. This result obtained in the study are in the same line with results reported by Tabaraki et al. [36], who used several extraction solvents such as methanol, ethanol, water, acetone, and ethyl acetate and stated that the methanolic extracts had the highest TPC, FRAP, and DPPH values. Saha et al. [1] also found that the extract obtained with 100% methanol from kinema had a higher TPC and antioxidant activity compared to the extract of kinema obtained with the methanol-ethanol mixture at various ratios.
Verification of predictive models
The verification of the validity and adequacy of the predictive model equations was realized in the optimum conditions of extraction temperature, extraction time, and solvent type. An optimum condition for TPC, TFC, DPPH, and FRAP as well as hydroxytyrosol, syringic acid, and oleuropein contents in the solid–liquid extraction of olive stone was determined by maximizing all responses. Simultaneous optimizations of the multiple responses were performed using Derringer’s desirability function method. The overall desirability value for the selected optimum condition was 0.982. In order to compare the experimental values with the predicted values of the responses using the developed empirical model equations [Eqs. (2–8)], extractions were conducted under the optimum conditions (extraction temperature of 40 °C, extraction time of 89.49 min, and methanol as the solvent type). The extractions were carried out in triplicates. The average values of experiments are given in Table 5. It was determined that there was no statistically significant difference between the predicted values and the mean values of TPC, TFC, DPPH, FRAP, hydroxytyrosol, syringic acid, and oleuropein obtained (p > 0.05) (Table 5). The values of percentage error were between 0.93 and 10.73% (Table 5). It was observed that the results of the verification experiments were close to the predicted values obtained from the optimization models. Briefly, the results provided through confirmation experiments clearly demonstrated the suitability of the developed models.
Conclusion
RSM was successfully implemented in this study to optimize the phenolic extraction from olive stones and to study the individual, quadratic, and interactive effects of process variables such as extraction temperature, extraction time, and solvent type on the solid–liquid extraction. The linear model, modified 2FI, and quadratic models were utilized in predicting all responses. The optimization demonstrated that the best conditions for obtaining high yields of TPC, TFC, DPPH, FRAP, hydroxytyrosol, syringic acid, and oleuropein were extraction temperature of 40 °C, extraction time of 89.49 min, and the use of methanol as the solvent. The results also proved that predicted values and experimental results were not significantly different from each other. Consequently, it can be recommended the models obtained is used to optimize the process conditions in the polyphenol extraction from olive stone. Olive stone, which is a by-product of the olive and olive oil industry, is a potential inexpensive source of natural antioxidants. Using the optimized extraction conditions, it can be possible to obtain the polyphenolic content efficiently and to use it in the food industry.
References
J. Saha, A. Biswas, A. Chhetri, P.K. Sarkar, Food Chem. 129(2), 507–513 (2011)
E.M. Silva, H. Rogez, Y. Larondelle, Sep. Purif. Technol. 55(3), 381–387 (2007)
M. Ranic, M. Milutinovic, A.K. Ristic, S.D. Brankovic, J. Clean. Prod. 80, 69–79 (2014)
N. Talhaoui, A. Taamalli, A.M. Gómez-Caravaca, A. Fernández-Gutiérrez, A. Segura-Carretero, Food Res. Int. 77, 92–108 (2015)
F. Rodrigues, F.B. Pimentel, M.B.P.P. Oliveira, Ind. Crops Prod. 70, 116–124 (2015)
E. Roselló-Soto et al., Trends Food Sci. Technol. 45(2), 296–310 (2015)
F.J. Gomez-de la Cruz, P.J. Casanova-Pelaez, J.M. Palomar-Carnicero, F. Cruz-Peragon, Energy 75, 146–152 (2014)
G. Rodríguez, A. Lama, R. Rodríguez, A. Jiménez, R. Guillén, J. Fernández-Bolaños, Bioresour. Technol. 99(13), 5261–5269 (2008)
G.A.R. Oliveira, A.E. Oliveira, E.C. De Oliveira, Da, Conceição, M.I.G. Leles, Multiresponse optimization of an extraction procedure of carnosol and rosmarinic and carnosic acids from rosemary. Food Chem 211, 465–473 (2016)
Y. Wang, V. Herdegen, X. Li, J.U. Repke, Sep. Purif. Technol. 204, 90–97 (2018)
Z. Ben Ahmed et al., Anal. Methods 8(31), 6107–6114 (2016)
M. Álvarez-Casas, C. García-Jares, M. Llompart, M. Lores, Food Chem. 157, 524–532 (2014)
N. De Zordi et al., J. Supercrit. Fluids 95, 491–498 (2014)
J.R. Sarkis, I. Michel, I.C. Tessaro, L.D.F. Marczak, Sep. Purif. Technol. 122, 506–514 (2014)
M. Brahim, F. Gambier, N. Brosse, Ind. Crops Prod. 52, 18–22 (2014)
V.C. Bochi, M.T. Barcia, D. Rodrigues, C.S. Speroni, M.M. Giusti, H.T. Godoy, Food Chem. 164, 347–354 (2014)
M. Chen, Y. Zhao, S. Yu, Food Chem. 172, 543–550 (2015)
M. Majeed et al., Saudi. J. Biol. Sci. 23(3), 389–396 (2016)
T. Belwal, P. Dhyani, I.D. Bhatt, R.S. Rawal, V. Pande, Food Chem. 207, 115–124 (2016)
B. Gullón, P. Gullón, T.A. Lú-Chau, M.T. Moreira, J.M. Lema, G. Eibes, Ind. Crops Prod. 108, 649–659 (2017)
J.A. Singleton, V.L. & Rossi, Am. J. Enol. Vitic. 16, 144–153 (1965)
Y. Li, C. Guo, J. Yang, J. Wei, J. Xu, S. Cheng, Food Chem. 96(2), 254–260 (2006)
D. Heimler, P. Vignolini, M.G. Dini, A. Romani, J. Agric. Food Chem. 53(8), 3053–3056 (2005)
Y.-H. Chu, C.-L. Chang, H.-F. Hsu, J. Sci. Food Agric. 80(5), 561–566 (2000)
L.M. Cheung, P.C.K. Cheung, V.E.C. Ooi, Food Chem. 81(2), 249–255 (2003)
C. Guo et al., Nutr. Res. 23(12), 1719–1726 (2003)
J.Z. Xu, S.Y.V. Yeung, Q. Chang, Y. Huang, Z.-Y. Chen, Br. J. Nutr. 91(6), 873–881 (2004)
Z. Erbay, N. Koca, F. Kaymak-Ertekin, M. Ucuncu, Food Bioprod. Process. 93, 156–165 (2014)
A.D. Assefa, R.K. Saini, Y.S. Keum, J. Food Meas. Charact. 11(2), 364–379 (2017)
C.Y. Gan, A.A. Latiff, Food Chem. 124(3), 1277–1283 (2011)
S. Mukherjee, N. Mandal, A. Dey, B. Mondal, J. Food Sci. Technol. 51(11), 3301–3308 (2014)
S. Butsat, S. Siriamornpun, Int. Food Res. J. 23(1), 180–187 (2016)
F. Spafiu, A. Mischie, P. Ionita, A. Beteringhe, T. Constantinescu, A.T. Balaban, Arch. Org. Chem. 10, 174–194 (2009)
Q.D. Do et al., J. Food Drug Anal. 22(3), 296–302 (2014)
J.S. Boeing, E.O. Barizão, B.C.E. Silva, P.F. Montanher, V. de Cinque, Almeida, J.V. Visentainer, Chem. Cent. J. 8(1), 48 (2014)
R. Tabaraki, E. Heidarizadi, A. Benvidi, Sep. Purif. Technol. 98, 16–23 (2012)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nakilcioğlu-Taş, E., Ötleş, S. The optimization of solid–liquid extraction of polyphenols from olive stone by response surface methodology. Food Measure 13, 1497–1507 (2019). https://doi.org/10.1007/s11694-019-00065-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-019-00065-z