Abstract
Polyphenol-containing extracts from olive (Olea europaea) leaves (OLL) were obtained using a glycerol-based deep eutectic solvent (DES) and a combination of DES with methyl β-cyclodextrin (m-β-CD). The extracts were stored at various temperatures for a period of 20 days and the reducing power (PR) was monitored to trace changes in the antioxidant potency of the extracts. Over the examination period and at every temperature tested, PR displayed a constant decline, which followed pseudo zero-order kinetics. The determination of the decay constants indicated that the presence of m-β-CD acted protectively, slowing down the progression of the PR decline. Examination of the polyphenolic profiles using liquid chromatography–diode array–mass spectrometry showed that after storage for 20 days at 50 °C, some major polyphenols occurring in OLL suffered extended degradation. The formation of a yellow pigment in the extracts stored in DES but not in aqueous ethanol suggested that polyphenol oxidation did occur during storage. It was concluded that the oxidation of some OLL components was rather responsible for the PR decline observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Olive leaves (OLL) are olive oil production residues, generated during olive tree pruning and in the early steps of olive cleaning. They are considered an important food industry by-product, because they bear a high load of polyphenolic substances, which have been shown to possess a versatile pharmacological potency [1]. This is the major reason for the development of a broad variety of methodologies of solid–liquid extraction, aiming at enhanced recovery of polyphenolic phytochemicals from OLL. These methodologies embrace primarily environmentally benign solvents, such as subcritical water and water/ethanol mixtures, but also eco-friendly effective technologies, such as microwave heating and ultrasonication [2].
The development and increasing use of the new-generation solvents, called deep eutectic solvents (DES) or low-transition temperature mixtures (LTTMs), has shifted research on the use of that kind of media for purposes of natural product extraction. The emerging scientific data provide solid evidence for the superiority of DES in processes pertaining to polyphenol recovery, compared with water and hydroethanolic solutions [3], opening new routes for the implementation of even more effective extraction procedures. In this frame, a recently designed novel DES, composed of glycerol and glycine, was shown to have significantly higher performance in the extraction of OLL polyphenols [4], as compared with aqueous ethanol. Following investigations revealed that incorporation of methyl β-cyclodextrin (m-β-CD) into the DES may act as an extraction booster, giving even higher extraction yield [5].
However, apart from the increasing demand for natural antioxidants in the food, cosmetics and pharmaceutical industries, which has led to the search for natural extracts, strategies with which to increase long-term storage stability of the extracts are also required. Stability in the extraction medium over time is an important issue associated with polyphenol extraction, considering that many polyphenols are inherently unstable molecules, owed to their susceptibility to oxidation. Therefore, the examination of their stability under specific conditions is imminent and requires detailed examination. On such a ground, this examination was undertaken to investigate the stability of OLL polyphenols in extracts generated using the above-mentioned extraction medium, composed of the DES and m-β-CD. Stability in the presence and absence of m-β-CD was assessed by monitoring the reducing power (PR) of the extracts over 20 days at various temperatures, and polyphenol transformations that might be linked with changes in PR were identified using liquid chromatography–diode array–mass spectroscopy.
Materials and methods
Chemicals
Solvents used for liquid chromatography were HPLC grade. Methyl β-cyclodextrin, glycerol (99%) and ethanol (99.8%) were from Acros Organics (Geel, Belgium). Anhydrous sodium carbonate was from Carlo Erba Reactifs (Val de Reuil, France). Glycine was from NeoLab Migge Laborbedarf-Vertiebs (Heildelberg, Germany). Ascorbic acid and ferric chloride hexahydrate were from Fluka (Steinheim, Germany). 2,4,6-Tripyridyl-s-triazine (TPTZ) was from Aldrich (Steinheim, Germany).
Preparation of the DES
The DES used was synthesised according to the optimised conditions described previously [4]. Briefly, glycerol (hydrogen bond donor—HBD) was mixed with an appropriate amount of glycine (hydrogen bond acceptor—HBA) and water to give a molar ratio HBD:HBA:water of 7:1:3, and the mixture was mildly heated under stirring until the formation of a transparent liquid. Aqueous solution 80% (w/v) of this DES was used for the extractions and stability tests.
Plant material
Dried and pulverised Olea europaea leaves (OLL) from Agrielia Kalamon variety, with average particle diameter of 0.5 mm, were used for all examinations performed. Details concerning collection and handling of the plant material have been analytically given elsewhere [4].
Batch extraction procedure and sample handling
Polyphenol extraction from OLL was carried out using the optimised methodology previously developed [5]. Amount of 2.5 g of dried plant material was mixed with 100 mL of 80% (w/v) aqueous DES containing 9% (w/v) methyl β-cyclodextrin (m-β-CD), to give a liquid-to-solid ratio of 32 mL g−1. Extractions were performed at 70 °C, under continuous stirring at 600 rpm, for 280 min. Extractions in the absence of m-β-CD were also repeated, under identical conditions. After the completion of each extraction, samples were centrifuged in a table centrifugator (Hermle, Wehingen, Germany) at 10,000×g for 10 min, and the clear extract was used for stability tests and analyses.
Stability test and determinations
The clear extracts were divided into aliquots of 30 mL, placed in screw-cap glass vials and stored in a freezer (4 °C), in a temperature-controlled dark chamber (22 °C) and in a thermostated water bath (50 °C). Sampling was randomly carried out to eliminate variance, at regular intervals, within a period of 20 days. Reducing power (PR) was estimated with the TPTZ assay and expressed as µmol AAE per g dry weight [6].
Qualitative liquid chromatography–diode array–mass spectrometry (LC–DAD–MS)
A Finnigan MAT Spectra System P4000 pump was used, coupled with a UV6000LP diode array detector and a Finnigan AQA mass spectrometer. A Fortis RP-18 column, 150 × 2.1 mm, 3 µm, was used, at 40 °C. The analytical methodology implemented was reported elsewhere [6].
Statistics
Extractions and stability tests were repeated at least twice and all analyses were performed in triplicate. Values reported are means. Linear regressions were established at least at a 95% significance level. For all statistics, Microsoft Excel™ 2010 and SigmaPlot™ 10 were used.
Results and discussion
Kinetics of P R evolution and the effect of m-β-CD
To assess extract stability at various temperatures, as well as to clarify the role of m-β-CD, PR of the OLL extracts was monitored over a period of 20 days. PR was chosen as a safe criterion to track changes because it is tightly associated with polyphenol structure [7] and it has been demonstrated to reflect changes related with redox phenomena [8]. In particular, PR has been shown to be inversely correlated with polyphenol oxidation in white wines, strong evidence that PR decline may stem from polyphenol loss or structure alteration as a result of oxidation. During the examination period, it was ascertained that PR exhibited a declining trend, which could be very effectively described as pseudo zero-order kinetics:
where PR is the reducing power, \(P_{{\text{R}}}^{0}\) the initial reducing power, k the pseudo zero-order decay rate constant (µmol AAE g−1 days−1), and t time (days). The decay constants (k) were calculated graphically from the slope of the regression lines, after plotting PR against t.
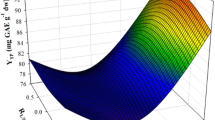
As can be seen in Fig. 1, kinetics at 4 °C showed that the presence of m-β-CD apparently accelerated PR decline. From the data given in Table 1, it was illustrated that the pseudo zero-order decay constant for the PR in the OLL extract obtained with DES/m-β-CD, at 4 °C, was higher than those determined for the extracts stored either in DES or in 60% aqueous ethanol. By contrast, PR exhibited higher stability in DES than in 60% aqueous ethanol. However, at both 22 and 50 °C, the extracts containing m-β-CD displayed lower k. For the extract containing m-β-CD, k was increased by almost 12% when storage temperature was increased from 4 to 50 °C, whereas the increase in the extracts lacking m-β-CD was approximately 46% and in aqueous ethanolic extracts 43%. This outcome highlighted the role of m-β-CD in PR stability.
Polyphenol stability in DES is an issue currently unexamined and the data available are too limited to draw safe conclusions. In a recent investigation, it was demonstrated that green tea polyphenols displayed higher stability in a DES than in conventional solvents, such as aqueous methanol and aqueous ethanol [9]. The exact mechanism for this effect could not be known, yet it could be assumed that extended hydrogen bonding between polyphenols and the HBA could confer some sort of stability. Polyphenols may develop this kind of interactions, since they may behave as HBDs [10]. Hydrogen bonding may also be developed between a polyphenol and cyclodextrins, and in this regard the presence of m-β-CD might be critical. Polyphenols such as flavonoid glycosides but also flavonoid aglycones have been shown to form complexes with various types of cyclodextrins, including β-cyclodextrin (β-CD) and 2-hydroxypropyl β-cyclodextrin (HP-β-CD) [11, 12]. The formation of flavonoid/HP-β-CD complexes contributed in higher stability of the encapsulated molecule, as opposed to the free (non-encapsulated) one [13], a fact attributed to the protective effect of HP-β-CD cavity [14]. Similar conclusion regarding stability was reached by studies on polyphenol-containing extracts [15, 16].
A hypothesis relating polyphenol stability with antioxidant activity has been proposed by examinations on rosmaric acid inclusion complexes by various cyclodextrins [17]. A polyphenol radical, deriving from a reaction with another radical species, once engulfed in m-β-CD hydrophobic cavity may be better stabilised through resonance, by intramolecular hydrogen bonding. Thus, the redox potential between the aroxyl radical and the reduced polyphenol may be lowered, rendering the polyphenol higher antioxidant potency. In fact, several studies have demonstrated that polyphenols such as quercetin and rutin [11], rosmarinic acid [17, 18], chlorogenic acid [19], and quercetin and glycosides therof [20], encapsulated in cyclodextrins may perform as more powerful antioxidants, compared with the non-encapsulated molecules. Therefore, the slower PR decay recorded for OLL extracts at 22 and 50 °C in the presence of m-β-CD might be ascribed to higher polyphenol stability, as a result of effective inclusion. On the other hand, the higher decay rate found for the extract stored in DES/m-β-CD at 4 °C would appear rather paradox and further studies are required to identify the phenomena implicated to yield this outcome.
Modifications in the polyphenolic profile
To clarify whether alterations in the polyphenolic profile accounted for the changes in PR observed, LC–DAD–MS investigation was undertaken for the samples stored at 50 °C, which displayed the most pronounced decline. In the trace recorded at 280 nm for the initial extract (day 0) obtained with the DES/m-β-CD (Fig. 2, upper chromatogram), eight polyphenolic substances could be tentatively identified (Table 2). The extract obtained only with the DES showed identical profile (data not shown), confirming previous results that stressed the importance of m-β-CD merely as an extraction booster [5]. On the basis of published data [21, 22], peak #1 was identified as hydroxytyrosol, peak #1b as tyrosol, peaks #5 and #8 as oleuropein and an isomer thereof, and peaks #2, 3, 6 and 7 as flavone glycosides. Furthermore, a flavonol glycoside was also detected (peak #4). When the extracts were analysed after 20 days of storage at 50 °C, some alterations in the polyphenolic profile were evident, the most prominent being an increase in hydroxytyrosol (peak #1), which pointed to oleuropein hydrolysis, and the drastic decrease in apigenin rutinoside (peak #6). Nevertheless, this decrease was not accompanied by the appearance of the apigenin aglycone; this fact raised suspicions for apigenin oxidative degradation.
HPLC traces of OLL extracts obtained with DES/m-β-CD, before (day 0) and after storage at 50 °C (day 20). The chromatogram was monitored at 280 nm. Peak assignment is as in Table 2
An important finding was the detection of a product identified only in the extracts stored in DES/m-β-CD or DES, but not in those stored in 60% ethanol (Fig. 3, lower chromatogram). This fact strongly emphasised the role of DES in its formation. The substance displayed λmax at 270 and 424 nm, which indicated that it was a yellow pigment (Fig. 4). Liquid chromatography–mass spectrometry examination showed a pseudo-molecular ion at m/z 503 amu and diagnostic fragments at m/z 439 amu [M − 64]+, m/z 309 amu [M − 194]+ and m/z 249 amu [M − 254]+ (Fig. 5). The fragment with m/z 439 could derive from dehydration and decarboxylation of the parent molecule, while further dehydration and fragmentation would yield the ion with m/z 308 amu, which in turn would provide the ion with m/z 249 amu. On the basis of these data, the product was identified as adduct of oleuropein aglycone with tyrosol quinone. A plausible pathway for the formation of such a product would embrace first oleuropein hydrolysis leading to the formation of the aglycone. One-electron oxidation of the hydroxytyrosyl moiety would give the semi-quinone derivative, which after nucleophilic attack by tyrosol, rearrangement and further oxidation, would yield the putatively proposed product (Fig. 6). This substance could possess yellow pigment-like UV features, as observed for other oxidised simple phenolics, such as chlorogenic acid [23]. Oxidation of oleuropein itself has also been shown to yield a product absorbing at 439 nm [24]. On the other hand, Fenton-type oxidation would be rather precluded, because oleuropein oxidation under such conditions was shown to give non-pigment products of higher molecular weight [25].
The evidence emerged from the putative structure of this compound suggested that some OLL constituents indeed underwent oxidation and this was the most probable cause of PR decline recorded during storage of the OLL extracts. Previous investigations on white wines demonstrated that browning, that is, the formation of yellow pigments, had a statistically significant correlation with a decrease in PR [8]. A following detailed kinetic investigation confirmed that browning development was indeed associated with a concomitant decline in PR [26], which was correlated with epicatechin, the major antioxidant constituent. Thus, the disappearance of certain OLL polyphenols due to oxidation would be very likely to bring about a drop in the reducing potency of the extracts.
Conclusions
DES are novel solvent of variable composition and for this reason they may present a broad spectrum of properties, whose characterisation is a matter of case experimentation. An issue of high importance in the extraction of antioxidant polyphenols using DES is their stability, since many polyphenols may readily undergo reactions, such as hydrolysis and oxidation. OLL extracts, obtained with either DES/m-β-CD or only DES were shown to suffer alterations regarding their polyphenolic profile, when stored for a period of 20 days at 50 °C. These alterations were mainly characterised by degradation of some glycosides and the formation of a yellow pigment as a result of polyphenol oxidation. It was, therefore, postulated that the decline in PR seen in the stored OLL extracts could be ascribed to the oxidation of some OLL constituents. However, the kinetic study demonstrated that the incorporation of m-β-CD in the extraction solvent may result in notable retardation of the PR decay. This finding highlighted the importance of such additives in stabilising polyphenol-containing extracts in DES. Further studies are required to clarify the exact mechanism of protection, which could lead to more efficient utilisation of m-β-CD in formulations related to food, pharmaceutical and cosmetic products.
Abbreviations
- P R :
-
Reducing power (µmol AAE g−1 dw)
- P R(0) :
-
Initial reducing power (µmol AAE g−1 dw)
- k :
-
Pseudo zero-order decay constant (μmol AAE g−1 days−1)
- t :
-
Time (days)
- DES:
-
Deep eutectic solvent
- m-β-CD:
-
Methyl β-cyclodextrin
- OLL:
-
Olive leaves
References
Obied HK, Prenzler PD, Omar SH et al (2012) Pharmacology of olive biophenols. Adv Mol Toxic 6:195–242
Roselló-Soto E, Koubaa M, Moubarik A et al (2015) Emerging opportunities for the effective valorization of wastes and by-products generated during olive oil production process: non-conventional methods for the recovery of high-added value compounds. Trends Food Sci Technol 45(2):296–310
Bakirtzi C, Triantafyllidou K, Makris DP (2016) Novel lactic acid-based natural deep eutectic solvents: efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J Appl Res Med Arom Plants 3:120–127
Athanasiadis V, Grigorakis S, Lalas S, Makris DP (2017) Highly efficient extraction of antioxidant polyphenols from Olea europaea leaves using an eco-friendly glycerol/glycine deep eutectic solvent. Waste Biomass Valor. https://doi.org/10.1007/s12649-017-9997-7
Athanasiadis V, Grigorakis S, Lalas S, Makris DP (2017) Methyl β-cyclodextrin as a booster for the extraction of Olea europaea leaf polyphenols with a bio-based deep eutectic solvent. Biomass Conver Bioref. https://doi.org/10.1007/s13399-017-0283-5
Blidi S, Bikaki M, Grigorakis S, Loupassaki S, Makris DP (2015) A comparative evaluation of bio-solvents for the efficient extraction of polyphenolic phytochemicals: apple waste peels as a case study. Waste Biomass Valor 6:1125–1133
Pulido R, Bravo L, Saura-Calixto F (2000) Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem 48(8):3396–3402
Sioumis N, Kallithraka S, Tsoutsouras E, Makris DP, Kefalas P (2005) Browning development in white wines: dependence on compositional parameters and impact on antioxidant characteristics. Eur Food Res Technol 220:326–330
Jeong KM, Ko J, Zhao J, Jin Y, Yoo DE, Han SY, Lee J (2017) Multi-functioning deep eutectic solvents as extraction and storage media for bioactive natural products that are readily applicable to cosmetic products. J Cleaner Prod 151:87–95
Bi W, Tian M, Row KH (2013) Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J Chrom A 1285:22–30
Alvarez-Parrilla E, Laura A, Torres-Rivas F, Rodrigo-Garcia J, González-Aguilar GA (2005) Complexation of apple antioxidants: chlorogenic acid, quercetin and rutin by β-cyclodextrin (β-CD). J Inclus Phenomena Macrocycl Chem 53:121–129
Jullian C, Miranda S, Zapata-Torres G, Mendizábal F, Olea-Azar C (2007) Studies of inclusion complexes of natural and modified cyclodextrin with (+)-catechin by NMR and molecular modeling. Bioorg Med Chem 15:3217–3224
Nguyen TA, Liu B, Zhao J, Thomas DS, Hook JM (2013) An investigation into the supramolecular structure, solubility, stability and antioxidant activity of rutin/cyclodextrin inclusion complex. Food Chem 136:186–192
Liu J, Qiu L, Gao J, Jin Y (2006) Preparation, characterization and in vivo evaluation of formulation of baicalein with hydroxypropyl-β-cyclodextrin. Inter J Pharmaceut 312:137–143
Kalogeropoulos N, Yannakopoulou K, Gioxari A, Chiou A, Makris DP (2010) Polyphenol characterization and encapsulation in β-cyclodextrin of a flavonoid-rich Hypericum perforatum (St John’s wort) extract. LWT Food Sci Technol 43:882–889
Mourtzinos I, Makris DP, Yannakopoulou K, Kalogeropoulos N, Michali I, Karathanos VT (2008) Thermal stability of anthocyanin extract of Hibiscus sabdariffa L. in the presence of β-cyclodextrin. J Agric Food Chem 56(21):10303–10310
Çelik SE, Özyürek M, Tufan AN, Güçlü K, Apak R (2011) Spectroscopic study and antioxidant properties of the inclusion complexes of rosmarinic acid with natural and derivative cyclodextrins. Spectrochim Acta Part A: Mol Biomol Spectr 78:1615–1624
Medronho B, Valente AJ, Costa P, Romano A (2014) Inclusion complexes of rosmarinic acid and cyclodextrins: stoichiometry, association constants, and antioxidant potential. Colloid Polymer Sci 292:885–894
Shao P, Zhang J, Fang Z, Sun P (2014) Complexing of chlorogenic acid with β-cyclodextrins: Inclusion effects, antioxidative properties and potential application in grape juice. Food Hydrocol 41:132–139
Çelik SE, Özyürek M, Güçlü K, Apak R (2015) Antioxidant capacity of quercetin and its glycosides in the presence of β-cyclodextrins: influence of glycosylation on inclusion complexation. J Inclus Phenomena Macrocycl Chem 83:309–319
Apostolakis A, Grigorakis S, Makris DP (2014) Optimisation and comparative kinetics study of polyphenol extraction from olive leaves (Olea europaea) using heated water/glycerol mixtures. Separ Purif Technol 128:89–95
Taamalli A, Arráez-Román D, Ibañez E, Zarrouk M, Segura-Carretero A, Fernández-Gutiérrez A (2012) Optimization of microwave-assisted extraction for the characterization of olive leaf phenolic compounds by using HPLC-ESI-TOF-MS/IT-MS2. J Agric Food Chem 60(3):791–798
Murata M, Sugiura M, Sonokawa Y, Shimamura T, Homma S (2002) Properties of chlorogenic acid quinone: Relationship between browning and the formation of hydrogen peroxide from a quinone solution. Biosci Biotechnol Biochem 66:2525–2530
Tzika ED, Papadimitriou V, Sotiroudis TG, Xenakis A (2008) Oxidation of oleuropein studied by EPR and spectrophotometry. Eur J Lipid Sci Technol 110:149–157
Antolovich M, Bedgood DR, Bishop AG, Jardine D, Prenzler PD, Robards K (2004) LC-MS investigation of oxidation products of phenolic antioxidants. J Agric Food Chemi 52:962–971
Sioumis N, Kallithraka S, Makris DP, Kefalas P (2006) Kinetics of browning onset in white wines: influence of principal redox-active polyphenols and impact on the reducing capacity. Food Chem 94:98–104
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Compliance with Ethics requirements
The authors declare that the study involved no Human Participants and/or Animals.
Rights and permissions
About this article
Cite this article
Athanasiadis, V., Grigorakis, S., Lalas, S. et al. Stability effects of methyl β-cyclodextrin on Olea europaea leaf extracts in a natural deep eutectic solvent. Eur Food Res Technol 244, 1783–1792 (2018). https://doi.org/10.1007/s00217-018-3090-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-018-3090-8