Abstract
Development of new strategies to add-value to agro-industrial by-products are of environmental and economical importance. Innovative and low-cost sources of protein and bioactive peptides have been explored worldwide. Spent brewer’s yeast (SBY) is the second most relevant by-product from the brewing industry, and despite its nutritional (about 50% protein, dry weight) and technological potential, it is still underused or needs to be disposed of. SBY cells need to be disrupted to release intracellular and cell wall proteins. This procedure has been performed using autolysis, glass bead milling, enzymatic hydrolysis and ultrasound processing. Enzymatic treatment is usually performed without prior purification and is a challenging process, which involves multiple factors, but has been successfully used as a strategy to add value to agro-industrial by-products. Scope and approach: in this review, we particularly focused on enzymatic hydrolysis as a strategy to promote SBY valorisation, illustrating the state-of-the-art processes used to produce protein extracts from this material as well as exploring fundamental concepts related to the particularities of yeast cell disruption and protein hydrolysis. Furthermore, innovative applications of value-added yeast by-products in food, biotechnological and pharmaceutical industries are presented and discussed. Key findings and conclusions: the discovery of valuable compounds found in spent yeasts as well as the development of new processing methodologies have been widening the possibilities of reuse and transformation of SBY as an ingredient and innovative matrix. Once released, yeast proteins and peptides may be applied as an innovative non-animal protein source or a functional and bioactive ingredient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Beer by-products into perspective: general aspects
Beer is one of the most consumed beverages in the world, with a global production of approximately 1.9 billion hL in 2018. The world’s beer market is growing slowly (about 1.4% in 2018), mostly represented by an increase in consumption reported in China. China, Europe and America play an important role in both production and consumption of beer. Brazil is one of the world’s largest beer producers and consumers, representing the third world’s largest beer market (Ziener and McNally 2019). According to the World Health Organization (2014), in 2010, beer represented 60% of Brazil’s alcohol consumption.
Beer is a beverage consisting essentially of barley malt, water, hops and yeasts. Barley might be partially replaced with unmalted cereals such as corn, rice, wheat, oats or sorghum, called adjuncts. This procedure is adopted because of either economic reasons (as in the case of corn) or the intention to produce beers with distinctive organoleptic characteristics (such as wheat, necessary in Weiss-type beers) (Ambrosi et al. 2014; Mussatto 2009).
Briefly, the brewing process is made up of 10 steps. Figure 1 shows a general scheme of the brewing process and the steps in which the main by-products are formed (Mussatto 2009). During milling, malt is ground to make its particles accessible to water. Next, mashing and lautering are performed when water is heated (78 \(^{\circ }\)C), activating endogenous malt enzymes. These enzymes start the process of hydrolysis, particularly that of complex carbohydrates. Grain husks form a natural filtration bed that promotes the separation of wort from solids. Finally, to complete the extraction of sugars, the grains are washed with hot water (78 \(^{\circ }\)C) until the filtrate has about 2% solid concentration, resulting in the brewer’s wort. At this stage, the solid by-product brewers’ spent grain (BSG) is generated, and it is the largest amount of waste generated from beer processing (140–200 g L−1). Then, the boiling step begins; the wort is boiled for a period of approximately 90 min and the hops are added. During this step, the wort is concentrated and sterilized, the enzymes are inactivated, the hop compounds are extracted and some proteins coagulate. In the whirlpool stage, some aggregated proteins, hops and other solids are separated. In this phase, residual hops (hot trub) are formed in a ratio of 1 to 4 g L−1. The wort is then cooled down to prevent oxidation when fermentation conditions are reached and yeasts are added, which initiates the fermentation process. Sugars are converted to ethyl alcohol and carbon dioxide and several other secondary compounds are produced. This step usually takes 3 to 15 days to be completed, depending on beer type. Some of the yeasts and other particles settle at the bottom of the fermentation tank or float to the surface, forming the second largest residue produced, the spent brewer’s yeast (SBY) in the ratio of 1.7 to 2.3 g L−1 (Ferreira et al. 2010; Kumar and Chandrasekaran 2016; Pinto et al. 2015). Finally, the “green” beer goes on to maturation. This step occurs at low temperatures, allowing flavour development and insolubilisation/deposition of proteins and polyphenols. Turbidity precursors are separated from the raw beer, which is then filtered, pasteurized and bottled (Mathias et al. 2015; Ambrosi et al. 2014; Huang et al. 2012).

Adapted from Mussatto (2009)
Schematic representation of beer processing and main by-products generated.
Among the three by-products, BSG is the most abundant side stream from the brewing industry. Most of the spent grain generated is reused either as animal feed, compost, fertiliser or sent to landfills. This by-product is rich in polysaccharides, is considered as a source of fermentable sugars, and contains a higher amount of proteins than many similar agro-industrial residues. Therefore, it is a potential ingredient for protein production (Ravindran et al. 2019; Qin et al. 2018). Indeed, a number of studies have investigated more efficient protein recovery methods from BSG and a number of applications have been reported, such as value-added protein-rich and carbohydrates-rich ingredients (Qin et al. 2018). Recently, the vermicomposting of BSG by Eisenia fetida was studied in order to convert this residue into a soil conditioner (Budroni et al. 2020; Saba et al. 2019). On the other hand, SBY and hot trub are still mostly underutilized or discarded because there are limited processes available that can handle their higher complexity and particularities. There is an unexplored potential to find new applications to them, which could reduce the disposal amount of such by-products, thus decreasing their environmental impact. Transforming them into value-added ingredients depends on the development of insightful, sustainable and economically-viable processing technologies that take into account their characteristics and constraints.
Although recycling of brewing yeasts in a new fermentation cycle (in a process known as “repitching”) is a common practice, the number of reuses is limited to maintain beverage quality. Excessive repitching can result in negative effects on fermentation performance and on sensorial profile, accumulation of haze-causing substances and undesirable secondary fermentation compounds. The number of times a specific culture can be repitched depends on the brewing fitness of the yeast population: their physiological state and fermentation performance in the previously defined brewing conditions. Measurement of yeast viability before fermentation can be checked to determine if further repitching is advised (Kalayu 2019). Even when repitching practices are performed, a great amount of SBY (a low-value brewing by-product with high organic load) is produced.
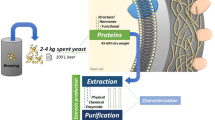
SBY is a low-cost, poorly reused by-product of nutrient-rich composition available in large amounts that could be used in more noble applications. This would reduce waste disposal, help to promote a sustainable economy and decrease the environmental impact of beer production. Thus, the aim of this review is to present the up-to-date applications for SBY and the related processing technologies that could turn SBY into value-added products and ingredients.
Potential of spent yeast as an alternative source of protein
Nutritional composition
SBY slurry is a moist (85–97%), organic-matter-rich residue, with a high chemical oxygen demand (COD, 1308 mg g−1) (Mathias et al. 2015). The final pH value of SBY is approximately 5.9 i.e., it is higher than the pH value of beer (between 4.2 and 4.5) (Mathias et al. 2015). Table 1 shows the ranges of macronutrients of both non-treated SBY (collected from the brewing industry) and yeast extracts, obtained after processing. SBY is rich in carbohydrates and proteins (40–50% dry weight, each) with lower amounts of ash, fibers, ribonucleic acids (RNAs) and lipids (Mathias et al. 2015; Mussatto 2009; Caballero-Córdoba and Sgarbieri 2000). After processing, protein, ash and free amino nitrogen contents are usually increased.
Tables 2 and 3, respectively, show the amino acid profile and the detailed content of vitamins and minerals of the yeast extracts. Spent yeast from brewing processes is reported as an excellent source of proteins with high biological value proteins with a well-balanced amino acids profile that meets Food and Agriculture Organization of the United Nations (FAO) and World Health Organization (WHO) recommendations (Jacob et al. 2019a). In SBY, acidic aminoacids (glutamic acid and aspartic acid) and the essential amino acids leucine and lysine are the most abundant, while sulfur-containing amino acids, such as methionine and cysteine, are the least (Jung et al. 2012, 2011; Lee et al. 2009; Pacheco et al. 1997; Halász and Lásztity 1991b). Amino acid composition may vary greatly with processing (Table 2). Spent yeasts contain significant amounts of tyramine, a derivative of tyrosine metabolism (Jach et al. 2015). With a high mineral content (up to 8.5%, d.w.), SBY contains relevant amounts of zinc and selenium with magnesium, potassium and phosphorus being the most abundant. Complex B vitamins are also present in important quantities (Huige 2006; Lewis and Young 2001). Less than 4% of yeast composition consists of lipids. Saturated fatty acids account for more than half of the lipidic composition of SBY, followed by monounsaturated and then polyunsaturated fatty acids (Caballero-Córdoba et al. 1997).
Chemical and nutritional composition of SBY is affected by its biodiversity: strain, operating conditions (of fermentation and the overall beer processing), how many times the yeast is reutilized (repitching), when it is collected and how the yeast extract is produced. The main yeast strains used for beer production are Saccharomyces cerevisiae and Saccharomyces pastorianus, but several non-Saccharomyces strains are also available. Recently, Jacob et al. (2019b) reported that the composition of yeast extracts was affected by different yeast strains (S. cerevisiae, S. pastorianus, Saccharomycodes ludwigii, Saccharomycopsis fibuligera, Brettanomyces bruxellensis and Torulaspora delbrueckii) for beers produced under the same conditions . Antioxidant properties were also dependent on which spent yeast was used in the brewing process. The influence of yeast strain on other biological or functional properties was not reported. Other recent studies assessed the effect of repitching, yeast strain and rupturing/extraction methods on final product composition (Marson et al. 2019; Mathias et al. 2015; Vieira et al. 2013). Therefore, the manufacturing industry needs to be robust enough to be able to take into account all these variations that are inherent in the brewing process (Jacob et al. 2019b; Marson et al. 2019).
Spent yeasts from alcohol distilleries (S. cerevisiae) can also be a potential by-product to process since they are also produced in large quantities in Brazil and in other ethanol-producing countries. They have a very similar overall composition but with higher RNA, ash and lipid contents. Those differences are due to the strain and fermentation conditions in use, which are different from those of brewing (Steckelberg et al. 2013; Yamada et al. 2010).
Challenges facing yeast-products processing
Saccharomyces cerevisiae extracts, including SBY, are considered safe (generally recognized as safe, GRAS; Jung et al. 2010; Chae et al. 2001). However, toxicity of yeast extracts and products depends on the method of protein extraction and processing. Some evidence of liver toxicity has been found for an yeast protein concentrate prepared with sodium perchlorate (Caballero-Córdoba and Sgarbieri 2000). On the other hand, yeast hydrolysate obtained from cultivated S. cerevisiae IFO 2346 cells hydrolysed with bromelain was investigated as a supplement for rats, but it showed no evidence of toxicity. Previous research has been conducted on both acute (single oral dose of 5000 mg kg\(^{{{ - }{\text{1}}}}\)) and subacute (dose of 1000 mg kg\(^{{{ - }{\text{1}}}}\) day\(^{{{ - }{\text{1}}}}\), for 14 days) toxicity of yeast hydrolysate samples with molecular weight fractions of 10–30 kg mol\(^{{{ - }{\text{1}}}}\) (Jung et al. 2010). Vieira et al. (2016c) also reported no cytotoxic effects of SBY autolysates after exposure in Caco-2 cells, in a concentration range of 0.5–3.0 mg\({}_{{{\text {peptides}}}}\) mL\(^{{{ - }{\text{1}}}}\). Chronic toxicity studies are still needed to evaluate further effects.
Some studies on the virulence of S. cerevisiae reported that, in high-risk immuno-compromised or critically ill patients (in intensive care, with intravascular catheters and in previous antibiotic therapy), some strains have the ability to translocate across the gastrointestinal mucosa and progress to an infection (Enache-Angoulvant and Hennequin 2005). Although invasive Saccharomyces sp. infections remain rare, workers that handle viable yeast cells (winemakers, bakers, pharmaceutical industry workers, researchers, etc.) should follow proper hygiene practices (Posteraro et al. 2018). In processed yeast products, after cell disruption or degradation, cells are not viable anymore, and the risk of opportunistic infection does not exist.
The inclusion of yeast in food products is usually limited by the high amount of nucleic acids (7–12% dry weight) present, mainly ribonucleic acid, which in humans is metabolized to uric acid and may progress to kidney stones or gout (Rajendran 2012; Huige 2006; Caballero-Córdoba and Sgarbieri 2000; Halász and Lásztity 1991b). The total content of nucleic acids in yeast products should be reduced to a final concentration of 1–3% (dry weight) before they can be used without the risk of increasing uric acid levels in blood and tissues in humans (Abou-Zeid et al. 1995). Nucleic acid content is higher when protein content and yeast growth rate are higher (Mathias et al. 2015; Vieira et al. 2013). In this context, processing is of great importance. After processing, nucleic acid content can be reduced by the action of enzymes, precipitation or formation of complexes with other molecules (Halász and Lásztity 1991b). Also, after hydrolysis by RNAses, RNA can be converted into flavouring molecules (Ferreira et al. 2010; Tangüler and Erten 2008; Huige 2006; Halász and Lásztity 1991b). Other processes, such as fractionation and purification by membranes or chromatography techniques, can promote the reduction and separation of nucleic acids from the protein-rich fraction (Marson et al. 2020).
Processing of SBY
SBY is essentially constituted of yeast cells. It is a perishable by-product that requires proper hygiene standards and practices during brewing, as well as handling and storage prior to yeast extract production. Contamination of spent yeasts by bacteria or other microorganisms has been reported (Barrette et al. 1999). The first step in yeast processing is to stabilize the material (Marson et al. 2020). Thermal treatments are the most commonly used, even though they might cause up to 58% losses in vitamin B2 content and 23% losses in vitamin B1 content, depending on heating conditions (Varga and Maráz 2002).
In addition to high nucleic acid content, which limits the use of yeast as food, yeast cells may also have low digestibility and a bitter taste when not processed. The thick cell walls of yeasts are resistant to digestive enzymes (Vilela et al. 2000). Such cell walls give yeast physical protection and elasticity, allowing SBY cells to maintain their shape. The cell wall consists predominantly of polysaccharides (85%, dry weight) and proteins (5 to 15%, dry weight) (Harrison 2011; Halász and Lásztity 1991b). Mannoproteins are glycosylated proteins found in the yeast cell wall, being responsible for the permeability and porosity of the cell, while \(\beta\)-glucans and chitin promote its mechanical rigidity (Paramera et al. 2014). Although glucose and mannose are the major components of the polysaccharide fraction, N-acetyl glucosamine is also found in small amounts (Halász and Lásztity 1991b). Thus, cell disruption is essential for the extraction of intracellular components and the proteins of the cell wall itself (Middelberg 1995).
Because spent yeasts from brewing have a bitter aroma when commercially produced as an extract or ingredient in foods, they can be subjected to a pretreatment called “debittering” to remove unwanted resins and tannins (In et al. 2005; Nand 1987) such as humulones and isohumulones (Shotipruk et al. 2005). These compounds, originally present in hops, are adsorbed on the yeast cell wall surface during fermentation and are responsible for the intense bitter taste of SBY products (Shotipruk et al. 2005; Nand 1987). The “debittering” process can be performed through successive washes with basic solutions, solvents or using adsorbents (In et al. 2005; Reed and Nagodawithana 1991; Nand 1987). The production of yeast extracts needs to overcome three major obstacles, namely yeast cell wall strength, which prevents yeast compounds from being released and transformed, as well as the high nucleic acid content and the bitter aroma of yeasts, all of which limit their application as ingredients. Spent yeast processing needs to handle those particular characteristics of yeast while creating a final ingredient that presents suitable technological, nutritional, functional, bioactive and sensorial properties. The next sections present processing technologies developed to disrupt the yeast cell wall and to produce yeast protein hydrolysates. The release of yeast compounds results in a complex pool of molecules of interest that need to be separated from the other compounds to ensure their specific application purpose.
Disruption of the yeast cell wall by physical and chemical methods
Disruption of the cell wall can occur through physical, chemical and enzymatic methods. Depending on the method of choice, there is a considerable impact on amino acid composition and yeast extract quality (Jacob et al. 2019a; Marson et al. 2019).
In physical processes, the destruction of the wall structure is carried out in a non-specific manner, involving, for example, agitation with glass beads, cavitation by high pressure or ultrasound and thermolysis (Jacob et al. 2019a; Liu et al. 2016, 2013; Harrison 2011; Middelberg 1995). Because of the amount of friction that is created during these processes, energy is greatly spent to keep the product temperature from rising (Asenjo and Dunnill 1981). The most common method involves glass bead agitation, sometimes referred to as milling. It allows to maintain the characteristics of the yeast intracellular components (including enzymes) stable if the temperature is kept low. Also, the process can be scaled up (Halász and Lásztity 1991a). The ultrasound technique, despite its effectiveness, still requires a large amount of energy, usually for a long period of time, which leads to the formation of high temperature outbursts. Molecules of interest might be deteriorated as a result of the formation of free radicals and other unsought chemical changes (Yusaf and Al-Juboori 2014; Bzducha-Wróbel et al. 2014).
Chemical disruption is performed using bases, acids, surfactants, detergents and solvents (Suwanapong et al. 2013; Harrison 2011; Middelberg 1995). Chemical methods act by permeabilizing chemical compounds from outside the cell wall, which allows intracellular products to pass through it. These methods can have great complexity during operation, limited potential for scaling up, low efficiency and low economic viability. In addition, chemical treatments can degrade compounds with biological properties and even introduce contaminants into the system, resulting in difficulties and the need for further processing steps (Liu et al. 2016).
Disruption of the yeast cell wall by enzymatic hydrolysis
Enzymatic processes occur in a predominantly targeted way (Middelberg 1995). Yeast autolysis, widely used by the industry to produce yeast extract, is classified as an enzymatic method, even though it is induced by adding chemicals or changing the temperature. Solvents (e.g. ethyl acetate) and salts (NaCl) are often added to increase efficiency. In autolysis, endogenous yeast enzymes are activated and degrade the cell wall from the inside out, causing rupture (Middelberg 1995). In a recent study that compared three processes in the disruption of SBY cells, autolysis was the most effective (98% of nitrogen released from the cells) (Jacob et al. 2019a). However, this process is not yet deeply understood and poorly controlled and the autolytic properties of the strain are decisive and can make the autolysis process non-viable (Jacob et al. 2019a; Marson et al. 2019; Bzducha-Wróbel et al. 2014). The addition of exogenous enzymes (mainly proteases) is also an option, as it releases components from the wall in a more controlled and efficient manner and the process can be easily scaled up (Bzducha-Wróbel et al. 2014; Halász and Lásztity 1991b; Asenjo and Dunnill 1981). Long hours of autolysis often result in losses of important components (e.g., antioxidants, polyphenols and vitamins) (Jacob et al. 2019c). Smaller concentrations of glutamic acid in autolysates (in comparison to mechanical disruption methods) have been reported recently. It can be hypothesised that glutamic acid takes part in a reaction catalysed by the enzyme glutamate decarboxylase, which is naturally present in yeast. As a result, a neuroactive compound known as \(\gamma\)-aminobutyric acid (GABA; Jacob et al. 2019a) is produced. In this case, losses in glutamic acid are advantageous because GABA can be applied in the pharmaceutical industry.
The disruption of yeast cell walls by enzymatic hydrolysis can be performed with different objectives involving carbohydrases, RNAses and mainly, proteases. Proteolytic hydrolysis is the most efficient method of solubilising, exposing and releasing yeast peptides (Marson et al. 2019; Chae et al. 2001), and this technology is widely used to improve and increase the functional, biological and nutritional properties of food proteins in various matrices (Phongthai et al. 2018; de Castro and Sato 2014; Yuan et al. 2008).
Enzymatic hydrolysis has been used independently or combined with traditional methods such as autolysis and mechanical rupture. Amorim et al. (2016a), for example, used autolysis, hydrolysis with a Cynara cardunculus extract as well as series of ultra and nanofiltration to obtain SBY yeast ingredients with different characteristics while Marson et al. (2020) developed a process, using commercial enzymes, to disrupt and hydrolyse yeast protein simultaneously. Proteolytic hydrolysis may not only disrupt the cell wall, causing the release of compounds, but also modify the existing proteins. Solubilization and modification of compounds inside and on the cell wall can be performed during or after cell wall disintegration.
The characteristics of the peptides released by the enzymatic treatment vary with the specificity and type of enzyme (Phongthai et al. 2018; de la Hoz et al. 2014), and the effectiveness of hydrolysis depends directly on the composition of the matrix to be hydrolysed and on the process variables (de Castro and Sato 2015). Optimization and development of the protein hydrolysis of yeast and yeast by-products has been performed for different purposes and using several strategies. Tables 4, 5 and 6 show the treatments and processing conditions used in the cell wall rupture, and in the release and modification of the biochemical components of yeasts. Every hydrolysis process was specially developed: to produce yeast extracts for nutritional purposes (Jacob et al. 2019a, c; Amorim et al. 2016b; Tangüler and Erten 2008); to promote the release of flavour-related \(5'\)-nucleotides (Xie et al. 2017; Cui et al. 2016; Chae et al. 2001); to release peptides with ACE-I activity (Amorim et al. 2019b; Vieira et al. 2017a; Mirzaei et al. 2015; Kanauchi et al. 2005), antioxidant activity (Marson et al. 2020, 2019; Podpora et al. 2016), iron-chelating ability (de la Hoz et al. 2014); or to release specific peptide sequences (Jung et al. 2011).
For SBY, a yield of 5% of peptides with angiotensin-converting enzyme inhibitory activity (ACE-I) was reported after hydrolysis with Alcalase\(^{{{\text {TM}}}}\) and subsequent purification (Kanauchi et al. 2005). In another study, Alcalase\(^{{{\text {TM}}}}\), Neutrase, Protamex\(^{{{\text {TM}}}}\), Flavourzyme\(^{{{\text {TM}}}}\) and ficin were used to release a peptide (Cyclo-His-Pro) from SBY. The hydrolysate obtained from Flavourzyme\(^{{{\text {TM}}}}\), an enzyme with exo and endoprotease activities, showed the highest recovery of the peptide analysed in a previous study (674.0 \(\upmu\)g g−1) (Jung et al. 2011). Chae et al. (2001) reported the use of Protamex\(^{{{\text {TM}}}}\) and two enzymes with exo and endoprotease activities, Flavourzyme\(^{{{\text {TM}}}}\) and Protein FN, to obtain yeast extract. The authors found that the dosage of exoprotease affected protein recovery, the degree of hydrolysis and sensorial characteristics to a greater extent than the dosage of endoprotease. The combined treatment of Protamex\(^{{{\text {TM}}}}\) and Flavourzyme\(^{{{\text {TM}}}}\) resulted in the greatest recovery of solids (50%) (Chae et al. 2001). Alcalase\(^{{{\text {TM}}}}\) and Protex 51FP were used to hydrolyse residual yeast from sugarcane processing. Using Alcalase\(^{{{\text {TM}}}}\) resulted in extracts with a higher degree of hydrolysis (de la Hoz et al. 2014). As for the hydrolysis of cultivated cells of S. cerevisiae, bromelain (Kim et al. 2009, 2004; Yu et al. 2002) is normally used.
Thus, so far, Alcalase\(^{{{\text {TM}}}}\), Protamex\(^{{{\text {TM}}}}\) and Flavourzyme\(^{{{\text {TM}}}}\) were the enzymes that obtained the highest yield and the greatest functional or biological activity for SBY during hydrolysis, as reported in recently published studies (Marson et al. 2020, 2019; de la Hoz et al. 2014; Jung et al. 2011). Alcalase\(^{{{\text {TM}}}}\) is a serine endoprotease, while the last two are mixtures of endo and exoproteases. The pH value of action of the enzymes usually ranges from 5.0 to 8.0. The adequate temperature range of the enzymes is quite wide, with maximum activity achieved at temperatures of 40–60 \(^{\circ }\)C (Nielsen and Olsen 2002). Indeed, these and other enzymes specially designed for yeast were tested for the proteolytic hydrolysis of SBY, and these tests showed different characteristics for them (Marson et al. 2020, 2019). A recent study has reported the production of an antioxidant SBY enzymatic hydrolysate with an optimised mixture of enzymes using a mixture design. It indicated synergistic effects of the simultaneous use of Brauzyn®, Protamex\(^{{{\text {TM}}}}\) and Alcalase\(^{{{\text {TM}}}}\) (Marson et al. 2020). A response surface methodology was used to maximize the production of ACE-I peptides from SBY using the C. cardunculus extract (Amorim et al. 2019b).
After enzymatic treatments are performed, broken yeast cells are separated by centrifugation, which results in two fractions (Lobo-Alfonso et al. 2010). Often referred to as yeast extract, the soluble fraction consists of yeast cell compounds, which can be processed in subsequent steps for the purpose of concentration, purification and fractionation, depending on the application intended for the ingredient (Nagodawithana et al. 2010). A yeast extract usually presents a reduced content of nucleic acids and polysaccharides, depending on the disruption treatment of choice (Kollar et al. 1992; Halász and Lásztity 1991a). The precipitated material, which is mostly insoluble in water, has potential as a carrier material for microencapsulation and as an emulsifying agent, since it is mainly composed of proteins and carbohydrates from the yeast cell wall. Mannoproteins and \(\beta\)-glucans are extracted from this fraction, through washing and precipitation steps, with yields of 4% and 10%, respectively (Melo et al. 2015; Araújo et al. 2014).
Current and potential applications of the processed spent yeast from brewing
In-depth knowledge of the rich macro and micronutrient composition of SBY, combined with the development of application-focused processes, are the key to extend the scope of application of this important brewing by-product and well as other yeast by-products. The next sections present up-to-date foreseen applications of SBY as an innovative ingredient in the food and pharmaceutical industries.
Uses of yeast biomass for human and animal nutrition: alternative source of proteins, bioplexes and vitamins
Among all possible applications, SBY is commonly used as feed for protein supplementation. As studies and technology progress, SBY feed supplements are investigated not only for their protein and vitamin-rich composition, but also for their bioactive effects in animals (Shurson 2018).
A yeast extract from hydrolysed cells of the IFO 2346 strain of S. cerevisiae was used to enrich pet food and showed anti-obesity effects in dogs (Kim et al. 2012). Supplementation of vitamin and mineral premix with brewer’s yeast (1% to 5%) in broiler diets reverted negative bone effects (diminished tibia ash amounts) in vitamin/mineral depleted diets (Sacakli et al. 2013).
Disrupted yeast cells from S. cerevisiae, Candida utilis and Kluyveromyces marxianus have been evaluated as sustainable protein sources for fish feed. A previous study proposed a sustainable way of cultivating yeast in lignocellulosic non-food biomass from forestry and agricultural industries, which resulted in a low-cost product. After cell disruption and processing to improve protein digestibility, those protein-rich yeast materials were used in aquaculture as feed, with various immunological and health benefits (Øverland and Skrede 2017).
Proteins from brewing yeasts have high biological quality, which makes SBY a sustainable alternative source of proteins, as well as a non-allergenic option for vegans/vegetarians. Because it comes from a by-product, it is also a better choice in comparison to higher-cost proteins from plants or animals (Bertolo et al. 2019). The digestibility of yeast proteins before and after disruption methods has been investigated recently and indicated high digestibility when processed (\(>95\)%), comparable to that of textured soy protein (Bertolo et al. 2019).
Yeast cells are researched as a vehicle to human mineral supplementation, in the form of bioplexes. Saccharomyces cerevisiae yeast cells have been reported to accumulate and readily adsorb minerals (Cr\({}^{{{\text {3+}}}}\), Se\({}^{{{\text {4+}}}}\), Mg\({}^{{{\text {2+}}}}\), Cu\({}^{{{\text {2+}}}}\), Cd\({}^{{{\text {2+}}}}\), Zn\({}^{{{\text {2+}}}}\), Mn\({}^{{{\text {2+}}}}\), Ca\({}^{{{\text {2+}}}}\), Fe\({}^{{{\text {3+}}}}\)) in amounts that exceed their physiological demand (Błażejak and Duszkiewicz-Reinhard 2004; Stehlik-Tomas et al. 2004; Park et al. 2003; Varga and Maráz 2002; Pacheco et al. 1999). The concentration of these microelements needs to be carefully selected before use to prevent them from being toxic to cells. The content of cations adsorbed by cell wall proteins (mainly mannoproteins) is proportional to the total surface area of the yeast cell. Cation content is also dependent on yeast strain, properties of cell morphology, composition and physiological state of yeast cells (Błażejak and Duszkiewicz-Reinhard 2004; Varga and Maráz 2002). The presence of phosphorylated mannans in the cell wall, cell size, thickness of the mannoprotein layer and the presence of free carboxyl, hydroxyl, phosphate and hydrosulfide groups in the surface proteins influence this bioaccumulation phenomena (Błażejak and Duszkiewicz-Reinhard 2004; Park et al. 2003). For the production of bioplexes, Saccharomyces sp. is cultivated in a medium supplemented with the cation of interest, and the enrichment of biomass takes place in two steps. Firstly, the adsorption in the cell wall occurs rapidly; it is referred to as biosorption. The second phase, chemisorption, is energy consuming and happens at a lower rate, resulting in the active transport of cations from the cell wall to the cytoplasmic membrane and then to the cell interior.
Magnesium ions were enriched 3-fold above the physiological demand in Saccharomyces sp. Authors reported that the mechanism of cation binding of Mg\({}^{{{\text {2+}}}}\) with yeast cells was chemisorption followed by accumulation inside the cell, forming bioplexes (Błażejak and Duszkiewicz-Reinhard 2004). The effect of enrichment of yeast (S. cerevisiae) with chromium, selenium and zinc did not influence the content and stability of complex B vitamins, but the iron-enriched yeast resulted in losses in vitamin B2 (Varga and Maráz 2002). Varga and Maráz (2002) also found that those four microelements were mainly present in yeast as undissolved, bound compounds. It is important to emphasize that the greater the viability of yeast cells, the higher the degree of cation binding (Błażejak and Duszkiewicz-Reinhard 2004).
SBY may present low viability, specially if it has been repitched several times, but Ca\({}^{{{\text {2+}}}}\) binding in a SBY protein concentrate is possible (Pacheco et al. 1999). Although the binding of several cations has still not been evaluated for spent yeasts, this may be a potential application, cations may be either readily adsorbed by the cell wall proteins or released inside the body. Humans can easily assimilate bioplexes (Błażejak and Duszkiewicz-Reinhard 2004). They deliver minerals along with the proteins they are bound to, and penetrate the intestinal wall. Bioplexes are also less susceptible to the formation of complexes with compounds such as phytic acids, commonly found in plants, which limit body absorption. The presence of other compounds of interest in yeasts, namely \(\beta\)-glucans, along with their high protein content and complex B vitamins, makes them an even more appealing vehicle for minerals supplementation.
Yeasts may also produce vitamins when grown in specific mediums. Saccharomyces sp. was reported to produce ergosterol, a precursor of vitamin D2 (ergocalciferol) that can be extracted from the lipid fraction of cell walls (Kollar et al. 1992). Genetically engineered S. cerevisiae was capable of producing minor amounts of l-ascorbic acid from l-galactose (Stahmann 2019).
Yeast extracts: from medium supplementation to flavouring compounds
The nutrient-rich composition of yeast extracts may be used to boost nutrient supply, thus improving the fermentation performance of various microorganisms, including the fermentation of Saccharomyces sp. in beer production (Jacob et al. 2019b). An SBY enzymatic hydrolysate produced with Alcalase\(^{{{\text {TM}}}}\) was used as a nitrogen source to enhance the succinic acid production by Actinobacillus succinogenes (Chen et al. 2011).
Yeasts are able to synthesize a myriad of flavour molecules such as alcohols and terpenoids, used in food flavouring. Naturally, during fermentation, some of those compounds are produced. Yeast flavour depends on a delicate balance among peptides, nucleotides (guanosine monophosphate and inosine monophosphate), carbohydrates and free amino acids. There is great interest in using yeast extracts as flavouring agents in foods (Rakowska et al. 2017; Pérez-Torrado et al. 2015).
When the envisaged application for SBY is the production of a flavouring yeast extract, the method chosen for disruption and processing of SBY is of great importance. The concentration of free amino acids in the extract plays a major role in the flavouring potency of the ingredient. Leucine, isoleucine, valine, histidine, proline, cysteine and glutamine greatly influence the aroma of the product. A recent study compared the amino acid composition of yeast extracts produced by mechanical disruption (by glass beads and ultrasound) and autolysis, and the latter resulted in a higher release of those target amino acids (Jacob et al. 2019a). Enzymatic hydrolysis with proper exogenous enzymes, such as RNAses, also promotes the release of 5\('\)-nucleotides (Xie et al. 2017; Vieira et al. 2013; Chae et al. 2001) and results in high quality yeast extracts that are suitable as flavouring ingredients.
Yeast proteins as non-synthetic food emulsifiers and functional ingredients
The use of SBY materials as a sustainable and technologically viable option to synthetic emulsifiers has been investigated lately. The emulsifying ability of cell wall components is often attributed to mannoproteins and \(\beta\)-d-glucans that have technological properties acting as water holding, thickening, emulsifying and stabilizing agents (Araújo et al. 2014; Kollar et al. 1992).
Mannoproteins extracted from SBY (Saccharomyces uvarum) showed potential as stabilizers and emulsifying agents when used in the formulation of mayonnaise (substituting xanthan gum) with no negative effects on the sensory attributes of the product (Araújo et al. 2014). Mannoproteins also demonstrated the ability to emulsify and stabilize French salad dressings, while improving their nutritional composition and sensorial acceptance (Melo et al. 2015). Use of inactivated high-pressure homogenized baker’s yeast dispersion for low-fat dressings indicated the potential of yeast biomass as an alternative emulsifier (Fernandez et al. 2012).
Gel stabilizing properties of an SBY extract produced by mechanical disruption using glass beads were detected in cooked hams. The addition of 1% of yeast extract resulted in increased hardness, chewiness, sliceability and water-holding capacity, in addition to the incorporation of amino acids and proteins with high biological value, with no sensorial differences detected in comparison to controls (Pancrazio et al. 2016). The substitution of meat by yeast extract (distillery spent yeast Saccharomyces sp.) up to 1.5% in Frankfurt type sausages did not result in any sensorial changes (aroma, flavour and texture) (Yamada et al. 2010).
Non-treated and ultrasound-treated SBY cells were reported to have emulsifying properties in model emulsions, but autolysed samples presented very poor emulsifying properties (Bertolo et al. 2019). Foaming ability and stability were also assessed, but lower values were found for autolysed samples. The water-holding properties of yeast cells worsened after the disruption treatments (autolysis and ultrasound), but the ultrasound treatment improved significantly the oil-holding capacity of the yeast biomass. The solubility of yeast ingredients was studied, and it was found that disruption processes and addition of salts can improve yeast proteins solubility (Bertolo et al. 2019).
Yeast materials have been also studied as new carrier agents for microencapsulation because of their interesting composition and functional properties (gel formation, stabilization, emulsification). Cells of S. cerevisiae after chemical treatment showed good encapsulation yields of chlorogenic acid, a natural hydrophilic antioxidant (Paramera et al. 2014; Shi et al. 2010).
Those results suggest that further research is necessary to improve the processing and incorporation of SBY and yeast-based products as functional additives in the food industry.
Yeast enzymes
Yeasts contain several enzymes with hydrolytic activity. Enzymatic extracts of SBY or cultivated S. cerevisiae cells were already employed alone (with low or medium protein conversion) or in combination with exogenous enzymes to produce protein hydrolysates (Vieira and Ferreira 2017; Vieira et al. 2017a, b, 2016a; Martínez-Alvarez et al. 2008). The SBY protein extract is usually produced by disruption of yeast cells using glass beads under refrigerating temperatures to minimize enzyme denaturation and loss of hydrolytic capacity. The reported activity of SBY proteases extract ranges from 0.2 to 1.0 U mL−1 at pH 6.0, and they have already been used to produce hydrolysates from by-products of sardine cannery and spent grains from brewing (BSG, Vieira and Ferreira 2017; Vieira et al. 2017a, b, 2016a). A full characterisation of SBY proteases are still not available, but Fukal et al. (1986) reported that SBY proteinases contain sulfhydryl and metallo-proteinases with thermostability up to 50 \(^{\circ }\)C and activity at pH values as low as 3.
The potential of SBY as a source of invertase (EC 3.2.1.26) is yet to be evaluated, but S. cerevisiae is a known source, and invertase has been extracted from baker’s yeast by autolysis and ultrafiltration (\(>20\) kDa membranes) with an activity higher than 4.0 \(\upmu\)kat mg\({}_{{{\text {protein}}}}\)−1 (Pérez-Torrado et al. 2015; Kollar et al. 1992).
SBY polyphenols
Yeasts are known to possess the ability to absorb polyphenols during fermentation processes or when grown in media containing high levels of those compounds (León-González et al. 2018; Rizzo et al. 2006). Some polyphenols from hops (humulones, iso-humulones, lupulones, chalcones and flavones) and malt are delivered to the medium during brewing and are adsorbed to different extent by yeast cells. According to the literature, total polyphenol content in SBY ranges from 1.2 to 375 mg of gallic acid equivalents g−1\({}{{{\text {(d.w.)}}}}\) (Jacob et al. 2019c; Vieira et al. 2016b; Podpora et al. 2016). A detailed study on polyphenols profile of SBY demonstrated the potential of SBY as a source of bioactive polyphenols such as (+)-catechin, gallic acid, protocatechuic acid, p-coumaric acid, ferulic acid, trans-ferulic acid, rutin, naringin, quercetin and kaempferol (León-González et al. 2018; Vieira et al. 2016b). Bryant and Cohen (2015) measured hop acid profiles of spent yeast and reported that the concentration of hop acids in spent yeasts was much higher than in respective beers. Hop acids in spent yeasts were mainly represented by \(\alpha\)-acids (humulones) and \(\beta\)-acids (lupulones). Five-fold higher total hop acid content in craft SBY samples in comparison to multinational SBY samples were detected. This result is probably related to the fact that craft beer formulations typically employs higher levels of hops than those used in multinational industries. Although the mechanisms underlying yeast adsorption of hop acids is still not elucidated, authors have hypothesised that hop acids were probably located in the cell wall or cell membrane and were mostly associated with dead yeast cells (Bryant and Cohen 2015).
\(\gamma\)-Aminobutyric acid (GABA) and kynurenic acid production
\(\gamma\)-Aminobutyric acid (GABA) is a neuroactive non-protein amino acid that is reported to have bioactive activities (Diana et al. 2014). This compound is involved in the metabolic Krebs cycle in plants, and in vertebrates, it is an important inhibitory neurotransmitter that reduces neuronal excitability throughout the nervous system. Changes in GABA concentrations in the brain and in GABA synthesis pathways are related to many mental and psychiatric disorders (Huntington’s disease, Parkinson’s disease, senile dementia, seizures, Alzheimer’s disease, stiff person syndrome and schizophrenia). Effects against hypertension, kidney diseases, diabetes and cancers were also reported (Diana et al. 2014). GABA may be synthesized from l-glutamic acid or its salts via the glutamic acid decarboxylase enzyme (GAD; EC 4.1.1.15) with vitamin B6 (pyridoxal phosphate) as a cofactor. It is found in plants, animals, microorganisms and foods, and it is even synthesized by the gut microbiota (Yılmaz and Gökmen 2020). The discovery of new high-GABA producing strains for high performance biotechnological production is of great interest. Recent studies have been investigating new microorganisms and biotechnological processes that are able to produce GABA (Diana et al. 2014). During autolysis of SBY, GABA may be produced because GAD as well as great amounts of glutamic acid and vitamin B6 naturally occur in SBY (Table 2) (Masuda et al. 2008). Jacob et al. (2019b) found 2-fold higher concentrations of GABA by S. cerevisiae (TUM 68) with the addition of glucose and monosodium glutamate by autolysis (pH 6.0, 37 \(^{\circ }\)C for 72 h), but further studies are still needed to confirm the feasibility of the commercial use of this technology. The concentration of GABA in the yeast extract was 10 mg g−1 (d.w.) (Jacob et al. 2019a). In tea, one of the most important food sources of GABA, the concentration ranges from 50 to 2000 \(\upmu\)g g−1 (d.w.).
The essential amino acid tryptophan is mainly metabolised through the kynurenine pathway. Some changes in this pathway, ultimately resulting in imbalances in tryptophan and kynurenines, is related to the pathogenesis of some disfunctions, such as Alzheimer’s disease, Huntington’s disease, dementia complex, acquired immune deficiency syndrome and schizophrenia (Chen and Guillemin 2009). For instance, quinolinic acid, a derivative from the kynurenine pathway, is an important factor of Alzheimer’s disease neuronal damage pathogenesis (Guillemin and Brew 2002). The neurotoxicity caused by quinolinic acid can be limited by kynurenic acid, its antagonist (Chen and Guillemin 2009). Kynurenic acid is also an inhibitor of the \(\alpha\)-7 nicotinic acetylcholine receptor and an antioxidant compound. Antagonists or agonists of nicotinic acetylcholine receptors are considered a therapeutic strategy to Alzheimer’s disease, because the interactions between those receptors and amyloid beta peptides are changed (Lombardo and Maskos 2015). So far, the content of tryptophan derivatives, among them kynurenic acid, has been determined in fermented foods and plants. Kynurenic acid was detected in fermented products (cheese, yoghurt, beer, wine) as well as in cacao powder, which presents the highest content, about 4500 \(\upmu\)g kg−1 (d.w.) (Yılmaz and Gökmen 2018). The synthesis of kynurenine and kynurenic acid by S. cerevisiae and S. pastorianus was confirmed during beer fermentation, summing up 0.02–0.05 \(\upmu\)g g−1 (d.w.) or 17–52 \(\upmu\)g L−1 (Yılmaz and Gökmen 2020, 2019). Their presence in SBY was still neither investigated nor reported, but it is a possibility. The discovery of those neuroactive tryptophan derivatives is still very recent and their production/detection in spent yeasts from brewing has still not been investigated.
Bioactive peptides
Some peptides in food that can exert functions in addition to their basic nutritional benefits are defined as bioactive peptides. Although they usually present biological properties to a lesser extent than synthetic drugs, they are less likely to accumulate in the body and have side effects (Li-Chan 2015). Several studies have been published reporting beneficial effects to the organism of protein hydrolysates and peptides from spent grains (BSG), SBY, barley and non-processed S. cerevisiae. There are studies reporting antioxidant activity (McCarthy et al. 2013), anti-inflammatory effects (Connolly et al. 2015, 2014; McCarthy et al. 2013) and effects against type II diabetes and hypertension (Connolly et al. 2014) of BSG hydrolysates. Evidence has also been reported for antimicrobial, antioxidant, and antihypertensive activities and effects of barley grain protein fractions on diabetes (\(\alpha\)-amylase inhibitory activity) (McClean et al. 2014; Ortiz-Martinez et al. 2014; Xia et al. 2012; Alu’datt et al. 2012).
Tables 7 and 8 show the up-to-date biological activities discovered in yeast hydrolysates detected by in vitro and in vivo tests. The investigation of biological properties of yeast hydrolysates is recent, with the majority of articles published in the last 10 years. The most studied yeast material as a bioactive source of peptides is cultivated non-residual yeast. Protein hydrolysates from S. cerevisiae showed beneficial effects on indicators of stress. Near to control levels of epinephrine and norepinephrine were found for rats after the ingestion of yeast extract for 8 days prior to a 48 h stress period (Kim et al. 2003). This yeast extract was reported to present immunomodular effects, by activating macrophage and interleukin-6 production (1.9-fold when given to rats at 2 g/kg/day). Rats bone marrow cells significantly proliferated 2.1-fold more than the control group (Yu et al. 2002). Cultivated yeast protein hydrolysates also were reported to decrease fat accumulation in rats. The alteration of the activity of enzymes involved in lipid regulation was evaluated. Rats fed with a high-fat diet supplemented with 0.5–1% yeast extract have shown decreased body weight gain, serum triglycerides and low-density lipoprotein cholesterol concentrations. Also, the yeast supplementation seemed to inhibit the activity of both hepatic glucose-6-phosphate dehydrogenase and malic enzymes (Jung et al. 2012; Kim et al. 2004). In another study, the anti-obesity activity of yeast hydrolysates was investigated through changes in the expression of neuropeptides Y and cocaine and amphetamine-regulated transcript, compared with a control group. Authors reported that the ingestion of yeast decreased body weight gain, and increased the expression of the mRNA of cocaine and amphetamine-regulated transcript, a neuropeptide with regulatory functions on feed intake and energy balance (Park et al. 2013). The anti-obesity effect of yeast hydrolysate consumption was confirmed in beagle dogs, for which a significant weight reduction was observed (Kim et al. 2012).
Weight reduction and decreased abdominal fat accumulation as a result of consumption of yeast hydrolysate were reported in a human study (Jung et al. 2014). Residual yeast peptides from sugarcane processing were able to bind iron (de la Hoz et al. 2014). The consumption of a S. cerevisiae-based fermentate in a randomised, double-blind and placebo-controlled trial reduced the incidence of cold and flu-like symptoms in a healthy population, regardless of vaccination history (Moyad et al. 2010). SBY protein hydrolysates showed blood pressure lowering effects (ACE-I, Amorim et al. 2019a; Vieira et al. 2017a; Kanauchi et al. 2005), antiulcer and antiproliferative activity (Amorim et al. 2016b), antioxidant activity (Marson et al. 2020, 2019; Jung et al. 2011) and effects against type II diabetes (Jung et al. 2011). The expression of biological activities in SBY are probably represented not only by peptides, but also by vitamins, phenolic components and enzymes (Jacob et al. 2019b).
Several studies have investigated the hydrolysis of purified matrices and their activity. It is extremely important to consider the use of complex matrices as a substrate for hydrolysis, and evaluate the potential for production of bioactive peptides from commercially relevant food by-products (Li-Chan 2015; Ortiz-Chao and Jauregi 2007), such as SBY (Marson et al. 2020; Xie et al. 2017; Mirzaei et al. 2015). Several studies have suggested that the biological activity of protein fractions depends on the sequence of peptides and their hydrophobicity (Phongthai et al. 2018), and the antioxidant activity is correlated with some other activities, such as antihypertensive activity (Zhang 2016; Garcia-Mora et al. 2015; Esteve et al. 2015; Ortiz-Chao and Jauregi 2007). Because of the usually complex composition of SBY extracts, fractionation and purification are necessary to separate compounds of interest (such as peptides) from the mixture. The peptides with the highest reported activities are often those with a molecular weight smaller than 10 kg mol−1; therefore, fractionation using chromatography or membrane technology is a very common practice in the production of peptides (Tables 7, 8).
SBY peptides with ACE-I activity and molecular weight smaller than 3 kg mol−1 maintained their activity following in vitro gastrointestinal digestion. More studies are needed to further investigate the effects of SBY on human health, but the potential of SBY-based products in medicine and heath is encouraging.
Conclusions and perspectives
Spent yeasts from brewing are a nutritional rich by-product with a lot of potential to be processed into value-added ingredients and products for the food and pharmaceutical industries. Several technologies to transform SBY are already available and should be developed considering the intended use of SBY for maximum yield and performance. Valorisation of SBY is of economical, environmental and technological interests, and the products developed using this material are already showing promising results. Yeast processing perspectives involve application-focused process development considering yeast variability, pilot and industrial scaling up needs, investigation of susceptibility to gastrointestinal digestion and bioaccessibility of SBY peptides and other compounds in humans, further characterisation of molecules extracted/synthesised by SBY and economical studies.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Abou-Zeid A, Jalaluddin AK, Abulnaja KO (1995) On methods for reduction of nucleic acids content in a single-cell protein from gas oil. Bioresour Technol 52(1):21–24. https://doi.org/10.1016/0960-8524(95)99782-Q
Alu’datt MH, Ereifej K, Abu-Zaiton A, Alrababah M, Almajwal A, Rababah T, Yang W (2012) Anti-oxidant, anti-diabetic, and anti-hypertensive effects of extracted phenolics and hydrolyzed peptides from barley protein fractions. Int J Food Prop 15(4):781–795. https://doi.org/10.1080/10942912.2010.503357
Ambrosi A, Cardozo NSM, Tessaro IC (2014) Membrane separation processes for the beer industry: a review and state of the art. Food Bioprocess Technol 7(4):921–936. https://doi.org/10.1007/s11947-014-1275-0
Amorim M, Pereira JO, Gomes D, Pereira CD, Pinheiro H, Pintado M (2016a) Nutritional ingredients from spent brewer’s yeast obtained by hydrolysis and selective membrane filtration integrated in a pilot process. J Food Eng 185:42–47. https://doi.org/10.1016/j.jfoodeng.2016.03.032
Amorim M, Marques C, Pereira J, Guardão L, Martins M, Osório H, Moura D, Calhau C, Pinheiro H, Pintado M (2019a) Antihypertensive effect of spent brewer yeast peptide. Process Biochem 76:213–218. https://doi.org/10.1016/j.procbio.2018.10.004
Amorim M, Pinheiro H, Pintado M (2019b) Valorization of spent brewer’s yeast: optimization of hydrolysis process towards the generation of stable ACE-inhibitory peptides. LWT 111:77–84. https://doi.org/10.1016/j.lwt.2019.05.011
Amorim MM, Pereira JO, Monteiro KM, Ruiz AL, Carvalho JE, Pinheiro H, Pintado M (2016b) Antiulcer and antiproliferative properties of spent brewer’s yeast peptide extracts for incorporation into foods. Food Funct 7:2331–2337. https://doi.org/10.1039/C6FO00030D
de Araújo VBS, de Melo ANF, Costa AG, Castro-Gomez RH, Madruga MS, D’Souza EL, Magnani M (2014) Followed extraction of \(\beta\)-glucan and mannoprotein from spent brewer’s yeast (Saccharomyces uvarum) and application of the obtained mannoprotein as a stabilizer in mayonnaise. Innov Food Sci Emerg Technol 23:164–170. https://doi.org/10.1016/j.ifset.2013.12.013
Asenjo JA, Dunnill P (1981) The isolation of lytic enzymes from cytophaga and their application to the rupture of yeast cells. Biotechnol Bioeng 23(5):1045–1056. https://doi.org/10.1002/bit.260230512
Barrette J, Champagne CP, Goulet J (1999) Development of bacterial contamination during production of yeast extracts. Appl Environ Microbiol 65(7):3261–3263. https://doi.org/10.3390/fermentation5020051
Bertolo AP, Biz AP, Kempka AP, Rigo E, Cavalheiro D (2019) Yeast (Saccharomyces cerevisiae): evaluation of cellular disruption processes, chemical composition, functional properties and digestibility. J Food Sci Technol 56(8):3697–3706. https://doi.org/10.1007/s13197-019-03833-3
Błażejak S, Duszkiewicz-Reinhard W (2004) Yeast cell biomass as a potential source of magnesium bioplexes—a review. Pol J Food Nutr Sci 54(3):223–232
Bryant RWR, Cohen SD (2015) Characterization of hop acids in spent brewer’s yeast from craft and multinational sources. J Am Soc Brew Chem 73(2):159–164. https://doi.org/10.1094/ASBCJ-2015-0315-01
Budroni M, Mannazzu I, Zara S, Saba S, Pais A, Zara G (2020) Composition and functional profiling of the microbiota in the casts of Eisenia fetida during vermicomposting of brewers’ spent grains. Biotechnol Rep 25:e00439. https://doi.org/10.1016/j.btre.2020.e00439
Bzducha-Wróbel A, Błażejak S, Kawarska A, Stasiak-Różańska L, Gientka I, Majewska E (2014) Evaluation of the efficiency of different disruption methods on yeast cell wall preparation for \(\beta\)-glucan isolation. Molecules 19(12):20941–20961. https://doi.org/10.3390/molecules191220941
Caballero-Córdoba GM, Sgarbieri VC (2000) Nutritional and toxicological evaluation of yeast (Saccharomyces cerevisiae) biomass and a yeast protein concentrate. J Sci Food Agric 80(3):341–351. 10.1002/1097-0010(200002)80:3\(<\)341::AID-JSFA533\(>\)3.0.CO;2-M
Caballero-Córdoba GM, Pacheco MT, Sgarbieri VC (1997) Composição química da biomassa de levedura integral (Saccharomyces sp.) e determinação do valor nutritivo da proteína em células íntegras ou rompidas mecanicamente. Food Sci Technol 17(2):102–106. https://doi.org/10.1590/S0101-20611997000200007
Chae HJ, Joo H, In MJ (2001) Utilization of brewer’s yeast cells for the production of food-grade yeast extract. Part 1: effects of different enzymatic treatments on solid and protein recovery and flavor characteristics. Bioresour Technol 76(3):253–258. https://doi.org/10.1016/S0960-8524(00)00102-4
Chen KQ, Li J, Ma JF, Jiang M, Wei P, Liu ZM, Ying HJ (2011) Succinic acid production by Actinobacillus succinogenes using hydrolysates of spent yeast cells and corn fiber. Bioresour Technol 102(2):1704–1708. https://doi.org/10.1016/j.biortech.2010.08.011
Chen Y, Guillemin GJ (2009) Kynurenine pathway metabolites in humans: disease and healthy states. Int J Tryptophan Res. https://doi.org/10.4137/IJTR.S2097
Connolly A, Piggott CO, FitzGerald RJ (2014) In vitro \(\alpha\)-glucosidase, angiotensin converting enzyme and dipeptidyl peptidase-IV inhibitory properties of brewers’ spent grain protein hydrolysates. Food Res Int 56:100–107. https://doi.org/10.1016/j.foodres.2013.12.021
Connolly A, O’Keeffe MB, Piggott CO, Nongonierma AB, FitzGerald RJ (2015) Generation and identification of angiotensin converting enzyme (ACE) inhibitory peptides from a brewers’ spent grain protein isolate. Food Chem 176:64–71. https://doi.org/10.1016/j.foodchem.2014.12.027
Cui C, Qian Y, Sun W, Zhao H (2016) Effects of high solid concentrations on the efficacy of enzymatic hydrolysis of yeast cells and the taste characteristics of the resulting hydrolysates. Int J Food Sci Technol 51(5):1298–1304. https://doi.org/10.1111/ijfs.13084
de Castro RJS, Sato HH (2014) Functional properties and growth promotion of bifidobacteria and lactic acid bacteria strains by protein hydrolysates using a statistical mixture design. Food Biosci 7:19–30. https://doi.org/10.1016/j.fbio.2014.05.004
de Castro RJS, Sato HH (2015) Biologically active peptides: processes for their generation, purification and identification and applications as natural additives in the food and pharmaceutical industries. Food Res Int 74:185–198. https://doi.org/10.1016/j.foodres.2015.05.013
de la Hoz L, Ponezi AN, Milani RF, da Silva VSN, de Souza AS, Bertoldo-Pacheco MT (2014) Iron-binding properties of sugar cane yeast peptides. Food Chem 142:166–169. https://doi.org/10.1016/j.foodchem.2013.06.133
de Mathias TRS, Alexandre VMF, Cammarota MC, Mello PPM, Sérvulo EFC (2015) Characterization and determination of brewer’s solid wastes composition. J Inst Brew 121(3):400–404. https://doi.org/10.1002/jib.229
de Melo ANF, de Souza EL, de Araujo VBS, Magnani M (2015) Stability, nutritional and sensory characteristics of French salad dressing made with mannoprotein from spent brewer’s yeast. LWT 62(1, Part 2):771–774. https://doi.org/10.1016/j.lwt.2014.06.050
Diana M, Quílez J, Rafecas M (2014) Gamma-aminobutyric acid as a bioactive compound in foods: a review. J Funct Foods 10:407–420. https://doi.org/10.1016/j.jff.2014.07.004
Enache-Angoulvant A, Hennequin C (2005) Invasive Saccharomyces infection: a comprehensive review. Clin Infect Dis 41(11):1559–1568. https://doi.org/10.1086/497832
Esteve C, Marina ML, García MC (2015) Novel strategy for the revalorization of olive (Olea europaea) residues based on the extraction of bioactive peptides. Food Chem 167:272–280. https://doi.org/10.1016/j.foodchem.2014.06.090
Fernandez VE, Palazolo GG, Bosisio NA, Martínez LM, Wagner JR (2012) Rheological properties and stability of low-in-fat dressings prepared with high-pressure homogenized yeast. J Food Eng 111(1):57–65. https://doi.org/10.1016/j.jfoodeng.2012.01.029
Ferreira IMPLVO, Pinho O, Vieira E, Tavarela JG (2010) Brewer’s saccharomyces yeast biomass: characteristics and potential applications. Trends Food Sci Technol 21(2):77–84. https://doi.org/10.1016/j.tifs.2009.10.008
Fukal L, Káš J, Rauch P (1986) Properties of yeast proteinases. J Inst Brew 92(4):357–359. https://doi.org/10.1002/j.2050-0416.1986.tb04423.x
Garcia-Mora P, Frias J, Nas EP, Zieliński H, Giménez-Bastida JA, Wiczkowski W, Zielińska D, Martínez-Villaluenga C (2015) Simultaneous release of peptides and phenolics with antioxidant, ACE-inhibitory and anti-inflammatory activities from pinto bean (Phaseolus vulgaris L. var. pinto) proteins by subtilisins. J Funct Foods 18:319–332. https://doi.org/10.1016/j.jff.2015.07.010
Guillemin GJ, Brew BJ (2002) Implications of the kynurenine pathway and quinolinic acid in Alzheimer’s disease. Redox Rep 7(4):199–206. https://doi.org/10.1179/135100002125000550
Halász A, Lásztity R (1991a) Introduction. In: Halász A, Lásztity R (eds) Use of yeast biomass in food production. CRC Press, Boca Raton, pp 1–10
Halász A, Lásztity R (1991b) Chemical composition and biochemistry of yeast biomass. In: Halász A, Lásztity R (eds) Use of yeast biomass in food production. CRC Press, Boca Raton, pp 23–41
Harrison STL (2011) Cell disruption. In: Moo-Young M (ed) Comprehensive biotechnology, 2nd edn. Academic, Burlington, pp 619–640. https://doi.org/10.1016/B978-0-08-088504-9.00127-6
Huang K, Gao JY, Ma S, Lu JJ (2012) Optimising separation process of protein and polysaccharide from spent brewer’s yeast by ultrafiltration. Int J Food Sci Technol 47(6):1259–1264. https://doi.org/10.1111/j.1365-2621.2012.02967.x
Huige NJ (2006) Chapter 18: brewery by-products and effluents. In: Stewart GG, Priest FG (eds) Handbook of brewing, 2nd edn. CRC Press, Boca Raton, pp 656–716
In MJ, Kim DC, Chae HJ (2005) Downstream process for the production of yeast extract using brewer’s yeast cells. Biotechnol Bioprocess Eng 10(1):85. https://doi.org/10.1007/BF02931188
Jach ME, Serefko A, Sajnaga E, Kozak E, Poleszak E, Malm A (2015) Dietary supplements based on the yeast biomass. Curr Top Nutraceutical Res 13(2):83–88. https://doi.org/10.1590/S1415-52732000000300005
Jacob FF, Hutzler M, Methner F-J (2019a) Comparison of various industrially applicable disruption methods to produce yeast extract using spent yeast from top-fermenting beer production: influence on amino acid and protein content. Eur Food Res Technol 245:95–109. https://doi.org/10.1007/s00217-018-3143-z
Jacob FF, Striegel L, Rychlik M, Hutzler M, Methner F-J (2019b) Spent yeast from brewing processes: a biodiverse starting material for yeast extract production. Fermentation 5(2):51 (special Issue “Food Wastes: Feedstock for Value-Added Products”). https://doi.org/10.3390/fermentation5020051
Jacob FF, Striegel L, Rychlik M, Hutzler M, Methner F-J (2019c) Yeast extract production using spent yeast from beer manufacture: influence of industrially applicable disruption methods on selected substance groups with biotechnological relevance. Eur Food Res Technol 245:1169–1182. https://doi.org/10.1007/s00217-019-03237-9
Jung EY, Lee HS, Chang UJ, Bae SH, Kwon KH, Suh HJ (2010) Acute and subacute toxicity of yeast hydrolysate from Saccharomyces cerevisiae. Food Chem Toxicol 48(6):1677–1681. https://doi.org/10.1016/j.fct.2010.03.044
Jung EY, Lee HS, Choi JW, Ra KS, Kim MR, Suh HJ (2011) Glucose tolerance and antioxidant activity of spent brewer’s yeast hydrolysate with a high content of cyclo-his-pro (CHP). J Food Sci 76(2):C272–C278. https://doi.org/10.1111/j.1750-3841.2010.01997.x
Jung EY, Hong YH, Kim JH, Park Y, Bae SH, Chang UJ, Suh HJ (2012) Effects of yeast hydrolysate on hepatic lipid metabolism in high-fat-diet-induced obese mice: yeast hydrolysate suppresses body fat accumulation by attenuating fatty acid synthesis. Ann Nutr Metab 61:89–94. https://doi.org/10.1159/000338441
Jung EY, Cho MK, Hong YH, Kim JH, Park Y, Chang UJ, Suh HJ (2014) Yeast hydrolysate can reduce body weight and abdominal fat accumulation in obese adults. Nutrition 30(1):25–32. https://doi.org/10.1016/j.nut.2013.02.009
Kalayu G (2019) Serial re-pitching: its effect on yeast physiology, fermentation performance, and product quality. Ann Microbiol 69:787–796. https://doi.org/10.1007/s13213-019-01493-4
Kanauchi O, Igarashi K, Ogata R, Mitsuyama K, Andoh A (2005) A yeast extract high in bioactive peptides has a blood-pressure lowering effect in hypertensive model. Curr Med Chem 12(26):3085–3090. https://doi.org/10.2174/092986705774933461
Kim JH, Jung EY, Hong YH, Bae SH, Kim JM, Noh DO, Nozaki T, Inoue T, Suh HJ (2012) Short communication: pet foods with yeast hydrolysate can reduce body weight and increase girth in beagle dogs. Can J Anim Sci 92(2):207–210. https://doi.org/10.4141/cjas2011-123
Kim JM, Lee SW, Kim KM, Chang UJ, Song JC, Suh HJ (2003) Anti-stress effect and functionality of yeast hydrolysate SCP-20. Eur Food Res Technol 217(2):168–172. https://doi.org/10.1007/s00217-003-0723-2
Kim JM, Kim S, Jung E, Bae SH, Suh HJ (2009) Yeast hydrolysate induces longitudinal bone growth and growth hormone release in rats. Phytother Res 23(5):731–736. https://doi.org/10.1002/ptr.2720
Kim KM, Chang UJ, Kang DH, Kim JM, Choi YM, Suh HJ (2004) Yeast hydrolysate reduces body fat of dietary obese rats. Phytother Res 18(11):950–953. https://doi.org/10.1002/ptr.1582
Kollar R, Sturdik E, Sajbidor J (1992) Complete fractionation of Saccharomyces cerevisiae biomass. Urol Nurs 6(3):225–237. https://doi.org/10.1080/08905439209549836
Kumar RS, Chandrasekaran M (2016) Beverages. In: Chandrasekaran M (ed) Valorization of food processing by-products. CRC Press, Boca Raton, pp 589–614
Lee H, Jung E, Bae SH, Kwon KH, Kim J, Suh HJ (2011) Stimulation of osteoblastic differentiation and mineralization in MC3T3 E1 cells by yeast hydrolysate. Phytother Res 25(5):716–723. https://doi.org/10.1002/ptr.3328
Lee HS, Jung EY, Suh HJ (2009) Chemical composition and anti-stress effects of yeast hydrolysate. J Med Food 12(6):1281–1285. https://doi.org/10.1089/jmf.2009.0098
León-González ME, Gómez-Mejía E, Rosales-Conrado N, Madrid-Albarrán Y (2018) Residual brewing yeast as a source of polyphenols: extraction, identification and quantification by chromatographic and chemometric tools. Food Chem 267:246–254. https://doi.org/10.1016/j.foodchem.2017.06.141
Lewis MJ, Young TW (2001) Brewing, 2nd edn. Springer. https://doi.org/10.1007/978-1-4615-0729-1
Li-Chan EC (2015) Bioactive peptides and protein hydrolysates: research trends and challenges for application as nutraceuticals and functional food ingredients. Curr Opin Food Sci 1:28–37. https://doi.org/10.1016/j.cofs.2014.09.005
Liu D, Zeng XA, Sun DW, Han Z (2013) Disruption and protein release by ultrasonication of yeast cells. Innov Food Sci Emerg Technol 18:132–137. https://doi.org/10.1016/j.ifset.2013.02.006
Liu D, Ding L, Sun J, Boussetta N, Vorobiev E (2016) Yeast cell disruption strategies for recovery of intracellular bio-active compounds—a review. Innov Food Sci Emerg Technol 36:181–192. https://doi.org/10.1016/j.ifset.2016.06.017
Lobo-Alfonso J, Price P, Jayme D (2010) Chapter 4: benefits and limitations of protein hydrolysates as components of serum-free media for animal cell culture applications. In: Pasupuleti VK, Demain AL (eds) Protein hydrolysates in biotechnology, 1st edn. Springer, New York, pp 55–78. https://doi.org/10.1007/978-1-4020-6674-0
Lombardo S, Maskos U (2015) Role of the nicotinic acetylcholine receptor in Alzheimer’s disease pathology and treatment. Neuropharmacology 96:255–262. https://doi.org/10.1016/j.neuropharm.2014.11.018
Marson GV, de Machado MTC, de Castro RJS, Hubinger MD (2019) Sequential hydrolysis of spent brewer’s yeast improved its physico-chemical characteristics and antioxidant properties: a strategy to transform waste into added-value biomolecules. Process Biochem 84:91–102. https://doi.org/10.1016/j.procbio.2019.06.018
Marson GV, de Castro RJS, de Machado MTC, Zandonadi FDS, de Barros HDFQ, Maróstica Júnior MR, Sussulini A, Hubinger MD (2020) Proteolytic enzymes positively modulated the physicochemical and antioxidant properties of spent yeast protein hydrolysates. Process Biochem 91:34–45. https://doi.org/10.1016/j.procbio.2019.11.030
Martínez-Alvarez O, Guimas L, Delannoy C, Fouchereau-Peron M (2008) Use of a commercial protease and yeasts to obtain CGRP-like molecules from saithe protein. J Agric Food Chem 56(17):7853–7859. https://doi.org/10.1021/jf801393r
Masuda K, Feng Guo X, Uryu N, Hagiwara T, Watabe S (2008) Isolation of marine yeasts collected from the Pacific Ocean showing a high production of \(\gamma\)-aminobutyric acid. Biosci Biotechnol Biochem 72(12):3265–3272. https://doi.org/10.1271/bbb.80544
McCarthy AL, O’Callaghan YC, Connolly A, Piggott CO, FitzGerald RJ, O’Brien NM (2013) In vitro antioxidant and anti-inflammatory effects of brewers’ spent grain protein rich isolate and its associated hydrolysates. Food Res Int 50(1):205–212. https://doi.org/10.1016/j.foodres.2012.10.022
McClean S, Beggs LB, Welch RW (2014) Antimicrobial activity of antihypertensive food-derived peptides and selected alanine analogues. Food Chem 146:443–447. https://doi.org/10.1016/j.foodchem.2013.09.094
Middelberg APJ (1995) Process-scale disruption of microorganisms. Biotechnol Adv 13(3):491–551. https://doi.org/10.1016/0734-9750(95)02007-P
Mirzaei M, Mirdamadi S, Ehsani MR, Aminlari M, Hosseini E (2015) Purification and identification of antioxidant and ACE-inhibitory peptide from Saccharomyces cerevisiae protein hydrolysate. J Funct Foods 19:259–268. https://doi.org/10.1016/j.jff.2015.09.031
Moyad MA, Robinson LE, Zawada ET, Kittelsrud J, Chen DG, Reeves SG, Weaver S (2010) Immunogenic yeast-based fermentate for cold/flu-like symptoms in nonvaccinated individuals. J Altern Complim Med 16(2):213–218. https://doi.org/10.1089/acm.2009.0310
Mussatto SI (2009) Biotechnological potential of brewing industry by-products. In: Nigam PSN, Pandey A (eds) Biotechnology for agro-industrial residues utilisation: utilisation of agro-residues, 1st edn. Springer, Dordrecht, pp 313–326. https://doi.org/10.1007/978-1-4020-9942-7_16
Nagodawithana TW, Nelles L, Trivedi NB (2010) Chapter 11: protein hydrolysates as hypoallergenic, flavors and palatants for companion animals. In: Pasupuleti VK, Demain AL (eds) Protein hydrolysates in biotechnology, 1st edn. Springer, New York, pp 191–208. https://doi.org/10.1007/978-1-4020-6674-0
Nand K (1987) Debittering of spent brewer’s yeast for food purposes. Food/Nahr 31(2):127–131. https://doi.org/10.1002/food.19870310208
Nielsen PM, Olsen HS (2002) Enzymic modification of food protein. In: Whitehurst RJ, Law BA (eds) Enzymes in food technology. Sheffield Academic Press, Sheffield, pp 109–143
Ortiz-Chao PA, Jauregi P (2007) Enzymatic production of bioactive peptides from milk and whey proteins. In: Rastall R (ed) Novel enzyme technology for food applications, 1st edn. Woodhead Publishing, Cambridge, pp 160–182
Ortiz-Martinez M, Winkler R, García-Lara S (2014) Preventive and therapeutic potential of peptides from cereals against cancer. J Proteomics 111:165–183. https://doi.org/10.1016/j.jprot.2014.03.044
Øverland M, Skrede A (2017) Yeast derived from lignocellulosic biomass as a sustainable feed resource for use in aquaculture. J Sci Food Agric 97(3):733–742. https://doi.org/10.1002/jsfa.8007
Pacheco MT, Caballero-Córdoba GM, Sgarbieri VC (1997) Composition and nutritive value of yeast biomass and yeast protein concentrates. J Nutr Sci Vitaminol 46(6):601–612. https://doi.org/10.3177/jnsv.43.601
Pacheco MTB, Carraro F, Sgarbieri VC (1999) Study of calcium binding to different preparations of yeast (Saccharomyces cerevisiae) protein by using an ion selective electrode. Food Chem 66(2):249–252. https://doi.org/10.1016/S0308-8146(98)00252-0
Pancrazio G, Cunha SC, de Pinho PG, Loureiro M, Meireles S, Ferreira IM, Pinho O (2016) Spent brewer’s yeast extract as an ingredient in cooked hams. Meat Sci 121:382–389. https://doi.org/10.1016/j.meatsci.2016.07.009
Paramera EI, Karathanos VT, Konteles SJ (2014) Chapter 23: yeast cells and yeast-based materials for microencapsulation. In: Gaonkar AG, Vasisht N, Khare AR, Sobel R (eds) Microencapsulation in the food industry, 1st edn, Academic, San Diego, pp 267–281. https://doi.org/10.1016/B978-0-12-404568-2.00023-6
Park JK, Lee JW, Jung JY (2003) Cadmium uptake capacity of two strains of Saccharomyces cerevisiae cells. Enzyme Microb Technol 33(4):371–378. https://doi.org/10.1016/S0141-0229(03)00133-9
Park Y, Kim JH, Lee HS, Jung EY, Lee H, Noh DO, Suh HJ (2013) Thermal stability of yeast hydrolysate as a novel anti-obesity material. Food Chem 136(2):316–321. https://doi.org/10.1016/j.foodchem.2012.08.047
Pérez-Torrado R, Gamero E, Gómez-Pastor R, Garre E, Aranda A, Matallana E (2015) Yeast biomass, an optimised product with myriad applications in the food industry. Trends Food Sci Technol 46(2, Part A):167–175. https://doi.org/10.1016/j.tifs.2015.10.008
Phongthai S, D’Amico S, Schoenlechner R, Homthawornchoo W, Rawdkuen S (2018) Fractionation and antioxidant properties of rice bran protein hydrolysates stimulated by in vitro gastrointestinal digestion. Food Chem 240:156–164. https://doi.org/10.1016/j.foodchem.2017.07.080
Pinto M, Coelho E, Nunes A, ao TB, Coimbra MA (2015) Valuation of brewers spent yeast polysaccharides: A structural characterization approach. Carbohydr Polym 116(Supplement C):215 – 222, https://doi.org/10.1016/j.carbpol.2014.03.010
Podpora B, Świderski F, Sadowska A, Rakowska R, Wasiakzys G (2016) Spent brewer’s yeast extracts as a new component of functional food. Czech J Food Sci 34(6):554–563 https://doi.org/10.17221/419/2015-CJFS
Posteraro B, Quarant G, Posteraro P, Sanguinetti M (2018) Chapter 48: Saccharomyces cerevisiae. In: Liu D (ed) Handbook of foodborne diseases. CRC Press, Boca Raton, pp 509–520. https://doi.org/10.1201/b22030
Qin F, Johansen AZ, Mussatto SI (2018) Evaluation of different pretreatment strategies for protein extraction from brewer’s spent grains. Ind Crop Prod 125:443–453. https://doi.org/10.1016/j.indcrop.2018.09.017
Rajendran RMNR (2012) Enzymatic conversion of RNA from yeast extract to guanosine monophosphate (a flavoring agent). Master’s Thesis, Chalmers University of Technology, Göteborg
Rakowska R, Sadowska A, Dybkowska E, Świderski F (2017) Spent yeast as natural source of functional food additives. Ann Natl Inst Hyg 68(2):115–121
Ravindran R, Sarangapani C, Jaiswal S, Lu P, Cullen P, Bourke P, Jaiswal AK (2019) Improving enzymatic hydrolysis of brewer spent grain with nonthermal plasma. Bioresour Technol 282:520–524. https://doi.org/10.1016/j.biortech.2019.03.071
Reed G, Nagodawithana TW (1991) Food and feed yeast. In: Yeast technology. Springer, Dordrecht, pp 413–440. https://doi.org/10.1007/978-94-011-9771-7
Rizzo M, Ventrice D, Varone M, Sidari R, Caridi A (2006) HPLC determination of phenolics adsorbed on yeasts. J Pharm Biomed Anal 42(1):46–55. https://doi.org/10.1016/j.jpba.2006.02.058
Saba S, Zara G, Bianco A, Garau M, Bononi M, Deroma M, Pais A, Budroni M (2019) Comparative analysis of vermicompost quality produced from brewers’ spent grain and cow manure by the red earthworm Eisenia fetida. Bioresour Technol 293:122019. https://doi.org/10.1016/j.biortech.2019.122019
Sacakli P, Koksal BH, Ergun A, Ozsoy B (2013) Usage of brewer’s yeast (Saccharomyces cerevisiae) as a replacement of vitamin and trace mineral premix in broiler diets. Rev Med Vet 164(1):39–44
Shi G, Rao L, Xie Q, Li J, Li B, Xiong X (2010) Characterization of yeast cells as a microencapsulation wall material by Fourier-transform infrared spectroscopy. Vib Spectrosc 53(2):289–295. https://doi.org/10.1016/j.vibspec.2010.04.007
Shotipruk A, Kittianong P, Suphantharika M, Muangnapoh C (2005) Application of rotary microfiltration in debittering process of spent brewer’s yeast. Bioresour Technol 96(17):1851–1859. https://doi.org/10.1016/j.biortech.2005.01.035
Shurson G (2018) Yeast and yeast derivatives in feed additives and ingredients: sources, characteristics, animal responses, and quantification methods. Anim Feed Sci Technol 235:60–76. https://doi.org/10.1016/j.anifeedsci.2017.11.010
Stahmann KP (2019) Vitamins and vitamin-like compounds: microbial production. In: Schmidt TM (ed) Encyclopedia of microbiology, 4th edn, Academic, Oxford, pp 569–580. https://doi.org/10.1016/B978-0-12-809633-8.13017-1
Steckelberg C, Andrietta MDGS, Andrietta SR, Stupielloé ENA (2013) Yeast proteins originated from the production of Brazilian bioethanol quantification and content. Int J Food Eng 9(1):141–146. https://doi.org/10.1515/ijfe-2012-0179
Stehlik-Tomas V, Zetić VG, Stanzer D, Grba S, Vahčić N (2004) Zinc, copper and manganese enrichment in yeast Saccharomyces cerevisiae. Food Technol Biotechnol 42(2):115–120
Suwanapong S, Khongsay N, Laopaiboon L, Jaisil P, Laopaiboon P (2013) Dried spent yeast and its hydrolysate as nitrogen supplements for single batch and repeated-batch ethanol fermentation from sweet sorghum juice. Energies 6(3):1618–1631. https://doi.org/10.3390/en6031618
Tangüler H, Erten H (2008) Utilisation of spent brewer’s yeast for yeast extract production by autolysis: the effect of temperature. Food Bioprod Process 86(4):317–321. https://doi.org/10.1016/j.fbp.2007.10.015
Varga E, Maráz A (2002) Yeast cells as sources of essential microelements and vitamins B1 and B2. Acta Aliment 31(4):393–405. https://doi.org/10.1556/AAlim.31.2002.4.8
Vieira E, Brandão T, Ferreira IMPLVO (2013) Evaluation of brewer’s spent yeast to produce flavor enhancer nucleotides: influence of serial repitching. J Agric Food Chem 61(37):8724–8729. https://doi.org/10.1021/jf4021619
Vieira E, Teixeira J, Ferreira I (2016a) Valorization of brewers’ spent grain and spent yeast through protein hydrolysates with antioxidant properties. Eur Food Res Technol 242:1975–1984. https://doi.org/10.1007/s00217-016-2696-y
Vieira EF, Ferreira IM (2017) Antioxidant and antihypertensive hydrolysates obtained from by-products of cannery sardine and brewing industries. Int J Food Prop 20(3):662–673. https://doi.org/10.1080/10942912.2016.1176036
Vieira EF, Carvalho J, Pinto E, Cunha S, Almeida AA, Ferreira IMPLVO (2016b) Nutritive value, antioxidant activity and phenolic compounds profile of brewer’s spent yeast extract. J Food Compos Anal 52:44–51. https://doi.org/10.1016/j.jfca.2016.07.006
Vieira EF, das Neves J, Vitorino R, Dias da Silva D, Carmo H, Ferreira IMPLVO (2016c) Impact of in vitro gastrointestinal digestion and transepithelial transport on antioxidant and ACE-inhibitory activities of brewer’s spent yeast autolysate. J Agric Food Chem 64(39):7335–7341. https://doi.org/10.1021/acs.jafc.6b02719
Vieira EF, Melo A, Ferreira IMPLVO (2017a) Autolysis of intracellular content of brewer’s spent yeast to maximize ACE-inhibitory and antioxidant activities. LWT 82:255–259. https://doi.org/10.1016/j.lwt.2017.04.046
Vieira EF, da Silva DD, Carmo H, Ferreira IM (2017b) Protective ability against oxidative stress of brewers’ spent grain protein hydrolysates. Food Chem 228:602–609. https://doi.org/10.1016/j.foodchem.2017.02.050
Vilela ESD, Sgarbieri VC, Alvim ID (2000) Determination of protein value of integral cells, total autolysate and yeast extract (Saccharomyces sp.). Braz J Nutr 13(3):185–192, https://doi.org/10.1590/S1415-52732000000300005
Williams R, Dias DA, Jayasinghe N, Roessner U, Bennett LE (2016) \(\beta\)-Glucan-depleted, glycopeptide-rich extracts from brewer’s and baker’s yeast (Saccharomyces cerevisiae) lower interferon-\(\gamma\) production by stimulated human blood cells in vitro. Food Chem 197:761–768. https://doi.org/10.1016/j.foodchem.2015.11.015
Xia Y, Bamdad F, Gänzle M, Chen L (2012) Fractionation and characterization of antioxidant peptides derived from barley glutelin by enzymatic hydrolysis. Food Chem. https://doi.org/10.1016/j.foodchem.2012.03.063
Xie J, Cui C, Ren J, Zhao M, Zhao L, Wang W (2017) High solid concentrations facilitate enzymatic hydrolysis of yeast cells. Food Bioprod Process 103:114–121. https://doi.org/10.1016/j.fbp.2017.03.004
Yamada EA, Cipolli KMAVB, Harada MM, Sgarbieri VC (2010) Utilização de extrato de levedura (Saccharomyces sp.) de destilaria de álcool em salsicha. Braz J Food Technol 13(3):197–204. https://doi.org/10.4260/BJFT2010130300026
Yu KW, Kim JM, Oh SH, Chang UJ, Suh HJ (2002) Physiological effects of yeast hydrolysate SCP-20. Food Res Int 35(9):879–884. https://doi.org/10.1016/S0963-9969(02)00097-2
Yuan X, Gu X, Tang J (2008) Optimization of the production of Momordica charantia l. var. abbreviata ser. protein hydrolysates with hypoglycemic effect using alcalase. Food Chem 111(2):340–344. https://doi.org/10.1016/j.foodchem.2008.03.070
Yusaf T, Al-Juboori RA (2014) Alternative methods of microorganism disruption for agricultural applications. Appl Energy 114:909–923. https://doi.org/10.1016/j.apenergy.2013.08.085
Yılmaz C, Gökmen V (2018) Determination of tryptophan derivatives in kynurenine pathway in fermented foods using liquid chromatography tandem mass spectrometry. Food Chem 243:420–427. https://doi.org/10.1016/j.foodchem.2017.10.004
Yılmaz C, Gökmen V (2019) Kinetic evaluation of the formation of tryptophan derivatives in the kynurenine pathway during wort fermentation using Saccharomyces pastorianus and Saccharomyces cerevisiae. Food Chem 297:124975. https://doi.org/10.1016/j.foodchem.2019.124975
Yılmaz C, Gökmen V (2020) Neuroactive compounds in foods: occurrence, mechanism and potential health effects. Food Res Int 128:108744. https://doi.org/10.1016/j.foodres.2019.108744
Zhang SB (2016) In vitro antithrombotic activities of peanut protein hydrolysates. Food Chem 202:1–8. https://doi.org/10.1016/j.foodchem.2016.01.108
Ziener S, McNally J (2019) Beverage trends: beer (market survey). https://www.braubeviale.de/en/news/press-releases/beverage-trends-beer-phs9j1b7cb_pireport
Acknowledgements
This work was supported by FAPESP (São Paulo Research Foundation, Grant Numbers #2016/18465-8, #2018/04067-6) and CNPq (Brazilian National Council for Scientific and Technological Development, Grant Number 306461-2017-0). The authors are also thankful to Food Engineering Doctoral Program of UNICAMP (Brazil), GAIA Doctoral School, Institut Européen des Membranes (Université de Montpellier, France), Tiago Afonso Marenda, Martin Hartinger and Philipp Hartinger from Brauerei Stierberg (Stierberg, Germany).
Author information
Authors and Affiliations
Contributions
All authors contributed to the idea and concept of the study. Literature search and data analysis was performed by GVM. The first draft of the manuscript was written by GVM and all authors reviewed and commented on previous versions of the manuscript. Funding acquisition, resources and supervision were done by M-PB and MDH. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marson, G.V., de Castro, R.J.S., Belleville, MP. et al. Spent brewer’s yeast as a source of high added value molecules: a systematic review on its characteristics, processing and potential applications. World J Microbiol Biotechnol 36, 95 (2020). https://doi.org/10.1007/s11274-020-02866-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-020-02866-7