Abstract
This study evaluates the content and formation of free and bound monochloropropanediol (MCPD) and bound glycidol (GE) in fine bakery wares from the German market. The analytical screening revealed, that free 3-MCPD can be quantified in products with low water and fat content, which are produced using high baking temperatures. Concentrations resulted to be 10.0 µg/kg (wafers), 14.1 µg/kg (crispbread) and 22.8 µg/kg (rusk) and are thus judged to be of minor significance when it comes to a potential introduction of a combined maximum level for free and bound species. As exception, considerable amounts of free 3-MCPD (maximum level of 265.4 µg/kg) were only quantified in cinnamon stars containing glycerine as additive. As expected, MCPDE were only quantified in products produced with refined vegetable oil (short bread, puff pastry, caramel biscuits, speculoos, cake, wafers, wholemeal biscuits and rusk). GE levels have already been significantly reduced compared to former studies, one cake showed a maximum content of 127.8 µg/kg. A model baking trial using 13C-3-MCPDE spiked butter as fat component in shortbread confirmed that free 3-MCPD and 2-MCPD, 3-MCPDE, 2-MCPDE or GE are not endogenously formed in marketable browned biscuits. A thermal or lipase-catalysed release of free 3-MCPD from its fatty acid esters can be excluded.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As an undesired foodborne toxicant, 3-monochloropropane-1,2-diol (3-MCPD) belongs to the class of chloropropanols and can on one hand occur as fatty acid ester (3-MCPDE) in various refined edible fats and oils and, on the other hand, is commonly found in its free form in a wide range of foods like coffee, cereals, roasted fish and meat [1]. Such foodborne toxicants may enter into finished foods in two different ways: “Exogenously” as a carry-over from ingredients and commodities or “endogenously” by formation during manufacturing processes applying high temperatures.
The presence of 3-MCPDE in fats and oils, as well as different food stuffs (e.g., salty crackers or fried potatoes) was first reported in 2004 [2], respectively, 2006 [3]. Today it is well known that 3-MCPDE, the fatty acid esters of the isomeric 2-monochloropropane-1,3-diol (2-MCPDE) and glycidyl esters (GE) will be formed during fat refining. Toxicological studies have shown that during digestion 3-MCPDE is completely converted into free 3-MCPD [4] and similarly, an almost complete release of glycidol from GE occurs during digestion, too [5]. Toxicological evaluation is, therefore, only based on their free forms. The International Agency for Research on Cancer (IARC) classified free 3-MCPD as a potential carcinogen for humans (category 2B) and the European Food Safety Authority (EFSA) published a tolerable daily intake (TDI) of 2 µg/kg bodyweight based on its updated risk assessment in the year 2018 [6, 7]. Glycidol is classified as a genotoxic carcinogen of category 2A by IARC [8] and due to its genotoxicity, for GE no TDI can be established. The Regulation (EU) 2018/290 sets maximum levels for GE in infant formulae and edible vegetable oils and fats. Accordingly, vegetable oils and fats should contain a maximum of 1000 μg/kg fat GE. Vegetable oils and fats used in the manufacture of foods for infants and young children are considered separately [9]. The currently discussed combined maximum levels for free and bound 3-MCPD for vegetable oils and fats are planned to be set for two categories. For unrefined oils, refined oils and fats from coconut, maize, rapeseed and olive (except olive pomace oil), sunflower, soybean and palm kernel, a maximum level of 1250 µg/kg fat is under discussion. Other refined vegetable oils, fish oil and oils of other marine organisms shall not exceed a maximum value of 2500 µg/kg fat [10].
The formation mechanisms of the three groups [free MCPD (2- and 3-MCPD), MCPDE (2- and 3-MCPDE) and GE] must be clearly distinguished. As pointed out above, the formation of 3-MCPDE and 2-MCPDE can be attributed to the refining of fats or oils, especially to the deodorisation, where undesirable substances are removed by steam distillation at 200–270 °C [3, 11]. Virgin, vegetable edible oils are, therefore, contaminated either not at all or only slightly by 3-MCPDE and 2-MCPDE. In general, MCPDEs are formed by the reaction of acylglycerols with naturally occurring or added chloride ions. Oil fruits, e.g., palm fruits, contain organic or inorganic chlorine compounds, which are decomposed at temperatures above 180 °C and release the necessary chloride ions [12]. Three mechanisms are currently proposed for the formation of MCPDE from acylglycerols: (1) by direct nucleophilic substitution in a SN2 reaction, (2) in a two-step process involving formation of an intermediate acyloxonium ion that is subsequently ring-opened by chloride or (3) via GE as an intermediate [13, 14]. The endogenous formation of free MCPD can occur via three different pathways: acid hydrolysis, enzymatic release from MCPDE and as a consequence of thermal treatment [13]. In the case of acid hydrolysis, such as the production of hydrolysed vegetable protein (HVP), glycerine, acylglycerols and phospholipids react with added hydrochloric acid. First MCPDE is formed and further hydrolysed into MCPD [14]. Furthermore, an enzymatic release of free MCPD from its fatty acid esters is possible, this cleavage can be traced back to a lipase-catalysed hydrolysis during the production [13, 15]. Lipases are often used to improve dough properties by releasing monoacylglycerols (MAG), diacylglycerols (DAG) and free fatty acids (FFA) from triacylglyceroles (TAG) [16, 17]. Glycerine has been identified to be an important precursor of MCPD [18]. Glycerine can be added to food as an intentional additive. According to Regulation (EC) No 1331/2008, its use in foodstuffs is generally permitted Quantum satis [19]. Glycerine can also be formed as a by-product of yeast fermentation [20]. The reaction of glycerine to free MCPD takes place either via the intermediate glycidol or via direct nucleophilic substitution (SN2) [14].
High temperatures are usually the most important factor in the formation of GE, which are also built to a significant extent during deodorisation of oils. DAGs were identified as the main precursors for GE formation: lipase-catalysed hydrolysis during ripening, harvest and transport of oil fruits might leads to sufficient DAG contents in vegetable oils. In particular, vegetable oils and fats have a high 1,2-DAG content, since TAG lipase has a specificity for the sn-1 and sn-3 position of TAG [21]. An intramolecular rearrangement followed by elimination of a fatty acid moiety forms GE above 230 °C [22,23,24].
Because of their formation, pathways MCPDE and GE are widespread in all refined vegetable oils and fats to a different extent, and will thus be transferred together with the ingredient into foods derived from them [1, 21]. Former studies showed that levels of MCPDE and GE in potato crisps or fine bakery wares are almost exclusively caused by used frying or baking fats [25, 26]. The aim of the study was to give an overview of free and bound MCPD and bound glycidol in fine bakery wares from the German market and to find out, whether free MCPD is generated within the industrial baking process. Additionally, a baking trial investigated the question, if free MCPD, MCPDE or GE are formed during baking of model biscuits (marketable browned) and if a possible formation of free MCPD can be traced back to a lipase-catalysed hydrolyses or thermal release from fatty acid esters.
Materials and methods
Materials
All samples were provided by different German industrial manufacturers of fine bakery wares in the period from December 2018 to March 2019. Table 1 shows the details of the samples (in total 94). Where possible, semi-finished fine bakery wares were sourced. This was done to focus on the baking process only without the influence of fillings or coating etc. To cover the whole range of the relevant portfolio, samples were completed with commercial products.
Preparation of short bread (model, laboratory scale)
For the model experiment, a short bread dough was prepared using the recipe given in Table 2. Before mixing the dough, 42 g butter were spiked with 3-MCPD-1,2-bis-palmitoylester-13C3 (13C-3-MCPDE) dissolved in toluene, pursuant to 2500 µg 3-MCPD per kg fat. The dough was mixed thoroughly with a household machine (Bosch MUM7 CONCEPT 7300 Edition 50), rolled out to a thickness of 5 mm and cookies with a diameter of 5.7 cm were prepared. The baking process was carried out in a laboratory oven (Heratherm OMH 100, Thermo Scientific) at three different temperatures 140 °C, 160 °C and 180 °C for 6, 12, 18, 24, 36 and 72 min. Until further analysis, the cookies were stored deep frozen and thoroughly homogenised using a standard lab mill (Retsch Grindomix GM 200) before analysis.
Reagents and chemicals
3-MCPD, 3-MCPD-d5 and Phenylboronic acid (PBA) were purchased from Merck (Darmstadt, Germany), 2-MCPD was supplied by Santa Cruz Biotechnology (Heidelberg, Germany). 2-MCPD-d5, 3-MCPD-1,2-bis-palmitoylester-d5, 3-MCPD-1,2-bis-palmitoylester, 2-MCPD-1,3-dipalmitoylester, 2-MCPD-1,3-dipalmitoylester-d5, glycidyloleat, glycidyloleat-d5 and 3-MCPD-1,2-bis-palmitoylester-13C3 were purchased from Toronto Research Chemicals (Toronto, Canada). All solvents, gases and reagents were of analytical grade.
Analysis of free MCPD, MCPDE and GE
Quantification of free MCPD, MCPDE and GE was carried out according to the AOAC official method 2018.12 (first action) with minor adaptions regarding the sample matrix [27]. In brief, the homogenised samples (2 g) were weighed in screw-cap vials, spiked with the two mixtures of internal standards free 2-MCPD-d5 and 3-MCPD-d5 (mixture 1: each 1 µg/mL in methanol, 100 µL) and 3-MCPD-1,2-bis-palmitoylester-d5, 2-MCPD-1,3-dipalmitoylester-d5 and glycidyloleat-d5 (mixture 2: pursuant to 5 µg/mL free 2-MCPD and glycidol equivalent and 10 µg/mL free 3-MCPD equivalent in toluene, 200 µL).
The Heat-Ultrasonic-Pressure-supported Solvent Extraction (HUPsSE) described by Kuhlmann allows the extraction of free and bound MCPD and glycidol, covering the range of polarity using methanol and tert-butyl-methyl-ether (tBME) [27]. To perform this HUPsSE, 5 mL methanol were added to each sample and the samples were extracted in an ultrasonic bath for 15 min at a starting temperature of 65 °C. After centrifugation, each supernatant was transferred into a new screw-cap vial and concentrated under nitrogen stream at 70 °C. The remaining residue was extracted a second time in the same way, using 5 mL methanol/tBME (1:1, v:v) instead. The resulting second supernatant was combined with the first one and further concentrated in the nitrogen stream. The insoluble residue was extracted once more with 5 mL tBME and again the corresponding supernatants were combined and concentrated in the nitrogen stream until dryness. Subsequently, 4 mL saturated sodium sulphate solution were added to the extracted and dried residue. The separation of the polar and non-polar phase was carried out by a double liquid/liquid extraction using twice 2.5 mL iso-hexane/tBME (4:1, v:v). In the following, the two obtained fractions are analysed separately.
After washing the polar phase (containing free MCPD) twice with 2.5 mL iso-hexane/tBME (4:1, v:v), each sample was extracted three times with 2 mL diethyl ether followed by the addition of phenylboronic acid (approx. 5 mg/mL in diethyl ether, 100 µL) to the combined organic extracts of each assay. Afterwards, the assays were evaporated to dryness (stream of nitrogen at 40–50 °C) and redissolved in iso-octane (300 µL).
After evaporating the non-polar phase (containing MCPDE and GE) under a stream of nitrogen at 43 °C, the residue was completely dissolved in 3 mL tBME. Subsequently, methanolic sodium hydroxid (0.6 g/100 mL in methanol, 1.4 mL, stored at − 25 °C) was added to each aliquot and a gentle ester cleavage was carried out at − 25 °C within 16 h. The reaction was terminated by addition of cooled (− 25 °C) acidified sodium bromide solution (ortho-phosphoric acid, 600 g/L, 2.4 mL). After removing the organic phase under nitrogen (43 °C), the samples were washed with iso-hexane (2 × 2.5 mL) and the remaining aqueous phase was extracted with diethyl ether (3 × 2 mL) followed by the addition of phenylboronic acid (approx. 5 mg/mL in diethyl ether, 100 µL) to the combined organic extracts of each assay. Afterwards, the assays were evaporated to dryness (stream of nitrogen at 40–50 °C) and redissolved in iso-octane (300 µL).
GC–MS analysis was carried out using an Agilent 7890A gas chromatograph equipped with an Agilent S/SL-injector coupled to an Agilent 5975C mass selective detector. Chromatographic separation was achieved using a Phenomenex ZB-50® column (50% phenyl-50% dimethylpolysiloxane: 30 m, id: 0.25 mm, film thickness: 0.25 µm) with 2.5 m pre-column: Rxi-5MS® (5% phenyl-95% dimethylpolysiloxane 30 m, id: 0.32 mm, film thickness: 0.25 µm) with helium as the carrier gas (1.2 mL/min). Samples (1 µL) were injected in pulsed splitless mode with an injection pulse pressure of 25 psi. The following temperature program was used: 60 °C for 1 min, 6 °C/min to 190 °C, 30 °C/min to 280 °C, isothermal 10 min. MS conditions were as follows: transfer line 280 °C, electron ionisation mode at 70 eV, ion source temperature 230 °C, quadrupole temperature 150 °C.
Our single laboratory validation showed a comparable method performance to the data given in the official method [27] and meets the legal requirements of the Commission Implementing Regulation (EU) 2019/2093 [28]. With the instrumentation used, the method achieved a limit of detection (LOD) of 3 µg/kg and a limit of quantification (LOQ) of 5 µg/kg for free MCPD. MCPDE and GE were detectable above 6 µg/kg (LOD) and quantifiable above 10 µg/kg (LOQ).
Analysis of acrylamide
The analysis of acrylamide was carried out according to the published method of Raters and Matissek 2018 [29].
Analysis of colour value (L-value)
To obtain comparable baking (browning) results, the colour value (L-value) of each biscuit was determined using a Chroma-Meter CR-300 (Minolta).
Results and discussion
Free MCPD in fine bakery wares
To evaluate levels of free MCPD in fine bakery wares, all samples listed in Table 1 were examined. Table 3 shows the results obtained in detail, Fig. 1 illustrates the results of free MCPD: Neither free 3-MCPD nor 2-MCPD were detected in butter biscuits, puff pastry, russian bread, wholemeal biscuits, caramel biscuits, “stollen” (loaf cake), cake or rice wafers. Detectable levels could be determined in nine different categories. Short bread, gingerbread Elisen [30], speculoos, brown gingerbread [30] and sponge biscuit showed levels close above LOQ (5 µg/kg).
Cinnamon stars (n = 8) showed unexpectedly high amounts of free 3-MCPD (max. 265.4 µg/kg) and even free 2-MCPD was quantified in significant levels (max. 142.8 µg/kg). Those results were substantial higher compared to free MCPD levels in the other categories. The observed ratio of 3-MCPD: 2-MCPD was 1.8–2.0, which is significantly lower than reported in other studies (3-MCPD:2-MCPD, 3:1) [20]. Based on the recipes, it was possible to distinguish between products with (n = 3) and without (n = 5) glycerine. These cinnamon stars containing glycerine (according to manufacturer’s specification approx. 2%) all showed quantifiable levels of free 3-MCPD and 2-MCPD, in glycerine free products, free MCPD was not detectable. It is well known that glycerine functions as a main precursor within the formation of free MCPD [18]. As described above, the reaction of glycerine to MCPD takes place either via the intermediate glycidol or via direct nucleophilic substitution (SN2). SN2 leads to a higher yield of 3-MCPD compared to 2-MCPD, which leads to a deviant ratio of 3-MCPD:2-MCPD of 2:1.[14]. In this context, increased levels can be explained and traced back to a formation via direct nucleophilic substitution (SN2). The formation of free MCPD can be avoided, e.g., using alternative additives.
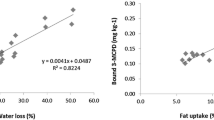
Significant amounts of free 3-MCPD were found in three categories: rusk, crispbread and wafers. Eight of nine analysed rusk samples showed results in the range of < LOD (3 µg/kg) and 22.6 µg/kg, with a median at 10.8 µg/kg. Two consecutive baking steps and low water as well as fat contents are characteristics of rusks. This high thermal input and the recipe-based use of yeasts as raising agent can be a possible explanation for the endogenous formation of free 3-MCPD. The main precursor glycerine can be formed as a by-product of yeast fermentation and thus promote the formation of free 3-MCPD.
The free 3-MCPD levels found in crispbread were slightly lower, five of ten samples showed measureable results, all ranging between < LOD (3 µg/kg) and 14.1 µg/kg, with a calculated median < LOQ (5 µg/kg). Crispbread is characterised by application of a high thermal load and also a low water and fat content. In contrast to most of the other fine bakery wares, the salt content of crispbread can be much higher (product dependent between approx. 1.3–3.0%). The use of yeast can vary; most crispbreads out of this study were produced without yeast, except that with the highest level of free 3-MCPD.
Four out of ten analysed wafer samples showed quantifiable results. The levels of free 3-MCPD ranged from < LOQ (5 µg/kg) to 10.0 µg/kg, with a median < 5 µg/kg (LOQ). Wafers are manufactured by applying high temperatures comparable to rusks and have a low water and fat content in the finished product leading to a potential for endogenous MCPD-formation.
Since in this part of the study, only dry and low-fat products showed quantifiable levels of free 3-MCPD, a release of 3-MCPD from its fatty acid esters as described by Sadowska-Rociek [13] as well as a lipase-catalysed formation are judged to be highly unlikely.
MCPDE and GE in fine bakery wares
Levels of fatty acid ester bound MCPD and glycidol were analysed in addition to the above-described results. Table 4 summarises the results in detail. As expected from the recipes, the contents of 2-MCPDE, 3-MCPDE and GE in butter biscuits, rice wafers, Russian bread and gingerbread Elisen were below LOD (6 µg/kg) in all cases. Usually, no tropical fats or even no additional fats are used in the production of these kinds of products. In contrast, all products produced with palm oil as ingredient (short bread, puff pastry, caramel biscuits, speculoos, cake, wafers, wholemeal biscuits and rusk) contain quantifiable results for 3-MCPDE and GE in varying amounts. Due to the typical ratio of 3-MCPDE to 2-MCPDE, 2-MCPDE was quantified in fewer cases. It is noticeable that GE levels have already been significantly reduced compared to former studies [26]. This reduction can be attributed to the commencement of the maximum level for GE in vegetable fats and oils since 2018 [9]. In two cases, significantly higher 3-MCPDE levels were detected: a cake sample with 1208 µg/kg and a short bread sample with 1365 µg/kg. A correlation between free and bound 3-MCPD could not be derived in both cases. It is obvious, that 3-MCPDE levels are depending on the types of fat used and their specifications as several fats can be mixed in one recipe.
Baking trial: laboratory scale
To verify above presented results, a baking trial (laboratory scale) was executed. For this purpose, a butter (spiked with 13C-3-MCPDE) based shortbread dough was prepared and biscuits were cut out as described above. Spiking ensures a differentiation between thermally generated free MCPD (endogenous formation from ingredients), lipase-catalysed release of free MCPD from MCPDE and thermally generated MCPDE. The analysis of acrylamide should be indicative for the increased thermal load. The biscuits were baked at six different times at 140 °C, 160 °C and 180 °C. Two biscuits were sampled at each time point and were analysed separately, results obtained as average.
Figure 2 displays the biscuits at several time points as overview. Marketable products with typical/similar degree of browning were obtained at 72 min at 140 °C, 24 min at 160 °C or 12 min at 180°. Table 5 lists all results in an overview. 13C-3-MCPD levels were found below LOD (3 µg/kg) at all control points and temperatures indicating that a release of 13C-3-MCPD from its ester has not occurred and can be excluded as formation mechanism. Similar findings were published by Hamlet et al. [16] using a different recipe based on baking fat [16]. As shown in Fig. 3, an endogenous formation of free 3-MCPD could be provoked in this model. Quantifiable results (> LOQ = 5 µg/kg) were obtained at 160 °C and 72 min baking time. At 180 °C, the levels of free 3-MCPD exceed the LOQ already after 24 min and reached a maximum value of 41.4 µg/kg at a maximal thermal load (72 min). The visual impression (see Fig. 2), supported by the level of acrylamide formed (up to 730 µg/kg) leads to the conclusion, that an endogenous formation of free 3-MCPD is possible only with very high thermal input. Again, marketable biscuits with typical browning level do not show detectable levels of free 3-MCPD.
In the same trial, an endogenous formation of 3-MCPDE, 2-MCPDE and GE were also examined. Levels of GE were found in all cases below the LOQ (= 10 µg/kg) due to the ingredient butter. As expected, the applied temperatures were not sufficient to form GE. A formation of GE is described above 230 °C [22]. Figure 4 lists all results for 3-MCPDE. Interestingly an endogenous formation of MCPDE could be enforced in the butter-based biscuit, attributable to 0.4% salt (see recipe in Table 2). This formation is likewise limited to model biscuits with the same increased thermal load. Quantifiable results (> LOQ = 10 µg/kg) were obtained at 160 °C and a baking time of 72 min. At an oven temperature of 180 °C, 3-MCPDE was measurable exceeding the LOQ after 24 min (20.8 µg/kg). A maximal thermal load (72 min, 180 °C) resulted in a 3-MCPDE content of 397.1 µg/kg. This model biscuit was the only sample in this baking trial showing 2-MCPDE (47.1 µg/kg). A former study of Mogol et al. showed comparable results [31], although the suggested removal of chloride is not necessary to minimise levels of MCPDE. Again, the following conclusion can be drawn: Marketable biscuits with typical browning levels do not show quantifiable amounts of the fatty acid esters (3-MCPDE, 2-MCPDE or GE).
Conclusion
This study provides an overview of free 3-MCPD, free 2-MCPD, 3-MCPDE, 2-MCPDE and GE levels in fine bakery wares. In most categories, free 3-MCPD and 2-MCPD were below LOD or LOQ, quantifiable amounts only reproducibly occurred in crisp bread, rusk or wafers. Common with these products are low water and fat contents as well as high baking temperatures. A release of free 3-MCPD from its esters can be excluded. Levels of endogenously formed free 3-MCPD are an order of magnitude below MCPDE brought in by added fats. Only glycerine containing cinnamon stars showed unexpectedly high amounts of free 3-MCPD and 2-MCPD, as glycerine is a potential precursor within the formation of free MCPD. It can be used as an additive or formed as a by-product of yeast fermentation and thus promote the formation of free 3-MCPD.
The additionally performed baking trial revealed that free 3-MCPD and 2-MCPD or 3-MCPDE, 2-MCPDE and GE are not endogenously formed in marketable browned biscuits. A thermal or lipase-catalyse release of free 3-MCPD from its fatty acid esters can be excluded in this case.
References
Hamlet CG, Sadd PA (2009) Chloropropanols and Chloroesters. In: Stadler RH, Lineback DR (eds) Process-induced food toxicants: occurence, formation, mitigation, and health risks. Wiley, Hoboken
Svejkovská B, Novotny O, Divinova V, Reblova Z, Doležal M, Velíšek J (2004) Esters of 3-chloropropane-1,2-diol in foodstuff. Czech J Food Sci 22(5):190–196
Zelinková Z, Svejkovská B, Velíšek J, Doležal M (2006) Fatty acid esters of 3-chloropropane-1,2-diol in edible oils. Food Addit Contam A 23(12):1290–1298
Abraham K, Appel KE, Berger-Preiss E, Apel E, Gerling S, Mielke H, Creutzenberg O, Lampen A (2013) Relative oral bioavailability of 3-MCPD from 3-MCPD fatty acid esters in rats. Arch Toxicol 87(4):649–659
Appel KE, Abraham K, Berger-Preiss E, Hansen T, Apel E, Schuchardt S, Vogt C, Bakhiya N, Creutzenberg O, Lampen A (2013) Relative oral bioavailability of glycidol from glycidyl fatty acid esters in rats. Arch Toxicol 87(9):1649–1659
International Agency for Research on Cancer (2016) Monographs on the evaluation of carcinogenic risk to humans: some chemicals present in industrial and consumer products, food and drinking-water. 3-Monochloro-1,2-propanediol. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono101-010.pdf. Accessed 06 Feb 2020
Knutsen HK, Alexander J, Barregård L, Bignami M, Brüschweiler B, Ceccatelli S, Cottrill B, Dinovi M, Edler L, Grasl-Kraupp B, Hoogenboom L, Nebbia CS, Oswald IP, Petersen A, Rose M, Roudot A-C, Schwerdtle T, Vleminckx C, Vollmer G, Wallace H, Lampen A, Morris I, Piersma A, Schrenk D, Binaglia M, Levorato S, Hogstrand C (2018) Update of the risk assessment on 3-monochloropropane diol and its fatty acid esters. EFSA J 16(1):407
International Agency for Research on Cancer (2000) Some industrial chemicals IARC monographs on the evaluation of carcinogenic risks to humans. https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Some-Industrial-Chemicals-2000. Accessed 06 Feb 2020
Commission E (2018) Commission Regulation (EU) 2018/290 of 26 February 2018 amending Regulation (EC) No 1881/2006 as regards maximum levels of glycidyl fatty acid esters in vegetable oils and fats, infant formula, follow-on formula and foods for special medical purposes intended for infants and young children. OJEU L55:27–29
European Commission (2020) DRAFT: ANNEX to the Commission regulation (EU) …/…amending Regulation (EC) 1881/2006 as regards maximum levels of 3-monochloropropanediol (3-MCPD), 3-MCPD fatty acid esters and glycidyl fatty acid esters in certain foods. https://data.consilium.europa.eu/doc/document/ST-7974-2020-ADD-1/en/pdf. Accessed 31 May 2020
Weißhaar R (2011) Fatty acid esters of 3-MCPD: overview of occurrence and exposure estimates. Eur J Lipid Sci Technol 113(3):304–308
Nagy K, Sandoz L, Craft BD, Destaillats F (2011) Mass-defect filtering of isotope signatures to reveal the source of chlorinated palm oil contaminants. Food Addit Contam A 28(11):1492–1500
Sadowska-Rociek A, Cieślik E (2016) Changes of 3-monochloropropane-1,2-diol levels in crackers and biscuits during storage. J Verbr Lebensm 11(4):317–324
Velíšek J, Doležal M, Crews C, Dvořák T (2002) Optical isomers of chloropropanediols: mechanisms of their formation and decomposition in protein hydrolysates. Czech J Food Sci 20(5):161–170
Yu L, Wang S, Sun B (2017) Food safety chemistry: toxicant occurrence, analysis and mitigation. CRC Press, Boca Raton
Hamlet CG, Asuncion L, Velíšek J et al. (2014) Investigation of the formation of 3-chloropropane-1–2-diol (3-MCPD) from mono- and di-esters of its fatty acids in foods (FS231006, FS231074, FS231075). https://www.food.gov.uk/sites/default/files/media/document/C04072_Final%2520report_July%25202014_151014.pdf. Accessed 29 Jan 2020
Paul R (2005) The origin and formation of 3-MCPD in foods and food ingredients. https://citeseerx.ist.psu.edu/viewdoc/download;jsessionid=E489E6F884B0694C94F70AFB43C292C1?doi=10.1.1.611.8907&rep=rep1&type=pdf. Accessed 09 Mar 2020
Hamlet CG, Sadd PA (2005) Effects of yeast stress and pH on 3-monochloropropanediol (3-MCPD)-producing reactions in model dough systems. Food Addit Contam A 22(7):616–623
The European Parliament and Council (2008) Regulation (EC) No 1331/2008 The European Parliament and Council of 16 December 2008 establishing a common authorisation procedure for food additives, food enzymes and food flavourings. OJEU L354:1–6
Hamlet CG, Sadd PA, Gray DA (2004) Generation of monochloropropanediols (MCPDs) in model dough systems. 1. Leavened doughs. J Agric Food Chem 52(7):2059–2066
Cheng W-w, Liu G-q, Wang L-q, Liu Z-s (2017) Glycidyl fatty acid esters in refined edible oils: a review on formation, occurrence, analysis, and elimination methods. Compr Rev Food Sci Food Saf 16(2):263–281
Destaillats F, Craft BD, Dubois M, Nagy K (2012) Glycidyl esters in refined palm (Elaeis guineensis) oil and related fractions Part I: Formation mechanism. Food Chem 131(4):1391–1398
Cheng W, Liu G, Liu X (2016) Formation of glycidyl fatty acid esters both in real edible oils during laboratory-scale refining and in chemical model during high temperature exposure. J Agric Food Chem 64(29):5919–5927
Craft BD, Nagy K, Seefelder W, Dubois M, Destaillats F (2012) Glycidyl esters in refined palm (Elaeis guineensis) oil and related fractions. Part II: practical recommendations for effective mitigation. Food Chem 132(1):73–79
Dingel A, Matissek R (2015) Esters of 3-monochloropropane-1,2-diol and glycidol: no formation by deep frying during large-scale production of potato crisps. Eur Food Res Technol 241(5):719–723
Dingel A, Matissek R (2017) No endogenous formation of MCPD fatty acid esters and glycidyl fatty acid esters during the baking process of fine bakery wares. Dtsch Lebensmitt Rundsch 113:511–515
Kuhlmann J (2019) 2-Monochloropropanediol (2-MCPD), 3-monochloropropanediol (3-MCPD), and glycidol in Infant and adult/pediatric nutritional formula: single-laboratory validation, first action 201812. J AOAC Int 102(4):1205–1220
European Commission (2019) Commission Implementing Regulation (EU) 2019/2093 of 29 November 2019 amending Regulation (EC) No 333/2007 as regards the analysis of 3-monochloropropane-1,2-diol (3-MCPD) fatty acid esters, glycidyl fatty acid esters, perchlorate and acrylamide. OJEU L317:96-101
Raters M, Matissek R (2018) Acrylamide in cocoa: a survey of acrylamide levels in cocoa and cocoa products sourced from the German market. Eur Food Res Technol 244(8):1381–1388
German Food Code (2010) Guideline for fine bakery products (Leitsätze für Feine Backwaren). GMBl(5/6): 120 ff
Mogol BA, Pye C, Anderson W, Crews C, Gökmen V (2014) Formation of monochloropropane-1,2-diol and its esters in biscuits during baking. J Agric Food Chem 62(29):7297–7301
Acknowledgements
We thank Nadine Karcher and Francesca Zamolo for their excellent technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Compliance with ethics requirements
This article does not contain any study with human or animal objects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stauff, A., Schneider, E. & Heckel, F. 2-MCPD, 3-MCPD and fatty acid esters of 2-MCPD, 3-MCPD and glycidol in fine bakery wares. Eur Food Res Technol 246, 1945–1953 (2020). https://doi.org/10.1007/s00217-020-03546-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-020-03546-4