Abstract
The increasing interest in quinoa in Europe has generated a large number of studies with this seed as a partial substitute for refined wheat flour in bakery products as a strategy to improve their nutritional value. However, the wide genetic diversity of this seed offers very different compositions in different varieties, which would lead to different technological behaviours in the breadmaking process. The aim of this work was to make a comparative study of the protein profile and rheological and thermal properties of three varieties of quinoa widely available commercially in Europe to study their technological potential as breadmaking ingredients with 25% replacement of wheat flour by whole quinoa flour. The results obtained during the analysis offered a view of the proteins present in the various quinoas, and of the processes of hydrolysis and generation of new bonds between wheat and quinoa proteins during the breadmaking process. The changes in the thermal and pasting properties of the bread doughs that included whole quinoa flour led to the development of baked products with different physico-chemical and textural properties, producing an increase on crumb staling. However, replacement of 25% of the wheat flour with whole quinoa flour produced only a slight decrease in the technological quality of the products. A significant increase (p < 0.05) in dietary fibre, minerals, lipids, and proteins in comparison with a whole wheat product, together with the overall consumer acceptance of the products that were developed, was conclusive for proposing replacement with quinoa flour as a strategy for nutritional improvement in the manufacture of bakery products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bread is one of the most common foods made with cereals in the world. However, the main cereal used for breadmaking is flour obtained by dry milling of wheat grain, which removes valuable nutrients and bioactive compounds [1]. Whole cereal and pseudocereal flours can be included in bakery products as a strategy to improve their nutritional profile without needing to use whole products completely [2,3,4]. Among the pseudocereals, quinoa (Chenopodium quinoa) is a dicotyledon originally from South America, although, because of its adaptation characteristics and wide genetic diversity, it is now grown in nearly every continent in the world, including Europe [5]. Because its composition is similar to that of cereals, it has a suitable balance of carbohydrates, proteins, lipids, and minerals, and it can be sold without restrictions in Europe in accordance with Regulation (EU) 2015/2283 [6], which means that a large number of varieties are marketed in countries of the European Union, all of which has created increasing interest in society. Moreover, unlike wheat, which contains gluten-forming proteins (gliadins and glutenins), the main proteins in quinoa are albumins and globulins, bound together by disulfide bridges [7]. The most abundant of these proteins is of type 11S, also known as globular chenopodin, with a molecular size of 50–60 kDa [8], followed by those of type 2S albumin, which are polypeptides of a relatively small size, about 9–10 kDa [9, 10]. The predominance of globulins and albumins in quinoa is technologically significant, because they have foaming, emulsifying, and gelling properties, which in some cases are similar to the techno-functional properties of soya or casein proteins [11].
Various studies show that the incorporation of whole quinoa flour in bread formulations causes technological changes produced by the dilution of gluten, inclusion of fibre, and/or lipids, or its starch characteristics [1, 12]. However, marked differences between varieties have been reported in recent years, regarding their chemical composition and physical properties, size of starch granules and amylose/amylopectin ratio, polyphenol content and antioxidant capacity, among other things [13,14,15,16].
Accordingly, the aim of this work was to make a comparative study of the protein profile and rheological and thermal properties of three varieties of quinoa widely available commercially in Europe to study their technological potential as breadmaking ingredients with 25% replacement of wheat flour by whole quinoa flour.
Materials and methods
Materials
Three types of commercial Bolivian quinoa seeds (Chenopodium quinoa) grown by members of ANAPQUI (La Paz, Bolivia) were purchased from Ekologikoak (Ondarroa-Bizkaia, Spain). Organic “quinoa real” (royal quinoa) (white, red, and black) was used to produce flour in a mill (Aromatic, Taurus, Oliana, Spain). The chemical composition of the white, red, and black quinoa flours according to the labelling was: 12.0, 11.0, and 11.2 g/100 g of moisture; 64.0, 56.7, and 57.2 g/100 g of carbohydrates; 6.0, 5.4, and 5.1 g/100 g of lipids; 4.0, 11.8, and 12.8 g/100 g of fibre; and 14.0, 15.1, and 13.7 g/100 g of proteins, respectively. Dehydrated yeast (Saccharomyces cerevisiae, Maizena, Spain) was used as starter for the breadmaking process. Commercial strong wheat flour (Carrefour, Madrid, Spain) was used for the bread formulation. The chemical composition of the wheat flour was: 12.6 g/100 g of moisture; 71 g/100 g of carbohydrates; 1.4 g/100 g of lipids; 3 g/100 g of fibre; and 12 g/100 g of proteins.
Breadmaking procedure
The control bread dough formula consisted of wheat flour (500 g), dehydrated yeast (1.0 g/100 g flour basis), sodium chloride (1.6 g/100 g flour basis), and distilled water (70.8 g/100 g flour basis). Whole quinoa flour was incorporated in the bread dough formula at 25 g/100 g on flour basis. The breadmaking procedure was performed in a breadmaker (BM 3989, Severin, Germany). The process variables consisted of the following steps: (a) kneading phase and rising phase for 9 min and 20 min, respectively; (b) kneading phase and rising phase for 14 min and 20 min, respectively; short stirring for 30 s; (c) rising phase for 4 min and 30 s; and (d) rising phase for 45 min, and lastly baking for 60 min. The breads obtained were cooled at room temperature for 2 h for subsequent analysis. The breadmaking process was performed in triplicate.
Chemical composition
Moisture content was determined by an official assay procedure [17]. Starch content was measured by an enzymatic procedure according to Method 996.11 [17]. Protein determination was carried out by the Dumas Combustion method (N conversion factor 5.7) according to ISO/TS 16634-2 (2016) [18]. Lipid content was extracted with petroleum ether under reflux conditions by the Soxhlet technique [19], whereas ash content was determined in a muffle furnace by incineration at 900 °C [19]. The dietary fibre content was measured by an enzymatic and gravimetric method [17]. The analyses were performed in triplicate.
Technological parameters
The technological parameters analysed were as follows: the height of the bread piece (cm) and the texture profile analysis using the TA.XT Plus Texture Analyser (Stable Micro Systems, Godalming, United Kingdom) with a 35 mm flat-end aluminium compression disc [20]. Each parameter was measured at least in triplicate in crumb of fresh bread and after 24 and 48 h of storage at room temperature in polyethylene bags. The experiments were conducted in triplicate.
Digital image analysis was used to measure the bread crumb structure. Images were taken at 600 pixels per cm with a scanner (HP Scanjet G2410, Hewlett Packard, USA) supported by HP Photosmart Essential 3.5 software. Data were processed using Fiji Image J (version 1.49q, National Institute of Health, USA) and NIS-Elements (Basic Research version, Nikon Instruments Inc., Amsterdam). The analysis was performed in triplicate.
Preliminary sensory analysis of the fresh breads was performed by a panel of 50 untrained tasters who usually consume bread, using a nine-point hedonic scale of overall acceptance (9. like extremely; 8. like very much; 7. like moderately; 6. like slightly; 5. neither like nor dislike; 4. dislike slightly; 3. dislike moderately; 2. dislike very much; 1. dislike extremely).
Protein profile
The sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) method was performed, based on the original procedure of Laemmli [21] modified by Fu & Sapirstein [22]. To obtain equal concentrations of proteins, the quinoa flours, wheat flour, wheat bread, and wheat and quinoa bread samples were weighed on the basis of their dry weight protein contents and mixed with 1 mL of sample buffer solution (pH 6.8) containing 0.063 mol/L Tris–HCl, 2% (w/v) SDS, 20% (w/v) glycerol (Merck, Germany), and 0.01% (w/v) Pyronine Y (Sigma–Aldrich, USA). The reduced samples were prepared using 7% (v/v) β-mercaptoethanol (2-ME, Sigma–Aldrich, USA) included in sample buffer. The blend was vortexed (Reax Top model, Heidolph, Germany) for 1 min every 10 min during 2 h. Extracted and dissolved samples were heated in a dry block heating thermostat (Bio TDB-120 model, BIOSAN, Latvia) for 3 min to denature proteins before analysing, and then applied (10 µL) to the SDS-PAGE, which was carried out in a cooled slab gel unit (Protean II xi Cell, Bio-Rad, CA, USA). The acrylamide concentrations of resolving gel and stacking gel were 12.5% and 5%, respectively. After concluding the electrophoresis, the gels were rinsed in rinsing solution [57% (v/v) water + 33% (v/v) methanol + 10% (v/v) trichloroacetic acid (100% w/v)] overnight to remove excess SDS from the surface of the gels. Then, the gels were stained overnight with Coomassie Brilliant Blue G-250 (Merck, Darmstadt, Germany) according to Ng and Bushuk [23]. Apparent molecular weights were determined using wide-range molecular weight protein markers (S8445, Sigma, MO, USA) as standards. The determination of the molecular weights of the protein bands in the quinoa flours, wheat flour, wheat bread, and wheat and quinoa breads was carried out using the Bio-Rad Image Lab 5.0 software after scanning from the gel imager (ChemiDoc MP Imaging System, Bio-Rad, USA).
Differential scanning calorimetry (DSC)
The thermal properties of the raw materials and during baking of the fermented dough as well as the amylopectin retrogradation induced during the bread storage were measured on a differential scanning calorimeter (DSC-7, PerkinElmer) according to the methodology described by Iglesias-Puig et al. [4] with modifications. The calorimeter was calibrated with indium (enthalpy of fusion 28.4 J/g and melting point 156.4 °C). Flours were weighed into DSC pans and mixed with Milli-Q water to obtain a water:flour ratio of 3:1. Samples were scanned at a rate of 10°C/min from 25 °C to 110 °C. Fermented dough samples (30–40 mg) were weighed directly in DSC stainless steel pans (LVC 0319-0218, PerkinElmer). After sealing, the pans were kept at 25 °C for 1 min, scanned at a rate of 10°C/min from 25 °C to 110 °C, kept at this temperature for 5 min, and cooled to 25 °C at 50°C/min. Afterwards, the pans were stored at 4 °C for 24 and 48 h and heated again in the calorimeter from 25 to 130 °C at 10°C/min to analyse amylopectin retrogradation. An empty pan was used as a reference, and three replicates of each sample were analysed. The parameters recorded were onset (To), peak (Tp), and conclusion (Tc) temperatures of gelatinization and retrogradation transitions. The starch gelatinization and amylopectin retrogradation (ΔHG and ΔHR, respectively) were calculated as the area enclosed between the straight line and the endotherm curve between To and Tc. They were expressed in joules per gram of starch and the experiments were conducted in triplicate.
Rapid visco analyser (RVA)
The pasting properties of samples were measured using a Rapid Visco Analyser (RVA-4; Newport Scientific, Warriewood, Australia) according to AACC Method 76 − 21 (1995) [19]. Distilled water (25 mL) was added to 3.0–3.5 g of sample placed into the aluminium RVA canister. The suspensions were stirred thoroughly at 160 rpm. The temperature was first maintained at 50 °C for 1 min to obtain a uniform temperature and then raised to 95 °C at a rate of 12°C/min, held at 95 °C for 2.5 min, cooled to 50 °C at a rate of 12°C/min, and finally held at 50 °C for 2 min. Pasting parameters evaluated included: pasting temperature (Ptemp), peak viscosity (PV), hot paste viscosity (HPV), final or cool paste viscosity (CPV), breakdown (PV–HPV), and setback (CPV – HPV). The RVA experiments were conducted in triplicate.
Statistical analysis
The data generated were analysed by ANOVA using SPSS Statistics Version 22 (International Business Machines Corporation, USA). Fisher’s least significant difference (LSD) test was used to determine statistically significant differences (p < 0.05) between mean values for different samples, at a 95% confidence level.
Results and discussion
SDS-PAGE protein profiles in reduced and unreduced forms
Total extractable proteins of whole quinoa flours, wheat flour, wheat bread, and wheat and quinoa breads in reduced form are shown in Fig. 1. There were a few differences among the protein patterns of the quinoa flours, such as a noticeable protein band with a molecular weight (MW) of 102 kDa in white quinoa flour, whereas red quinoa flour and black quinoa flour did not have this protein band (Lanes 1, 2, and 3); there was also a clear protein band with 38 kDa MW (Lane 3). Otherwise, the protein band profiles of the quinoa flours were generally very similar in reduced form (Fig. 1).
SDS-PAGE patterns of the total extractable proteins of quinoa flours and wheat and quinoa bread samples. All samples were reduced with 7% β-mercaptoethanol. Lane M wide-range protein markers (Sigma S8445). Lane 1 white quinoa flour. 2 Red quinoa flour. 3 Black quinoa flour. 4 Wheat flour. 5 Wheat bread. 6 Wheat bread with white quinoa. 7 Wheat bread with red quinoa. 8 Wheat bread with black quinoa. MW molecular weight
The main protein fractions in quinoa grain are albumins and globulins (chenopodin) which are stabilized by disulfide bonds. The globulins, also called chenopodin or 11S-type proteins, consist of two subunits which are acidic subunits (30–40 kDa MW) and basic subunits (20–25 kDa MW). Lower MW (8–11 kDa) proteins of quinoa grain are called 2S-type proteins [8, 9, 24, 25]. These proteins are also indicated in Figs. 1 and 2. The effects of the breadmaking process on quinoa flour proteins were also investigated in reduced form. The composition of individual proteins in the quinoa flours was significantly modified during both fermentation and baking processes. It was found that, during the breadmaking process, the mixing, fermentation, and baking processes caused some changes in quinoa flour proteins, such as protein hydrolysis by proteases that caused breaking of proteins [26] or disulfide formation through oxidation causing polymerization of proteins which could not enter into the gel. These changes are mainly responsible for the flavour during the fermentation and baking stages [27, 28]. Ingredients notably influence aromatic compounds, and flours usually have distinct aromatic characteristics [29]. In contrast, a small number of protein bands were observed in wheat and quinoa bread samples when compared with those found in the corresponding flours. In all the quinoa flours, a double protein band around 79 kDa MW seemed to be hydrolysed and then smaller fragments may have been polymerized with other wheat proteins (Lanes 1, 2, and 3; Lanes 6, 7, and 8). The intensities of the protein bands with MW of 50, 52, 58, and 62 kDa decreased considerably after the breadmaking process (Lanes 1, 2, and 3; Lanes 6, 7, and 8). These protein bands might be hydrolysed and then polymerized with wheat proteins, and conclusively an intense protein band around 41 kDa MW appeared in wheat and quinoa bread samples (Lanes 6, 7, and 8). Similarly, the protein bands at 35 and 37 kDa in the quinoa flours were hydrolysed via protease attack and then accumulated as a protein band at 34 kDa that appeared very intensely on gel. In addition, the intensity of the binary protein band around 30 kDa in the quinoa flours (Lanes 1, 2, and 3) decreased substantially after the breadmaking process (Lanes 6, 7, and 8). The protein bands located below 25 kDa MW in all the quinoa flours also did not appear after the breadmaking process, owing to protein hydrolysis or polymerization with higher MW wheat proteins.
SDS-PAGE patterns of the total extractable proteins of quinoa flours and wheat and quinoa bread samples prepared without using any reducing agent. Lane M wide-range protein markers (Sigma S8445). Lane 1 white quinoa flour. 2 Red quinoa flour. 3 Black quinoa flour. 4 Wheat flour. 5 Wheat bread. 6 Wheat bread with white quinoa. 7 Wheat bread with red quinoa. 8 Wheat bread with black quinoa. MW molecular weight
A protein band that did not appear in the protein profile of wheat flour (Lane 4) was detected at 110 kDa MW in the profile of wheat bread (Lane 5). The protein bands detected in wheat flour at 13, 28, and 58 kDa did not appear after breadmaking owing to protein hydrolysis and subsequent polymerization with other wheat proteins by formation of cross-linking via disulfide linkages.
Total extractable proteins of quinoa flours, wheat flour, wheat bread, and wheat and quinoa bread samples were investigated without using reducing agent (2-ME), and the SDS-PAGE results of the unreduced samples are shown in Fig. 2. The protein patterns of the quinoa flours in unreduced form were generally found to be similar (Lanes 1, 2, and 3). However, some changes were observed that were due to varietal differences in the quinoa flours. For example, white quinoa flour and red quinoa flour had a thin protein band at 103 kDa MW, whereas the black quinoa flour did not have this protein band in the unreduced form (Lanes 1, 2, and 3). Similarly, intense protein bands between 34 and 37 kDa MW were observed in the white quinoa flour and red quinoa flour, but these protein bands were not detected in the black quinoa flour. Furthermore, protein bands around 21.5 kDa and 30 kDa were detected in the white and red quinoa flours, but were not detected in the black quinoa flour. Double protein bands around 84 kDa in the white quinoa flour were also not detected in the red and black quinoa flours in unreduced form (Fig. 2).
After the breadmaking process, a few faint bands of proteins were detected in the wheat bread and wheat and quinoa breads in unreduced form (Fig. 2). The higher MW protein bands above 49 kDa in the quinoa flours did not appear in unreduced form, probably owing to protein polymerization, because they could not enter into the gel. The intense protein bands at 49, 57, and 60 kDa MW were probably hydrolysed by proteases or may have been polymerized with other proteins, and finally, they did not appear on gel after breadmaking. Similarly, the protein bands between 30 and 37 kDa MW and the protein bands lower than 29 kDa MW did not appear on gel in unreduced form after breadmaking (Lanes 6, 7, and 8; Fig. 2). When the protein profiles of the wheat flour and its bread were examined (Lanes 4 and 5 in Fig. 2), it was seen that the intensities of the protein bands between 42 and 62 kDa decreased after breadmaking. In addition, the intensity of the protein band at 28 kDa decreased in unreduced form as well (Lanes 4 and 5).
The results presented in Fig. 1 indicated that during thermal processing, owing to Maillard and protein cross-linking reactions, the structure of the dough proteins might have changed. This could cause formation of aggregates or protein cross-linking through the formation of disulfide bonds, resulting in the creation of high MW insoluble proteins. Since MWs higher than 200 kDa could not enter into the gel, they could not be detected on the gel. Similar findings have been reported previously in several studies [26, 30, 31]. Singh [30] explained that a low degree of protein extraction from bread samples was due to differences in rate of temperature change and in moisture content in different parts of the bread, and disulfide bonds were the major cross-links formed in bread crusts during baking and they were responsible for protein insolubility.
Thermal properties
The thermal properties of the raw materials, analysed in the differential scanning calorimeter (DSC), are shown in Table 1. These properties are influenced by the protein and lipid contents, the granule structure (amorphous/crystalline structure relationship), and the molecular structure of the amylopectin, such as its branching, chain length and molecular weight, among other things [32]. The starch gelatinization onset temperature (To) of the quinoa flours presented lower values than those of the wheat flour, and this difference was significantly lower (p < 0.05) in black quinoa. In addition, lower peak temperature (Tp) values were observed in the white quinoa flour than in the wheat flour (p < 0.05). Lower gelatinization temperatures indicate shorter amylopectin chains, because they need lower temperatures to dissociate completely [33, 34]. The conclusion temperature (Tc) and gelatinization enthalpy (ΔHG) were significantly higher (p < 0.05) in the red and black quinoa flours than in the wheat and white quinoa flours, owing to the high crystallinity of the starch granules in the quinoa [35].
In varieties from Peru, Repo-Carrasco-Valencia and Valdez-Arana [51] reported ΔHG values similar to those observed in the present work, but the gelatinization temperatures were slightly higher. These differences are basically due to the variability between cultivars.
The thermal properties of the bread doughs during the simulation of baking are shown in Table 1. With regard to gelatinization, a general increase in the To and Tp temperatures was observed in the formulations with quinoa in comparison with the control sample, but this increase was only significant (p < 0.05) in the formulations with white or black quinoa. Furthermore, there was a general decrease in the Tc and ΔHG values in comparison with the control dough, and they were significantly lower (p < 0.05) in the doughs with white quinoa. This behaviour is due to the inclusion of fibre from the whole quinoa flour. During the cooking stage, when the gelatinization of the starch takes place, the water is less available in the formulations with quinoa, basically because of the presence of fibre, so the ungelatinized granules would need higher temperatures and less energy to gelatinize, producing increases in To and Tp and decreases in Tc and ΔHG [37].
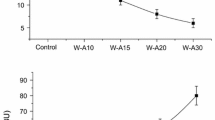
A significant increase (p < 0.05) in the enthalpy of the amylopectin retrogradation (ΔHR) was observed during storage in all the formulations (Fig. 4a), as reported by other authors in studies on retrogradation kinetics [38, 39]. No significant changes in ΔHR were observed during the first 24 h of storage. However, the incorporation of quinoa in the doughs produced a significant reduction (p < 0.05) of this parameter with respect to the control after 48 h. The replacement of wheat flour with red or black quinoa caused a significant increase (p < 0.05) in the retrogradation temperatures with respect to the control and the formulation with white quinoa during storage (data not shown).
Pasting properties
The pasting properties of the raw materials and the bread mixtures were analysed (Table 2). The pasting temperature (Ptemp) of the quinoa flours was significantly higher (p < 0.05) than that of the control flour, which might lead to poor cooking characteristics [40], although the inclusion of 25% of whole quinoa flour did not alter this parameter significantly. The quinoa flours presented significantly higher (p < 0.05) peak time (Ptime) values than the control (Table 2). However, the inclusion of these flours in the formulation produced a significant decrease (p < 0.05) in the time needed for peak formation, denoting a non-additive behaviour and suggesting the appearance of physico-chemical interactions between the components of the flours. The differences in size and structure of the starch granules cause unequal distribution of moisture during heating, and therefore, the behaviour of the doughs is different from that of the individual flours [41]. On the other hand, it is worth noting that the peak viscosity (PV) and breakdown values were significantly lower (p < 0.05) in the quinoa flours than in the wheat flour, which caused a corresponding decrease in these parameters in the analysis of the breadmaking mixtures. Hot paste viscosity (HPV) is related to the final volume of the loaf after baking, owing to its effect on the incorporation and capacity of movement of CO2 in the dough [42, 43]. This might indicate that the lower HPV shown by the quinoa flours with respect to the wheat flour might lead to an increase in the volume of the final product [44, 45]. However, the incorporation of quinoa flours in the breadmaking mixtures led to a general increase in HPV, which was significant (p < 0.05) in the mixtures with white or red quinoa. Setback is the stage in which there is a regrouping and/or reordering of starch molecules and it is associated with the texture of bakery products [46]. The analysis of the raw materials showed significantly lower (p < 0.05) setback values in the quinoa flours than in the control sample. However, the only significant reduction (p < 0.05) in the breadmaking mixtures was in the one with black quinoa.
In general, the values of the pasting properties of the quinoa flours were lower than those of the wheat flour. This can be explained by the characteristics of the starch granules of the various raw materials with regard to their degree of crystallinity and amylopectin chain length and by the higher fibre content in the quinoa flours, reducing the availability of water in the breadmaking mixtures and consequently affecting the pasting properties [47]. In general, the results obtained for the royal quinoa flours in the present study fit within the results reported by Wu et al. [48] after analysing 13 varieties of quinoa.
Effect of incorporation of quinoa on bread performance
The physico-chemical parameters of the wheat bread and the bakery products incorporating whole quinoa flour are shown in Table 3. A significant decrease (p < 0.05) in loaf height was observed in the breads made with black quinoa in comparison with the control sample (~ 6.5%). Although the incorporation of white or red quinoa did not lead to significant differences with respect to the control, the value of this parameter tended to decrease. The reduction in loaf height was similar to the loss of volume reported by other authors [12, 49], basically affected by the dilution of gluten and the higher fibre concentration in the quinoa flours. However, there were no significant changes in loaf weight between the breads that incorporated quinoa and the control bread (Table 3). The moisture content of the samples with quinoa, except the one with red quinoa, increased significantly (p < 0.05), basically owing to the use of whole quinoa flours. The protein content tended to increase, and this increase was statistically significant (p < 0.05) in the formulations with white and red quinoa. It is worth noting that the replacement of wheat flour with whole quinoa flour not only increases the protein content but also produces an improvement in the biological value of the proteins in these formulations, because quinoa proteins are more digestible than wheat proteins and they provide essential amino acids that are limiting in wheat flours [50, 51]. There was also a significant increase (p < 0.05) in the dietary fibre and mineral contents in the formulations with white and red quinoa in comparison with the control, thus contributing to a suitable intake of fibre and minerals such as Ca, Fe and Zn in the diet [42, 52].
The results of the digital image analysis of the crumb of the products developed are shown in Table 3. There was a significant increase (p < 0.05) in the value of the cell area/total area parameter in the crumb of breads that included red or black quinoa in comparison with the bread with white quinoa and the control (Fig. 3). Although significant changes were not seen in the cells/cm2 parameter, a decreasing tendency was observed in the sample with black quinoa. It is worth noting that there was a very significant increase (p < 0.05) in the maximum cell area in the crumb of the breads with various varieties of quinoa in comparison with the control bread. These differences may be due to greater α-amylase activity in the quinoa, leading to an increase in the quantity of fermentable sugars produced from the starch [53, 54]. Although the maximum cell area increased in the crumb of the breads with quinoa, there was a decrease in the median cell area of those breads, most probably due to the formation of large gas cells which compressed the other gas cells, reducing the median cell area.
With regard to texture, the parameters analysed are shown in Table 3. A significant increase (p < 0.05) was observed in the firmness parameter of the breads with white or black quinoa in comparison with the control, basically due to the reduction in the percentage of gluten. The incorporation of quinoa in the bread formulations also led to significant increases (p < 0.05) in the gumminess and chewiness parameters, whereas there was a significant decrease (p < 0.05) in cohesiveness with respect to the control sample. In general, during storage, there were significant changes in all the texture parameters of the products developed (data not shown). However, a very marked increase was observed in the firmness values of the products formulated with quinoa in comparison with the control sample during 2 days of storage (Fig. 4b). This crumb hardening can be explained partly by the phenomenon of amylopectin retrogradation (Fig. 4a). Retrogradation is a complex phenomenon that depends on many factors, such as the size and structure of the starch granules, and it involves phenomena such as the formation of bonds with proteins and/or the presence of lipids with surfactant properties that can cause differences in the migration of water molecules between gluten and starch during storage [55]. Accordingly, the significant increase (p < 0.05) in the crumb firmness during storage of the products with quinoa may be due to a greater loss of moisture generated by an irregular dough, with layers of gluten surrounding conglomerates of starch granules [56].
Firmness and amylopectin retrogradation of control and wheat and quinoa bread samples (n = 3): unfilled square, day 1; grey colored filled square, day 2; black colored filled square, day 3. Mean ± Standard Deviation, n = 3. Values followed by the same letter in the same line are not significantly different at 95% confidence level
The preliminary sensory analysis indicated that partial replacement of wheat flour with 25% of whole quinoa flour did not significantly affect the general acceptability of the products developed. However, the breads with quinoa were given slightly better scores than the control sample, with the exception of the bread with red quinoa, which received slightly less acceptance. The acceptance of products made with quinoa might be due, among other things, to the formation of aromatic compounds, such as pyridines, characteristic of quinoa flours, generating flavours accepted by consumers [28].
Conclusions
The global proteomic approach offered a general view of the various proteins in the different quinoas and the changes that took place during the breadmaking process, which included hydrolysis and formation of bonds between quinoa proteins and wheat proteins, modifying the protein structure of the doughs formulated. In general, the three varieties of quinoa presented a similar behaviour in terms of pasting properties, thermal characteristics and proximal composition that were different if comparing to wheat flour. The gelatinization thermal transition of starch from red and black quinoa flours appeared in a greater temperature range than white quinoa flour. The replacement of 25% of the wheat flour with whole quinoa flour in making bakery products caused a change in the thermal and pasting properties of the bread doughs, which led to the development of baked products with different physico-chemical and textural characteristics. However, a significant increase (p < 0.05) in the nutritional profile together with the overall consumer acceptance of the products developed was conclusive for proposing replacement with quinoa flour as a strategy for nutritional improvement in the manufacture of bread with refined wheat despite the slight decrease in the technological quality of the products developed. Therefore, black quinoa bread presented a higher amount of dietary fibre/ash and a lower amount of starch compared to white and red quinoa breads. These differences produced breads with a lower loaf height and higher crumb firmness, chewiness, and resilience with a similar acceptability by consumers regardless the different formulations.
References
Haros M, Sanz-Penella JM (2017) Food uses of whole pseudocereals. In: Haros M, Schoenlechner R (eds) Pseudocereals: chemistry and technology. Wiley, Oxford
Sanz-Penella JM, Laparra JM, Sanz Y, Haros M (2012) Bread supplemented with amaranth (Amaranthus cruentus): effect of phytases on in vitro iron. Plant Foods for Hum Nutr 67:50–56
Garcia-Mantra I, Monedero V, Haros M (2014) Application of phytases isolated from bifidobacteria in the development of cereal-based products with amaranth. Eur Food Res Techn 238:853–862
Iglesias-Puig E, Monedero V, Haros M (2015) Bread with whole quinoa flour and bifidobacterial phytases increases dietary mineral intake bioavailability. LWT Food Sci Technol 60:71–77
Ruiz KB (2013) Quinoa biodiversity and sustainability for food security under climate change: a review. Agron Sustain Dev. https://doi.org/10.1007/s13593-013-0195-0
Regulation (EU) 2015/2283 (2015) Official J Eur Union. 25 Nov 2015
D´Amico S, Schoenlechner R, Tömököszi S, Langó B (2017) Proteins and amino acids of kernels. In: Haros M, Schoenlechner R (eds) Pseudocereals: chemistry and technology. Wiley, Oxford
Brinegar C, Goundan S (1993) Isolation and characterization of chenopodin, the 11S seed storage protein of quinoa (Chenopodium quinoa). J Agr Food Chem 41:182–185
Brinegar C, Sine B, Nwokocha L (1996) High-cysteine 2S seed storage proteins from quinoa (Chenopodium quinoa). J Agr Food Chem 44:1621–1623
Hager AS, Wolter A, Jacob F, Zannini E, Arendt EK (2012) Nutritional properties and ultra-structure of commercial gluten free flours from different botanical sources compared to wheat flours. J Cereal Sci 56:239–247
Janssen F, Pauly A, Rombouts I, Jansens KJA, Deleu LJ, Delcour JA (2017) Proteins of amaranth (Amaranthus spp.), buckwheat (Fagopyrum spp.), and quinoa (Chenopodium spp.): a food science and technology perspective. Compr Rev Food Sci F 16:39–58
Park SH, Maeda T, Morita N (2005) Effect of whole quinoa flours and lipase on the chemical, rheological and breadmaking characteristics of wheat flour. Jpn Soc Appl Glycosci 52:337–343
Lindeboom N, Chang P, Falk K, Tyler R (2005) Characteristics of starch from eight quinoa lines. Cereal Chem 82:216–222
Aluwi NA, Murphy KM, Ganjyal GM (2017) Physicochemical characterization of different varieties of quinoa. Cereal Chem 94:847–856
Reguera M, Haros M (2017) Structure and composition of kernels. In: Haros M, Schoenlechner R (eds) Pseudocereals: chemistry and technology. Wiley, Oxford
Ballester-Sanchez J, Gil JV, Haros M, Fernandez-Espinar MT (2019) Effect of incorporating white, red or black quinoa flours on the total polyphenol content, antioxidant activity and colour of bread. Plant Food Hum Nutr. https://doi.org/10.1007/s11130-019-00718-w
Association of Official Analytical Chemist (1996) Method 925.09, 996.11., 985.29, in official methods of analysis, 15th edn. AOAC, Arlington
ISO/TS (2016) Food products. Determination of the total nitrogen content by combustion according to the Dumas principle and calculation of the crude protein content. Part 2: Cereals, pulses and milled cereal products. International Organization for Standardization, Geneva, p 25
Association of Analytical Cereal Chemists (1995) Method 30–20, 08–03, 76–21 (9th edn). Method, in approved methods of American Association of Cereal Chemistry, Saint Paul
Gámbaro A, Fiszman S, Giménez A, Varela P, Salvador A (2004) Consumer acceptability compared with sensory and instrumental measures of white pan bread: sensory shelf-life estimation by survival analysis. J Food Sci 69:401–405
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of Bacteriophage T 4. Nature 227:680–685
Fu BX, Sapirstein HD (1996) Procedure for isolating monomeric proteins and polymeric glutenin of wheat flour. Cereal Chem 73:143–152
Ng PKW, Bushuk W (1987) Glutenin of marquis wheat as a reference for estimating molecular weights of glutenin subunits by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Cereal Chem 64:324–327
Abugoch L, Romero N, Tapia CA, Silva J, Rivera M (2008) Study of some physicochemical and functional properties of quinoa (Chenopodium quinoa Willd) protein isolates. J Agr Food Chem 56:4745–4750
Abugoch L, Castro E, Tapia C, Anon MC, Gajardo P, Villarroel A (2009) Stability of quinoa flour proteins (Chenopodium quinoa Willd.) during storage. Int J Food Sci Tech 44:2013–2020
Horszwald A, Troszynska A, Del Castillo MD, Zielinski H (2009) Protein profile and sensorial properties of rye breads. Eur Food Res Technol 229:875–886
Martínez-Anaya MA (1996) Enzymes and bread flavor. J Agric Food Chem 44:2470–2480
Hansen A, Schieberle P (2005) Generation of aroma compounds during sourdough fermentation: applied and fundamental aspects. Trends Food Sci Technol 16:85–94
Di Renzo T, Reale A, Boscaino F, Messia MC (2018) Flavoring production in kamut®, quinoa and wheat doughs fermented by lactobacillus paracasei, lactobacillus plantarum, and lactobacillus brevis: a SPME-GC/MS study. Front Microbiol 9:429
Singh H (2005) A study of changes in wheat protein during bread baking using SE-HPLC. Food Chem 90:247–250
Miller AG, Gerrard JA (2005) Assessment of protein function following cross-linking by α-dicarbonyls. Ann Ny Acad Sci 1043:195–200
Tester RF (1997) In: Frazier PJ, Richmond P, Donald AM (eds) Starch, structure, functionality. Roy Soc Ch, London
Yamin FF, Lee M, Pollak LM, White PJ (1999) Thermal properties of starch in corn variants isolated after chemical mutagenesis of inbred line B73. Cereal Chem 76:175–181
Jane J, Chen YY, Lee LF, McPherson AE, Wong KS, Radosavljevic M, Kasemsuwan T (1999) Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem 76:629–637
Steffolani ME, Leon AE, Perez GT (2013) Study of the physicochemical and functional characterization of quinoa and kañiwa starches. Starch 65:976–983
Repo-Carrasco-Valencia RAM, Serna LA (2011) Quinoa (Chenopodium quinoa, Willd.) as a source of dietary fiber and other functional components. Food Sci Technol 31:225–230
Santos E, Rosell CM, Collar C (2008) Gelatinization and retrogradation kinetics of high-fiber wheat flour blends: a calorimetric approach. Cereal Chem 85:455–463
Haros M, Rosell CM, Benedito C (2002) Effect of different carbohydrases on fresh bread texture and bread staling. Eur Food Res Technol 215:425–430
Ribotta PD, León AE, Añon MC (2003) Effect of frozen storage on the gelatinization and retrogradation of amylopectin in dough baked in a differential scanning calorimeter. Food Res Int 36:357–363
Hoseney RC (1984) Gas retention in bread doughs. Cereal Food World 29:305–306
Waterschoot J, Gomand SV, Fierens E, Delcour JA (2014) Starch blends and their physicochemical properties. Starch 66:1–13
Bath DE, Shelke K, Hoseney KC (1992) Fat replacers in high-ratio layer cakes. Cereal Food World 37:495–500
Kim HYL, Yeom HW, Lim HS, Lim ST (2001) Replacement of shortening in yellow layer cakes by corn dextrins. Cereal Chem 78:261–271
Lee S, Kim S, Inglett GE (2006) Effect of shortening replacement with oat rim on the physical and rheological properties of cakes. Cereal Chem 82:120–124
Onyango C, Mutungi C, Unbehend G, Meinolf G, Lindhauer MG (2010) Rheological and baking characteristics of batter and bread prepared from pregelatinised cassava starch and sorghum and modified using microbial transglutaminase. J Food Eng 97:465–470
Michiyo W, Tomoko M, Kikuchi T, Hiroshi K, Naofumi M (2004) Application of pregerminated brown rice for breadmaking. Cereal Chem 81:450–455
Bulut-Solak B, Alonso-Miralles L, O´Mahony JA (2016) Composition, morphology and pasting properties of Orchis anatolica tuber gum. Food Hydrocoll 69:483–490
Wu G, Morris CF, Murphy KM (2014) Evaluation of texture differences among varieties of cooked quinoa. J Food Sci 79:2337–2345
Wang S, Opassathavorn A, Zhu F (2015) Influence of quinoa flour on quality characteristics of cookie, bread and Chinese steamed bread. J Texture Stud 46:281–292
Vega-Gálvez AM, Miranda J, Vergara J, Uribe J, Puente L, Martinez EA (2010) Nutrition facts and functional potential of quinoa (Chenopodium quinoa Willd.), an ancient Andean grain: a review. J Sci Food Agric 90:2541–2547
Repo-Carrasco-Valencia R, Valdez-Arana J (2017) Carbohydrates of kernel. In: Haros M, Schoenlechner R (eds) Pseudocereals: chemistry and technology. Wiley, Oxford
Stikic R, Glamoclija D, Demin M, Vucelic-Radovic B, Jovanovic Z, Milojkovic-Opsenica D, Jacobsen SE, Milovanovic M (2012) Agronomical and nutritional evaluation of quinoa seeds (Chenopodium quinoa Willd.) as an ingredient in bread formulations. J Cereal Sci 55:132–138
Lorenz K, Coulter L (1991) Quinoa flour in baked products. Plant Food Hum Nutr 41:213–223
Caussette M, Kershaw JL, Sheltod DR (1997) Survey of enzyme activities in desaponified quinoa Chenopodium quinoa Willd. Food Chem 60:587–592
Gray JA, Bemiller JN (2003) Bread staling: molecular basis and control. Compr Rev Food Sci F 2:1–21
Morita N, Hirata C, Park SH, Mitsunaga T (2001) Quinoa flour as a new foodstuff for improving dough and bread. J Appl Glycosci 48:263–270
Acknowledgements
This work was financially supported by grants QuiSalhis-Food (AGL2016-75687-C2-1-R) from the Ministry of Economy, Industry and Competitiveness (MEIC-Spain), la ValSe-Food CYTED-119RT0567 and LINCE (PROMETEO/2017/189) from the Generalitat Valenciana, Spain. The contract given to J. Ballester-Sánchez by MEIC-Spain is gratefully acknowledged. The authors express their sincere appreciation to J. M. Coll Marqués for his aid in the digital image analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ballester-Sánchez, J., Yalcin, E., Fernández-Espinar, M.T. et al. Rheological and thermal properties of royal quinoa and wheat flour blends for breadmaking. Eur Food Res Technol 245, 1571–1582 (2019). https://doi.org/10.1007/s00217-019-03265-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-019-03265-5