Abstract
In the present work, different foods including banana, potato, cassava, onion, garlic, polenta, rice balls and beef patties were investigated in relation to the possible endogenous formation of 3-monochloropropane-1,2-diol fatty acid esters (bound 3-MCPD) and carry-over of these contaminants from the oil due to fat uptake during frying. For that, the samples were fried in two different types of oil and bound 3-MCPD was determined by using an indirect method based on acid transesterification and gas chromatography coupled to mass spectrometry analysis. The compounds were not detected in the fried foods when corn oil containing non-significant levels of bound 3-MCPD (<0.05 mg kg−1) was used, indicating no endogenous formation during frying. On the other hand, when the same foods were fried in palm oil containing 1.64 mg kg−1 of bound 3-MCPD, the mean concentrations ranged from 0.12 to 0.25 mg kg−1, indicating a clear carry-over of the contaminants. In this case, a good correlation was observed between the levels of the compounds in fried samples and water loss/fat uptake.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It has been known since 2006 that high concentrations of 3-monochloropropane-1,2-diol fatty acid esters (bound 3-MCPD) can be found in edible oils and fats, especially in palm oil and derived products [1–4]. These compounds are formed during the refining process when lipids react with chlorides at high temperatures, such as those employed in the deodorization step [5, 6]. Polar chlorinated compounds have been found to be the main chloride donors [7], but the identification of the most important lipid precursors still remains a challenge. Acidity may also play an important role contributing to the formation of bound 3-MCPD when oil is heated at a high temperature [8].
Bound and free 3-MCPD are process contaminants and their occurrence in the diet may represent a public health concern. Free 3-MCPD, which can be released from these esters by enzymatic hydrolysis in the gastrointestinal tract, is classified as a possible human carcinogen (group 2B) by the International Agency for Research on Cancer [9]. Moreover, its ability to induce nephrotoxicity and affect male fertility is well-known and a provisional maximum tolerable daily intake (PMTDI) of 2 µg kg−1 body weight (bw) was established by the Joint FAO/WHO Expert Committee on Food Additives considering kidney proximal tubule hyperplasia in rats [10, 11]. Recently, the European Food Safety Authority [12] derived a more conservative tolerable daily intake (TDI) for 3-MCPD of 0.8 µg kg−1 bw and showed that the mean exposure to this contaminant was above the TDI for several population groups, including infants, toddlers, and other children.
Refined oils and fats are used in many processes and food formulations and, as expected, some previous investigations showed that fried foods may also contain significant levels of these compounds. In fried potato products, concentrations between 0.10 and 0.26 mg kg−1 in French fries and 0.10–2.20 mg kg−1 in potato chips were already reported [13, 14]. Levels below 0.60 mg kg−1 were observed in fish fried products (fish fingers and fried fish) by other authors [15]. Arisseto et al. [16] evaluated 85 samples of commercial fried foods collected in the Brazilian market and verified a high number of positive samples (75%), with concentrations up to 0.99 mg kg−1.
The contamination of fried foods with bound 3-MCPD has been mainly attributed to the use of contaminated oil during frying [13, 17]. However, no comprehensive study has been carried out so far to confirm this hypothesis. Only a recent publication showed that no endogenous formation of bound 3-MCPD occurred during frying of potato crisps when using high-oleic sunflower oils under typical large-scale frying conditions, which was attributed to the fact that the thermal input during the industrial frying of potato crisps (160–188 °C) is notably lower than temperatures during deodorization (up to 270 °C) [18].
Since the consumption of fried foods may represent an important route of human exposure to 3-MCPD [12], the factors affecting their contamination should be better understood in order to allow the development of appropriate mitigation strategies to reduce dietary intake. In this way, the objective of this work was to provide new insights in relation to the contamination of fried foods by bound 3-MCPD, considering: (1) different raw materials (meat, cereals, fruit, bulbs and tubers); (2) the levels of potential precursors and the possibility of endogenous formation in these foods; and (3) the type of oil, its initial content of bound 3-MCPD and carry-over due to fat uptake during frying.
Materials and Methods
Standards and Chemicals
Analytical standards of 3-MCPD-1,2-dipalmitoyl ester (PP-3-MCPD) and 3-MCPD-1,2-dipalmitoyl-d5 ester (PP-3-MCPD-d5) at a purity higher than 98% were purchased from Toronto Research Chemicals (North York, ON, Canada). Stock solutions at a concentration of 0.5 mg ml−1 were prepared by dissolving the standards in tetrahydrofuran (THF, analytical grade).
Other chemicals used in the experiments included: acid phenylboronic (PBA, 97% purity), supplied by Sigma-Aldrich Corp. (Steinheim, Germany); methanol (HPLC grade), acquired from Tedia Company Inc. (Fairfield, OH, USA); tetrahydrofuran (HPLC grade), supplied by JT Baker (USA); hexane, acetone, petroleum ether, diethyl ether, concentrated hydrochloric acid, sulfuric acid, sodium bicarbonate, sodium sulfate, and ammonium sulfate (analytical grade), purchased from Labsynth (Diadema, SP, Brazil); silver nitrate (99.0–100.5% purity) and potassium chromate (≥99.5% purity), acquired from Merck (Darmstadt, Germany); and ultrapure water, obtained from a Milli-Q Plus system (Millipore, Bedforte, MA, USA).
Samples
The experiments were carried out using banana, potato, cassava, onion, garlic, corn flour, rice and meat, acquired in the local market and prepared according to common culinary practices. Banana (variety terra) was peeled and sliced diagonally with a thickness of 0.5 cm. Potato (cultivar Asterix) was peeled and cut into sticks of about 1 × 1 cm cross-section and 5–10 cm length. Cassava (yellow root) was peeled, cut in half lengthwise, cooked in hot water (80 °C) for 35 min, and then cut into sticks of about 5–10 cm length. Onion (brown type) was peeled and cut into rings of about 0.5 cm thickness. Garlic (purple stripe) was separated into cloves, soaked in water for a few minutes, peeled and sliced 0.1–0.2 cm thickness. Corn flour (600 g) was mixed with water (3 l), cooked for 30 min at 70 °C until a firm consistency, and used to prepare polenta sticks of about 3 × 1.5 cm after cooling. Carnaroli rice (600 g) was mixed with boiling water (2 l), cooked for 25 min, and hand-molded into balls of about 3 cm diameter. Ground beef (chuck) was molded into patties of 60 g approximately, and then frozen.
Frying
Two experiments were conducted for each food prepared as previously described. In the first one, the samples were fried in corn oil with no detected levels of bound 3-MCPD (limit of detection, LOD = 0.05 mg kg−1) in order to verify the potential endogenous formation of the contaminants. In the second experiment, the samples were fried in palm oil containing 1.64 ± 0.15 mg kg−1 of bound 3-MCPD (n = 10) in order to evaluate the carry-over of the compounds due to fat uptake.
Frying was carried out in an electric fryer (Orcil, Rio de Janeiro, RJ, Brazil) using a proportion of oil/food 5:1 (w/w). The initial oil temperature and frying time were fixed and ranged from 160 to 190 °C and 2 to 9 min, respectively, depending on the product (Table 1). The criteria used for choosing these temperatures took into account the acceptance of the different products in terms of their sensory characteristics and not the possible formation of other contaminants, such as acrylamide. All frying experiments were performed in duplicate and the oil was not reused in any process.
In the present work, the fat uptake was estimated from the difference between the lipid content of the samples after frying and the lipid content of the raw samples. Therefore, the possible mass transfer mechanism between lipid components from the matrix to the frying medium was not taken into account.
Moisture and Volatile Compounds
The content of moisture and volatile compounds were determined by gravimetry according to Zenebon and Pascuet [19] and Horwitz et al. [20] (Method 984.25), in duplicate. Homogenized samples of raw food (3–4 g) were weighed into tared aluminum capsules containing treated sand, homogenized and placed in a vacuum oven (50 mmHg) at a temperature of 70–100 °C, until constant weight. Homogenized samples of fried foods (10 g) were also weighed into tared aluminum capsules containing treated sand, homogenized and placed in a convection oven at 103 °C until constant weight. The results were expressed in g per 100 g of sample.
Total fat
Total fat was determined by gravimetry after acid hydrolysis according to Zenebon and Pascuet [19] and Horwitz et al. [20] (Method 963.15), in duplicate. The samples (5 g) were homogenized, mixed with 100 ml of ultrapure water and 60 ml of concentrated hydrochloric acid. They were then hydrolyzed for 30 min under heating. After cooling, the solutions were filtered on double filter paper. The paper containing the lipids was placed in a ventilated oven at 80 °C for 2 h. The lipids were extracted in a Butt type extractor with petroleum ether under reflux for 6–8 h, the solvent removed in a rotary evaporator at 45 °C and the flask with the residue weighed. The results were expressed in g per 100 g of sample.
Chloride Content
Chloride ion concentration was determined by Mohr’s method, according to Zenebon and Pascuet [19], in triplicate. Briefly, 10 g of the homogenized sample were weighed into porcelain crucibles and incinerated in a muffle furnace at 450 °C for 6 h. The crucibles were removed from the furnace directly into a desiccator. The ashes were solubilized with 100 ml deionized water into a 250-ml conical flask and 2 ml of chromate indicator were added (5% K2CrO4 solution). The samples were titrated with 0.01 mol l−1 silver nitrate solution. The endpoint of the titration was identified as the first appearance of a red-brown color of silver chromate. The results were expressed in mg per 100 g of sample.
Determination of Bound 3-MCPD
To determine the levels of bound 3-MCPD in the samples, an in-house validated indirect method based on acid transesterification and gas chromatography-mass spectrometry (GC–MS) was applied according to Arisseto et al. [16]. Briefly, the homogenized sample (5 g) was weighed into a 50-ml centrifuge tube and 100 µl of a solution of PP-3-MCPD-d5 at 50 μg ml−1 was added as internal standard. Extraction was performed with diethyl ether (20 ml) in an ultrasound bath for 5 min and, after centrifugation (at 3000 rpm for 5 min), an aliquot of the supernatant (4 ml) was evaporated under a N2 flow at 25–30 °C. The residue was dissolved in THF (1 ml) before acid transesterification (1.8 ml of sulfuric acid in methanol 1.8%, v/v) and derivatization with phenylboronic acid (PBA). The analyses were conducted on a HP 7890A gas chromatograph coupled to a MSD 5975C mass spectrometer (Agilent Technologies, Palo Alto, CA, USA). The total concentration of bound 3-MCPD was expressed as free 3-MCPD equivalent. The analytical method (including the extraction of bound 3-MCPD from the matrix and subsequent steps) was not validated for free 3-MCPD.
Results and Discussion
Potential Endogenous Formation of Bound 3-MCPD During Frying
As previously mentioned, bound 3-MCPD is a group of process contaminants formed mainly in vegetable oils and fats during the deodorization step of the refining process in which temperatures above 200 °C are used [5, 6]. However, as the formation of bound 3-MCPD was also verified in products treated at high temperatures, such as dark malt, roasted coffee and biscuits [21–23] as well as in the pomace drying process to obtain pomace olive oil [24], it is necessary to investigate whether an endogenous formation during frying is significant.
For that, selected foods were fried in corn oil with undetected levels of bound 3-MCPD (<0.05 mg kg−1). In order to ensure that there would be no formation of bound 3-MCPD in the oil during heating and to avoid a possible contamination of the food due to the frying medium, the oil used in the experiments was first heated (without sample) to 180 °C for 7 min. No increase in the concentration of bound 3-MCPD was observed after heating, suggesting that the frying medium does not represent a source of possible contamination in the case of these experiments.
As a potential precursor of the contaminants, chloride was evaluated in the selected foods before and after frying. According to Table 2, chloride was found in all tested samples at mean levels varying from 4.9 to 119.0 mg per 100 g before frying and from 5.9 to 153.5 mg per 100 g after frying. The highest concentration was verified in banana, followed by potato. However, although the presence of this potential precursor and the use of high temperatures, the levels of bound 3-MCPD were below the analytical limit of detection (<0.04 mg kg−1) in all evaluated samples before and after frying (Table 2), showing that there was no formation of the contaminants under the applied conditions. The content of bound 3-MCPD in the oil after frying the foods was also measured, but non quantifiable concentrations were found (all samples were below the limit of quantification = 0.10 mg kg−1), suggesting that chloride of the foods was not able to produce measurable levels of bound 3-MCPD in the oil.
Despite previous studies performed in model systems about possible reaction mechanisms having demonstrated the possibility of bound 3-MCPD formation at temperatures from 100 °C [25], the results reported here do not support the same evidence when real foods are used. Besides the temperature and heating time applied in this study, which may not have been sufficient to promote the formation of bound 3-MCPD, the presence of chloride in the raw samples before frying may not ensure that the necessary reactive species, which are still unknown, would be present in sufficient amounts to form the contaminants.
Dingel and Matissek [18] investigated the possibility of endogenous formation of bound 3-MCPD during the deep frying of potato crisps in large-scale industrial production when using high-oleic sunflower oils (HOSO). For that, potato crisps, fresh frying oil and used frying oil were taken from different production lines at 0 (fresh oil, no crisps), 2, 4, 8, 12, 16, 20 and 24 h. The median levels of bound 3-MCPD in the frying oils and in the corresponding crisps varied between 0.4 and 0.5 mg kg−1 (expressed as free 3-MCPD equivalent). Since no significant change was observed over the duration of the whole experiment, the authors concluded that no endogenous formation of bound 3-MCPD occurs during frying of potato crisps when using HOSO under typical large-scale frying conditions.
By using a different approach, the results presented here confirm that bound 3-MCPD is not endogenously formed in foods under typical frying conditions. This was observed not only for potato but also for other foods, such as cassava, banana, onion, garlic, polenta, rice ball and beef patty, which were firstly investigated in this work.
Potential Carry-Over of Bound 3-MCPD Due to Fat Uptake During Frying
As an endogenous formation of the contaminants during frying was excluded according to previous results, the same foods were then fried in palm oil containing 1.64 ± 0.15 mg kg−1 of bound 3-MCPD (mean ± standard deviation of ten samples analyzed from five containers of 10 kg each) in order to evaluate the carry-over of the compounds due to fat uptake. The results concerning the content of moisture, fat and bound 3-MCPD, before and after frying, are shown in Table 3.
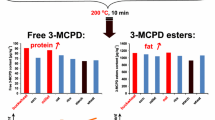
Mean moisture contents varied from 63.9 to 90.2 g per 100 g before frying and from 14.6 to 57.5 g per 100 g after frying. There was a clear moisture decrease for all evaluated foods during the process. The highest water losses were observed for garlic and onion. As for fat, mean contents ranged from not detected (<0.10 g per 100 g) to 7.20 g per 100 g before frying and from 7.2 to 17.7 g per 100 g after frying. Total fat clearly increased with frying, especially for onion and garlic. Moisture decrease and fat increase are both expected when frying foods since this process involves a simultaneous water loss and fat uptake [26, 27].
According to Gamble et al. [28], the increase in oil content during the frying of potato was influenced by the moisture loss as the vapors start to form inside the matrix during the process and, as a result, pores and cracks are formed through which vapors escape. In order to compensate for the water loss, oil penetration occurs. Other authors showed that transport phenomena of oil during frying were also considerably affected by the changes in matrix microstructure. From pore size distribution analysis, Alam and Takhar [29] observed that porosity increased with frying time, which was attributed to lower tortuosity values. As a result, more oil was absorbed into the potato matrix.
In relation to bound 3-MCPD, Table 3 shows that concentrations were below the analytical limit of detection (0.04 mg kg−1) in all raw samples, but varied between 0.12 and 0.25 mg kg−1 in the fried samples, which clearly shows the carry-over of the compounds from the contaminated palm oil. The highest concentrations were observed in garlic and onion, which are in accordance with our previous data on commercial fried foods that showed the highest level of contamination (0.99 mg kg−1) in a sample of fried chopped garlic [16].
Garlic and onion also presented the highest water loss and fat uptake, suggesting a good correlation between these variables and the levels of bound 3-MCPD, as can be seen in Fig. 1, which also includes the other evaluated foods. A positive correlation between the weight of oil uptake and the weight of water removed has already been observed by other authors [30]. However, the positive correlation between these variables and the levels of bound 3-MCPD in fried foods, as observed in Fig. 1, had not been demonstrated so far.
The expected concentration of bound 3-MCPD in the fried foods, considering the levels of the compounds in the oil (1.64 ± 0.15 mg kg−1) and the fat uptake, was also estimated and compared with the measured concentration (Table 4). As can be observed, similar amounts were verified in banana, levels above the expected concentration were obtained in potato and beef patty while cassava, onion, garlic, polenta and rice ball were below the expected levels of bound 3-MCPD. For most of the samples, the observed differences may be considered low and could be attributed to variations in the recovery rates of the analytical method (generally acceptable between 70 and 120%) and the use of an average value of bound 3-MCPD in the oil before frying. Other factors such as possible irreversible reactions involving bound 3-MCPD and hydrolysis to free 3-MCPD, not yet investigated, could have some influence in foods that presented higher differences, as polenta.
Conclusion
In the present work, the possible sources of fried foods contamination by bound 3-MCPD during frying were investigated. Common temperatures used during frying may not be sufficient to induce an endogenous formation of the contaminants in fried foods. On the other hand, the carry-over of the compounds due to the use of contaminated oil was clearly observed, indicating that fat uptake constitutes the most important route of contamination in the frying process. In this case, the highest concentrations of bound 3-MCPD were observed in fried garlic and onion, i.e. foods that presented the highest water loss and fat uptake. The data reported here provide new insights in relation to the contamination of fried foods by bound 3-MCPD and may stimulate further studies on the mitigation of these chemical contaminants.
References
Zelinková Z, Svejkovská B, Velíšek J, Doleža M (2006) Fatty acid esters of 3-chloropropane-1,2-diol in edible oils. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 23:1290–1298
Kuhlmann J (2011) Determination of bound 2,3-epoxy-1-propanol (glycidol) and bound monochloropropanediol (MCPD) in refined oils. Eur J Lipid Sci Technol 113:335–344
MacMahon S, Begley TH, Diachenko GW (2013) Occurrence of 3-MCPD and glycidyl esters in edible oils in the United States. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 30:2081–2092
Arisseto AP, Marcolino PFC, Vicente E (2014) Determination of 3-monochloropropane-1,2-diol fatty acid esters in Brazilian vegetable oils and fats by an in-house validated method. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 31:1385–1392
Franke K, Strijowski U, Fleck G, Pudel F (2009) Influence of chemical refining process and oil type on bound 3-chloro-1,2-propanediol contents in palm oil and rapeseed oil. LWT—food. Sci Technol 42:1751–1754
Hrncirik K, Van Duijn G (2011) An initial study on the formation of 3-MCPD esters during oil refining. Eur J Lipid Sci Technol 113:374–379
Ermacora A, Hrncirik K (2014) Influence of oil composition on the formation of fatty acid esters of 2-chloropropane-1,3-diol (2-MCPD) and 3-chloropropane-1,2-diol (3-MCPD) under conditions simulating oil refining. Food Chem 161:383–389
Ramli MR, Siew WL, Ibrahim NA, Kuntom A, Abd Razak RA (2015) Other factors to consider in the formation of chloropropandiol fatty esters in oil processes. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 32:817–824
IARC (2012) Monographs on the evaluation of carcinogenic risks to humans 101:349–374
FAO/WHO (2002) Safety evaluation of certain food additives and contaminants: 3-chloro-1,2-propanediol. WHO Food Addit Ser 48:401–438
FAO/WHO (2007) Safety evaluation of certain food additives and contaminants: 3-chloro-1,2-propanediol. WHO Food Addit Ser 58:239–267
EFSA (2016) Risks for human health related to the presence of 3- and 2-monochloropropanediol (MCPD), and their fatty acid esters, and glycidyl fatty acid esters in food. EFSA J 14:4426
Zelinková Z, Doležal M, Velíšek J (2009) 3-Chloropropane-1,2-diol fatty acid esters in potato products. Czech J Food Sci 27:S421–S424
Ilko V, Zelinková Z, Doležal M, Velíšek J (2011) 3-Chloropropane-1,2-diol fatty acid esters in potato products. Czech J Food Sci 29:411–419
Merkle S, Karl H, Fritsche J (2014) Development of an analytical method for the determination of 2- and 3-MCPD fatty acid esters in fish and fish products. Paper presented at 12th Euro Fed Lipid Congress “Oils, Fats and Lipids: From Lipodomics to Industrial Innovation”, 14–17 September 2014. Montpellier, France. http://www.hsfs.org/download/FSMA2014/P1_Merkle_FSMA-2014.pdf. Accessed 17 Jan 2017
Arisseto AP, Marcolino PFC, Vicente E (2015) 3-Monochloropropane-1,2-diol fatty acid esters in commercial deep-fat fried foods. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 32:1431–1435
Weisshaar R (2011) Fatty acid esters of 3-MCPD: overview of occurrence and exposure estimates. Eur J Lipid Sci Technol 113:304–308
Dingel A, Matissek R (2015) Esters of 3-monochloropropane-1,2-diol and glycidol: no formation by deep frying during large-scale production of potato crisps. Eur Food Res Technol 241:719–723
Zenebon O, Pascuet NS (2005) Métodos físico-químicos para análise de alimentos. Instituto Adolfo Lutz, São Paulo
Horwitz W, JR Latimer, George W (2012) Official methods of analysis of the association of official analytical chemists. AOAC, Maryland
Svejkovská B, Novotny O, Divinová V, Réblová Z, Doležal M, Velíšek J (2004) Esters of 3-chloropropane-1,2-diol in foodstuffs. Czech J Food Sci 22:190–196
Doležal M, Chaloupská M, Divinová V, Svejkovská B, Velíšek J (2005) Occurrence of 3-chloropropane-1,2-diol and its esters in coffee. Eur Food Res Technol 221:221–225
Mogol BA, Pye C, Anderson W, Crews C, Gökmen V (2014) Formation of monochloropropane-1,2-diol and its esters in biscuits during baking. J Agric Food Chem 62:7297–7301
Özdikicierler O, Yemisçioglu F, Gümüskesen AS (2016) Effects of process parameters on 3-MCPD and glycidyl ester formation during steam distillation of olive oil and olive pomace oil. Eur Food Res Technol 242:805–813
Svejkovská B, Doležal M, Velíšek J (2006) Formation and decomposition of 3-chloropropane-1,2-diol esters in models simulating processed foods. Czech J Food Sci 24:172–179
Mellema M (2003) Mechanisms and reduction of fat uptake in deep-fat fried foods. Trends Food Sci Technol 14:364–373
Ziaiifar AM, Achir N, Courtois F, Trezzani I, Trystram G (2008) Review of mechanisms, conditions, and factors involved in the oil uptake phenomenon during the deep fat frying process. Int J Food Sci Technol 43:1410–1423
Gamble MH, Rice P, Selman D (1987) Relationship between oil uptake and moisture loss during frying of potato slices from cv. Record UK tubers. Int J Food Sci Technol 22:233–241
Alam T, Takhar PS (2016) Microstructural characterization of fried potato disks using X-ray micro computed tomography. J Food Sci 81:E651–E664
Diaz A, Trystam G, Vitrac O, Dufour D, Raoult-Wack AL (1999) Kinetics of moisture loss and fat absorption during frying for different varieties of plantain. J Sci Food Agric 79:291–299
Acknowledgments
This work was supported by the São Paulo Research Foundation (FAPESP) under Grant Numbers 2011/08936-0 and 2011/19043-6. The authors also thank Dr. Ana Lúcia da Silva C. Lemos and Márcia Mayumi H. Haguiwara for the meat processing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
About this article
Cite this article
Arisseto, A.P., Marcolino, P.F.C., Augusti, A.C. et al. Contamination of Fried Foods by 3-Monochloropropane-1,2-diol Fatty Acid Esters During Frying. J Am Oil Chem Soc 94, 449–455 (2017). https://doi.org/10.1007/s11746-017-2951-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-017-2951-9