Abstract
Prosecco wine is one of the most important products of the Italian oenological landscape. Its production is strictly regulated by several disciplinary. Thus, it is important to verify the quality of the final product, to defend the uniqueness of this wine. This work describes a rapid method to discriminate among varieties of Italian Prosecco wine using the volatile-fraction distribution as an untargeted fingerprint. The volatile profile corresponds to gas-chromatograms obtained in head-space mode. Principal components analysis of chromatograms allows discriminating the Prosecco samples depending on geographical origin, cultivation practices, and wine-making technologies. In particular, conventional vs. biological agriculture and manual vs. mechanical harvesting give well-separated clusters when projected on a scores plot. Influence plots allow evaluating which variables are the most effective to describe the differences between oenological classes, which are declared in the label and coded in the disciplinary of origin denomination. The identification of discriminating molecules in the volatile profile is also performed by Kovats indexes. Thus, possible chemical markers for the classification of Italian Prosecco wines are appointed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The creation of an exclusive and inimitable wine is strictly linked to the grape variety, the climate, the soil, the environment, the viticultural and oenological practices. Every single choice defines the qualitative expression of wine and makes it unique in the competition with other cultures [1,2,3]. In the literature, there are several studies about markers to assess wine origin [4, 5], variety and age [6, 7], to detect frauds and ensure authenticity [5]. These concern also the aromatic profile [8,9,10].
The power of the synergy between grape, environment and human work is well represented by Prosecco wine. The grape variety of Prosecco wine is Glera, that constitutes almost 85% of the final wine [11, 12]. Two Italian designations certify the excellence of Prosecco: the designation of controlled origin (Denominazione di Origine Controllata, DOC) and the designation of controlled and guaranteed origin (Denominazione di Origine Controllata e Garantita, DOCG). Such designations set wine productions and protect it from frauds and unfair competition.
Prosecco wine is an excellence in the Italian viticultural landscape. It is produced only in few provinces of the Veneto and Friuli-Venezia Giulia regions (north-eastern Italy), which are characterized by different morphology, climate, and origin: details are reported in the relevant disciplinary [11, 12].
A zonation study [2] evaluated the aromatic composition of Glera grape, throughout a chemical analysis of the volatile compounds in the must. That study analyzed three areas: West of Valdobbiadene, East of Conegliano and the zone between the two. The predominant aromatic molecules are the benzenoids, to which balsamic and spice notes are associated. Monoterpenes are the second most important class, and floral and citrus notes characterize them. The norisoprenoids are the last studied class and give to the wine notes of tropical and citrus fruits.
Even compounds with concentration ranging from ng/L to mg/L considerably influence the aromatic profile of wine [13,14,15,16]. These are generally divided into three classes [17]. The primary, or varietal aromas, typical of the grape variety, is linked to the viticultural ecosystem, like grape, rootstock, climate, soil, cultural technique [13]. It is mainly constituted by terpenes [18, 19], norisoprenoids, benzenoids, pyrazine, ketones, and aldehydes deriving from the pulp, leaves, skins, and berries [1, 13, 14]. The secondary aromas are produced during crushing and the different fermentation processes. These are produced by bacteria and yeast metabolism [13, 14]. The tertiary aromas are produced during aging, secondary fermentation, and oxidation–reduction reactions [14].
Further studies have demonstrated that the number of grapes per stump, the soil, the water availability [20], and the sunlight affect the aromatic profile [1]. The right equilibrium between the leaf wall and the number of grapes is given by optimal light and temperature conditions. For the Glera, a great amount of grapes causes a low concentration of monoterpenes and norisoprenoids [2]. The soil physically sustains the crops and shares water and mineral salts with them. The grain size and water properties affect the wine aroma [1].
Cultivation practices can also influence the composition of wine aroma [1]. First of all, the exposure of the vineyard to the sunlight has a strong influence on the final product. The sun is responsible for the synthesis of numerous volatile compounds [1, 21, 22]. Irrigation affects water conditions, which, as previously said, greatly affect the wine aroma [20]. The presence of fungicides causes a marked change in the aromatic profile, both because they seem to persist, even if in traces, in must and wine, and because they alter the kinetics of fermentation [23,24,25]. Even nitrogen fertilization seems to change the volatile component, as it increases the precursors of the amino acids [1].
Among the many factors influencing the wine aroma, the contact with the lees after alcoholic fermentation may modify the volatile profile. In the production of sparkling wine, some companies use to put the lees in contact with the wine. The wine lees are “the residue that forms at the bottom of the recipient containing wine, after fermentation, during storage or after authorized treatments, as well as the residue obtained following the filtration or centrifugation of this product” (EEC regulation No. 337/79). The lees contain microorganisms, yeasts, and, in less amount, inorganic matter and tartaric acid. Their composition is variable and depends on many factors [26]. When alcoholic fermentation ends, yeast autolysis frees several substances into the wine [27, 28]. Then, some volatile compounds are released and some are fixed [28]. This phenomenon could reduce or increase some compounds changing the wine aroma [28].
An important role in the creation of wine aromatic profile is played by the harvest techniques and by the period in which they are performed [29, 30]. The manual harvest involves a selective choice of ripe grapes, and, if necessary, the application of scalar harvest (collection of ripe bunches) is performed at a later time. In mechanical harvest, all grapes are picked together indistinctively, thus increasing the percentage amount of not maturated grapes. It is a cheap and fast method, but it could cause a greater release of must from grape or crushing caused by the collection [31,32,33]. The must formed is exposed to oxidative phenomena thus promoting, in extreme cases, the growth of the spontaneous flora that gives organoleptic defects to the wine [32, 34]. The defects are due both to an increase in protein content and to an increase of phenolic compounds extracted from the skin and seeds, especially when grapes are transported from the harvesting to the processing place [34, 35]. As a consequence of the mechanically harvested grapes, also the increase of two thiol compounds has been observed [30]: 3-mercapto-hexanol and 3-mercapto-hexyl-acetate. These two molecules contribute to the fruity and tropical aroma of white wine [30]. Furthermore, a slight increase in esters, such as ethyl hexanoate, ethyl octanoate, ethyl acetate, and isoamyl acetate has been associated with manual harvesting [29]. Grapes mechanically harvested and transported for a long time contain a high concentration of thermo-unstable proteins and, consequently, twice the amount of bentonite will be required to guarantee wine stability [34]. The bentonite is a chemically treated clay used in winemaking to reduce undesirable constituents and to prevent proteins haze formation after bottling, but it could compromise the wine quality [36, 37].
Temperature is a fundamental parameter in alcoholic fermentation, because it affects the fermentative kinetics, the cell metabolic activity in general and the concentration of yeast metabolism products. It considerably affects the organoleptic characteristics of the wine [38].
In the literature, many examples of analytical methods to study wines quality and authenticity are present: LC–MS [4], HPLC–DAD–MS [39], HPLC-Q/TOF–MS [40], ESI-FT–MS [41], LC–MS–MS [42], NMR [6, 43], FT-Raman [5], SERS technology [44, 45], and vibrational spectroscopy [46,47,48].
In this study, we applied an analytical approach based on the combined use of a gas-chromatographic technique (GC) [49,50,51] and multivariate analysis. The aim was to discriminate samples of Prosecco wine based on their denomination, origin area, and method of production, and to obtain useful analytical information about these products. An identification work was carried out to search for possible molecular markers that can describe the authenticity and the typicality of Prosecco.
Materials and methods
Wine samples
In this work, two types of Prosecco were studied: 14 samples of Prosecco DOC and 19 of Prosecco DOCG “Conegliano-Valdobbiadene”. Among the DOC samples, eight are produced in the province of Treviso and are called “DOC Treviso”, four are produced in the Veneto and Friuli-Venezia Giulia regions and are called “DOC Veneto-Friuli” (VF), and two comes from biological agriculture (BIO). Among the four Prosecco DOC Veneto-Friuli samples, one is not filtered after the alcoholic fermentation and the wine remains in contact with yeast. This type of wine is called wine with lees or unfiltered (UNF) wine. The Prosecco DOCG samples due their names to the provinces of the region Veneto in which they are produced: Conegliano and Valdobbiadene.
Samples were purchased by the quality-control laboratory of COOP Italia, an Italian consortium that acts as a central retailer and one of the most important supermarket chains in Italy; samples were taken before the distribution in supermarket chains.
Each sample was recorded and tagged by a progressive number. Hence, traceability is always verifiable.
Sample preparation and analysis
For head-space (HS) GC analysis, an aliquot of wine was degassed with an ultrasonic bath, to remove the excess of CO2. A constant quantity of 2 g ± 1% of each degassed sample was directly placed in a vial, without any chemical pre-treatment. Each vial was closed with a special magnetic hermetic (air-tight) plug. For each wine sample, four vials were prepared, to obtain replicated analyses. The vials were put in a sample holder rack. Samples were prepared immediately after bottle opening.
Volatile compounds were analyzed by a gas chromatograph called Heracles II (Alpha M.O.S., Tolosa, France). Chromatographic separations were carried out in two columns working in parallel mode: a non-polar column MXT-05 (Column a, 5% di-phenyl, 95% methyl-poly-siloxane, 10 m length, 180 μm diameter) and a slightly polar column MXT-1701 (Column b, 14% cyano-propyl-phenyl, 86% methyl-poly-siloxane, 10 m length, 180 μm diameter). Each of them was connected to a flame ionization detector (FID). The carrier gas was hydrogen, which is self-produced by a module of Heracles II. Each vial was taken by the auto-sampler and put in a shaker oven, where it remained for 1200 s at 40 °C, shaken at 500 rpm. In this way, the volatile compounds were concentrated in the headspace. Then, the vial silicone septum was pierced by a syringe, and 5 ml of the headspace were sampled and adsorbed on a CARBOWAX trap (at 40 °C for 60 s) before chromatographic analysis. The gas carrier flows inside such a trap to remove the excess of air and moisture and to concentrate the analytes. When the temperature of the trap reached 240 °C, this temperature was kept for 93 s. Then, the sample was desorbed and injected. The chromatographic thermal program started at a temperature of 40 °C, held up for 2 s, and then increased to 270 °C (3 °C s−1), and finally held up for 21 s. The total separation time was of 100 s. The signal was acquired and digitized every 0.01 s.
Volatile compounds identification
An alkane solution (from n-hexane to n-hexadecane) (Merck, Darmstadt, Germany) was used to convert the retention time to Kovats index using the following expression:
where RI is the Kovats retention index, RT is the retention time, x is the unknown compound, z is the number of carbon atoms of the n-alkane eluted before the unknown compound, z + 1 is the number of carbon atoms of the n-alkane eluted after the unknown compound.
Four replicated measurements of the n-alkane standard were injected.
The volatile compounds were tentatively identified by the database of the Heracles software, AroChemBase (Alpha M.O.S.), by Flavornet database [52], NIST database, and based on articles in the literature.
Data acquisition and software
The chromatograms were processed by Alpha Soft software (Alpha M.O.S.), which integrates the chromatogram peaks, transcribes peak-areas in a data matrix and calculates the Kovats indexes from the retention times.
The rows of the data matrix (objects) represent the replicates of analyses performed on the samples, while the columns of the data matrix represent the peak areas (variables). The data matrix is the dataset for multivariate data processing. The dimension was: 132 rows (33 samples × 4 replicates) and 37 columns (integrated HS-GC peak areas).
Data processing was performed by the software CAT [53], based on the software R (R Core Team, Vienna, Austria).
Principal component analysis
Data processing was based on principal component analysis (PCA). PCA is a well-known chemometric procedure based on a linear transformation of the original variables into a new reference system whose axes are the principal components (PCs), orthogonal to each other [54, 55] and oriented along the most informative directions of the mathematical space. PCA is mainly used to perform an explorative analysis of data through scores plots, representing the objects in the principal components space, and loadings plots, describing the role of the original variables in the new reference system [54, 55].
Influence plots describe how the samples are compliant with the built model; in this work, they were created using Q and Hotelling T2 statistics [56]. The two statistics have independent confidence intervals and limits [52,53,54].
T2 statistic measures the distances between samples and the centroid of the PCA model, then it compares these distances with the model limits. The parameter T2 is defined as [55]:
where ti,m is the score value of i-th sample on m-th component, λm are the eigenvalues of the m considered principal components (PCs).
Q statistic quantifies the distance between the original data and the PCA model: it is the measure of residuals [57]. The Q value is computed as [55]:
where ei is the i-th row of residuals matrix E [55].
The higher confidence limits for T2 and Q depend on the significant number of components retained in the PCA model and on the level of significance.
Results and discussion
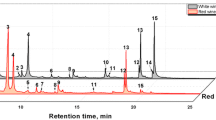
Complete aroma compounds and the RI of analyzed Prosecco wines are reported in Table 1.
The identification obtained is the best with the use of the FID detectors.
The complete dataset of 129 objects (after removing three evident outliers) and 37 columns (integrated HS-GC peak areas) was studied by PCA. Figure 1 shows the scores plot. Figure 1 shows an overlap zone between DOC and DOCG samples, which is probably due to the closeness of the production areas of these two classes. The samples are visualized in the plane of the first two principal components, which explain the 43.2% of the total variance.
The general dataset was then divided into two parts: Prosecco DOC and Prosecco DOCG to make further exploration of these two classes.
Prosecco DOC
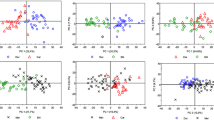
This dataset is composed of 56 objects and 37 variables (HS-GC integrated peak areas). The objects are visualized in the plane of the first two principal components (Fig. 2), which explains 42.0% of the total variance. The VF samples (in red) are located on the right. This group includes the UNF samples (in pink). The DOC Treviso samples, in green, are clustered in the center of the score plot. The BIO samples (in black) are clustered on the left. The scores plot shows a good separation between the three classes (VF, Treviso, BIO).
These results could be attributed to the different geographical origins of the products, which is in agreement with several studies [62,63,64].
Indeed, the DOC Treviso samples are produced in the same province of Veneto; VF are produced either in other provinces of the same region Veneto or in a different region (Friuli-Venezia Giulia). As for BIO samples, in the literature, in-depth studies about volatile compounds in biological wine are missed; however, since during the production of biological wine many compounds and oenological practices are forbidden [65], this could cause a variation in volatile profiles.
To examine in depth the study of BIO and UNF samples and to highlight potential differences from the VF and the DOC Treviso wines, further evaluations are performed.
Projection of BIO samples on the model of DOC Veneto-Friuli and DOC Treviso
A new PCA model (named DOC VF-T model) including only VF and DOC Treviso samples has been recalculated. For each BIO sample, an “average sample” has been computed as a sample whose variables are the mean values of the four replicates. The averaged samples are projected on the DOC VF-T model, which explains 70% of the total variance with five PCs. Significant differences are shown. The corresponding influence plot reporting Q and T2 statistics is reported in Fig. 3. It shows how much the BIO samples are compliant with the model. The BIO samples are located far beyond the limits of both the statistics at 0.1% of significance level, indicating a strong difference with the other DOC samples. This evidence may be ascribed to specific rules that the European Regulation CE 203/2012 establishes for the production of a biological wine. In particular, in this production, the use of some oenological practices is forbidden: partial cold concentration, the addition of sulfur dioxide with physical proceedings, tartaric stabilization with electro-dialysis or cationic exchangers, and wine partial de-alcoholization. Other practices are limited or only allowed under certain conditions.
Normalized Q and T2 indices have been calculated for each variable of each sample (Fig. 4). The red horizontal line in Fig. 4 is the boundary corresponding to 5% significance level [66]: variables overcoming this threshold make the corresponding samples not compliant with the DOC model. These parameters indicate which variables are mostly responsible for the discrimination of biological wines.
The influence plot in Fig. 3 and the Q and T2 statistics in Fig. 4 allow to identify the following potential volatile molecules that stave off the BIO samples from the DOC VF-T model. The corresponding RTs are specified among brackets. Alcohols: ethanol (472.41a, 563.59b), butanol (672.77a), 2-methyl-butanol (877.69a, 840.45b), 3-methyl-butanol (840.45b), Hexanol (877.69a, 980.23b); aldehydes and ketones: acetaldehyde (438.41a, 491.69b) and butane-2,3-dione (546.60a); esters-acids: isoamyl acetate (877.69a, 945,12b), isobutyric acid (877.69a, 945.12b), hexyl acetate (996.14 a, 1075.23b), ethyl hexanoate (996.14 a, 1075.23b), (Z)-hexen-1-ol-acetate (996.14 a, 1075.23b), ethyl octanoate (1207.67a, 1272.54b), ethyl cinnamate (1479.64a, 1626.85b); terpenes: 2-methylisoboreol (1207.67a, 1272.54b); norisoprenoids: α-ionone (1418.01a, 1549.81b), (E)-damascone (1418.01a), β-ionone (1479.64a, 1626.85b); benzenoids: vanillin (1479.64a).
Projection of UNF samples on the model of VF and DOC Treviso
Unfiltered samples are “wine on lees”. After alcoholic fermentation, these samples lie on yeasts. When autolysis occurs the cell wall degrades and releases enzymes and mannoproteins [27]. Some volatile compounds are absorbed from cell walls and volatile compounds precursors increase [28]. The volatile profile is affected also by the time of contact and the type of yeast [28, 67].
To explore the volatile species characterizing the UNF samples, the same analysis as in the previous paragraph 3.1.1 has been carried out for these samples. The “average UNF sample” has been projected on the same PCA VF-T model. The influence plot is calculated with five principal components, explaining about 70% of the total variance (Fig. 5).
The sample is located out of Q statistics confidence limits at 0.1% of significance level.
Normalized Q indices of all variables of the sample have been evaluated (Fig. 6).
Figure 6 shows a difference between the UNF projected sample and the DOC VF-T model, in particular in the amount of acetaldheyde (438.41a, 491.69b), ethyl acetate (614.99a, 675.91b), butane-2,3-dione (546.60a), 3-methyl-butanal (653.94a, 722.14b), 2-methyl-butanal (653.94a), 2-methyl-propanal (653.94a), acetoin (719.12a, 857.10b), 3-methyl-butanol (840.45b), hexyl acetate (996.14a, 1075.23b), ethyl hexanoate (996.14a, 1075.23b), (Z)-hexen-1-ol-acetate (996.14a, 1075.23b).
These results are in agreement with the study conducted by Bueno et al. [27], who analyzed the effect on the volatile profile of two wines (Airen and Macabeo) due to short contact time with lees. This study shows how the contact with lees changes the concentration of ethyl acetate, hexyl acetate, ethyl-hexanoate, 3-methyl-butanol, and others.
Prosecco DOCG
An analogous study has been carried out for Prosecco DOCG. After removing three evident outliers (data not reported), the model shown in Fig. 7 has been computed. It is created basing on 73 objects and 37 variables. The first two principal components explain 44.2% of the total variance. Figure 7 shows a group samples from a particular company (C. Company) laying on the left side of the score plot, while the other samples lay on the right side.
The only difference declared by the C. Company is that it performs the manual harvesting of grapes. The harvesting type could influence the quality of a wine, as reported in several studies [30, 34, 68]. Such difference encouraged performing a T2 and Q analysis, to understand how manual harvesting affects the volatile fraction of wine.
A PCA model (DOCG model) has been computed with Prosecco DOCG samples excluding those produced from the C. Company.
For all the samples produced by the C. Company, the replicates average samples have been calculated. These samples have been projected on the DOCG model, as it is shown in the influence plot in Fig. 8. The influence plot is calculated with seven PCs explaining about 75% of the total variance.
Also in this case, the influence plot indicates a strong difference between C. Company samples and the other DOCG, as the former are far beyond the confidence limits at 0.1% significance level. Thus, for each variable of each sample, normalized Q and T2 indices have been calculated; they are displayed in Fig. 9.
The volatile compounds that discriminate the C. Company samples from the other ones are the following. Alcohols: ethanol (472.74a, 563.59b), butanol (672.77a), 2-methyl-1-propanol (614.99a), 2-methyl-butanol (877.69a, 840.45b), 3-methyl-butanol (840.45b); esters: ethyl acetate (614.99a, 675.91b), isopropyl acetate (719.12a, 840.45b), methyl butanoate (719.12a, 771.48b), ethyl butyrate (783.85a), ethyl isobutyrate (813.74b), isoamyl acetate (877.69a, 945.12b), hexyl acetate (996.14a, 945.12b), ethyl hexanoate (996.14a), hexyl ottanoate (996.14a), methyl decanoate (1329.04a), methyl butanoate (771.48b), ethyl propionate (771.48b); other compounds: acetic acid (614.99a, 771.48b), 2,3-butendiol (798.35a), vanillin (1479.64a), ethyl cinnamate (1479.64a), β-ionone (1479.64a).
Comparison with previous studies
A study led by Olejar et al. [29] showed a difference between hand-picked wine and machine harvested wine in ethyl and acetate esters content. Arfelli et al. [69] showed a difference in higher alcohols, volatile acids, esters and other volatile compounds between hand-picked grapes and mechanically harvested grapes. Pocock et al. [35] argued that mechanical harvesting and long-distance transport may influence the extraction of haze-forming proteins into juice. In particular, some proteins derived from mechanically damaged grapes are precursors of specific volatile compounds.
Our study confirms the differences reported in these studies and shows that there could be other molecules influenced by the manual harvesting.
Conclusions
This work demonstrates that the head-space gas-chromatograms of Italian Prosecco wines are suitable as untargeted fingerprints, allowing to evaluate how the oenological practices and the different areas of production can affect the aroma of the final products obtained from the same grape (Glera). Therefore, the volatile profile has been used to create multivariate models allowing to study the differences between several categories of Italian Prosecco: DOC, DOCG, biological, unfiltered, manual harvested. Moreover, the application of T2 and Q statistics to PCA models allowed to identify some volatile species as possible markers for the discrimination among these categories.
The PCA showed that DOC wines produced in Treviso can be distinguished from the ones produced in other provinces of the region of Veneto and another region (Friuli Venetia Giulia). Moreover, the biological samples are well discriminated from the other DOC samples. About DOCG wines, discrimination between the wines produced by hand-picked harvesting and those obtained by mechanical harvesting is shown.
The influence plot resulted to be a useful tool to find which variables are responsible for the different behavior of BIO, UNF, and manually harvested samples.
The method here proposed provides a low-cost screening method for the authentication of Italian Prosecco wine, based on the combined application of multivariate statistics and flash HS-GC-FID.
Availability of data and material
Data are not available.
References
González-Barreiro C, Rial-Otero R, Cancho-Grande B, Simal-Gándara J (2015) Wine aroma compounds in grapes: a critical review. Crit Rev Food Sci Nutr 55:202–218
Tomasi D, Gaiotti F, Jones GV (2013) The power of the terroir: the case study of prosecco wine. ISBN 9783034806282
Moret I, Scarponi G, Cescon P (1994) Chemometric characterization and classification of five Venetian white wines. J Agric Food Chem 42(5):1143–1153
Pisano PL, Silva MF, Olivieri AC (2015) Anthocyanins as markers for the classification of Argentinean wines according to botanical and geographical origin. Chemometric modeling of liquid chromatography-mass spectrometry data. Food Chem 175:174–180
Magdas DA, Guyon F, Feher I, Pinzaru SC (2018) Wine discrimination based on chemometric analysis of untargeted markers using FT-Raman spectroscopy. Food Control 85:385–391
Geana EI, Popescu R, Costinel D, Dinca OR, Ionete RE, Stefanescu I, Artem V, Bala C (2016) Classification of red wines using suitable markers coupled with multivariate statistic analysis. Food Chem 192:1015–1024
Francioli S, Torrens J, Riu-Aumatell M, López-Tamames E, Buxaderas S (2003) Volatile compounds by SPME-GC as age markers of sparkling wines. Am J Enol Vitic 54:158–162
Perestrelo R, Barros AS, Câmara JS, Rocha SM (2011) In-depth search focused on furans, lactones, volatile phenols, and acetals as potential age markers of Madeira wines by comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry combined with solid phase microextraction. J Agric Food Chem 59(7):3186–3204
Nasi A, Ferranti P, Amato S, Chianese L (2008) Identification of free and bound volatile compounds as typicalness and authenticity markers of non-aromatic grapes and wines through a combined use of mass spectrometric techniques. Food Chem 110(3):762–768
Fabani MP, Ravera MJA, Wunderlin DA (2013) Markers of typical red wine varieties from the Valley of Tulum (San Juan-Argentina) based on VOCs profile and chemometrics. Food Che 141(2):1055–1062
Disciplinare di Produzione dei Vini a Denominazione di Origine Controllata e Garantita “Conegliano Valdobbiadene - Prosecco.” pp 1–15
Disciplinare di Produzione della Denominazione di Origine Controllata dei Vini “Prosecco.”
Robinson AL, Boss PK, Solomon PS, Trengove RD, Heymann H, Ebeler SE (2014) Origins of grape and wine aroma. Part 1. Chemical components and viticultural impacts. Am J Enol Vitic 65:1–24
D’Onofrio C (2011) Caratterizzazione funzionale della biosintesi degli aromi delle uve durante lo sviluppo dell’acino e controllo della qualità aromatica delle uve. Italus Hortus 18:39–61
Noguerol-Pato R, González-Barreiro C, Cancho-Grande B, Simal-Gándara J (2009) Quantitative determination and characterisation of the main odourants of Mencía monovarietal red wines. Food Chem 117(3):473–484
Bosch-Fusté J, Riu-Aumatell M, Guadayol JM, Caixach J, López-Tamames E, Buxaderas S (2007) Volatile profiles of sparkling wines obtained by three extraction methods and gas chromatography-mass spectrometry (GC-MS) analysis. Food Chem 105(1):428–435
Cabrita MJ, Costa Freitas AM, Laureano O, Borsa D, Di Stefano R (2007) Aroma compounds in varietal wines from Alentejo, Portugal. J Food Compos Anal 20(5):375–390
Vilanova M, Sieiro C (2006) Determination of free and bound terpene compounds in Albariño wine. J Food Compos Anal 19(6-7):694–697
Marais J (2017) Terpenes in the aroma of grapes and wines: a review. S Afr J Enol Vitic 4(2):49–58
Chapman DM, Roby G, Ebeler SE, Guinard JX, Matthews MA (2005) Sensory attributes of Cabernet Sauvignon wines made from vines with different water status. Aust J Grape Wine Res 11:339–347
Belancic A, Agosin E, Ibacache A, Bordeu E, Baumes R, Razungles A, Bayonove C (1997) Influence of sun exposure on the aromatic composition of chilean Muscat grape cultivars Moscatel de Alejandria and Moscatel rosada. Am J Enol Vitic 48:181–186
Bureau SM, Razungles AJ, Baumes RL (2000) The aroma of Muscat of Frontignan grapes: effect of the light environment of vine or bunch on volatiles and glycoconjugates. J Sci Food Agric 80:2012–2020
Noguerol-Pato R, González-Rodríguez RM, González-Barreiro C, Cancho-Grande B, Simal-Gándara J (2011) Influence of tebuconazole residues on the aroma composition of Mencía red wines. Food Chem 124(4):1525–1532
González Álvarez M, Noguerol-Pato R, González-Barreiro C, Cancho-Grande B, Simal-Gándara J (2012) Changes of the sensorial attributes of white wines with the application of new anti-mildew fungicides under critical agricultural practices. Food Chem 130(1):139–146
González-Rodríguez RM, Noguerol-Pato R, González-Barreiro C, Cancho-Grande B, Simal-Gándara J (2011) Application of new fungicides under good agricultural practices and their effects on the volatile profile of white wines. Food Res Int 44(1):397–403
Pérez-Serradilla JA, de Castro MDL (2008) Role of lees in wine production: a review. Food Chem 111(2):447–456
Bueno JE, Peinado RA, Medina M, Moreno J (2006) Effect of a short contact time with lees on volatile composition of Airen and Macabeo wines. Biotechnol Lett 28:1007–1011
Bautista R, Fernández E, Falqué E (2007) Effect of the contact with fermentation-lees or commercial-lees on the volatile composition of white wines. Eur Food Res Technol 224(4):405–413
Olejar KJ, Fedrizzi B, Kilmartin PA (2015) Influence of harvesting technique and maceration process on aroma and phenolic attributes of Sauvignon blanc wine. Food Chem 183:181–189
Allen T, Herbst-Johnstone M, Girault M, Butler P, Logan G, Jouanneau S, Nicolau L, Kilmartin PA (2011) Influence of grape-harvesting steps on varietal thiol aromas in sauvignon blanc wines. J Agric Food Chem 59(19):10641–10650
Clary C, Steinhauer R, Frisinger J, Peffer T (1990) Evaluation of machine- vs. hand-harvested chardonnay. Am J Enol Vitic 41:176–181
Morris JR (1998) Factors influencing grape juice quality. HortTechnology 8(4):471–478
Carrara M, Catania P, Pipitone F, Vallone M, Salvia M (2007) Assessment of the pedicel detaching and crushing forces of grape berries to determine the optimal mechanical harvesting time. J Agric Eng 3:23–37
Pocock KF, Waters EJ (1998) The effect of mechanical harvesting and transport of grapes, and juice oxidation, on the protein stability of wines. Aust J Grape Wine Res 4(3):136–139
Pocock KF, Hayasaka Y, Peng Z, Williams PJ, Waters EJ (1998) The effect of mechanical harvesting and long-distance transport on the concentration of haze-forming proteins in grape juice. Aust J Grape Wine Res 4(1):23–29
Catarino S, Madeira M, Monteiro F, Rocha F, Curvelo-Garcia AS, De Sousa RB (2008) Effect of bentonite characteristics on the elemental composition of wine. J Agric Food Chem 56(1):158–165
Lira E, Salazar FN, Rodríguez-Bencomo JJ, Vincenzi S, Curioni A, López F (2014) Effect of using bentonite during fermentation on protein stabilisation and sensory properties of white wine. Int J Food Sci Technol 49(4):1070–1078
Molina AM, Swiegers JH, Varela C, Pretorius IS, Agosin E (2007) Influence of wine fermentation temperature on the synthesis of yeast-derived volatile aroma compounds. Appl Microbiol Biotechnol 77:675–687
Fraige K, Pereira-Filho ER, Carrilho E (2014) Fingerprinting of anthocyanins from grapes produced in Brazil using HPLC-DAD-MS and exploratory analysis by principal component analysis. Food Chem 145:395–403
Vaclavik L, Lacina O, Hajslova J, Zweigenbaum J (2011) The use of high performance liquid chromatography-quadrupole time-of-flight mass spectrometry coupled to advanced data mining and chemometric tools for discrimination and classification of red wines according to their variety. Anal Chim Acta 685(1):45–51
Villagra E, Santos LS, Vaz BG, Eberlin MN, Felipe Laurie V (2012) Varietal discrimination of Chilean wines by direct injection mass spectrometry analysis combined with multivariate statistics. Food Chem 131(2):692–697
Hermann G, Jaitz L, Schmölzer C, Koellensperger G, Eder R, Hann S (2012) Analysis of (poly-)phenols in commercially available red wines by means of LC-MS|Analyse von (Poly-)Phenolen in kommerziell erhältlichen Rotweinen via LC-MS. Mitteilungen Klosterneubg 62(1):13–20
Godelmann R, Fang F, Humpfer E, Schütz B, Bansbach M, Schäfer H, Spraul M (2013) Targeted and nontargeted wine analysis by 1H NMR spectroscopy combined with multivariate statistical analysis. Differentiation of important parameters: grape variety, geographical origin, year of vintage. J Agric Food Chem 61(23):5610–5619
Magdas DA, Cinta Pinzaru S, Guyon F, Feher I, Cozar BI (2018) Application of SERS technique in white wines discrimination. Food Control 92:30–36
de Almeida MP, Leopold N, Franco R, Pereira E (2019) Expedite SERS fingerprinting of Portuguese white wines using plasmonic silver nanostars. Front Chem 7:368
Bevin CJ, Dambergs RG, Fergusson AJ, Cozzolino D (2008) Varietal discrimination of Australian wines by means of mid-infrared spectroscopy and multivariate analysis. Anal Chim Acta 621(1):19–23
Banc R, Loghin F, Miere D, Fetea F, Socaciu C (2014) Romanian wines quality and authenticity using FT-MIR spectroscopy coupled with multivariate data analysis. Not Bot Horti Agrobot Cluj-Napoca 42(2):556–564
Culbert J, Cozzolino D, Ristic R, Wilkinson K (2015) Classification of sparkling wine style and quality by MIR spectroscopy. Molecules 20(5):8341–8356
Antoce AO (2012) Influence of maceration enzyme treatment on the colour and volatile profile of two red Romanian wines. Rev Chim 63(9):859–864
Antoce AO, Cojocaru GA (2015) Effects of blending and co-inoculation on the aromatic profile of wines. Rev Chim 66(10):1567–1571
Cojocaru GA, Antoce AO (2019) Influence of glutathione and ascorbic acid treatments during vinification of feteasca regala variety and their antioxidant effect on volatile profile. Biosensors 9(4):140
Arn H, Acree TE (1998) Flavornet: a database of aroma compounds based on odor potency in natural products. Dev Food Sci 40:27
R. Leardi, C. Melzi, G.P. CAT (chemometric agile tool). https://gruppochemiometria.it/index.php/software. Accessed Mar 2020
Bro R (2014) Principal component analysis. Anal Methods 6:2812–2831
Ballabio D (2015) A MATLAB toolbox for principal component analysis and unsupervised exploration of data structure. Chemom Intell Lab Syst 149:1–9
Mujica LE, Rodellar J, Fernández A, Güemes A (2011) Q-statistic and t2-statistic pca-based measures for damage assessment in structures. Struct Health Monit 10:539–553
Ballabio D, Consonni V (2013) Classification tools in chemistry. Part 1: linear models. PLS-DA. Anal Methods 5:3790–3798
Rottiers H, Tzompa Sosa DA, Van de Vyver L, Hinneh M, Everaert H, De Wever J, Messens K, Dewettinck K (2019) Discrimination of cocoa liquors based on their odor fingerprint: a fast GC electronic nose suitability study. Food Anal Methods 12:475–488
Frauendorfer F, Schieberle P (2006) Identification of the key aroma compounds in cocoa powder based on molecular sensory correlations. J Agric Food Chem 54(15):5521–5529
Fang Y, Qian M (2005) Aroma compounds in Oregon Pinot Noir wine determined by aroma extract dilution analysis (AEDA). Flavour Fragr J 20(1):22–29
Schieberle P (1991) Primary odorants of pale lager beer. Zeitschrift fǘr Leb Und-Forshcung 193:558–565
Buratti S, Benedetti S, Scampicchio M, Pangerod EC (2004) Characterization and classification of Italian Barbera wines by using an electronic nose and an amperometric electronic tongue. Anal Chim Acta 525(1):133–139
Schlesier K, Fauhl-Hassek C, Forina M, Cotea V, Kocsi E, Schoula R, van Jaarsveld F, Wittkowski R (2009) Characterization and determination of the geographical origin of wines. Part I: overview. Eur Food Res Technol 230:1
Antoce AO, Namolosanu I (2011) Rapid and precise discrimination of wines by means of an electronic nose based on gas-chromatography. Rev Chim 62(6):593–595
(2012) Regolamento di esecuzione (UE) N.203/2012 della commissione dell’8 marzo 2012. Gazz. Uff. dell’Unione Eur
Conlin AK, Martin EB, Morris AJ (2000) Confidence limits for contribution plots. Proc J Chemom 14:725–736
Riu-Aumatell M, Bosch-Fusté J, López-Tamames E, Buxaderas S (2006) Development of volatile compounds of cava (Spanish sparkling wine) during long ageing time in contact with lees. Food Chem 95(2):237–242
Santos AO, Wample RL, Sachidhanantham S, Kaye O (2012) Grape quality mapping for vineyard differential harvesting. Braz Arch Biol Technol 55:193–204
Arfelli G, Sartini E, Bordini F, Caprara C, Pezzi F (2010) Mechanical harvesting optimization and postharvest treatments to improve wine quality. J Int des Sci la Vigne du Vin 44(2):101–115
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization: FG, DM; methodology: FG; formal analysis and investigation: TF, AZ; writing—original draft preparation: TF, AZ; writing—review and editing: FG, DM; funding acquisition: FG; resources: FG; supervision: DM.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest associated with this publication.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Forleo, T., Zappi, A., Gottardi, F. et al. Rapid discrimination of Italian Prosecco wines by head-space gas-chromatography basing on the volatile profile as a chemometric fingerprint. Eur Food Res Technol 246, 1805–1816 (2020). https://doi.org/10.1007/s00217-020-03534-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-020-03534-8