Abstract
This study describes the possibilities of valorising a waste stream that originates from apple wood by mapping the reducing capacity and phenolic profile from extracts derived from apple tree (Malus domestica). This study evaluated the efficiency of warm solvent extraction (WSE) and ultrasound-assisted extraction (UAE) techniques for extracting antioxidant phenolic compounds from the bark and core wood of an apple tree cultivated in the north-eastern part of Belgium. Furthermore, the influence of the pre-treatment technique, namely, fresh, oven-dried, and freeze-dried samples, respectively, on the yield of polyphenols was studied. Fresh bark extract obtained by UAE—the most efficient extraction technique—employing acetone 60% v/v contains the highest levels of phenolic compounds as well as the highest antioxidant activity. High-performance liquid chromatographic analysis shows that phloridzin is the major compound of the identified polyphenol markers present in bark and core wood extracts. Based on the obtained results, it may be possible to produce a polyphenolic extract from apple wood at an industrial scale without extensive costs or altering the antioxidant properties. This study reveals the potential of apple tree wood residues valorisation through the recovery of phenolic compounds for food, pharmaceutical, and cosmetic applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyphenols are the most abundant secondary metabolites of plants and do not play an essential role in the primary processes of a plant. This does not mean polyphenols are insignificant for the development of a plant, since certain polyphenolic compounds act as a natural defence against diseases [1,2,3] or as a natural colour pigment [4, 5]. Besides their biological function, natural phenols have been reported to possess good properties as food preservatives and are interesting compounds to be used as antioxidant agents in, e.g., cosmetic preparations [6].
Recently, the number of studies concerning the extraction of polyphenols from inexpensive and renewable sources has been increasing [7,8,9,10,11]. Fruit growers annually have hundreds of tons of wood waste. Despite their potential for chemical recycling, residues from the harvest of apple woods or wood processing are frequently used for applications with low added value [8, 12]. Certain companies already developed products out of wood, such as smoke chips and smoke planks used for barbeques and smoke ovens. Apple tree wood residues, such as bark, can be an important resource for the extraction of bioactive molecules [13, 14]. In fact, the recovery of phenolic compounds from wood wastes is gaining increased attention [13, 15]. Recently, Moreira et al. [11] have shown the potential of apple tree wood residues as a source of phenolic compounds. In another study, the presence of one important bioactive polyphenol, phloretin, in the bark of apple trees was reported [16].
The extraction of bioactive compounds from natural products is an important step to enable utilization of residues can be used in food, pharmaceutical, or cosmetic products. Different techniques for the extraction of polyphenols from solid samples in several waste streams of plant origin have been reviewed [1]. Conventional techniques, such as warm solvent extraction (WSE) and Soxhlet extraction, are still widely used for the recovery of phenolic compounds from tree wood, but new techniques, such as ultrasound-assisted extraction (UAE) and microwave-assisted extraction (MAE), are gaining considerable attention because of their environmentally friendly character [10, 11]. A number of reported applications have shown that UAE is a green and economical alternative to conventional extraction when working with natural products [8, 17, 18].
Besides the extraction technique used to recover polyphenols, the type of extraction solvent has been investigated in several studies [11, 19, 20]. Moreover, the methods used for the preservation of samples represent another parameter which deserves attention, especially in the case of apple wood. Due to the high moisture content and microbial activity, apple wood is unstable and can degrade rapidly. There are several methods for sample preservation. Bartolomé et al. [21] evaluated three methods, namely, freeze drying, oven drying, and freezing of fresh material, for the preservation of brewing spent grains, which is a major by-product of large and small breweries. Freezing of fresh wood material is not suited for large-scale use, because the volumes to be stored are too large. On the other hand, drying or freeze drying reduces the volume of the product, which makes it more suitable for storage [22, 23]. In general, these preservation methods are considered as the best treatments for high-quality dry matter, even though they are not the best option from an economical point of view [23]. In addition, it may also lead to loss in bioactive compounds, despite maintaining the sensory attributes [24]. In the case of the oven-drying process, the main disadvantage is the exposure to oxygen and high temperatures, which may influence the chemical composition. Nevertheless, the oven-drying process is frequently used because of its low cost, as opposed to freeze drying, which is less attractive from the economical point of view.
This study will examine the influence of sample pre-treatment applied on apple wood. More specifically, the technique of oven-drying and freeze drying will be compared with freshly ground wood on the amount of available polyphenols. As described in the literature [22,23,24], a sharp decrease in the availability of polyphenols is expected when the wood is oven-dried before the extraction process.
In addition to the influence of pre-treatment, the effect of the extraction process was evaluated by the total polyphenol content. The first parameter of this process, the composition of the solvent, is a frequently investigated parameter, knowing that it strongly influences the extraction efficiency [7, 8, 11, 14]. Different solvent mixtures on the extraction of polyphenols from Malus domestica ‘King Jonagold’ were investigated. Namely, methanol, ethanol, acetone, and water were used for the extraction of phenolic compounds. The solvents were used in the pure form and also in mixtures with water (20%, 40%, 60%, and 80%, v/v). In correlation with recent studies [7, 10], a higher yield in polyphenols is expected for extraction produced in the presence of a mixture of organic solvent and water than when pure solvent is used. To achieve extraction of bioactive compounds, both WSE and UAE were employed as extraction techniques, the second parameter of the extraction process. This study aims to demonstrate that ultrasonic extraction can lead to a reduced use of solvents without a loss of yield in correlation with the conventional solvent-based technology [8, 17, 18].
Extraction efficiency of both techniques with the different solvent mixtures on the pre-treated bark and core wood was evaluated by the total phenolic content (TPC) and total flavonoid content (TFC), as well as by the antioxidant activity assays which were determined via measurement of 2,2′-diphenyl-1-picrylhydrazyl radical scavenging activity (DPPH-RSA) and Ferric Reducing Antioxidant Power (FRAP) assays. Moreover, the characterization of the polyphenols from apple tree bark and core wood by high-pressure liquid chromatography equipped with a photodiode array detector (HPLC–PDA) was employed for the identification and quantification of the extracted phenolic compounds to see which phenolic compound contributes most to the antioxidant activity of the obtained extracts.
Materials and methods
Processing of apple wood
The wood from Malus domestica ‘King Jonagold’ was used during this project. The apple tree, with 15 years, was felled at 18th of March 2016 and sampled at Haspengouw, a region in the county Limburg, northeast Belgium. Different parts, namely, the bark and core wood, from apple tree were separated for further investigation. All the parts were shredded at the place, where they were felled, and grinded to fine particles (< 1.2 mm) with a hammer mill. After grinding, a part of the wood was subjected to a drying process and another part was directly vacuum sealed. The part of the wood subjected to a drying process was dehydrated either in a freeze dryer or in an oven (60 °C) until constant weight. All the samples were vacuum sealed and stored at − 32 °C until further extraction.
Reagents
Aluminium chloride, ethanol 99%, sodium acetate, and sodium carbonate (anhydrous) were purchased from Chem-Lab. Acetic acid, 2,4,6′-tris(1-pyridyl)-5-triazine (TPTZ), hydrochloric acid, iron (II) sulphate heptahydrate, Folin–Ciocalteu reagent, quercetin (≥ 95%), gallic acid (GA) (≥ 97.5%), and DPPH were obtained from Sigma-Aldrich. Iron (III) chloride hexahydrate was purchased from Acros. Barium chloride and the solvents for extraction—ethanol, methanol, and acetone—were purchased from VWR Chemicals. Potassium acetate was purchased from Merck and sulphuric acid from Fisher chemicals.
The analytical reference compounds phloridzin (> 99%), phloretin (≥ 99%), (+)-catechin (≥ 99%), (−)-epicatechin (≥ 97.9%), kaempferol-3-glucoside (≥ 95%), (−)-epicatechin gallate (≥ 98%), p-coumaric acid (≥ 98%), rutin (> 95%), naringin (≥ 90%), (1)-naringenin (≥ 95%), procyanidin B1 (≥ 90%), and procyanidin B2 (≥ 90%) for the calibration of the HPLC were acquired from Sigma-Aldrich. Caffeic (≥ 99%) and ferulic acids (≥ 90%) were acquired from extrasynthese, and vanillic acid was supplied by EFBT (Lab of Enzyme, Fermentation and Brewing Technology—KU Leuven, Ghent, Belgium). Ultra-pure water was obtained from a Milli-Q System (Millipore).
Extraction technique
Different solvents, namely, methanol, ethanol, acetone, and water, were used for the extraction of phenolic compounds from the grinded wood material. The solvents were used in the pure form and also in mixtures with water (20%, 40%, 60%, and 80%, v/v).
Warm solvent extraction (WSE)
The method described by Meneses et al. [20] was used for WSE. For that, 1.0 g of sample was mixed with 100.0 mL of solvent in a 250 mL closed conical flask, and maintained during 30 min on a heating plate at 60 °C with magnetic stirring [25]. There was no cooling system on the closed flask. The extraction took place at temperatures near the boiling point of the solvents employed. The produced extracts were filtered through filter paper and 0.22 µm polyvinylideenfluoride membranes (purchased from Phenomenex) and stored at − 32 °C until analyses. All the steps were conducted in the absence of oxygen.
Ultrasound-assisted extraction (UAE)
The method used for the UAE was based on the results of a study described by Lazar et al. [10]; 0.5 g of grinded wood material and 50.0 mL of solvent were mixed into 250 mL reactive vessels and placed in an ultrasonic thermostatic bath (VWR ultrasonic cleaner 800 W). The extraction process was performed at 60 ± 3 °C for 30 min. Stirring was performed every 5 min for 30 s. Afterwards, the extracts were centrifuged using a Eppendorf centrifuge 5810 at 4000 rpm for 6 min. The produced extracts were treated analogously as was the case for WSE.
Determination of moisture content
The moisture content of fresh and pre-treated apple wood was determined by weight difference before and after heating at 103 °C for 4 h. After the heating process, the samples were placed in a desiccator for 30 min before weighing. The moisture content of bark and core wood is 46.9% and 43.5%, respectively. After the pre-treatment employed, the moisture content is lower than 10%. Dry matter for bark and core wood were 100% and 96.3%, respectively.
Total phenolic content (TPC)
TPC was evaluated by a modified Folin–Ciocalteu method [26] described by Moreira et al. [11]. The method involves the reduction of Folin–Ciocalteu reagent by phenolic compounds, with the simultaneous formation of a blue complex. The calibration curve was made with standard solution of GA and measurements were carried out at 740 nm in a Shimadzu UV-1800 spectrophotometer. TPC was expressed as milligram of GA equivalents per gram of dry matter (DM) of the sample (mg GAE/g DM). All measurements were done in triplicate.
Total flavonoid content (TFC)
Flavonoids were measured by a colorimetric method described by Chang et al. [27]. The principle of the aluminium chloride colorimetric method is based on the formation of a stable complex by the aluminium chloride with the C4 keto group and the C3 or C5 hydroxyl group of the flavones and flavonols. The measurement of the extract was compared to a standard curve prepared with quercetin standard. Absorbances were measured at 415 nm with a Shimadzu UV-1800 spectrophotometer. TFC was expressed as milligram of quercetin equivalents per gram of DM of the sample (mg QE/g DM). All measurements were done in triplicate.
Determination of antioxidant potential of extracts
The antioxidant activity of the extracts obtained from the apple tree material was evaluated by the DPPH-RSA and FRAP assays, according to the procedures described by Brand-Williams et al. [28] and Benzie and Strain [29], respectively.
DPPH-RSA assay
DPPH is a stable free radical and is used to test phenolic compounds containing antioxidant potential [29, 30]. The extract (150 µL) was added to 2.85 mL of 130 µmol/L daily made methanol DPPH solution, and was mixed. After an incubation period of 90 min at room temperature in the dark, the decrease in absorbance was determined at 515 nm against a blank. Methanol was used as a blank or control solution. The decrease of the absorbance is a degree for the antioxidative potential of the sample. The radical scavenging activity was expressed as a percentage of inhibition calculated by
where A0 and AE are the absorbance of the blank solution and the extract, respectively.
FRAP assay
100 µL of extract was mixed with 3.00 mL of FRAP reagent (300 mmol/L acetate buffer—pH 3.6, 10 mmol/L TPTZ in 40 mmol/L HCl, 20 mmol/FeCl3.6H2O in a ratio of 10:1:1) and 300 µL of milli-Q water. This mixture was incubated at 37 °C for 15 min. After that, the absorbance was determined at 593 nm against a blank prepared with milli-Q water. Aqueous solutions of FeSO4.7H2O in a range from 200 to 1000 µmol/L were used for generating a calibration curve. FRAP values were expressed as mmol of ferrous equivalent per gram of DM of the sample (mmol Fe(II)/g DM).
HPLC–PDA analysis
The instrument used to analyze the apple wood extracts was a Shimadzu system consisting of a low-pressure quaternary gradient unit (model LC-20AT) with an in-line degasser (model DGU-20A5R) and an auto-sampler (model SIL-20AT). The system is equipped with a column oven (model CTU-20AC) and a photodiode array detector (model SPD-M20A High-Performance Liquid Chromatography PDA detector). The phenolic composition of the extracts obtained from the apple wood was analyzed by the HPLC method described by Rubilar et al. [31] with minor modification. A Phenomenex Gemini C18 column (250 mm × 4.6 mm, 5 μm) and a guard column with the same characteristics maintained at 40 °C with a gradient program were used for the separation of phenolic compounds. Mobile phase A (HPLC grade methanol) and mobile phase B (ultra-pure water) both with 0.1% formic acid were used for elution at a flow rate of 1.0 mL/min. The injection volume for samples and reference compounds was 20 µL. The following gradient was applied: 0–20 min: 15–30% A; 20–40 min: 30–45% A; 40–45 min: 45–50% A; 45–50 min: 50–55% A; 50–65 min: 55–70% A; 65-75 min: 70–100% A, followed by 100% A for 5 min and back to 15% A in 20 min followed by 5 min of reconditioning before the next injection. UV spectra were recorded in a range of 190 to 800 nm, and the quantification was made at 260, 270, 280, 308, 320, 350, and 368 nm depending on the maximum absorption from the phenolic compound. The following polyphenols were identified and quantified: the monomeric flavan-3-ols: (+)-catechin, (−)-epicatechin, and (−)-epicatechingallate; the flavanones: naringin and naringenin; the dihydrochalcones: phloretin and phloredzin; the flavonols: rutin, quercetin, and kaempherol; the hydroxybenzoic acids: gallic and vanillic acid; the derivatives of cinnamic acid: p-coumaric, ferulic, and caffeic acids and the proanthocyanidins: procyanidin B1 and procyanidin B2 [32, 33]. Calibration curves were made by dilution of the stock solutions, and injecting them into the HPLC–PDA system. Table 1 gives the analytical parameters of the obtained calibration graphs. The polyphenols in the obtained extracts from apple wood were identified by the comparison of the retention times and the spectral data with the ones obtained from the analysis of the reference compounds. The identified compounds were quantified using the calibration curves of the authentic reference compounds. For the apple wood extracts, the concentrations were calculated based on triplicate injections and the results were expressed as mg/g DM.
Statistical analysis
The results of the analyses reported in this work are the average of three measurements and expressed as mean ± standard deviation (SD). Data analysis was carried out with the software IBM SPSS Statistics 23. A two-way between-groups analysis of variance (ANOVA) was conducted by the Tukey test to explore the impact of the two different independent variables, namely, the pre-treatment and the extraction technique, on a dependent variable. The pre-treatment techniques were divided into three groups (Group 1: fresh material; Group 2: freeze-dried material; and Group 3: oven-dried material), and the extraction techniques in two groups (Group 1: WSE; Group 2: UAE). Using the two-way ANOVA not only the main effect of each independent variable, but also the interaction between both independent variables can be shown. The p value less than 0.05 (p < 0.05) was considered statistically significant. In most cases, the interaction effect did not reach statistical significance (p > 0.05), which makes it possible to safely interpret the main effects: pre-treatment technique and the extraction technique. In case of a significant result for the interaction effect, an analysis of simple effects was conducted. This means that the results for each of the subgroups were studied separately. This involves splitting the sample into groups according to one of the independent variables and running separate one-way ANOVAs by Games–Howell post hoc test to explore the effect of the other variable. For this, the SPSS Split File option was used.
Results and discussion
Preliminary assays
Influence of the solvent mixture
Alcoholic solvents are commonly used to extract phenolics from natural sources because of the high yield reported [19]. In the present study, different pure solvents, i.e., water, acetone, methanol, and ethanol, and solvent mixtures in the ratios of 20%, 40%, 60%, and 80%, v/v (organic solvent/water) were tested by applying the WSE and UAE techniques [20]. Recently, Moreira et al. [11] reported that the yield of polyphenols extracted from apple wood bark was highest when applying conventional extraction at 55 °C with ethanol/water (1/1; v/v) for 2 h. In another study, however, it has been recommended to perform the extraction at a temperature not higher than 50 °C due to risk of polyphenol oxidation [10]. A recent study by Naima et al. [14] reveals, however, that increasing the temperature from 40 to 60 °C improves the yield of polyphenols recovered from Moroccan Acasia mollissima barks. Therefore, taking into consideration data from the literature and the possible degradation of phenolic compounds [8, 10], extractions were carried out at 60 °C, respectively, for 30 min.
TPC of the obtained extracts was used to evaluate the effect of the solvent composition. Three woods are composed by different molecular composition and polarities, which makes the solvent composition an important parameter for comparison. Table 2 shows the impact of the solvent mixture on TPC available in extracts derived from apple tree bark and core wood with WSE and UAE, respectively. From this table, it was observed that the addition of water to organic solvents, such as acetone, methanol, and ethanol, creates a more polar medium, which facilitates the extraction of phenolic compounds. The results of our study on apple tree bark and core wood indicate that extracts obtained with 40% up to 80% of organic solvent in the mixture contain the highest amount of total polyphenols, with no significant difference between the compositions. This is in agreement with recent findings [9, 10, 14, 20], which show that the yield of phenolic compound improves up to 80% (v/v). However, in combination with UAE, less solvent is required to achieve the same return in TPC as with WSE. The combination 40 v/v% organic solvent mixture with UAE almost always results in a higher yield of polyphenols (Table 2) than in any of the WSE technique combinations with organic solvent mixtures.
In case of UAE, when looking at the results in more detail (Table 2), extractions performed with ethanol mixtures show no significant (p > 0.05) differences in TPC values, compared to the amounts recovered with acetone or methanol mixtures, which is very interesting, since the use of ethanol is preferable to methanol or acetone in view of food and pharmaceutical applications of the apple tree bark and core wood extracts. Moreover, ethanol is recommended as an eco-friendly and safe solvent [5].
Influence of extraction technique
Next to the solvent mixture, the extraction techniques WSE and UAE, will also impacts the extraction yield of polyphenolic compounds recovered from apple tree woods. Taking into consideration the results (Table 2) described in the chapter concerning the Influence of the solvent mixture, independent variables for both extraction techniques were defined. Regarding the comparison of both extraction techniques, bark and core wood extracts obtained with UAE show significant higher amount of polyphenols. There is a statistically significant main effect for the extraction technique (p < 0.05). Based on the estimated marginal means, pairwise comparisons showed a significant difference between WSE and UAE, because the mean differences were significant at the 0.05 level. According to several studies, UAE has been applied successfully to obtain valuable compounds from food and plant materials. The results obtained in this study are in accordance with other recent studies [8, 10, 17] which reveals that ultrasounds promote the release of intracellular substances and intensify the extraction of phenolic compounds.
Influence of the pre-treatment technique
In an ideal situation, the extraction of polyphenols should be performed using fresh samples, although it is not the ideal process due to the large volumes that need to be stored. Because of perishability, shelf life, and quality, several techniques to preserve plant material are used. TPC of extracts obtained from fresh, oven-, and freeze-dried apple bark samples were used to evaluate the effect of the pre-treatment technique (Table 3). When comparing the technique of oven- and freeze drying, there can be seen that extracts obtained from the several pre-treated wood samples by UAE, a significant difference is noticed in the amount of polyphenols (p < 0.05). When comparing the fresh samples with bark pre-treated by freeze drying, there is no significant difference (p > 0.05). The same tendency was noticeable for WSE obtained extracts from the several fresh and pre-treated wood samples. In all cases, an independent sample t test showed that TPC was significantly influenced (p < 0.05) by oven drying. In general, it can be seen (Table 3) that the quantities of total polyphenols in the oven-dried bark samples are significantly lower than in freeze-dried and fresh wood samples and oven drying can, therefore, lead to a significant loss in polyphenols.
Characterization of apple core wood and apple bark extracts
Phenolic content and antioxidant activity of apple tree bark and core wood
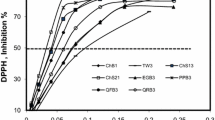
Both WSE and UAE extracts from pre-treated apple wood (core wood and bark) were characterized by TPC, TFC, and antioxidant activity, measured by the DPPH-RSA and FRAP assays (Figs. 1, 2, 3, 4).
Influence of the composition of the solvent mixture and the pre-treatment technique on apple tree core wood extracts obtained by warm solvent extraction for: a total phenolic content (mg gallic acid equivalent/g dry matter); b total flavonoid content (mg quercetin equivalent/g dry matter); c FRAP, ferric reducing antioxidant power (mM FeSO4·7H2O/g dry matter); and d DPPH-RSA, 2,2′-diphenyl-1-picrylhydrazyl radical scavenging activity (% reduction/g dry matter)
Influence of the composition of the solvent mixture and the pre-treatment technique on apple tree bark extracts obtained by warm solvent extraction for: a total phenolic content (mg gallic acid equivalent/g dry matter); b total flavonoid content (mg quercetin equivalent/g dry matter); c FRAP, ferric reducing antioxidant power (mM FeSO4·7H2O/g dry matter); and d DPPH-RSA, 2,2′-diphenyl-1-picrylhydrazyl radical scavenging activity (% reduction/g dry matter)
Influence of the composition of the solvent mixture and the pre-treatment technique on apple tree core wood extracts obtained by ultrasound-assisted extraction for: a total phenolic content (mg gallic acid equivalent/g dry matter); b total flavonoid content (mg quercetin equivalent/g dry matter); c FRAP, ferric reducing antioxidant power (mM FeSO4·7H2O/g dry matter); and d DPPH-RSA, 2,2′-diphenyl-1-picrylhydrazyl radical scavenging activity (% reduction/g dry matter)
Influence of the composition of the solvent mixture and the pre-treatment technique on apple tree bark extracts obtained by ultrasound-assisted extraction for: a total phenolic content (mg gallic acid equivalent/g dry matter); b total flavonoid content (mg quercetin equivalent/g dry matter); c FRAP, ferric reducing antioxidant power (mM FeSO4·7H2O/g dry matter); and d DPPH-RSA, 2,2′-diphenyl-1-picrylhydrazyl radical scavenging activity (% reduction/g dry matter)
In general, the spectrophotometric assays employed demonstrate that (pre-treated) bark and core wood extracts present the highest amount of total polyphenols, flavonoids, and reducing power when acetone/water mixtures are used for extraction. In addition, it can be noticed that extractions performed with acetone/water mixtures show a higher extraction efficiency than those conducted with pure acetone or pure water. The highest values on polyphenols were obtained using mixtures of solvents with concentrations of 40% up to 80%. These extracts also show the highest antioxidant activity, suggesting that the phenolic compounds recovered from apple tree residue can be linked to the high antioxidant properties [34, 35].
As preliminary results revealed that TPC obtained from oven-dried samples is significantly lower than that obtained from fresh and freeze-dried samples, the same trend is observed concerning the results of reducing power obtained via the DPPH-RSA and FRAP assays. Extracts obtained from oven-dried samples differ significantly from extracts made from both fresh and freeze-dried samples (p < 0.05). The extracts from fresh or freeze-dried samples show the highest reducing capacity without a significant difference (p > 0.05). The results obtained are in agreement with literature data for grape skin, tomatoes, ginger, and citrus fruits, which show that freeze drying leads to higher contents of phenolic compounds than oven-drying pretreatments [22, 36, 37]. The results from the present study enable us to conclude that the pre-treatment applied to the samples can influence the amount of phenolic compounds recovered.
Concerning the differences between the wood samples (Figs. 1, 2, 3, 4), the highest phenolic and flavonoid content, as well as the highest antioxidant capacity are found in bark extracts. The bark, which shields the core, needs to offer protection from insects and possible infections, which explains the higher amount of phenolic compounds compared to core wood. In the present study, TPC using the WSE technique varies from 3.5 ± 0.1 to 28.5 ± 1.3 mg GAE/g DM for oven-dried core wood and freeze-dried bark extracts, respectively. Using the UAE technique, TPC ranged between 4.9 ± 0.2 to 29.0 ± 1.2 mg GAE/g DM for oven-dried core wood and fresh bark, respectively. Independent of the extraction technique applied, these results demonstrate the huge variability in TPC between bark and core wood extracts. Large differences were also found in a study on MAE and conventional extraction [11], which reported that bark extracts prepared by MAE show at least a twofold higher amount of TPC and TFC, as well as higher antioxidant activity compared to core extracts.
Identification and quantification of phenolic compounds in apple core wood and apple bark
The HPLC–PDA analysis offers a detailed phenolic profile of the different extracts. During a preliminary study, all extracts were analyzed by HPLC–PDA. Next, some selected extracts, based on the pre-treatment procedure, extraction solvent employed, and extraction technique, were quantified using the parameters, as reported in Table 1. These selected extracts were analyzed three times. Figure 5 gives a graphical representation of the total amount of polyphenol marker compounds detected in treated apple wood.
The freeze-drying process did not generate significant declines in the amount of polyphenol markers quantified in this study (Fig. 5) compared to fresh samples (p = 0.551). On the other hand, as shown in Fig. 5 and Table 5, the oven-drying process causes a loss of phenolic compounds. Table 4 shows the total amounts of quantified phenolic marker compounds by HPLC–PDA in a selection of apple tree core wood and bark extracts obtained by WSE and UAE of fresh and freeze-dried samples. Despite the presentation of the total amount of polyphenols in oven-dried samples in Fig. 5, the detailed view of these samples is not discussed, as they show significantly lower amounts in polyphenols compared to fresh (p < 0.001) and freeze-dried samples (p < 0.05). This corresponds with the findings from the preliminary study discussed in the chapter Influence of the pre-treatment technique, where a significant decrease in total polyphenols was observed for oven-dried wood samples. These results are in agreement with a similar study on the phenolic composition of grape skin, which also showed that the freeze-drying process maintained the phenolic composition in comparison with fresh samples, whereas the oven-dried samples showed a large decrease in the content of phenolic compounds [22].
Concerning the differences between the samples, the achieved results (Fig. 5, Table 4) show that the amount of marker compounds in the extracts derived from bark are significantly higher (p < 0.001) with levels more than twofold that of similar extracts produced with core wood.
Taking into consideration the results obtained in the Preliminary assays, and the results already discussed in this chapter, only a detailed representation of fresh and freeze-dried bark derived UAE extracts is displayed in Table 5. Regarding the amount of flavonoids in comparison with the phenolic acids, the flavonoid content represents at least 93% of the total amount of polyphenol markers in the extracts. Another pertinent conclusion that can be drawn from the data presented in Table 5 is that the total amount of polyphenol marker compounds in the extracts obtained using ethanol mixtures as extracting solvent is similar to the content obtained using acetone mixtures. The acetone mixtures show slightly higher values, but there is no significant difference (p > 0.05). This finding is promising, since the use of ethanol is preferable to the other tested solvents when it comes to application in the food industry. Despite the considerable amount of marker compounds in extracts produced in the presence of pure water, there is a significant loss (p < 0.05) compared to those produced with acetone/water mixtures. However, the difference with ethanol/water mixtures (p = 0.126) is not significant.
In a previous study, Xü et al. [16] showed that phloretin, a bioactive dihydrochalcone known mostly as an ingredient for cosmetics, is present in the bark of apple trees. However, this compound was not detected in our extracts obtained by WSE and UAE at 60 °C. On the other hand, phloridzin, the 2′-glucoside of phloretin, appears to be the major compound identified in both fractions of apple tree wood, with an amount ranging from 22.168 ± 0.015 to 37.480 ± 0.094 mg/g DM. Ehrenkranz et al. [38] have intensively investigated the effect of phloridzin on the glucose uptake and its effect on diabetes because of the ability of phloridzin, to lower glucose plasma concentrations independent of insulin. The highest phloridzin content found in their study was 37.48 ± 0.10 mg/g DM for fresh bark extracted with 60% acetone by UAE, which is quite similar to the value reported in this study and the value reported recently by Moreira et al. [11] (29.4 mg/g DM). Following phloridzin, kaempferol-3-glucoside accounts 10.6% up to 14.8% of the total amount of phenolic compounds quantified in bark extracts. Furthermore, in this study, the condensed tannin procyanidin B2 was also identified and quantified in the extracts. This compound has been linked to the antioxidative activity of apples, and therefore, it deserves further attention. The content of procyanidin B2 varies from 0.057 ± 0.004 to 1.135 ± 0.007 mg/g DM, which is higher than values reported by Lee et al. [39] in several apple cultivars (0.034 to 0.217 mg/g fresh apple). In addition to the flavonoids already described, (−)-epicatechin gallate, (−)-epicatechin, (+)-catechin, naringin, and rutin were also detected and quantified. Bioactive phenols, such as gallic acid, catechin, caffeic acid, vanillic acid, epicatechin, p-coumaric acid, ferulic acid, rutin, quercetin, naringenin, and phloretin, have been detected previously in apple bark extracts [30, 40]. Some of the reported phenolic acids were also found in our bark extracts, i.e., vanillic, gallic, ferulic, p-coumaric, and caffeic acid. Gallic and vanillic acid was only identified in some extracts prepared with pure water. Another phenolic compound quantified in the majority of extracts was p-coumaric acid, ranging from 0.045 ± 0.001 to 0.224 ± 0.002 mg/g DM. Ferulic acid is also present with the highest amount detected in a freeze-dried sample extracted with 60% ethanol (0.184 ± 0.002 mg/g DM).
Conclusion
In summary, the present study demonstrates that apple wood extracts contain a considerable amount of phenolic compounds with antioxidant activity. In fact, the relatively high content of phenolic compounds recovered by WSE and UAE opens the possibility of applying these types of extraction techniques at pilot scale, aiming at valorisation of vegetable by-products as natural sources of functional substances for further use in different fields, such as, e.g., food, feed, and cosmetics. In general, core wood and bark extracts obtained with 40 up to 80% v/v solvent/water mixture contain the highest amounts of total polyphenols and flavonoids. From the analyzed samples, bark extracts obtained after the application of UAE presented the highest yield in polyphenols. This study favors the UAE technique when extracting polyphenols from apple wood. This technique is affordable and pragmatic and yields anti oxidative extracts that can be used in the food industry, pharmaceutics, and cosmetics. In addition to finding the most economically and ecologically beneficial extraction method, this study shows that the pretreatment techniques used on apple wood influence the phenolic composition. Although fresh wood residue is no ideal starting material due to the large volumes for storage, it is, however, the most interesting time to perform extractions on freshly rooted trees at an industrial level, since fresh samples provide the highest amount of polyphenols. Nevertheless, it should be noted that extracts based on freeze-dried samples hold the same phenolic profile as those based on fresh samples. The least favorable starting material is that of samples dried at 60°, where the amount of polyphenols are significantly lower. In general, the combination of UAE with fresh and freeze-dried bark in the presence of ethanol yields the highest amount of polyphenols. In addition, when looking at the number of polyphenols via HPLC detection, UAE extraction in the presence of water shows similar efficiency with a WSE extraction in the presence of any type of solvent mixture.
References
Ignat I, Volf I, Popa VI (2011) A critical review of methods for characterization of polyphenolic compounds in fruits and vegetables. Food Chem 126:1821–1835. https://doi.org/10.1016/j.foodchem.2010.12.026
Mikulic-Petkovsek M, Stampar F, Veberic R (2009) Accumulation of phenolic compounds in apple in response to infection by the scab pathogen, Venturia inaequalis. Physiol Mol Plant Pathol 74:60–67. https://doi.org/10.1002/jsfa.4093
Pontais I, Treutter D, Paulin JP, Brisset MR (2008) Erwinia amylovora modifies phenolic profiles of susceptible and resistant apple through its type III secretion system. Physiol Plant 132:262–271. https://doi.org/10.1111/j.1399-3054.2007.01004.x
Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med Cell Longev 2:270–278. https://doi.org/10.4161/2Foxim.2.5.9498
Mumper RJ, Dai J (2010) Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15:7313–7352. https://doi.org/10.3390/molecules15107313
Gasser P, Lati E, Peno-Mazzarino L, Bouzoud D, Allegaert L, Bernaert H (2008) Cocoa polyphenols and their influence on parameters involved in ex vivo skin restructuring. Int J Cosmet Sci 30:339–345. https://doi.org/10.1111/j.1468-2494.2008.00457.x
Bouras M, Chadni M, Barba FJ, Grimi N, Bals O, Vorobiev E (2015) Optimization of microwave-assisted extraction of polyphenols from Quercus bark. Ind Crops Prod 77:590–601. https://doi.org/10.1016/j.indcrop.2015.09.018
Ghitescu RE, Volf I, Carausu C, Bühlmann AM, Andrei GL, Valentin IP (2015) Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason Sonochem 22:535–541. https://doi.org/10.1016/j.ultsonch.2014.07.013
Hofmann T, Nebehaj E, Albert L (2015) The high-performance liquid chromatography/multistage electrospray mass spectrometric investigation and extraction optimization of beech (Fagus sylvatica L.) bark polyphenols. J Chromatogr A 1393:96–105. https://doi.org/10.1016/j.chroma.2015.03.030
Lazar L, Talmaciu AI, Volf I, Popa VI (2016) Kinetic modeling of the ultrasound-assisted extraction of polyphenols from Picea abies bark. Ultrason Sonochem 32:191–197. https://doi.org/10.1016/j.ultsonch.2016.03.009
Moreira M, Barroso FM, Boeykens A, Withouck H, Morais S, Delerue-Matos C (2017) Valorization of apple tree wood residues by polyphenols extraction: comparison between conventional and microwave-assisted extraction. Ind Crops Prod 104:210–220. https://doi.org/10.1016/j.indcrop.2017.04.038
Dedrie M, Jacquet N, Bombeck PL, Hébert J, Richel A (2015) Oak barks as raw materials for the extraction of polyphenols for the chemical and pharmaceutical sectors. A regional case study. Ind Crops Prod 70:316–321. https://doi.org/10.1016/j.indcrop.2015.03.071
Stevanovic T, Diouf PN, Garcia-Perez ME (2009) Bioactive polyphenols from healthy diets and forest biomass. Curr Nutr Food Sci 5:264–295. https://doi.org/10.2174/157340109790218067
Naima R, Oumam M, Hannache H, Sesbou A, Charrier B, Pizzi A, Charrier-El Bouhtoury F (2015) Comparison of the impact of different extraction methods on polyphenols yields and tannins extracted from Moroccan Acasia mollissima barks. Ind Crops Prod 70:245–252. https://doi.org/10.1016/j.indcrop.2015.03.016
Kammerer DR, Kammerer J, Valet R, Carle R (2014) Recovery of polyphenols from the by-products of plant food processing and application as valuable food ingredients. Food Res Int 65(1):2–12. https://doi.org/10.1016/j.foodres.2014.06.012
Xü K, Lü H, Qü B, Shan H, Song J (2010) High-speed counter-current chromatography preparative separation and purification of phloretin from apple tree bark. Sep Purif Technol 72:406–409. https://doi.org/10.1016/j.seppur.2010.02.020
Chemat F, Rombaut N, Sicaire AG, Meullemiestre A, Fabiano-Tixier AS, Abert-Vian M (2017) Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason Sonochem 34:540–560. https://doi.org/10.1016/j.ultsonch.2016.06.035
Pradal D, Vauchel P, Decossin S, Dhulster P, Dimitrov K (2016) Kinetics of ultrasound-assisted extraction of antioxidant polyphenols from food by-products: extraction and energy consumption optimization. Ultrason Sonochem 32:137–146. https://doi.org/10.1016/j.ultsonch.2016.03.001
Spingo G, Tramelli L, De Faveri DM (2007) Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J Food Eng 81:200–208. https://doi.org/10.1016/j.jfoodeng.2006.10.021
Meneses GTN, Martins S, Teixeira JA, Mussatto SI (2013) Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep Purif Technol 108:152–158. https://doi.org/10.1016/j.seppur.2013.02.015
Bartolomé B, Santos M, Jiménez JJ, Del Nozal MJ, Gomez-Cordovés C (2002) Pentoses and hydroxycinnamic acids in Brewer’s Spent Grain. J Cereal Sci 36:51–58. https://doi.org/10.1006/jcrs.2002.0442
De Torres C, Díaz-Maroto MC, Hermosín-Gutiérrez I, Pérez-Coello MS (2010) Effect of freeze-drying and oven-drying on volatiles and phenolics composition of grape skin. Anal Chim Acta 660:177–182. https://doi.org/10.1016/j.aca.2009.10.005
Nunes JC, Labo MG, Catelo-Branco VN, Oliveira FR, Guedes Torres A, Perrone D, Monteiro M (2016) Effect of drying method on volatile compounds, phenolic profile and antioxidant capacity of guava powders. Food Chem 197:881–890. https://doi.org/10.1016/j.foodchem.2015.11.050
Aydin E, Gocmen D (2015) The influences of drying method and metabisulfite pre-treatment on the color, functional properties and phenolic acids contents and bioaccessibility of pumpkin flour. LWT Food Sci Technol 60:385–392. https://doi.org/10.1016/j.lwt.2014.08.025
Dorta E, Lobo MG, Gonzalez M (2012) Reutilization of mango by-products: study of the effect of extraction solvent and temperature on their antioxidant properties. J Food Sci 71:80–88. https://doi.org/10.1111/j.1750-3841.2011.02477.x
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Chang CC, Yang MH, Wen HM, Chern JC (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:178–182
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28:25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Benzie FFI, Strain J (1996) The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: the FRAP assay. Anal Biochem 239:70–76. https://doi.org/10.1006/abio.1996.0292
Moreira MM, Morais S, Carvalho DO, Barros AA, Delerue-Matos C, Guido LF (2013) Brewer’s spent grain from different types of malt: evaluation of the antioxidant activity and identification of the major phenolic compounds. Food Res Int 54:382–388. https://doi.org/10.1016/j.foodres.2013.07.023
Rubilar M, Pinelo M, Shene C, Sineiro J, Nunez MJ (2007) Separation and HPLC-MS identification of phenolic antioxidants from agricultural residues: almond hulls and grape pomace. J Agric Food Chem 55:10101–10109. https://doi.org/10.1021/jf0721996
Jakobek L, Barron RA (2016) Ancient apple varieties from Croatia as a source of bioactive polyphenolic compounds. J Food Compos Anal 45:9–15. https://doi.org/10.1016/j.jfca.2015.09.007
Tsao R, Yang R, Young C, Zhu H (2003) Polyphenolic profiles in eight apple cultivars using high-performance liquid chromatography. J Agric Food Chem 51:6347–6353. https://doi.org/10.1021/jf0346298
Iglesias I, Echeverria G, Soria Y (2008) Differences in fruit colour development, anthocyanin content, fruit quality and consumer acceptability of eight ‘Gala’ apple strains. Sci Hortic 119:32–40. https://doi.org/10.1016/j.scienta.2008.07.004
Vieira FGK, Borges GSC, Copetti C, Amboni RDMC, Denardi F, Fett R (2009) Physico-chemical and antioxidant properties of six apple cultivars (Malus domestica Borkh) grown in southern Brazil. Sci Hortic 122:421–425. https://doi.org/10.1016/j.scienta.2009.06.012
Sun Y, Shen Y, Liu D, Ye X (2015) Effects of drying methods on phytochemical compounds and antioxidant activity of physiologically dropped un-matured citrus fruits. LWT Food Sci Technol 60:1269–1275. https://doi.org/10.1016/j.lwt.2014.09.001
Gümüșay OA, Borazan AA, Ercal N, Demirkol O (2015) Drying effects on the antioxidant properties of tomatoes and ginger. Food Chem 173:156–162. https://doi.org/10.1016/j.foodchem.2014.09.162
Ehrenkranz JRL, Lewis NG, Ronald KC, Roth J (2005) Phlorizin: a review. Diabetes/Metab Res Rev 21:31–38. https://doi.org/10.1002/dmrr.532
Lee KW, Kim YJ, Kim DO, Lee HJ, Chang Y (2003) Major phenolics in apple and their contribution to the total antioxidant capacity. J Agric Food Chem 51:6516–6520. https://doi.org/10.1021/jf034475w
Hong-Zhen S, Hai-Tao L, Su-Hua B, Bao-Hua L, Jun Z, Hong-Yi D (2012) Determination method of 12 phenolic compounds in apple bark by HPLC. J Fruit Sci 5:929–935
Acknowledgements
The authors are grateful to Valerie Van Vooren for the provided language help and writing assistance regarding this paper.
Funding
Annick Boeykens is a beneficiary of a PWO (‘Projectmatig Wetenschappelijk Onderzoek’) Grant, provided to Odisee by the Flemish Government, for the investigation project ‘Phenolic compounds in by-products’. Manuela M. Moreira (SFRH/BPD/97049/2013) wishes to acknowledge Fundo Social Europeu and Ministério da Ciência, Tecnologia e Ensino Superior for funding her postdoctoral fellowship by means of a POPH-QREN—Tipologia 4.1—Formação Avançada. The financial support from FCT/MEC through national funds and cofinanced by FEDER, under the Partnership Agreement PT2020 through the project UID/QUI/50006/2013—POCI/01/0145/FEDER/007265 and the project 6818—Transnational Cooperation, Agreement between Portugal (FCT) and Serbia (MSTD) are also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest to disclose.
Compliance with Ethics requirements
The authors state that the present study complies with the ethical requirements and this study does not include studies on human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Withouck, H., Boeykens, A., Vanden Broucke, M. et al. Evaluation of the impact of pre-treatment and extraction conditions on the polyphenolic profile and antioxidant activity of Belgium apple wood. Eur Food Res Technol 245, 2565–2578 (2019). https://doi.org/10.1007/s00217-019-03373-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-019-03373-2