Abstract
The use of general and specific exopeptidases is of great interest for the hydrolysis of food proteins. Protein hydrolysates with a high degree of hydrolysis and, therefore, a reduced bitterness and improved antioxidative capacity can be produced due to the synergistic specificities of the general aminopeptidase PepN and the proline-specific peptidase PepX. These two activities were previously combined in a fusion protein and the latter showed both specific activities. However, due to its solubility an application of the fusion protein in continuous processes will be complicated in the future. Therefore, the aim of this study was the production, characterization and use of cross-linked enzyme aggregates (CLEAs) from the fusion protein (FUS-PepN_PepX CLEAs). The FUS-PepN_PepX CLEAs produced had activity for both specific enzymes. The biochemical characteristics determined (e.g., pH and temperature optima, environmental conditions) showed that the CLEAs are suitable for application in a complex matrix, such as food protein hydrolysates. The relative degree of hydrolysis of a prehydrolyzed casein solution was increased by 100% and the hydrolysate obtained showed a strong antioxidative capacity (ABTS-IC50 value: 7.85 µg mL−1). The stability against NaCl and the possibility of using ethanol as a microbial hurdle as well as the size of the FUS-PepN_PepX CLEAs seem promising for an application in an enzyme membrane reactor in the future. In summary, using these CLEAs, casein hydrolysates with a high degree of hydrolysis, a potentially reduced bitterness and high antioxidative capacity can be produced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of general and specific aminopeptidases in the food industry is widely spread. Cheese-making, baking and meat tenderization are examples of their application [1]. However, peptidases are also used to produce protein hydrolysates with different functionalities. The hydrolysates can be used, for example, for emulsification, foam forming, gelatinization or as seasoning [2, 3], and the protein hydrolysis can also be performed to improve digestibility, modify sensory quality, such as texture or taste, improve antioxidative capacity or reduce allergenic compounds [4–6]. An issue of many protein hydrolysates is that they have a bitter taste resulting from peptides with a low molecular weight, composed mainly of hydrophobic amino acids [7]. A possibility to overcome the bitterness is the use of exopeptidases [7–11], such as the proline-specific X-prolyl-dipeptidyl aminopeptidase (PepX; EC 3.4.14.11) in combination with a general aminopeptidase N (PepN; EC 3.4.11.2) [8]. Our group studied these two enzymes as soluble single enzymes (PepX and PepN) [12], and as a combined fusion protein (FUS-PepN_PepX; molecular mass: 188.3 kDa) [5]. The fusion protein was produced by adding a linker of ten amino acids (SSGLVPRGSH) between both enzymes and, therefore, combined both activities in one single protein [5]. Consequently, it was found that the relative degree of hydrolysis of a casein hydrolysate was increased in both cases by approximately 130% [5, 12], and the bitterness was decreased (unpublished data). An immobilization method seemed promising because the reuse of soluble enzymes is challenging. One of the simplest methods for immobilization is the adsorption of the enzyme on the surface of a carrier [13]. The disadvantage of this method is the weak binding, caused by electrostatic, hydrophobic or ionic interactions. Thus, the enzymes can be easily released from the surface by changing the environmental conditions, such as ion composition, pH value and temperature. The formation of covalent bonds between the enzyme and the carrier increases the stability, but can also reduce its activity [14]. In addition to many other immobilization approaches, enzymes can be immobilized by cross-linking, as was done in the current study. The first generation of so-called cross-linked enzyme aggregates (CLEAs) was described in 2000 [15]. Briefly, the enzymes are first precipitated by the addition of, for example, salts or organic solvents [16], followed by the cross-linking of the enzymes precipitated by glutaraldehyde [17]. The advantages of CLEAs are that they are insoluble, the superstructure of the enzyme is conserved and, therefore, the catalytic activity is retained [18]. In addition, compared with free enzymes, CLEAs can be more stable to denaturation by heat or organic solvents and proteolysis than the particular soluble enzyme [19–21]. However, CLEAs can also have some disadvantages, such as a diffusion limitation, mainly with large substrates [22, 23]. For example, this was shown for an immobilized α-amylase using starch as a substrate [24]. Here, the K M value increased from 3.5 mg mL−1 (free enzyme) to 10.5 mg mL−1 (CLEAs). A further example is CLEAs prepared from the lipase CalB [25]. It was found that the small substrate p-nitrophenyl propionate was hydrolyzed faster than the large substrate triacetin. Further disadvantages of CLEAs are that they are small and fragile. Thus, they can form clumps during centrifugation and filtration treatments, which can cause internal mass-transfer limitations and difficulties in handling and full recovery (recycling) of the CLEA particles [21, 26–29]. Nevertheless, due to the simplicity of the preparation of CLEAs, we produced “combi-CLEAs” previously, which consisted of single PepX and PepN [30]. The advantage of “combi-CLEAs”, compared to CLEAs produced from one single enzyme, is generally that a so-called “catalytic cascade process” can be realized [22, 31, 32]. The goal of these “PepN/PepX combi-CLEAs” was to produce hydrolysates with a high degree of hydrolysis and, thus, a reduced bitterness, by combining the two synergistic activities of PepX and PepN [30]. Unfortunately, as shown in that study, the relative degree of hydrolysis was increased by approximately 52% and not 130% as it was for the soluble enzymes [5, 12, 30]. This was most probably caused by the accessibility of the casein-derived peptides to the active sites [30]. A hypothesis to overcome this issue is the production of CLEAs from the fusion protein FUS-PepN_PepX. In theory, such CLEAs should show a better accessibility, caused simply by their more unrestricted structure. Therefore, the aim of the current study was the production of FUS-PepN_PepX CLEAs and their biochemical characterization. In addition, their versatility should be shown during a casein hydrolysis.
Materials and methods
Materials
All chemicals were of analytical grade and obtained from Sigma-Aldrich GmbH (Schnelldorf, Germany), Carl Roth (Karlsruhe, Germany), AppliChem GmbH (Darmstadt, Germany) or Merck AG (Darmstadt, Germany). The chromogenic peptides H-Ala-pNA and H-Ala-Pro-pNA were purchased from Bachem AG (Bubendorf, Switzerland). Sodium caseinate powder was purchased from FrieslandCampina (Amersfoort, Netherlands). Alcalase® 2.5 L was acquired from Novozymes (Bagsværd, Denmark).
Recombinant production and purification of FUS-PepN_PepX
The expression and purification of the fusion protein FUS-PepN_PepX were carried out as described previously [5]. In short, transformed E. coli BL21(DE3) cells containing a pET-20b(+)_pepN_L1_pepX vector were cultivated in a tabletop bioreactor system (Minifors, Infors AG, Bottmingen/Basel, Switzerland) at 37 °C. The recombinant protein expression was induced at OD600 = 5 using 0.5 mM IPTG (isopropyl-β-D-1-thiogalactopyranoside) and the temperature was reduced to 30 °C to minimize the formation of inclusion bodies. The culture was harvested after 9.5 h of cultivation and stored at −20 °C. Due to the His6-tag attached, FUS-PepN_PepX was purified by Ni2+-charged immobilized metal affinity chromatography (IMAC; IDAlow, KNAUER Wissenschaftliche Geräte GmbH, Berlin, Germany; 1 column volume = 18.5 mL) using an ÄKTA-FPLC system (GE Healthcare, München, Germany). Increasing the concentration of imidazole to 500 mM eluted FUS-PepN_PepX. Afterwards, the fusion protein was desalted in Na2HPO4/KH2PO4 buffer (50 mM; pH 6.5).
Standard assay for the determination of PepN and PepX activity
The particular activity of the soluble fusion protein and the CLEAs was determined as described previously [5, 12, 30], with minor modifications. For the standard assay, 50.5 µL of the enzyme solution was incubated with 177 µL Na2HPO4/KH2PO4 buffer (50 mM; pH 6.5) for 5 min at 37 °C. To start the reaction, 12.5 µL of the specific chromogenic substrate (FUS-PepN-activity: 7.5 mgH-Ala-pNA/mLDMSO; FUS-PepX-activity: 5 mgH-Ala-Pro-pNA/mLDMSO) was added to the samples. The reaction was stopped by adding 50 µL acetic acid (50% (v/v)) to the mixture. The samples were centrifuged at 20,000× g for 5 min and 240 µL of the supernatant was transferred to a microtiter plate. The absorbance of the samples was measured (Multiskan FC, Thermo Scientific, Braunschweig, Germany) at 405 nm. One katal (kat) of FUS-PepN or FUS-PepX activity was defined as the release of 1 mol p-nitroaniline per s.
The particular activity of the CLEAs (FUS-PepNCLEA or FUS-PepXCLEA) was measured in the same manner, except buffer was added to the samples instead of enzyme solution. Thus, 227.5 µL Na2HPO4/KH2PO4 buffer (50 mM; pH 6.5) was added to the CLEAs after washing (see below).
FUS-PepN_PepX CLEA preparation
The protein concentration (10.1–114 µg mL−1), precipitant (acetone, ethanol, 1-butanol, 2-butanol, 1-propanol, 2-propanol, ammonium sulfate), precipitant concentration (ammonium sulfate; 0.1–4 M), cross-linking time (0.25–24 h), and cross-linking agent concentration (glutaraldehyde; 10–200 mM) was investigated in preliminary studies concerning the most suitable conditions for the FUS-PepN_PepX CLEAs preparation protocol. Subsequently, the influence of casein as a proteic feeder was tested. The most suitable parameters for the preparation regarding the activity yieldCLEA obtained [30] are summarized in Table 1. The activity yieldCLEA was expressed as residual activity of the CLEAs compared to the free enzyme.
Thus, the standard procedure for the preparation of FUS-PepN_PepX CLEAs was performed as follows: after cooling the precipitating agent (ammonium sulfate, 3 M; 199.5 µL) on ice for 15 min, the purified and diluted FUS-PepN_PepX solution (50.5 µL; 31.3 µgprotein mL−1) was added. After a further cooling period of 15 min, the solution was centrifuged (20,000× g, 4 °C, 5 min) and glutaraldehyde (final concentration: 50 mM) was added to induce cross-linking. Afterwards, the samples were stored on ice for 1 h and, subsequently, centrifuged (20,000× g, 4 °C, 5 min). The supernatant was discarded and the pellet was washed twice with Na2HPO4/KH2PO4 buffer (50 mM; pH 6.5; 1 mL).
Biochemical characterization of FUS-PepN_PepX CLEAs
The FUS-PepN_PepX CLEAs prepared were investigated regarding application-relevant characteristics. The standard assay was used unless otherwise stated.
Influence of pH and temperature on the initial activity of FUS-PepN_PepX CLEAs and temperature stability
The CLEAs were prepared as described above for determining the influence of the pH, but the specific buffer was used for washing instead of the standard buffer. The pH was varied in the range between 5.0 and 9.0 and all buffers had a concentration of 50 mM. The following buffers were used: Na/K phosphate (pH 5.0–7.5), Bis–Tris/HCl (pH 6.5–7.5) and Tris/HCl (pH 7.5–9.0). Incubation was performed at 30–75 °C to determine the influence of the temperature, in contrast to the standard assay. The CLEAs were suspended in standard buffer (227.5 µL) containing 0.1% (w/v) sodium azide to prevent microbial growth, and incubated at 0, 37 and 50 °C for up to 2 weeks for the temperature stability. Samples were taken several times for determination of the activity.
Influence of organic solvents, CaCl2, NaCl and EDTA on FUS-PepN_PepX CLEAs
The assay conditions were identical to the standard assay, except that the CLEA pellets were suspended in 203.5 µL buffer instead of 227.5 µL. In addition, 24 µL of the test substance was added. The concentration of the organic solvents was set to 10% (v/v) in the final assay. The concentration of CaCl2 and ethylenediaminetetraacetic acid (EDTA) in the final assay varied between 0.1 and 10 mM and 0.0001 and 0.01 mM, respectively. Both compounds were dissolved in H2Odd.
For the investigation of the NaCl influence standard CLEA production and activity assay conditions were used except that the standard buffer (see above) was used for CLEA washing and activity determination contained different NaCl concentrations (final concentration: 0–2.5 M).
Determination of the kinetic parameters of FUS-PepN_PepX CLEAs
The kinetic parameters of the FUS-PepN_PepX CLEAs were individually determined using H-Ala-pNA (FUS-PepNCLEA activity) and H-Ala-Pro-pNA (FUS-PepXCLEA activity) as substrates. Standard activity assay conditions were used, in which the final substrate concentration ranged from 0.01 to 6.2 mM depending on the particular enzyme activity and substrate. The results were plotted according to Michaelis–Menten and the kinetic parameters were calculated either, as described previously [12], by Hanes linearization or by nonlinear regression fitting using SigmaPlot 12.5 (Systat Software, Inc,. San Jose, CA) [5, 33].
Hydrolysis of casein with Alcalase® and the FUS-PepN_PepX CLEAs
At first, casein was prehydrolyzed using the endopeptidase preparation Alcalase® 2.5 L (Novozymes, Bagsvaerd, Denmark). In a second step, the prepared peptides were further hydrolyzed by the CLEAs produced from fusion protein FUS-PepN_PepX.
Determination of the initial activity of Alcalase® with o-phthaldialdehyde (OPA)
The initial enzymatic activity of Alcalase® 2.5 L was determined as described previously [5, 12, 30] using casein (1% (w/v)) dissolved in Na/K phosphate buffer (50 mM; pH 6.5) as a substrate and, subsequently, derivatized with o-phthaldialdehyde [34]. One katal of proteolytic activity was defined as the amount of enzyme required to release 1 mol of l-serine equivalent amino groups per s.
Prehydrolysis of casein with Alcalase®
A prehydrolysis of casein using Alcalase® 2.5 L was performed, as described previously [5, 12, 30]. Samples (450 µL) were taken at various times during hydrolysis until no increase in serine equivalents was observed. The samples were added to 50 µL of sodium dodecyl sulfate (SDS; 10% (w/v)) and heated to 80 °C for 10 min for enzyme inactivation. After centrifugation (8000× g, 5 min), the degree of hydrolysis (DH) of the samples was analyzed and calculated using the OPA assay, as described previously [5, 12, 30]. In addition, samples (1 mL) were taken and directly inactivated (80 °C, 10 min) without the addition of SDS. These samples were used for the determination of the antioxidative effect and the gas chromatographic analysis (see below). All inactivated samples were stored at −20 °C for analysis conducted later. At the end, the hydrolyzed casein solution was heated to 85 °C for 30 min and stored at −20 °C.
Application of FUS-PepN_PepX CLEAs on a prehydrolyzed casein solution
The prehydrolyzed casein solution (see above) was used as a substrate for further hydrolysis with FUS-PepN_PepX CLEAs. Sodium azide (0.1% (w/v)) was added to the prehydrolyzed casein solution (40 mL). The solution was incubated at 37 °C for 30 min. Afterwards, FUS-PepN_PepX CLEAs were added with a standardized FUS-PepNCLEA activity of 200 nkatH-Ala-pNA. Samples were taken over a period of 48 h and treated as described above. Finally, the hydrolyzed casein solution was heated to 85 °C for 30 min and stored at −20 °C.
Gas chromatographic analysis of the casein hydrolysates
The analysis of the casein hydrolysates (determination of amino acids released) was realized using a GC-2010Plus (Shimadzu, Kyoto, Japan) with an AOC-20i autoinjector, a flame ionization detector (FID) and a Zebron ZB-1701 column (30 m × 0.25 mm × 0.25 µm; Phenomenex, Torrance, USA). The injection (1 µL) was carried out splitless. Helium was used as a carrier gas with a column flow rate of 3.33 mL min−1. The injector temperature was 250 °C and the temperature of the FID was 375 °C. A temperature program was carried out for the separation of the amino acids. The initial column temperature was set to 140 °C and held for 2.5 min. Afterwards, the temperature was increased at a rate of 40 °C min−1 to a final temperature of 300 °C. The final temperature was held for a further 2.5 min to elute all sample substances from the column. Before the samples were injected into the GC, derivatization of the amino acids was performed [35, 36]. At first, the samples (without SDS) from the casein hydrolysis were diluted (1:12.5) and an amount of 120 µL was combined with 20 µL HCl (1 M) containing the internal standard (ISTD; L-norvaline, 10 mM). Afterwards, 60 µL of the sample prepared was transferred into a glass vial and 80 µL of ethanol/pyridine (ratio 4:1) and 10 µL of ethyl chloroformate were added. Subsequently, the samples were mixed at 900 rpm (ThermoMixer comfort, Eppendorf, Hamburg, Germany) for 5 min and 150 µL chloroform containing 1% (v/v) ethyl chloroformate were added to the sample. After another shaking step (900 rpm, 5 min), the samples were left to stand for 10 min without shaking, and 100 µL of the lower phase was transferred to a GC vial. The assignment of the peaks detected was realized over the retention time of reference amino acids.
Antioxidative effect of the casein hydrolysates
The antioxidative effect of the different samples during the hydrolysis was determined using the ABTS∙+ [2,2-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt] decolorization assay [37–39]. Therefore, an ABTS∙+ stock solution was prepared with ABTS (7 mM) and ammonium peroxodisulfate (APS; 2.45 mM) in H2Odd and was left to react for 12–16 h in the dark. Before usage, the stock solution was diluted with phosphate-buffered saline (pH 7.4) to an absorption of 0.700 ± 0.05 at 734 nm. The diluted ABTS∙+ solution (1000 µL) was mixed with the specific diluted samples (without SDS) from the casein hydrolysis (10 µL; 5 µgcasein mL−1) and incubated at 30 °C in the dark for 15 min. Afterwards, the samples were measured at 734 nm. Na/K phosphate buffer (50 mM; pH 6.5) instead of the sample was used as a reference.
The ABTS-IC50 value was defined as the amount of hydrolysate required to reduce the absorbance of ABTS∙+ to 50%. Therefore, the final sample after FUS-PepN_PepX CLEA treatment was diluted to final concentrations between 0.5 and 25 µgcasein mL−1 and used in the ABTS∙+ assay as described above.
Statistical analysis
The standard deviation was used for data evaluation and calculated with Excel (Microsoft, Redmond, USA). All experiments were conducted at least in duplicate, with three independent measurements. The standard deviation was always below 5%.
Results
The current study deals mainly with the characterization and application of CLEAs produced from the fusion protein consisting of the exopeptidases PepN and PepX (FUS-PepN-L1-PepX) [5]. The exopeptidases originated from Lb. helveticus ATCC 12046 [12]. The CLEAs of the fusion protein will be abbreviated as FUS-PepN_PepX CLEAs in the following. The PepN activity in the CLEAs will be called FUS-PepNCLEA activity and the PepX activity, FUS-PepXCLEA activity.
Biochemical characterization of FUS-PepN_PepX CLEAs
To the best of our knowledge, no CLEAs have ever been produced from a fusion protein in general, or using FUS-PepN_PepX specifically. However, the CLEA productions of either PepN or PepX and the production of so-called “combi-CLEAs” of PepN/PepX are described [30]. A suitable CLEA production protocol was evaluated before the biochemical characteristics of FUS-PepN_PepX CLEAs were determined and compared to the others. The activity yieldCLEA obtained using the conditions optimized for FUS-PepNCLEA and FUS-PepXCLEA activity were approximately 15 and 50%, respectively.
Influence of pH and temperature on the initial activity of FUS-PepN_PepX CLEAs
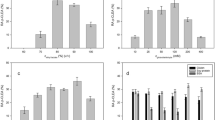
All buffers used for determining the optimum pH (Fig. 1a, b) of the FUS-PepNCLEA and FUS-PepXCLEA activity had a concentration of 50 mM. The highest values for both activities were detected at a pH of 7.0. It was shown that the buffer substance used had no influence on the particular CLEA activity. In addition, the initial FUS-PepXCLEA activity showed a broad optimal pH range (Fig. 1b). The activity was over 70% in a pH range between 5.5 and 8.0. By contrast, the FUS-PepNCLEA activity was sharper (Fig. 1a). The initial activity decreased directly to 70% or below using buffers with pH values of 6.5 and 7.5. The optimum temperature for the FUS-PepNCLEA activity was determined at 40 °C (Fig. 1c). At a higher temperature (45 °C), 74% of the maximum activity was measured. At the highest temperature tested (60 °C), the activity was still 11%. The highest value for the initial FUS-PepXCLEA was determined at 60 °C (Fig. 1d). However, only a minor lower activity (97%) was detected at 55 °C. A residual activity of 46 and 21% was measured at 70 and 75 °C, respectively. The temperature stability of FUS-PepN_PepX CLEAs was determined at 0, 37 and 50 °C. At 0 °C (on ice), both activities were relatively stable over the analysis time (14 days) with a residual activity of 50 and 60% for FUS-PepNCLEA and FUS-PepXCLEA, respectively. At 37 °C, near the optimum temperature of FUS-PepNCLEA, the residual activity after 2 days was 70 and 91% for FUS-PepNCLEA and FUS-PepXCLEA, respectively. Finally, after 14 days at this temperature, the residual activity decreased to 5 and 27% for FUS-PepNCLEA and FUS-PepXCLEA, respectively. At 50 °C the residual activity after 2 days was 13 and 61% for FUS-PepNCLEA and FUS-PepXCLEA, respectively. After 14 days, the residual activity was approximately 1% for FUS-PepNCLEA and 13% for FUS-PepXCLEA. In addition to the temperature stability, also the storage stability of the FUS-PepN_PepX CLEAs was determined. Therefore, the CLEAs were stored at −20 °C and the activity was tested several times. As a result, it was found that the residual FUS-PepNCLEA activity (26%) was less compared to the residual FUS-PepXCLEA activity (71%) after 4 weeks of storage.
Influence of organic solvents, CaCl2, NaCl and EDTA on the FUS-PepN_PepX CLEA activity
The pNA substrates have a low solubility in water, as described previously [5], and, therefore, were dissolved in dimethyl sulfoxide (DMSO). Thus, the pNA standard assay always contained 5.2% (v/v) DMSO. The influence of different organic solvents on the FUS-PepN_PepX CLEA activity was measured in the presence of an additional 10% (v/v) of the specific solvent (Table 2). The activity value after the addition of 10% (v/v) H2Odd was used as a reference (100%). The FUS-PepNCLEA activity was reduced to 73% by additional DMSO, whereas the FUS-PepXCLEA activity was reduced to 53%. However, overall, the other solvents tested (ethanol, acetone and dimethylformamide (DMF)) had a stronger negative effect on the enzyme activity of FUS-PepN_PepX CLEAs. Thus, DMSO is the organic solvent of choice for substrates that are not soluble in water. In addition, the influence of the metal chelate building reagent EDTA (ethylenediaminetetraacetic acid) on the FUS-PepN_PepX CLEAs was analyzed. Due to the fact that PepN is a metallopeptidase [12], it was expected that EDTA would reduce the FUS-PepNCLEA activity remarkably. However, a reduction of FUS-PepNCLEA activity was determined (residual activity: 25%) only at the highest concentration tested (0.01 mM). As expected, the FUS-PepXCLEA activity was not affected, because PepX is a serine peptidase [12]. Finally, two different metal salts were investigated for their influence on FUS-PepN_PepX CLEAs. Both metal salts were used as chlorides to prevent any influence of the anion. Interestingly, the addition of CaCl2, up to a concentration of 10 mM, had no remarkable influence on either the FUS-PepNCLEA or FUS-PepXCLEA activity. Due to the fact that NaCl is sometimes used in food protein hydrolysis processes as an antimicrobial additive [5], its effect on the FUS-PepN_PepX CLEA activity was investigated in more detail (Fig. 2). The FUS-PepNCLEA activity decreased directly with the addition of NaCl. The residual activity was decreased to 93%, even at low concentrations (0.04 M). However, at the highest concentration tested (2.5 M), the residual FUS-PepNCLEA activity was still 13%. By contrast, the FUS-PepXCLEA activity increased up to 112% at NaCl concentrations between 0.16 and 0.32 M. At higher concentrations, the activity decreased to a final value of 80% residual activity at 2.5 M.
Determination of the kinetic parameters of FUS-PepN_PepX CLEAs
The kinetic parameters of the FUS-PepN_PepX CLEAs (V max , K M and K IS) were determined using H-Ala-pNA and H-Ala-Pro-pNA as specific substrates. A valid protein determination of the supernatant retained was not possible due to the high ammonium sulfate content during CLEA preparation. Therefore, it cannot be ensured that all the protein applied was incorporated in the CLEAs under the conditions used for the CLEA preparation. Consequently, the V max values cannot be given as specific values and the definitive volumetric activities were plotted according to Michaelis–Menten (Fig. 3). The kinetic parameters were calculated similarly (Table 3). A strong substrate inhibition (K IS the substrate concentration at half V max inhibition [12]) was determined for the FUS-PepNCLEA activity using the substrate H-Ala-pNA (Fig. 3a). By contrast, no substrate inhibition was determined for the FUS-PepXCLEA activity with H-Ala-Pro-pNA as a substrate (Fig. 3b). No remarkable differences in the kinetic parameters were observed using the Michaelis–Menten plot/Hanes linearization and nonlinear regression fitting using SigmaPlot (Table 3).
Hydrolysis of casein with Alcalase® and the FUS-PepN_PepX CLEAs
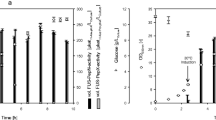
Both PepN and PepX are exopeptidases; therefore, they cannot act on intact proteins. Consequently, the casein solution (2.5% (w/v)) was first hydrolyzed with the commercial endopeptidase preparation Alcalase® 2.5 L, until no increase in the degree of hydrolysis was observed (5 h; Fig. 4). The corresponding amount of serine equivalent (degree of hydrolysis) was defined as 100% relative degree of hydrolysis. After heat inactivation of the Alcalase®, the FUS-PepN_PepX CLEAs were added. The relative degree of hydrolysis increased without delay. This showed that the FUS-PepN_PepX CLEAs could hydrolyze the peptides produced during the Alcalase® treatment. The relative degree of hydrolysis was 193% after 24 h of hydrolysis with the FUS-PepN_PepX CLEAs. During an additional 24 h of hydrolysis, the relative degree of hydrolysis increased only sparsely and achieved a relative degree of hydrolysis of 201% (Fig. 4). Thus, the relative degree of hydrolysis could be increased by approximately 100% by the action of FUS-PepN_PepX CLEAs.
The amino acids released were analyzed by GC-FID (Fig. 5). The casein substrate solution showed no detectable amino acids before the hydrolysis with Alcalase® (Fig. 5a). The peak between 0.8 and 2 min corresponded with the solvent chloroform. The peak at 3.8 min was the ISTD added and the small peak at 4.6 min was an artifact of the derivatization reagents. The same picture was obtained after the prehydrolysis with Alcalase®. This is caused by the fact that only amino acids and small peptides (2–5 amino acids; depending on the composition) are vaporizable after derivatization and these were not produced by the action of the endopeptidase preparation Alcalase®. An increase in the peak number and heights was observed after the application of the FUS-PepN_PepX CLEAs (Fig. 5a). The increase of the relative degree of hydrolysis and the peak number and heights resulted from the release of amino acids, and the short vaporizable peptides from the prehydrolyzed casein were caused by the action of the FUS-PepN_PepX CLEAs. The sample after 48 h of FUS-PepN_PepX CLEA application is shown separately in Fig. 5b for a better visualization. However, it is worth mentioning that the peaks labeled correspond to the retention times of the standard amino acids analyzed. Thus, it cannot be excluded that short peptides with a similar retention time were also present underneath the peaks. The fact that short peptides were present in the hydrolysate was visible due to the small peaks between the peaks labeled. Thus, we decided not to quantify the amino acids released.
Antioxidative effect of the casein hydrolysates
The ABTS∙ + decolorization assay was used to investigate the antioxidative effect at different stages of the casein hydrolysis. All samples tested were diluted to a casein content of 5 µg mL−1. This range was required for a linear relationship between the casein content and antioxidative effect. The antioxidative effect of the non-hydrolyzed casein solution was approximately 13% (Fig. 6a). After the prehydrolysis using Alcalase®, the antioxidative effect increased to approximately 23%. The application of the FUS-PepN_PepX CLEAs increased the antioxidative effect to over 38% at the end of the hydrolysis process. However, the ABTS-IC50 value is a more important value for the evaluation of the hydrolysate antioxidativity. This value was evaluated for the final sample after FUS-PepN_PepX CLEA application. The ABTS-IC50 value of this hydrolysate was 7.85 µg mL−1 (Fig. 6b), which indicated a strong antioxidative capacity.
Course of the antioxidative effect at different stages of the casein hydrolysis with Alcalase® and the FUS-PepN_PepX CLEAs (a), and the relationship between the hydrolysate concentration of the final hydrolysate and the antioxidative effect (b). In a, the casein(hydrolysate) concentration was standardized to 5 µg mL−1, and in b, the hydrolysate concentration varied between 0.5 and 25 µg mL−1. The ABTS∙+ decolorization assay was used for both
Discussion
In this work, CLEAs were produced from the fusion protein FUS-PepN_PepX. These FUS-PepN_PepX CLEAs showed activity for both enzymes. Altogether, this study reported the first production of CLEAs from the fusion protein of two exopeptidases, and their characterization and application in casein hydrolysis. In general, to the best of our knowledge, this is the first time that the same enzymes (here: PepN and PepX) have been characterized, applied for hydrolysis and compared as single soluble enzymes [12], as a soluble fusion protein [5], as CLEAs prepared from single enzymes [30], as “combi-CLEAs” [30], and finally, as CLEAs from the fusion protein (current study).
Enzyme immobilization by cross-linking
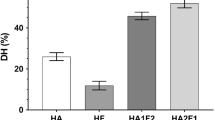
The use of soluble enzymes in industrial applications leads to high costs because they cannot be reused. In addition to many other immobilization approaches (such as carrier-binding, encapsulation or inclusion/entrapment), enzymes can be immobilized by cross-linking [40]. In general, CLEAs are produced without any additional carrier and, thus, without additional costs. Due to this reason CLEAs are ideal catalysts for industrial applications [41]. However, it is always worth keeping in mind that every aspect of the CLEA formation process influences the final structure of the CLEAs prepared and, therefore, the activity retained and accessibility of the active site. Differences became obvious by comparing the activity yields obtained of CLEAs produced (1) out of the single enzymes PepN or PepX [30], (2) the so-called “PepN/PepX combi-CLEAs” [30], and (3) the FUS-PepN_PepX (current study). An activity yieldCLEA of 8 and 16% was obtained for PepN and PepX, respectively, for (1) [30]. These yields were similar for (2), where the activity yieldCLEA was 9 and 18% for the PepN and PepX activity, respectively [30]. However, the activity yieldCLEA was markedly increased for (3), where the yields obtained were 15 and 50% for the PepN and PepX activity, respectively. These results indicated that the structure of FUS-PepN_PepX affects the CLEA formation in a positive way concerning the activity retained after CLEA formation. However, it was still unclear at this point whether the change in structure resulted only in an increased activity yieldCLEA or if it additionally influences the biochemical and kinetic characteristics as well as the versatility during protein hydrolysis.
Comparison of biochemical characteristics
The great advantage of the fusion protein was that both enzyme activities were combined in one single molecule [5]. However, FUS-PepN_PepX was still soluble and, therefore, a reuse for future studies is challenging. Thus, the fusion protein was immobilized by cross-linking, but it was not clear how the biochemical characteristics changed compared to the single enzymes [12], the fusion protein [5], and the CLEAs prepared previously from the single enzymes [30]. It was found during the characterization of the FUS-PepN_PepX CLEAs that some of the characteristics changed. Some selected characteristics are summarized in Table 4. Regarding the optimum pH values, similar optima were obtained, whereas it should be mentioned that the optima were slightly higher for both the single CLEAs [30], and the FUS-PepN_PepX CLEAs compared to the soluble enzymes. The improved pH stability described already for FUS-PepX [5] was also retained for the FUS-PepXCLEA. This is a great advantage compared to the single PepX, because food generally has an acid or neutral pH value. Regarding the optimum temperature, an increase was determined for both FUS-PepNCLEA and FUS-PepXCLEA activity. The highest FUS-PepNCLEA activity was determined at 40 °C, whereas it was 35 °C for FUS-PepN [5] and 30 °C for single PepN [12]. A temperature optimum of 40 °C was also measured for the single PepN-CLEAs [30]. The optimum temperature for FUS-PepXCLEA activity was determined at 60 °C. This was 10 °C higher than measured for FUS-PepX and single PepX activity [5, 12]. An optimum between 50 and 60 °C was determined previously for single PepX-CLEAs [30]. However, the FUS-PepXCLEA activity at 70 °C was still 46%, whereas the single PepXCLEA activity was approximately 10% [30]. A disadvantage of the FUS-PepN_PepX CLEAs was observed concerning temperature stability. Although the temperature for the initial activity was higher than for the soluble enzymes, the stability was similar or lower. The residual activity at 37 °C, the temperature of the later casein hydrolysis, after two days (the time of the casein hydrolysis) was 70 and 91% for the FUS-PepNCLEA and FUS-PepXCLEA, respectively. By contrast, the soluble single enzymes and FUS-PepN_PepX both showed activity at about 90% each [5, 12]. The reason for this is still unclear, but the stability of the FUS-PepN_PepX CLEAs was sufficient for the later application. The microbial stability is a requirement for protein hydrolysis processes. One method in the industry is the application of NaCl to the hydrolysis process [42]. However, the application of NaCl in combination with FUS-PepN_PepX was not possible, because a concentration of 2.5 M reduced the FUS-PepN activity to approximately 0.5% [5]. An improvement of the FUS-PepN_PepX CLEAs was observed here. The FUS-PepN CLEAs were still 13% active at a NaCl concentration of 2.5 M. As an alternative, ethanol can be added to ensure microbial stability [42]. The residual FUS-PepN_PepX CLEA activities obtained with 10% (v/v) ethanol were at 33% (FUS-PepNCLEA) and 54% (FUS-PepXCLEA) comparable to the residual activity of 28% for FUS-PepN and 64% for FUS-PepX [5]. Thus, ethanol seems to be a feasible compound to ensure microbial stability. Concerning the sensibility against the metal ion-chelating reagent EDTA, differences were found for FUS-PepNCLEA compared to FUS-PepN and single PepN. Even a very low concentration of 0.001 mM EDTA reduced the activity of single PepN to 8% [12], whereas the residual activities of FUS-PepN and FUS-PepNCLEA were still 84% [5] and 99%, respectively. At a tenfold increased EDTA concentration (0.01 mM), hardly any activity (1%) was measurable for single PepN [12], and a very low activity for FUS-PepN (6%) [5], while FUS-PepNCLEA activity was still 25%. Differences were also found concerning calcium, which is a common metal ion in milk and casein. Single PepN and FUS-PepN had a residual activity of 8 and 5%, respectively, at the highest CaCl2 concentration tested (10 mM) [5, 12]. By contrast, the FUS-PepNCLEA activity was not influenced by the addition of CaCl2 and showed a residual activity of 95%. In summary, the FUS-PepNCLEA activity was more robust against environmental influences than the single PepN and FUS-PepN and will, therefore, be more suitable for an application in complex matrices such as food. Concerning the kinetic values (K M and K IS), no noteworthy changes were observed between the single PepN [12], FUS-PepN [5], and FUS-PepNCLEA. In comparison to single PepX, the K M value using H-Ala-Pro-pNA as a substrate was reduced for FUS-PepXCLEA. This had already been observed for FUS-PepX [5]. Consequently, the cross-linking of the fusion protein did not negatively influence the kinetic parameters. However, it is always worth keeping in mind that synthetic substrates were used and not original peptide substrates. A change of the kinetic parameters by the use of original peptide substrates was shown previously for single PepX [43], but was not in the focus of the current study.
Application of the FUS-PepN_PepX CLEAs in protein hydrolysis
A possible application of the FUS-PepN_PepX CLEAs is the hydrolysis of food proteins (e.g., casein). This should lead ideally to a product with a high degree of hydrolysis and, therefore, a reduced bitterness and an increased antioxidative capacity. The relative degree of hydrolysis was increased by approximately 130% in our previous studies (Table 4) using the soluble single enzymes PepN and PepX [12] and the fusion protein FUS-PepN_PepX [5]. By contrast, the use of the FUS-PepN_PepX CLEAs reached an increased degree of hydrolysis of about 100%. This indicates that the FUS-PepN_PepX CLEAs are suitable for protein hydrolysis, but the accessibility to the active sites is probably limited. However, compared to the “combi-CLEAs” consisting of PepN/PepX produced previously [30], the increase of the relative degree of hydrolysis was nearly doubled (52% vs. 100%). This means that the accessibility of the fusion protein to the active sites in the CLEAs was better than for the “combi-CLEAs”. The minor lower relative degree of hydrolysis using the FUS-PepN_PepX CLEAs compared to the soluble fusion protein resulted in a slightly lower antioxidative effect of the resulting hydrolysate. However, both hydrolysates showed an inhibition of approximately 38% by comparing the inhibition of both hydrolysates (standardized hydrolysate concentration: 5 µg mL−1) at the point of 100% increased relative degree of hydrolysis (data not shown in our previous study [5]). The ABTS-IC50 value of the final casein hydrolysate obtained with FUS-PepN_PepX CLEAs was 7.85 µg mL−1 compared to 5.81 µg mL−1 for the hydrolysate produced with the soluble fusion protein FUS-PepN_PepX [5]. The minimal lower final antioxidative capacity determined in the current study can probably be explained by a lower release of tyrosine (Fig. 5b) and/or YP dipeptides. The strong antioxidative effect of tyrosine and tyrosine-containing dipeptides has been described previously [38, 39, 44, 45].
Conclusion
It was demonstrated that the CLEAs produced from the fusion protein FUS-PepN_PepX exhibited both enzyme activities. In addition, the activity yieldCLEA obtained was markedly increased compared to the previous “combi-CLEA” preparation. The biochemical characteristics of the FUS-PepN_PepX CLEAs obtained, such as the increased stability against NaCl and the possibility of using ethanol as a microbial hurdle, indicated that they are suitable for application in food protein hydrolysis. In addition, the application in an enzyme membrane reactor in the future seems promising because the CLEAs should not penetrate the membrane. This should be verified in a further study. Finally, the CLEAs were suitable to produce casein hydrolysates with a high antioxidative capacity, which can protect products against oxidative stress. However, it is worth mentioning that the accessibility to the active sites and, therefore, the relative degree of hydrolysis was lower than for the soluble fusion protein, but markedly increased compared to the “combi-CLEA”. Thus, it depends on the specific application and demand of the hydrolysate whether the enzyme of choice is the soluble fusion protein or the FUS-PepN_PepX CLEAs.
References
Rao MB, Tanksale AM, Ghatge MS, Deshpande VV (1998) Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev 62:597–635
Sprössler BG (2003) Enzymanwendungen in der Lebensmittelindustrie und Entwicklungstrends. In: Heiss R (ed) Leb. Biotechnol. Mech. und thermische Verfahren der Lebensmittelverarbeitung. Springer, Berlin, Heildelberg, pp 479–488
Ewert J, Claaßen W, Glück C, Zeeb B, Weiss J, Hinrichs J, Stressler T, Fischer L (2016) A non-invasive method for the characterisation of milk protein foams by image analysis. Int Dairy J 62:1–9
Tavano OL (2013) Protein hydrolysis using proteases: an important tool for food biotechnology. J Mol Catal B Enzym 90:1–11
Stressler T, Pfahler N, Merz M, Hubschneider L, Lutz-Wahl S, Claaßen W, Fischer L (2016) A fusion protein consisting of the exopeptidases PepN and PepX—production, characterization, and application. Appl Microbiol Biotechnol 100:1–17
Merz M, Kettner L, Langolf E, Appel D, Blank I, Stressler T, Fischer L (2015) Production of wheat gluten hydrolysates with reduced antigenicity employing enzymatic hydrolysis combined with downstream unit operations. J Sci Food Agric 96:3358–3364
Saha BC, Hayashi K (2001) Debittering of protein hydrolyzates. Biotechnol Adv 19:355–370
FitzGerald RJ, O’Cuinn G (2006) Enzymatic debittering of food protein hydrolysates. Biotechnol Adv 24:234–237
Nishiwaki T, Yoshimizu S, Furuta M, Hayashi K (2002) Debittering of enzymatic hydrolysates using an aminopeptidase from the edible basidiomycete Grifola frondosa. J Biosci Bioeng 93:60–63
Raksakulthai R, Haard NF (2003) Exopeptidases and their application to reduce bitterness in food: a Review. Crit Rev Food Sci Nutr 43:401–445
Tan PST, Van Kessel TAJM, Van de Veerdonk FLM, Zuurendonk PF, Bruins AP, Konings WN (1993) Degradation and debittering of a tryptic digest from β-casein by aminopeptidase N from Lactococcus lactis subsp. cremoris WG2. Appl Environ Microbiol 59:1430–1436
Stressler T, Eisele T, Schlayer M, Lutz-Wahl S, Fischer L (2013) Characterization of the recombinant exopeptidases PepX and PepN from Lactobacillus helveticus ATCC 12046 important for food protein hydrolysis. PLoS ONE 8:e70055
Buchholz K, Kasche V, Bornscheuer UT (2012) Biocatalysts and enzyme technology. Wiley, New York
Mateo C, Palomo JM, Fernandez-Lorente G, Guisan JM, Fernandez-Lafuente R (2007) Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb Technol 40:1451–1463
Cao L, van Rantwijk F, Sheldon RA (2000) Cross-linked enzyme aggregates: a simple and effective method for the immobilization of penicillin acylase. Org Lett 2:1361–1364
Hofland GW, de Rijke A, Thiering R, van der Wielen LA, Witkamp GJ (2000) Isoelectric precipitation of soybean protein using carbon dioxide as a volatile acid. J Chromatogr B Biomed Sci Appl 743:357–368
Cao L, Van Langen LM, Van Rantwijk F, Sheldon RA (2001) Cross-linked aggregates of penicillin acylase: robust catalysts for the synthesis of β-lactam antibiotics. J Mol Catal B Enzym 11:665–670
Sheldon RA (2011) Characteristic features and biotechnological applications of cross-linked enzyme aggregates (CLEAs). Appl Microbiol Biotechnol 92:467–477
Yu HW, Chen H, Wang X, Yang YY, Ching CB (2006) Cross-linked enzyme aggregates (CLEAs) with controlled particles: application to Candida rugosa lipase. J Mol Catal B Enzym 43:124–127
Cui JD, Jia SR (2015) Optimization protocols and improved strategies of cross-linked enzyme aggregates technology: current development and future challenges. Crit Rev Biotechnol 35:15–28
Cui J, Zhao Y, Feng Y, Lin T, Zhong C, Tan Z, Jia S (2017) Encapsulation of spherical cross-linked phenylalanine ammonia lyase aggregates in mesoporous biosilica. J Agric Food Chem 65:618–625
Hwang ET, Gu MB (2013) Enzyme stabilization by nano/microsized hybrid materials. Eng Life Sci 13:49–61
Garcia-Galan C, Berenguer-Murcia Á, Fernandez-Lafuente R, Rodrigues RC (2011) Potential of different enzyme immobilization strategies to improve enzyme performance. Adv Synth Catal 353:2885–2904
Dalal S, Kapoor M, Gupta MN (2007) Preparation and characterization of combi-CLEAs catalyzing multiple non-cascade reactions. J Mol Catal B Enzym 44:128–132
Schoevaart R, Wolbers MW, Golubovic M, Ottens M, Kieboom APG, van Rantwijk F, van der Wielen LAM, Sheldon RA (2004) Preparation, optimization, and structures of cross-linked enzyme aggregates (CLEAs). Biotechnol Bioeng 87:754–762
Pchelintsev NA, Youshko MI, Švedas VK (2009) Quantitative characteristic of the catalytic properties and microstructure of cross-linked enzyme aggregates of penicillin acylase. J Mol Catal B Enzym 56:202–207
Kopp W, Da Costa TP, Pereira SC, Jafelicci M Jr, Giordano RC, Marques RFC, Araújo-Moreira FM, Giordano RLC (2014) Easily handling penicillin G acylase magnetic cross-linked enzymes aggregates: catalytic and morphological studies. Process Biochem 49:38–46
Cui J, Jia S, Liang L, Zhao Y, Feng Y (2015) Mesoporous CLEAs-silica composite microparticles with high activity and enhanced stability. Sci Rep 5:14203
Yamaguchi H, Miyazaki M, Asanomi Y, Maeda H (2011) Poly-lysine supported cross-linked enzyme aggregates with efficient enzymatic activity and high operational stability. Catal Sci Technol 1:1256–1261
Stressler T, Ewert J, Eisele T, Fischer L (2015) Cross-linked enzyme aggregates (CLEAs) of PepX and PepN - production, partial characterization and application of combi-CLEAs for milk protein hydrolysis. Biocatal Agric Biotechnol 4:752–760
Bruggink A, Schoevaart R, Kieboom T (2003) Concepts of nature in organic synthesis: cascade catalysis and multistep conversions in concert. Org Process Res Dev 7:622–640
Mateo C, Chmura A, Rustler S, Van Rantwijk F, Stolz A, Sheldon RA (2006) Synthesis of enantiomerically pure (S)-mandelic acid using an oxynitrilase-nitrilase bienzymatic cascade: a nitrilase surprisingly shows nitrile hydratase activity. Tetrahedron Asymmetry 17:320–323
Murado MA, Prieto MA (2013) Dose-response analysis in the joint action of two effectors. A new approach to simulation, identification and modelling of some basic interactions. PLoS ONE 8:e61391
Nielsen PM, Petersen D, Dambmann C (2001) Improved method for determining food protein degree of hydrolysis. J Food Sci 66:642–646
Hušek P (1991) Amino acid derivatisation and analysis in five minutes. FEBS Lett 280:354–356
Stressler T, Eisele T, Schlayer M, Fischer L (2012) Production, active staining and gas chromatography assay analysis of recombinant aminopeptidase P from Lactococcus lactis ssp. lactis DSM 20481. AMB Express 2:39
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Stressler T, Eisele T, Fischer L (2013) Simultaneous monitoring of twelve angiotensin I converting enzyme inhibitory peptides during enzymatic β-casein hydrolysis using Lactobacillus peptidases. Int Dairy J 30(96–102):39
Eisele T, Stressler T, Kranz B, Fischer L (2013) Bioactive peptides generated in an enzyme membrane reactor using Bacillus lentus alkaline peptidase. Eur Food Res Technol 236:483–490
Matijošytė I, Arends IWCE, de Vries S, Sheldon RA (2010) Preparation and use of cross-linked enzyme aggregates (CLEAs) of laccases. J Mol Catal B Enzym 62:142–148
Rehman S, Bhatti HN, Bilal M, Asgher M (2016) Cross-linked enzyme aggregates (CLEAs) of Penicillium notatum lipase enzyme with improved activity, stability and reusability characteristics. Int J Biol Macromol 91:1161–1169
Merz M, Eisele T, Claaßen W, Appel D, Rabe S, Stressler T, Fischer L (2015) Continuous long-term hydrolysis of wheat gluten using a principally food-grade enzyme membrane reactor system. Biochem Eng J 99:114–123
Stressler T, Eisele T, Kranz B, Fischer L (2014) PepX from Lactobacillus helveticus: automated multi-step purification and determination of kinetic parameters with original tripeptide substrates. J Mol Catal B Enzym 108:103–110
van Overveld FWPC, Haenen GRMM, Rhemrev J, Vermeiden JPW, Bast A (2000) Tyrosine as important contributor to the antioxidant capacity of seminal plasma. Chem Biol Interact 127:151–161
Torkova A, Koroleva O, Khrameeva E, Fedorova T, Tsentalovich M (2015) Structure-functional study of tyrosine and methionine dipeptides: an approach to antioxidant activity prediction. Int J Mol Sci 16:25353–25376
Acknowledgements
The authors thank Nina Pfahler and Wolfgang Claaßen (University of Hohenheim, Institute of Food Science and Biotechnology, Department of Biotechnology and Enzyme Science) for their help during the bioreactor cultivation. Additionally, the authors thank Alena Kussler and Lena Nesensohn (University of Hohenheim, Institute of Food Science and Biotechnology, Department of Biotechnology and Enzyme Science) for performing preliminary experiments. Finally the authors thank Prof. Dr. Lutz Fischer (University of Hohenheim, Institute of Food Science and Biotechnology, Department of Biotechnology and Enzyme Science) for giving the opportunity to perform this work in his department.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human participants or animals.
Rights and permissions
About this article
Cite this article
Braun, C., Ewert, J. & Stressler, T. Characterization of cross-linked enzyme aggregates (CLEAs) of the fusion protein FUS-PepN_PepX and their application for milk protein hydrolysis. Eur Food Res Technol 243, 1815–1828 (2017). https://doi.org/10.1007/s00217-017-2885-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-017-2885-3