Abstract
Spent brewer’s yeast is an excellent source of a variety of bioactive substances. In this study, for the first time, the focus was solely on investigating the influence of three industrially applicable cell disruption methods (cell mill, sonotrode, and autolysis) on selected substance groups relevant for physiology and process technology. A consistent spent yeast (Saccharomyces cerevisiae TUM 68) produced in a standardized industrial pilot top-fermenting process was used as a raw material. Using mechanical methods, i.e., cell mill and sonotrode, the protein content (as not hydrolyzed in free amino acids), the trehalose and the total fat content in the yeast extract were increased compared with those produced in the autolytic method. The analyzed B vitamin levels were also higher, the biologically active 5-CH3-H4folate in particular had the greatest proportion in the folate vitamer distribution of the mechanically produced yeast extracts. An increased level of non-fragmented genomic and mitochondrial DNA could also be found in the yeast extract produced via the mechanical methods. The antioxidative and reduction potential was decreased by the degradation of polyphenols and glutathione in the yeast extract following autolysis. The mineral, RNA, glycogen, glucose, fructose and ash contents did not differ significantly. Therefore, the cell mill and sonotrode offered a good alternative method to conventional autolytic procedures, especially to transfer physiologically relevant substance groups in higher concentrations to the yeast extract.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The widely used term “yeast extract” in food processing describes the soluble contents of a yeast cell produced following destruction and removal of the yeast cell membrane [1, 2]. There are various disruption methods to access the valuable ingredients in yeast and make these available. On an industrial scale, mainly autolytic methods using solvents, enzyme additives, and salts [2], or mechanical methods such as cell mills are applied [1, 3]. The selection of method depends partly on the substances that need to be extracted from the yeast cell and partly on the disruption effectiveness and efficiency. In the laboratory, an ultrasonic sonotrode is often used to disrupt cells. The principle behind this is based on the implosion of cavitation bubbles, which applies shear forces to the cell walls and disrupts them [4]. The up-scaling potential of this disruption method for industry has already been shown using Barbell Horn Ultrasonic Technology [5].
A particularly cost-effective starting product used to produce yeast extract is spent yeast, which occurs in vast quantities (15 kg/10 hL beer) during the beer manufacturing process, as only part of it is reused for the next batch [6, 7]. The spent yeast or the yeast extract produced from this varies greatly in its physical composition as a result of the yeast strain [8] or the yeast cell growth phase [9]. This is particularly reflected, for example, in the key functional substance groups of the food industry such as the mineral [10], vitamin [11], RNA [12], protein content [8] or the enzymatic activity [13] of the yeast cells [14]. In addition to the diversity of the yeast starting product, the cell disruption method used when producing the yeast extract also plays an important role [15]. For instance Jacob et al. showed that the content of amino acids can differ according to the manufacturing process [15]. The specified diversity of the spent yeast that is used as the starting material and the manifold influences of the manufacturing methods mean that the data relating to the yeast extracts’ physical composition vary in the literature and can hardly be compared with each other [16,17,18,19]. In addition, extracting the β-glucan from the cell walls of the yeast using a cell mill generates a yeast extract which can be reused to increase profit and can compete with extracts obtained in autolytic processes [1].
In this study, we compared mechanical and autolytical disruption methods for yeast as well as the effect of using an ultrasonic sonotrode, a novel option for yeast extract production with proven up-scaling potential. We chose the yeast strain Saccharomyces cerevisiae TUM 68, which is a dominant brewing yeast strain used worldwide. Furthermore, this is the first time this strain is used as a starting material for a yeast extract following fermentation in a closed cylindro-conical tank (CCT), which is the more frequently employed fermentation process compared with open top-fermentation methods and where the resulting spent yeast is only reused to a minor extent [7]. Since the particular focus of prior research has been on amino acid composition [15], this study investigated the key functional substance groups of the food industry such as B vitamins, minerals, DNA and RNA content, as well as the general nutritional composition of the yeast extract. Furthermore, we correlated the antioxidative potential as a function of the substances responsible for this such as polyphenols and glutathione.
Materials and methods
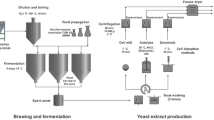
A graphical overview and correlations between the applied methods are depicted in Fig. 1.
Yeast propagation and fermentation
A sterilized, hopped, and standardized all malt wort concentrate was used as the propagation and fermentation medium (N53940; Döhler GmbH, Darmstadt, Germany). For the precise composition refer to Table 1. The all malt wort concentrate was diluted to an original wort content of 12.4 °P [d = (a × p) + b where d = specific weight (in kg/m3), a = 4.13 kg/(m3 °P), b = 997 kg/m3, p = original wort content (in °P)] and heat-treated before use at 100 °C for 10 min.
The top-fermenting brewer’s yeast Saccharomyces cerevisiae LeoBavaricus TUM 68 (hereinafter TUM 68) on agar slant was sourced from the Yeast Center at the Weihenstephan Research Center for Brewing and Food Quality of the Technical University of Munich (RCW). This yeast strain has already been characterized in detail by the RCW [20]. An inoculation loop of an agar slant culture was transferred to 40 mL standardized wort and incubated for 48 h at 20 °C on a shaker (80 rpm). The 40 mL was then expanded to 400 mL with standard wort and incubated again for 48 h at 20 °C on a shaker (80 rpm). This incubation process was repeated again after the 400 mL was made up to 4 L standardized wort.
The propagated yeast was used to ferment 50 L standardized and aerated (10 mg O2/L) wort in a CCT with a living cell count of 10 million/mL. Fermentation took place unpressurized for 5 days at 18 °C. The viscous core yeast, with a volume of approx. 1 L, was cropped on the 6th day via the CCT cone. The residual extract from the fermented wort was 3.6% and revealed an alcohol content of 5.2 vol.% (measured using the Anton Paar DMA 5000 Density Meter with Alcolyzer Plus measuring module).
Yeast pre-treatment
After the yeast was cropped (70% live, methylene blue method [21]), it was immediately subjected to three washing processes to remove residual wort components. Each washing process was performed as follows: the viscous cropped spent yeast was diluted with distilled water to 10% dry matter, passed through a yeast sieve (mesh size 0.5 mm), centrifuged (1000g, 5 min, 18 °C, 500 mL centrifuge tube) and the liquid supernatant was discarded. The sedimented yeast in the centrifuge tube was then resuspended with distilled water for 5 min to 10% dry matter and the washing procedure was started afresh. The washed yeast was subsequently collected in a single container and diluted to 7% dry matter in distilled water before being fed into the disruption process as a consistent starting material.
Yeast quality control
The propagation yeast, the spent yeast following fermentation, the washed spent yeast before disruption and the macerated yeast suspension were only used after passing quality control. The following tests also had to give negative results for foreign yeasts and microorganisms: visual detection of non-starter culture microorganisms (examination under microscope), detection of wild yeast species/strains (qualitative detection; YM medium + CuSO4; specification: negative per 1 mL) [22], detection of beer-spoilage organisms (qualitative detection, medium NBB-broth, specification: negative per 1 mL) [23], detection of bacteria (qualitative detection, yeast extract agar + cycloheximide, specification: negative per 0.1 mL), detection of bottom-fermenting yeast based on specific real-time PCR [22], PCR screening of beer-spoilage organisms [foodproof beer screening kit (Biotecon Diagnostics)] [24].
Yeast cell disruption methods
The disruption methods described and implemented here are used both academically and in industry [1, 4, 5, 12, 25,26,27,28].
Cell mill
The mechanical disruption method was carried out using a Vibrogen cell mill VI 6 (Edmund Bühler GmbH). The stroke frequency of the shaker was 75 Hz. The process volume was 200 mL. Glass beads with a diameter of 0.5 mm were used for cell disruption with a weight ratio of 1:2 (glass beads/yeast suspension). The mill was cooled by a water–glycol mixture flowing through the jacket of the beaker so that the suspension temperature was held constant at 7 °C. The procedure took 15 min per sample.
Ultrasonic sonotrode
Cell disruption using cavitation was carried out using the ultrasonic homogenizer SONOPLUS HD 3400 (Bandelin). The sonotrode diameter was 25 mm with an operating frequency of 20 kHz. The process suspension volume of 200 mL was subjected for 30 min to a constant ultrasonic output of 400 W without pulsation. The resulting process heat was removed by means of a cooling bath to maintain a constant temperature of 7 °C.
Autolysis
To autolyze the yeast, 200 mL of the suspension was heated in a reaction vessel for 24 h at 50 °C with constant stirring (100 rpm). Sodium chloride (0.086 mol/L) and ethyl acetate (0.051 mol/L) were also added at the start of the process.
Generation of yeast extract
In accordance with the literature [2, 12, 29], the cell wall components first had to be separated from the cell extract in the samples. The disrupted yeasts were centrifuged for 20 min at 10,000 g and 4 °C. The supernatant was carefully pipetted into the centrifuge tubes. Following centrifuging, a freeze-dryer was used (condenser temperature: − 50 °C, vacuum: 0.1 mbar, ice condenser capacity: 3 kg/24 h) to gently remove water from the liquid yeast extract (cytosol extract). In this way, a yeast extract powder was produced for the subsequent analyses that had a residual water content of 10% and offered a constant basis for comparison.
Analysis
The dry yeast extracts enabled the disruption methods to be directly compared in terms of the following analysis without the need to consider the effectiveness of the different methods [28]. Results about the effectiveness of the three disruption methods can be seen in a previous work of Jacob et al. [15]. Depending on the limit of quantification of the chosen analytical method, the dried yeast extract sample was resuspended in distilled water and analyzed directly. A commercially available industrial yeast extract produced by autolysis was included in all analyses to provide a practical comparison. For manufacturing and technical reasons, the relevant starting yeast for this yeast extract product could not be precisely determined in terms of either its physiological status or its previous fermentation medium.
Protein
The protein content in the yeast extract was determined by measuring the absorption of the color complex formed between the yeast protein and Bradford reagent at 595 nm according to the Bradford method [30].
The nitrogen content in the yeast extract was determined using the Kjeldahl method described in the MEBAK (Central European Brewing Technology Analysis Commission) brewing technology analysis methods (Method 2.6.1.1) [31].
The free amino acids (except proline and cysteine) were quantified using high-performance liquid chromatography (HPLC) according to MEBAK Method 2.6.4.1 [31]. The detailed procedure for proline and cysteine (Method 4.11.1) was taken from Buch für chemische Untersuchung von Futtermitteln (the chemical analysis of feedstuffs) [32]. To determine all free and protein-bound amino acids (total amino acid quantity), the resuspended yeast extracts underwent acid hydrolysis before measurements according to Method 4.11.1 of the chemical analysis of feedstuffs [32].
Free alpha-amino nitrogen (FAN) was determined photometrically (570 nm) according to MEBAK Method 2.6.4.1 (Ninhydrin Method) [31].
Carbohydrates
Glycogen was determined enzymatically using amyloglycosidase according to the description by Schulze et al. [33]. The glucose released as per the specification was determined using HPLC and can be taken from the MEBAK Method 2.7.1 [31].
Trehalose was determined using a modified method described by Mahmud et al. in which the absorption of complexes formed using anthrone reagent was measured spectrophotometrically at 620 nm [34].
The β-glucan was measured enzymatically according to MEBAK Method 2.5.1, glucose, fructose, maltose, sucrose, and maltotriose according to MEBAK Method 2.7.1 using HPLC [31].
Fat
The crude fat was determined according to Method 5.1.1 from the Methodenbuch für chemische Untersuchung von Futtermitteln (the chemical analysis of feedstuffs) [32].
The long-chained fatty acids were determined using gas chromatography (GC) according to MEBAK Method 2.23.2 [31].
Water and ash content
The water content was determined using MEBAK Method 2.2 [35], the ash content was determined similarly according to Method 8.1 from the Methodenbuch für chemische Untersuchung von Futtermitteln (the chemical analysis of feedstuffs) [32].
Vitamins
Vitamin B1 (DIN EN 14122), B2 (DIN EN 14152), and B6 (DIN EN 14663) were determined according to DIN standards using HPLC [36]. Vitamin B3 (AOAC 944.13), B5 (AOAC 945.74), and B12 (AOAC 952.20/986.23) were determined according to AOAC methods using microbiological processes [37]. Biotin (vitamin B7) was determined based on Mücke, also microbiologically, using Lactobacillus plantarum (ATCC 8014) [38]. Vitamin B9 (total folate) and the folate derivatives were analyzed according to the recently published method by Striegel et al. by means of LC–MS/MS [39].
Minerals
The micro and macro minerals in the yeast extract underwent multi-element determination using inductively coupled plasma optical emission spectroscopy (ICP-OES) as per MEBAK Method 2.24.12 [31].
DNA and RNA
The total DNA and total RNA concentration in the yeast extract was determined photometrically (260 nm) using the NanoDrop3300 spectrophotometer (Thermo Fisher Scientific). The DNA was purified according to Hutzler using a InstaGene® Matrix (Bio-Rad, Hercules, CA, USA), the RNA was purified using a PureLink® RNA Mini Kit (Thermo Fisher Scientific) and PureLink® DNase (Thermo Fisher Scientific) [22]. Real-time PCR (polymerase chain reaction) assay (PCR temperature protocol, primers, probes and Master Mix composition) for gene Sc-GRC3 (hereinafter GRC3) and OG-COXII (hereinafter COX2) was performed according to Hutzler [22].
Polyphenols
The determination of the total polyphenol content followed Method 2.16.1 of the MEBAK analysis specifications [31]. In this method, the polyphenols reacted with iron (III) ions in an alkaline solution, forming colored iron complexes which were spectrophotometrically measured at 600 nm [31].
Glutathione
The glutathione content was measured according to an analytical specification from the patent of Ortiz-Julien, in which, after reacting with Ellman’s reagent [5,5′-dithiobis(2-nitrobenzoic acid), DTNB], the absorption was measured spectrophotometrically at 412 nm [40].
EBC-bittering units
Bittering compounds (mainly iso-α acids) were determined photometrically (275 nm) according to MEBAK Method 2.17.1 and expressed as EBC-bittering units [31].
Antioxidative and reduction potential
The antioxidative potential of the yeast extract was measured using a Sigma-Aldrich kit, in which the antioxidants from a sample inhibit the formation of radical cations [41]. Spectrophotometry was used to measure this inhibition proportionally by means of a color reaction. Trolox (TE), a vitamin E analog, was used as the control antioxidant.
The reduction potential of the yeast extract can be determined as per MEBAK Method 2.15.2 [31]. The reductones of the sample reduce a certain quantity of Tillmann’s reagent (2,6-dichlorophenolindophenol, DPI) within a certain period, which can be measured spectrophotometrically (520 nm) [31].
Statistical evaluation
All experiments were performed in triplicate and the relevant results given as arithmetic means. At a confidence level of 95%, the expected range (confidence interval) for each mean was calculated from the variance using Student’s t distribution. A single-factor analysis of variance (ANOVA) was conducted to show a difference between all disruption methods. A paired t test was also performed to demonstrate the difference between two disruption methods. “Significant” differences were described as having a p value < 0.05. A test by Dixon was used to evaluate the results.
Results and discussion
General nutritional compositions
The greatest proportion of the dry mass of yeast extract is formed by the proteinogenic substances. In the literature, a broad range (50–80% of dry mass of yeast extract) of values can be found, the determination of which is based on various analytical methods and calculations [1, 2, 8, 29, 42, 43]. In many publications, this is performed by ascertaining the total nitrogen according to Kjeldahl and multiplying by a factor based on the amino acid spectrum, the number of amino groups and the molecular weight of the respective amino acids. The high RNA content (up to 11%) [12] in the yeast extract casts severe doubts on this method as it would also detect the nitrogen of the nucleobases and this will influence the result. Ihl et al. have already referred to differences between the Kjeldahl method and other methods of determination [44]. The term “protein” is often described in many publications as the total of all proteinogenic substances. However, the use of yeast extract as a source of nitrogen in microorganism culture media or in industrial fermentations such as beer production from unmalted grain worts, necessitates precise differentiation between free amino acids and the amino acids bound via amide bonds in oligopeptides or protein macromolecules [16, 45]. The reason for this is that for example free amino acids are preferentially utilized by brewing yeasts [16, 45]. Therefore, we compared the proteinogenic material determination in all three disruption methods used to produce the yeast extract (Table 2). Those yeast extracts produced using the cell mill and sonotrode, revealed that the mathematical sum of the total of all free amino acids and the protein content according to Bradford including all protein molecules greater than 3 kDa [46] corresponds approximately to the total quantity of all the amino acids found (total amino acids, determination following hydrolytic disruption). This result did not hold true for the autolytically produced yeast extract as virtually all amino acids present here were free or bound in protein molecules smaller than 3 kDa and were consequently not recorded using the Bradford method. By multiplying the yeast extract nitrogen content by the widely used factor 6.25, a higher value for the proteinogenic material was reflected in all the investigated production methods than the value which resulted from the total quantity of all the amino acids found in the yeast extract (total amino acids, following hydrolytic disruption). In comparison, the factor 5.5 also used in the literature gave a lower value for the extracts produced in the laboratory. In the analyzed industrial product, the starting material of which could not be identified in detail, a higher value in contrast was also established for the proteinogenic material using the factor 5.5. This could presumably be attributed to factors including the higher RNA content (5.5%). For the yeast material used in this study, the factor 5.8 was calculated for evaluating the proteinogenic material in the produced yeast extract using the Kjeldahl method. As a result of the variable composition of the yeast extract, due to the disruption method and the yeast starting material, an analysis of the individual amino acids using HPLC and the subsequent total sum for the protein determination is more practical than multiplying the nitrogen content by a certain factor. A method using the Fourier-transform infrared spectrometer, which directly measures the peptide bonds in the protein, also offers a very precise method to differentiate the bound from the free amino acids [8]. The determined values for the proteinogenic material lie in the broad range of existing literature data [1, 2, 8, 29, 42, 43, 47]. However, it is difficult to make a direct comparison with values from previous studies due to the varying information on the yeast strain [48], fermentation medium [8, 47] or yeast extract production parameters [29]. In terms of the content of free amino acids in the yeast extract, the two mechanical methods gave a significantly lower result (t test p value < 0.05) than autolysis. The total protein content did not differ significantly between the three disruption methods investigated in this study (t test p value > 0.05).
The carbohydrate composition of the yeast varied depending on the growth phase of the yeast population and based on the nutrient supply. In beer manufacture, the yeast cells increasingly store glycogen and trehalose at the end of fermentation due to a lack of various nutrients such as carbohydrates, nitrogen, sulfur, and phosphorus [49]. At this point, almost all of the sugars that the yeast can quickly utilize, such as glucose, fructose and maltose, are consumed from the medium [50]. The yeast extract obtained in the three disruption methods also had a correspondingly low content of 0.3% glucose and 0.09% fructose on average, with no significant difference being established between the different disruption methods (Table 2). Similar values were previously determined by Münch in her study on autolytically produced beer yeast extract and it was concluded that those quantities were insufficient for a targeted yeast extract aroma formation by heating [28]. Trehalose also has the property to protect the cell from dehydration, heat, cold or osmotic stress by stabilizing the cell membrane and yeast proteins [51]. Furthermore, trehalose contributes to sweetness (45% relative to sucrose), is very pH- and heat-stable, inhibits the degradation of proteins and lipids in food and cosmetic industry products [51]. The content of trehalose in the yeast extract following the two mechanical processes compared with the autolytic method was significantly increased by more than 17 mg/g dry mass (t test p value < 0.05). Based on the high stability of this carbohydrate, even at temperatures of up to 100 °C and a pH range of 3.5–10 [51], it was considered likely that enzymatic degradation took place in the yeast cell during the autolysis phase. In the literature, a large accumulation of trehalose under temperature stress of 37–45 °C and osmotic stress is reported, although it is also stated that a degradation of this carbohydrate always takes place as a result of various concurrent metabolic pathways [52]. Presumably, however, in the disruption conditions in this study trehalose is degraded by trehalase in the course of the autolytic process. This presumption corresponds to the results of Yoshikawa et al., who could increase the extraction yield of trehalose from yeast cells by means of preliminary thermal denaturation of trehalase [53]. The trehalose content of Saccharomyces cerevisiae can reach up to 25% of the dry mass under relevant environmental conditions (e.g., temperatures above 25 °C) [52], although these are not normal conditions in brewery practice [49]. At 44 mg/g yeast extract, the yields of the mechanical methods cell mill and sonotrode were also correspondingly low; however, they were significantly above the investigated autolytic method (t test p value < 0.05) or, for example, a high-intensity pulsed electric field (PEF) process [54]. An optimized fractionation process of baker’s yeasts according to Pastinen et al. gave a trehalose content of 5.4% [55]. In comparison, the glycogen content of the yeast extract was not significantly changed by the three disruption methods and was approximately 26%, which constituted the second-largest proportion (after the protein fraction) of the extract’s dry mass and corresponded to a common harvested yeast following a beer fermentation process [49]. The considerably lower value in the commercially available industrial product is presumably due to a different glycogen content in the starting yeast, which does not come from the brewing process. The β-glucan content in mechanically and autolytically produced yeast extract was on average 1.4 mg/g dry mass. The disruption methods did not differ significantly (ANOVA p value > 0.05) in the latter content. Since the industrially produced yeast extract also contains this quantity, this small proportion of β-glucan being primarily bound in the cell walls of the yeast and insoluble in water [56] is presumably not fully recovered during extraction due to process technology reasons.
The ash content of the yeast extracts made up 13.3% of the dry mass on average and did not depend on the used manufacturing method. This value corresponds with a deviation of 1% to the results of Vieira et al. and Yamada et al., who investigated both Saccharomyces cerevisiae and Saccharomyces pastorianus strains [1, 42]. The total fat content of the yeast extracts produced using the cell mill and sonotrode were for both just 1.1%, and just 0.5% following autolysis. Following both mechanical disruption methods, the total content of free long-chain (C8–C18) fatty acids at 80 µg on average (with a variance of 10 µg) compared with autolysis at 5 µg (with a variance of 2 µg) was higher per g of dry mass (t test p value < 0.05) (data of long-chain fatty acids are not shown in detail). Therefore, using the cell mill and sonotrode, a larger proportion of fat-containing substances remained in the yeast extract. The insoluble and separated fraction in the autolytic yeast extract manufacture is called “hulls” or “ghosts” [49]. Adding this fraction to must fermentation (wine production) improves the yeast activity, for example, by releasing unsaturated fatty acids [57]. The proportion of fat-containing substances, of “hulls”, after mechanical production may differ compared with the autolytic process and should be investigated in more detail.
Vitamins
Brewing yeasts are commonly known as a vitamin B-rich foodstuff. In the human body, these vitamins catalyze key reactions in the amino acid and protein metabolism or have detoxifying or antioxidative functions [1]. Vieira et al. analyzed vitamin B contents for a yeast extract produced using a cell mill from a Saccharomyces pastorianus yeast and reported that their values differed from the results of other research papers and suggested that this was mainly due to the yeast extract production methods [1]. Based on the consistent yeast starting material for producing the yeast extract in the present study, the speculation about the impact of practical industrial processes (cell mill, sonotrode, and autolysis) was investigated on a small scale. Using the example of vitamin B9 (folic acid group) it was shown that not only the total folate content was influenced by the production method, but also the distribution of the various biologically active vitamers was impacted and could be determined using a method recently published by Striegel et al. [39]. The results of total folate content, as well as the vitamer distribution of the most abundant vitamers 5-CH3-H4folate, 5-CHO-H4folate, 10-CHO-PteGlu, H4folate, and PteGlu are shown in Table 3 and Fig. 2. Whereas the total folate contents of the yeast extracts after mechanical disruption methods were not significantly different (t test p value > 0.05), the total folate content after autolysis was significantly lower compared with sonotrode and cell mill (t test p value < 0.05). Moreover, the total folate content of the yeast extract after autolysis was significantly lower compared with the industrial product (t test p value < 0.05). The main vitamers were 5-CH3-H4folate and 5-CHO-H4folate, minor vitamers were H4folate, PteGlu, and 10-CHO-PteGlu. The yeast extracts produced by cell mill and sonotrode revealed to be very high in the biological active folate form 5-CH3-H4folate. However, a closer look into the vitamer distribution in the yeast extract after autolysis showed a large decrease in 5-CH3-H4folate and H4folate, compared to the yeast extract of the cell mill and sonotrode. The decrease of 5-CH3-H4folate after thermal autolysis can be explained by its degradation during thermal treatment [58]. Comparing our results with literature data, Hjortmo et al. analyzed 44 yeast strains and found substantial differences of total folate between 4000 and 14,500 µg/100 g dry matter [11]. Except for the yeast extract from the thermal autolysis, our results are at the lower end of this range. However, the latter author only reported the methylated vitamer 5-CH3-H4folate and H4folate, whereas we also detected 5-CHO-H4folate, 10-CHO-H4folate and PteGlu. Vieira et al. analyzed the nutritional value of brewer’s spent yeast extract and found a mean folate content of 3010 µg/100 g. However, they did not look at the vitamer distribution. With respect to our results, the yeast extract produced by thermal autolysis tremendously affected the folate content and therefore decreased the nutritional value of the yeast extract. For the other analyzed vitamins (B1, B2, B3, B5, B6), with the exception of biotin (B7) and cobalamin (B12), the autolytically produced yeast extracts also showed significantly lower values in each case (t test p value < 0.05) than the results of the two mechanically produced yeast extracts. It is likely that the high temperature (50 °C) combined with the long contact period (24 h) in autolysis also cause a partial degradation of the vitamins. No significant difference was determined between the sonotrode and cell mill disruption methods for vitamins B1, B2, B3, B5, B6 (t test p value > 0.05). The quantity of vitamin B3 after the cell mill production process (94.19 mg/100 g) was slightly higher than that reported by Vieira et al. (77.2 mg/100 g), though substantially higher than for Pinto et al. (0.79 mg/100 g) [1, 59]. However, for pyridoxine (B6) (4.86 mg/100 g), the value was slightly lower than that revealed by Pinto et al. (9.99 mg/100 g), and ten times lower than that reported by Vieira et al. (55.1 mg/100 g) [1, 59]. Vitamin B2 and B12 were not found by Vieira et al. [1]. Compared with the absolute contents given by Narziss for brewer’s yeasts (taking into account a cell wall amount of approximately 30%), the contents of all B vitamins of the mechanically produced yeast extracts were in his specified range. With the exception of vitamin B7 and B12, the methods using sonotrode and cell mill are preferred to ensure higher B vitamin contents in the yeast extract [6]. A simultaneous and complete release of all B vitamins, though, is almost impossible and requires an extraction method tailored to the relevant vitamin in each case and using enzyme combinations or acid hydrolysis [60]. The higher vitamin values (except for B12) of the autolytically produced industrial product compared with the autolytically produced yeast extract in this paper were presumably caused by higher vitamin starting values in the yeast, for example related to a different yeast strain and fermentation medium [11].
Minerals
All the disruption methods investigated here destroyed the yeast cell structure in different ways. For instance, the yeast cells in the cell mill disruption process were compressed by the dynamic of the glass pearls in the contact zones and mechanically sheared, which ultimately caused a large number of small individual cell fragments and released the entire cell content in this way [3]. Using the ultrasonic sonotrode also mechanical shear forces acted on the yeast cell surfaces as a result of the cavitation bubbles imploding. The cell wall and plasma membrane are thereby damaged to such an extent that the cytoplasm was released. The yeast cells displayed various fracture points [61]. However, the yeast cells were not completely crushed as was the case in the cell mill. In the performed autolysis, a multitude of various biochemical processes that occur simultaneously ultimately induced cell death based on the cell’s own autolytic enzymes, their activity being supported by the high temperature, the plasmolytically acting salt and the lipid solvent ethyl acetate. At the end of the process, the minimized dead yeast cell “hulls” or “ghosts”, remained [49, 56, 62]. Despite the different processes of cell destruction and yeast extract release described above, it is striking that the zinc content in the yeast extract did not differ significantly following the three disruption methods (ANOVA p value > 0.05). According to Nicola et al., the mineral zinc was bound at 30% in the yeast cell wall mannoprotein fractions and is present at 56% in the vacuole, at 5% in the cytosol and the rest in other organelles [10]. The total zinc content per 100 g yeast dry mass was 3.5 mg. The three methods released an average of 2.2 mg zinc per 100 g yeast extract dry mass (Table 4), which, for a cell wall proportion of approx. 30% [56], corresponds to a release rate of 63%. It is therefore likely that the three disruption methods used in this study only released the zinc from the vacuole and cytoplasm, while the zinc bound to the mannoprotein was separated with the cell walls via centrifugation. In addition, autolysis in which the yeast’s own enzymes take effect, does not appear to cause any additional dissolution of the zinc into the yeast extract. This claim is supported by the results of Gaudreau et al., who were able to increase the zinc content in yeast extract using cell wall-cleaving β-1,3-glucanase in autolysis [63]. The content of all the other analyzed minerals in the three yeast extracts produced in this study did not differ significantly either (Table 4). Consequently, the disruption method makes no difference to the release of the other minerals analyzed. Comparing the values determined here with data from Vieira et al. [1] and taking into account fluctuations in the mineral content of the yeast that relate to the yeast strain, fermentation medium and physiological condition of the yeast [64], the respective values are comparable. Only the zinc content reported by Vieira et al. is three times higher than the values determined in this study, and equivalent to that of the industrial product [1], which could be due to a zinc-enriched fermentation medium. The increased content of boron, cobalt, and strontium in the industrial product deviated from the investigated yeast extracts, the reasons for which are a matter of speculation. As Vieira et al. previously discussed the minerals chromium, molybdenum, zinc, iron, and manganese in a yeast extract produced via cell mill from spent brewer’s yeast, the yeast extracts produced via autolysis and sonotrode are also suitable as a nutritional supplement according to the standards of the National Academy of Science [1, 65].
DNA and RNA
DNA (deoxyribonucleic acid) is found in the cell nucleus and in the mitochondria. In transcription, the information stored in the individual DNA genes are transferred to mRNA (messenger ribonucleic acid) as required for translation (protein biosynthesis). If the yeast is fed into an autolytic process, the yeast’s own enzymes cause the RNA to degrade and produce, for instance, taste-active substances referred to as 5′-nucleotides which can be perceived as an umami taste [12]. The chemical formation and the process engineering extraction of the 5′-nucleotides have already been discussed in detail. Vieira et al. described the influence of various hydrolytic processes on the nucleotide composition of a yeast extract previously obtained using a cell mill, as well as the impact of the yeast starting material [12]. Zhao et al. investigated the impact of manufacturing parameters such as pH and temperature on the RNA-degradation products during autolysis [66]. In the yeast extracts produced in different ways in this study, only the total RNA content was determined photometrically and the extensive differentiation of the degradation products was omitted as these have already been sufficiently discussed by Vieira et al. and Zhao et al. Total RNA in the yeast extracts amounted to an average of 50 mg/g dry mass for the two mechanical processes, and 48.84 mg/g of the dry mass following autolysis. Based on statistical variations in the production processes, this difference was not significant (t test p value > 0.05). For a yeast extract obtained using a cell mill, Vieira et al. determined a total RNA content of 4% [1]. Additional hydrolytic treatment produced values between 5.06 and 5.93% [12]. Following autolysis (50 °C, pH 7), Zhao et al. identified a degradation of the total RNA into ribonucleotides (88.8%), ribonucleosides (8.8%), bases (1.4%) and oligonucleotides (1%) in a yeast extract [66]. The total RNA content of the industrial product investigated in this study was 5.5% (55.18 mg/g yeast extract).
If the yeast extract is used as a culture medium to enrich microorganisms from which DNA should be subsequently extracted, DNA fragments from the original yeast extract culture medium may contaminate the extracted target DNA in any PCR analysis. It is also interesting to what extent the DNA from the cell nucleus is released and present in the yeast extract, compared with the DNA from the mitochondria, as a result of using the different disruption methods. The reason for this is, for example, that specific genes need to be present and extracted in advance to identify Saccharomyces cerevisiae (top-fermenting beer yeasts) in the chromosomes of the mitochondria and of the cell nucleus. In his study, Hutzler developed methods to identify and differentiate yeasts (relevant for beverages), which were primarily based on detecting certain DNA target sequences using PCR systems (polymerase chain reaction) [22]. To detect the industrially used yeast species Saccharomyces cerevisiae and Saccharomyces pastorianus (lager yeast strain), one option is a Real-Time PCR system, which uses the gene GRC3 (protein encoding, SGD name: YLL035W) as a target region, which is on chromosome XII of the cell nucleus. The PCR system GRC3 is specific for the species Saccharomyces cerevisiae and Saccharomyces pastorianus. Another option is a Real-Time PCR system that uses gene COX2 (gene encoding for cytochrome c oxidase subunit II, SGD name: Q0250) from the mitochondrial DNA as a target region [22]. The PCR system COX2 is specific for the species Saccharomyces cerevisiae. During real-time PCR, PCR-products were amplified using specific primers and fluorescent probes, and the amplification curve intersects at a defined fluorescence threshold, which is described as a Ct-value (threshold cycle number) and is indirectly proportional to the starting copy number of the DNA target sequence [22]. If the Ct-values of the disruption methods are compared (Fig. 3), the Ct-values differ significantly in both the PCR systems GRC3 and the COX2 of the two mechanically produced yeast extracts compared with the autolysis Ct-value. No significant differences were detected between cell mill and sonotrode. As the Ct-value for the mechanically produced yeast extracts was lower than that for the autolytically obtained yeast extract, a larger starting quantity of DNA must therefore have been present in the mechanically produced yeast extract. In contrast, a portion of the DNA appeared to have been lost during autolysis, which presumably may be due to the degradation of the DNA by DNases. Using published PCR efficiencies for the PCR systems GRC3 (86.9%) and COX2 (88.5%) a \({1869^{(~{\text{C}}{{\text{t}}_{{\text{autolysis}}}} - {\text{C}}{{\text{t}}_{{\text{cell}}\,{\text{mill}}\,{\text{or}}\,{\text{sonotrode}}}})}}\) fold higher target DNA content can be shown for GRC3 and a \({1885^{(~{\text{C}}{{\text{t}}_{{\text{autolysis}}}} - {\text{C}}{{\text{t}}_{{\text{cell}}\,{\text{mill}}\,{\text{or}}\,{\text{sonotrode}}}})}}\) fold higher target DNA content can be shown for COX2 according to calculations of Hutzler [22]. As a result, there was a 418-fold or 357-fold higher target DNA content of the cell nucleus (GRC3) and a 477-fold or 600-fold higher target mitochondrial (COX2) DNA content in the yeast extract after cell mill or sonotrode, respectively, in contrast to autolysis. Comparing the total DNA content of the yeast extracts after the different disruption methods using the NanoDrop method (spectrophotometrical determination) significantly more was measured in the mechanically produced yeast extracts, too. An average of 20.56 mg DNA per gram yeast extract could be released following disruption with the cell mill. For the sonotrode method and after autolysis, the figures were 19.67 mg/g and 2.30 mg/g, respectively. Zhao et al. previously stated degradation of the total DNA during autolysis [66]. The total DNA content in an intact yeast cell is given as 0.1–1.5% of the cell dry mass [66]. At a cell wall content of approx. 30% [56], virtually the total DNA content was released from the cell in both the mechanical processes. The low Ct-values of the PCR systems COX2 and GRC3 for both mechanical methods and therefore the high DNA release rates show that non-fragmented DNA (PCR product length) from the nucleus and DNA from the mitochondria are in higher concentrations in the yeast extract compared to that manufactured using the autolysis disruption method.
Glutathione, polyphenol content, and antioxidative potential
In addition to enzymatic components of yeast such as superperoxide dismutase, catalase or glutathione reductase, the antioxidative potential of the yeast can be attributed in particular to various non-enzymatic antioxidants such as vitamins, glutathione, mineral ions, phenolic constituents or sulfurous amino acids [67]. For a homogenate of Saccharomyces cerevisiae, 85–95% of the radical scavenging activity is based on a thermostable non-enzymatic fraction [67]. Vieira et al. were able to increase the antioxidative potential of the yeast extract produced via cell mill by heating, whereby they determined 6 h and 40 °C to be the optimum processing parameters using response surface methodology (RSM) and traced this back to the release of bound phenolic components [68]. For longer exposure times under higher temperatures however, a negative quadratic effect was detected in the contour plots of the RSM model for the antioxidative potential. As a result of this, the antioxidative potential was further reduced, presumably due to degradation of various antioxidative substances such as vitamins, phenolic components, amino acids and bioactive peptides [68]. This process might also have occurred in our study as the autolytically produced extracts in this paper were exposed to a temperature of 50 °C for 24 h and have correspondingly lower values for the antioxidative potential than the yeast extracts using the cell mill or sonotrode (t test p value < 0.05) (Fig. 4). The quantity of the bioactive peptide glutathione and the total polyphenol content was significantly reduced for the autolytically generated yeast extract compared with the mechanically produced extract (t test, p value < 0.05). Given a consistent starting yeast, this is likely to have been caused by enzymatic degradation. The increased release of sulfurous amino acids in autolysis presumably only supplied a small proportion of the total antioxidative potential as, according to Jacob et al., these are present to a greater extent in a yeast extract produced autolytically than mechanically (cell mill, sonotrode) [15]. The mechanically (vortexer) produced yeast extract in the studies by Vieira et al. displayed, according to the determination method (FRAP, DPPH, ferricyanide reducing power), an antioxidative activity of 1.3–2.6 mg TE/g dry mass and was approximately doubled following autolytic optimization [1, 68]. These values therefore are around ten times lower than the values determined in this study. The various yeast washing methods before the extraction process might explain the differences in the antioxidative activity of the yeast extracts. Vieira et al. debittered the yeast cells three times with a phosphate buffer (pH 7) and the total polyphenol content was three times lower than in our study [1, 68]. The reduction potential of the yeast extract was also tested along with the antioxidative potential. In each case, both potentials displayed a significantly positive correlation (R2 = 0.968).
Polyphenolic compounds, glutathione, antioxidative potential, and reduction potential in yeast extract according to yeast extraction method (dotted lines between data points only for graphical support); significant differences between cell mill vs. sonotrode vs. autolysis (p value ANOVA < 0.01) for all tested components; for results of the pairwise t test, see text
Summary
In conclusion, the release of the functional substance groups investigated in this study was dependent upon the performed disruption method (cell mill, sonotrode, and autolysis). The protein content (as not hydrolyzed in free amino acids) and the trehalose, fat, B vitamin and DNA contents were significantly lower following autolysis compared with the mechanical process. The antioxidative and reduction potential was also significantly lower following autolysis as a result of the degradation of polyphenols and glutathione. The mineral, RNA, glycogen, glucose, fructose and ash contents did not differ significantly. Therefore, the two mechanical methods offer a good alternative to transfer especially valuable nutritional substance groups to the yeast extract with low degradation rates compared with the autolytic methods. The spent yeast Saccharomyces cerevisiae TUM 68 presented a suitable starting product to produce the yeast extract using the top-fermenting fermentation process performed in this study. To what extent the variation of fermentation medium and parameters during beer manufacture influences the potential of the spent yeast as a starting product for producing yeast extract will be addressed in further studies. The potential of the cell wall constituents separated from the yeast extract and referred to as “hulls” should also be evaluated.
References
Vieira F, Carvalho J, Pinto E, Cunha S, Almeida A, Ferreira I (2016) Nutritive value, antioxidant activity and phenolic compounds profile of brewer’s spent yeast extract. J Food Compos Anal 52:44–51
Sommer R (1998) Yeast extracts: production, properties and components. Food Aust 50(4):181–183
Liu D, Ding L, Sun J, Boussetta N, Vorobiev E (2016) Yeast cell disruption strategies for recovery of intracellular bio-active compounds—a review. Innov Food Sci Emerg Technol 36:181–192
Apar DK, Özbek B (2008) Protein releasing kinetics of bakers’ yeast cells by ultrasound. Chem Biochem Eng Q 22(1):113–118
Bystryak S, Santockyte R, Peshkovsky AS (2015) Cell disruption of S. cerevisiae by scalable high-intensity ultrasound. Biochem Eng J 99:99–106
Narziß L, Back W, Gastl M, Zarnkow M (2017) Abriss der Bierbrauerei, 8. vollständig überarbeitete und erweiterte Auflage. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
Quain DE (2006) Yeast supply and propagation in brewing. In: Brewing: new technologies. Woodhead, Cambridge, pp 167–182
Berlowska J, Dudkiewicz-Kołodziejska M, Pawlikowska E, Pielech-Przybylska K, Balcerek M, Czysowska A, Kregiel D (2017) Utilization of post-fermentation yeasts for yeast extract production by autolysis: the effect of yeast strain and saponin from Quillaja saponaria. J Inst Brew 123:396–401
Martinezforce E, Benitez T (1995) Effects of varying media, temperature, and growth-rates on the intracellular concentrations of yeast amino-acids. Biotechnol Prog 11(4):386–392
De Nicola R, Hall N, Melville S, Walker G (2009) Influence of zinc on distiller’s yeast: cellular accumulation of zinc and impact on spirit congeners. J Inst Brew 115(3):265–271
Hjortmo S, Patring J, Jastrebova J, Andlid T (2005) Inherent biodiversity of folate content and composition in yeasts. Trends Food Sci Technol 16(6–7):311–316
Vieira E, Brandão T, Ferreira IMPLVO (2013) Evaluation of Brewer’s spent yeast to produce flavor enhancer nucleotides: influence of serial repitching. J Agric Food Chem 61:8724–8729
Alexandre H (2011) 2.45 autolysis of yeasts. Compr Biotechnol (Second Ed) 2:641–649
Kieliszek M, Kot AM, Bzducha-Wrobel A, Blazejak S, Gientka I, Kurcz A (2017) Biotechnological use of Candida yeasts in the food industry: a review. Fungal Biol Rev 31(4):185–198
Jacob FF, Hutzler M, Methner F-J (2019) Comparison of various industrially applicable disruption methods to produce yeast extract using spent yeast from top-fermenting beer production: influence on amino acid and protein content. Eur Food Res Technol 245(1):95–109
Ingledew WM, Sosulski FW, Magnus CA (1986) An assessment of yeast foods and their utility in brewing and enology. Am Soc Brew Chem 44(4):166–170
López-Solís R, Duarte-Venegas C, Meza-Candia M, Barrio-Galán R, Peña-Neira A, Medel-Marabolí M, Obreque-Slier E (2017) Great diversity among commercial inactive dry-yeast based products. Food Chem 219:282–289
Spearman M, Chan S, Jung V, Kowbel V, Mendoza M, Miranda V, Butler M (2016) Components of yeast (Sacchromyces cervisiae) extract as defined media additives that support the growth and productivity of CHO cells. J Biotechnol 233:129–142
Zhang J, Reddy J, Buckland B, Greasham R (2003) Toward consistent and productive complex media for industrial fermentations: studies on yeast extract for a recombinant yeast fermentation process. Biotechnol Bioeng 82(6):640–652
Research Center Weihenstephan (2018) Stammbeschreibung LeoBavaricus—TUM 68® Saccharomyces cerevisiae, obergärige Weizenbierhefe. http://www.blq-weihenstephan.de/tum-hefen/hefen-und-bakterien.html. Accessed 1 Apr 2018
Pfenninger H (1996) MEBAK Brautechnische Analysemethoden Band 3 Selbstverlag der MEBAK, Freising
Hutzler M (2009) Entwicklung und Optimierung von Methoden zur Identifizierung und Differenzierung von getränkerelevanten Hefen. Technischen Universität, München
Back W (2008) Ausgewählte Kapitel der Brauereitechnologie. Fachverlag Hans Carl GmbH, Nürnberg
Biotecon Diagnostics (2018) Foodproof beer screening kit. https://www.bc-diagnostics.com/products/kits/real-time-pcr/spoilage-organisms/foodproof-beer-screening-kit/. Accessed 1 Apr 2018
Bronn WK (1996) Hefe und Hefeextrakte. In: Heiss R (ed) Lebensmitteltechnologie. Biotechnologische, chemische, mechanische, und thermische Verfahren der Lebensmittelverarbeitung. Springer, Berlin, pp 336–343
Jazwinski SM (1990) Preparation of extracts from yeast. Methods Enzymol 182:154–174
Kim KS, Yun HS (2006) Production of soluble beta-glucan from the cell wall of Saccharomyces cerevisiae. Enzyme Microbial Technol 39:496–500
Münch P (1999) Aromastoffe in thermisch behandelten Hefeextrakten. Verlag Dr. Hut, München
Podpora B, Świderski F, Sadowska A, Piotrowska A, Rakowska R (2015) Spent Brewer’s yeast autolysates as a new and valuable component of functional food and dietary supplements. J Food Process Technol. https://doi.org/10.4172/2157-7110.1000526
Bradford MM (1976) A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Jacob F (2012) MEBAK Brautechnische Analysemethoden Würze Bier Biermischgetränke. Selbstverlag der MEBAK, Freising-Weihenstephan
Naumann C, Bassler R, Seibold R, Barth C (1976) Methodenbuch Band 3, die chemische Untersuchung von Futtermitteln. VDLUFA-Verlag, Darmstadt
Schulze U, Larsen ME, Villadsen J (1995) Determination of intracellular trehalose and glycogen in Saccharomyces cerevisiae. Anal Biochem 228(1):143–149
Mahmud SA, Nagahisa K, Hirasawa T, Yoshikawa K, Ashitani K, Shimizu H (2009) Effect of trehalose accumulation on response to saline stress in Saccharomyces cerevisiae. Yeast 26(1):17–30
Anger M (2006) Brautechnische Analysenmethoden Rohstoffe. Selbstverlag der MEBAK, Freising-Weihenstpahn
Beuth Verlag GmbH (2018) DIN-Normen. https://www.beuth.de. Accessed 1 Apr 2018
AOAC International (2018) Official methods of analysis of AOAC International. http://www.aoac.org. Accessed 1 Apr 2018
Mücke D (1957) Einführung in Mikrobiologische Bestimmungsverfahren. Quantitative Bestimmung von Aminosäuren und Vitaminen des B-Komplexes. Thieme Verlag, Leipzig
Striegel L, Chebib S, Netzel ME, Rychlik M (2018) Improved stable isotope dilution assay for dietary folates using LC-MS/MS and its application to strawberries. Front Chem 6:11–11
Ortiz-Julien A (2012) Method for preventing defective ageing of white wines. United States of Amercia Patent
Sigma-Aldrich (2018) Antioxidant Assay Kit. https://www.sigmaaldrich.com/catalog/product/sigma/cs0790?lang=de®ion=DE&gclid=EAIaIQobChMI6rTnrqKr3AIVi7gbCh1YwgLTEAAYASAAEgJKQPD_BwE. Accessed 1 Apr 2018
Yamada EA, Sgarbieri VC (2005) Yeast (Saccharomyces cerevisiae) protein concentrate: Preparation, chemical composition, and nutritional and functional properties. J Agric Food Chem 53(10):3931–3936
Amorim M, Pereira JO, Gomes D, Pereira CD, Pinheiro H, Pintado M (2016) Nutritional ingredients from spent brewer’s yeast obtained by hydrolysis and selective membrane filtration integrated in a pilot process. J Food Eng 185:42–47
Ihl M, Tagle MA (1974) Estimation of protein in yeast. J Sci Food Agric 25(5):461–464
Lei H, Zheng L, Zhao H, Zhao M (2013) Effects of worts treated with proteases on the assimilation of free amino acids and fermentation performance of lager yeast. Int J Food Microbiol 161:76–83
G-Biosciences (2018) Protein assays technical guide and handbook. https://info2.gbiosciences.com/complete-protein-assay-guide. Accessed 1 Apr 2018
Kieliszek M, Błażejak S, Bzducha-Wróbel A, Kot AM (2019) Effect of selenium on lipid and amino acid metabolism in yeast cells. Biol Trace Elem Res 187:316–327
Chae HJ, Joo H, In MJ (2001) Utilization of brewer’s yeast cells for the production of food-grade yeast extract. Part 1: effects of different enzymatic treatments on solid and protein recovery and flavour characteristics. Biores Technol 76(3):253–258
Bokulich NA, Bamforth CW (eds) (2017) Brewing microbiology current research, omics and microbial ecology. Caister Academic Press, Norfolk
Annemüller G, Manger HJ (2013) Gärung und Reifung des Bieres 2. überarbeitete Auflage 2013 edn. VLB Berlin, Berlin
Higashiyama T (2002) Novel functions and applications of trehalose. Pure Appl Chem 74(7):1263–1269
Parrou JL, Teste MA, Francois J (1997) Effects of various types of stress on the metabolism of reserve carbohydrates in Saccharomyces cerevisiae: Genetic evidence for a stress-induced recycling of glycogen and trehalose. Microbiol Uk 143:1891–1900
Yoshikawa Y, Matsumoto K, Nagata K, Sato T (1994) Extraction of trehalose from thermally-treated bakers-yeast. Biosci Biotechnol Biochem 58(7):1226–1230
Jin Y, Wang M, Lin SY, Guo Y, Liu JB, Yin YG (2011) Optimization of extraction parameters for trehalose from beer waste brewing yeast treated by high-intensity pulsed electric fields (PEF). Afr J Biotechnol 10(82):19144–19152
Pastinen O, Nyyssola A, Pihlajaniemi V, Sipponen MH (2017) Fractionation process for the protective isolation of ergosterol and trehalose from microbial biomass. Process Biochem 58:217–223
Walker GM (1998) Yeast physiology and biotechnology. Wiley, Chichester
Munoz E, Ingledew WM (1989) Effect of yeast hulls on stuck and sluggish wine fermentations—importance of the lipid component. Appl Environ Microbiol 55(6):1560–1564
Nguyen MT, Hendrickx M (2003) Model studies on the stability of folic acid and 5-methyltetrahydrofolic acid degradation during thermal treatment in combination with high hydrostatic pressure. J Agric Food Chem 51(11):3352–3357
Pinto L, Lopes M, Carvalho Filho C, Alves L, Benevides C (2013) Determinação do valor nutritivo de derivados de levedura de cervejaria (Saccharomyces spp.). Revista Brasileira de Produtos Agroindustriais 15(1):7–17
Halvin K, Paalme T, Nisamedtinov I (2013) Comparison of different extraction methods for simultaneous determination of B complex vitamins in nutritional yeast using LC/MS-TOF and stable isotope dilution assay. Anal Bioanal Chem 405(4):1213–1222
Bermudez-Aguirre D (ed) (2017) Ultrasound advances in food processing and preservation. Elsevier Science, London
Middelberg APJ (1995) Process-scale disruption of microorganisms. Biotechnol Adv 13(3):491–551
Gaudreau H, Conway J, Champagne CP (2001) Production of zinc-enriched yeast extracts. J Food Sci Technol Mysore 38(4):348–351
Aleksander P, Piotr A, Tadeusz T, Makarewicz M (2009) Accumulation and release of metal ions by Brewer’s yeast during successive fermentations. J Inst Brew 115(1):78–83
National Academies of Sciences Engineering Medicine (2011) Dietary reference intakes tables and application. http://nationalacademies.org/hmd/Activities/Nutrition/SummaryDRIs/DRI-Tables.aspx. Accessed 23 Jan 2019
Zhao J, Fleet GH (2005) Degradation of RNA during the autolysis of Saccharomyces cerevisiae produces predominantly ribonucleotides. J Ind Microbiol Biotechnol 32(9):415–423
Santiago LA, Mori A (1993) Antioxidant defenses of bakers-yeast against free-radicals and lipid peroxides in rat-brain. Arch Biochem Biophys 306(1):16–21
Vieira EF, Melo A, Ferreira IMPLVO (2017) Autolysis of intracellular content of Brewer’s spent yeast to maximize ACE-inhibitory and antioxidant activities. LWT Food Sci Technol 82:255–259
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Friedrich Felix Jacob, Lisa Striegel, Michael Rychlik, Mathias Hutzler and Frank-Jürgen Methner declare that they have no conflict of interest.
Compliance with ethics requirements
The authors Friedrich Felix Jacob, Lisa Striegel, Michael Rychlik, Mathias Hutzler and Frank-Jürgen Methner hereby confirm that this manuscript is performed according and follows the COPE guidelines and has not already been published nor is it under consideration for publication elsewhere. This article does not contain any studies with human or animal subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jacob, F.F., Striegel, L., Rychlik, M. et al. Yeast extract production using spent yeast from beer manufacture: influence of industrially applicable disruption methods on selected substance groups with biotechnological relevance. Eur Food Res Technol 245, 1169–1182 (2019). https://doi.org/10.1007/s00217-019-03237-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-019-03237-9