Abstract
Protein glycosylation of human serum exosomes can reveal significant physiological information, and the development of large-scale identification strategies is crucial for the in-depth investigation of the serum exosome glycoproteome. In this study, using surface functionalization techniques, an ultra-hydrophilic mesoporous silica magnetic nanosphere (denoted as Fe3O4-CG@mSiO2) was synthesized for the quick and accurate detection of glycopeptides from HRP digests. The Fe3O4-CG@mSiO2 nanospheres demonstrated outstanding enrichment capability, high sensitivity (5 amol/μL), good size exclusion effect (HRP digests/BSA proteins, 1:10,000), stable reusability (at least 10 times), and an excellent recovery rate (108.6 ± 5.5%). Additionally, after enrichment by Fe3O4-CG@mSiO2, 156 glycopeptides assigned to 64 proteins derived from human serum exosomes were successfully identified, which demonstrates that the nanospheres have great potential for the research of the large-scale serum exosome glycoproteome.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exosomes are a kind of extracellular vesicle including the cellular components (proteins, DNA, and RNA) that secrete them [1]. According to research, the post-translational modification of exosome proteins is closely linked to the incidence and progression of disease, and many exosome surface proteins and marker proteins are glycoproteins [2]. As a result, further research into the glycosylation modification of exosome proteins is critical. N-glycosylation plays important physiological and biological roles in the immune response, molecular recognition, cell adhesion, and signal transmission, among others [3,4,5]. Previous studies have found that aberrant glycosylation can influence the onset and progression of neurodegenerative diseases, diabetes, and various other conditions [6, 7]. Therefore, the investigation of protein glycosylation of exosomes is of great practical value and may have application in the clinical area.
In recent decades, mass spectrometry (MS) has become an efficient analysis tool for in-depth investigation of the glycoproteome [8, 9]. Nevertheless, MS-based methods still face some challenges, including low ionization efficiency, the occurrence of high-abundance non-glycopeptides, and the interference caused by salt, which severely suppresses the mass spectrometry signal of low-abundance glycopeptides [10]. Directly employing MS to characterize glycopeptides may produce poor results. Therefore, prior to MS analysis, it is important to design a method for specifically enriching glycopeptides from complex biological sample systems.
To date, substantial efforts have been devoted to developing strategies for glycopeptide enrichment before MS detection, including chemical hydrazide, boronate affinity chromatography, lectin affinity chromatography, and hydrophilic interaction liquid chromatography (HILIC) [11, 12]. Because of their simple operation, good reproducibility, superior compatibility with MS, and selective enrichment of multiple glycopeptides, the HILIC-based strategies have become the most commonly used methods [13,14,15,16,17]. Zwitterionic hydrophilic (ZIC-HILIC) materials stand out among a multitude of HILIC stationary phases because they include both positive and negative groups, which increases their hydrophilicity significantly [18,19,20,21,22,23]. Previously, our group employed a one-step hydrothermal method to prepare magnetic ZIC-HILIC Fe3O4-CG composites, which exhibited good glycopeptide enrichment capacity. However, the composites do not reveal effective size exclusion properties, so we have been eager to design a ZIC-HILIC material with good size exclusion capacity for the specific identification of glycopeptides [24].
Mesoporous materials have seen significant development in recent years due to their structural advantages, including huge specific surface area, simple synthesis methods, tunable pore size, and homogeneous pore channels [25, 26]. As a result, various functional mesoporous materials have been employed to adsorb low-abundance target peptides and exclude high-abundance proteins [27, 28]. For example, Zheng’s group coated mesoporous polydopamine on a graphene oxide substrate, and then modified it with arginine. The composites produced have high glycopeptide enrichment capacity and can be efficiently used to enrich glycopeptides from biological samples [29]. Additionally, Wang's group developed a zwitterionic hydrophilic mesoporous silica material (denoted as Fe3O4@SiO2@Au@mSiO2@L-Cys) as a hydrophilic platform for glycopeptide enrichment [30]. However, the small specific surface area, cumbersome synthesis steps, large steric hindrance, and poor hydrophilicity of these HILIC mesoporous materials limit their further application. Thus, to boost glycopeptide enrichment efficiency, HILIC mesoporous materials with facile synthesis methods, large specific surface area, small steric hindrance, and strong hydrophilicity are urgently needed.
Herein, we designed an ultra-hydrophilic mesoporous silica magnetic nanosphere (denoted as Fe3O4-CG@mSiO2, where C refers to cysteine and G refers to glutathione) for highly selective and sensitive glycopeptide enrichment. Firstly, ultra-hydrophilic bifunctional Fe3O4-CG nanospheres were synthesized through Fe–S interaction, which provides superparamagnetic properties for fast and efficient solid–liquid separation [31]. In addition, the nanospheres uniquely combine the properties of L-cysteine (Cys) and reduced glutathione (GSH), which overcomes the limitation of large steric hindrance and greatly enhances the hydrophilicity of the material. Afterwards, an ordered mesoporous silica layer was modified on the surface of the Fe3O4-CG to exclude large proteins and for specific capture of glycopeptides. The Fe3O4-CG@mSiO2 nanospheres show great potential for use in large-scale glycoproteomics research and may contribute to the clinical diagnosis of illness in the future.
Materials and methods
Materials and chemicals
Ethylene glycol, iron chloride hexahydrate (FeCl3·6H2O), sodium acetate (CH3COONa), acetonitrile (ACN), ammonium bicarbonate (NH4HCO3), phosphoric acid (H3PO4), and hydrochloric acid (HCl) were purchased from Aladdin. Bicarbonate (TEAB), CD2O (20 wt%, 98% D), formaldehyde (CH2O, 37%), cyanoborohydride (NaBH3CN), horseradish peroxidase (HRP), bovine serum albumin (BSA), 2,5-dihydroxybenzoic acid (DHB), dithiothreitol (DTT), and iodoacetamide (IAA) were purchased from Sigma-Aldrich. Peptide-N-glycosidase F (PNGase F) was purchased from Genetimes Technology. Hexadecyl trimethyl ammonium bromide (CTAB), formic acid, sodium hydroxide (NaOH), Cys, GSH, and tetraethyl orthosilicate (TEOS) were purchased from Macklin Biochemical Co.. Human serum samples were acquired from the Affiliated Hospital of Medical School, Ningbo University. All deionized water was processed using a Milli-Q system.
Pretreatment of standard protein and serum
In 100 μL of deionized water, HRP (1 mg) and BSA (2 mg) were dissolved, respectively. The proteins were denatured in boiling water at 100 °C for 10 min after ultrasonication for 5 min. After cooling the denatured protein sample at room temperature, 100 μL of ammonium bicarbonate (50 mmol/L) was added along with the addition of trypsin at a protein-to-trypsin ratio of 40:1. The mixture was incubated for 16 h at 37 °C. The resultant solutions were stored at −20 °C.
To reduce viscosity, the serum sample was diluted with an equal volume of phosphate-buffered saline (PBS) solution. The diluted serum sample was centrifuged for 3 min at 4 °C at 3000×g. The supernatant was placed into 1 mL tubes and centrifuged for 45 min at 4 °C at 12,000×g. To obtain the serum exosomes, the supernatant was filtered through a 0.22 μm filter. The serum exosomes were suspended in an ice-cold buffer containing 50 mM Tris–HCl and 8 M urea and sonicated for 30 min for lysis. The exosome protein solution was mixed with 10 mM DTT and incubated at 37 °C for 4 h before alkylation in 20 mM IAA for 1 h in the dark. The proteins were then digested overnight at 37 °C with trypsin (enzyme/protein ratio 1:40). Finally, the tryptic digests were desalted, lyophilized, and stored for further use.
Preparation of Fe3O4-CG
Bifunctional hydrophilic Fe3O4-CG nanospheres were synthesized using a one-step solvothermal technique. The precise synthesis processes were consistent with those reported previously [32]. To begin, 1.35 g of iron chloride hexahydrate was dispersed in 75 mL of ethylene glycol. The blended solution was then mechanically agitated for 30 min, after which it was ultrasonically dispersed for a few minutes with 3.6 g anhydrous sodium acetate. Following that, 30.733 mg GSH and 6.058 mg Cys were simultaneously added to the combined solution and agitated for 2 h; the solution was then transferred to a reaction vessel. Finally, the solution was heated for 16 h at 200 °C. The product was cleaned three times with ethanol and deionized water followed by vacuum drying overnight.
Preparation of Fe3O4-CG@mSiO2 nanospheres
The specific synthesis steps for Fe3O4-CG@mSiO2 nanospheres were consistent with those reported previously, with minor revisions [33]. Briefly, 100 mg Fe3O4-CG nanospheres and 1 g CTAB were added to 100 mL deionized water and ultrasonically dispersed for 30 min until they were dispersed completely. Next, 100 mL of NaOH solution (10 mM) and 800 mL of deionized water were added to the solution, and the liquid obtained was stirred for 0.5 h at 60 °C. Afterwards, 2.5 mL of TEOS/ethanol (volume ratio of 1:4) was added to the solution and heated for 12 h at 60 °C. The finished product was washed three times each with deionized water and ethanol and then vacuum-dried for an entire night. To remove CTAB, the nanospheres were transferred to a muffle furnace and calcined for 4 h at 350 °C.
The procedure for glycopeptide enrichment from standard proteins and serum exosomes
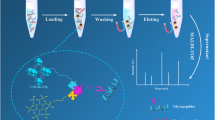
The procedure for the enrichment of glycopeptide from standard proteins is exhibited in Fig. 1. Briefly, 0.25 mg of Fe3O4-CG@mSiO2 was dissolved in 200 μL of loading buffer (95% ACN/3% TFA, V/V) containing 100 fmol/μL HRP digestion. Afterwards, the mixed solution was vibrated for 30 min at 37 °C. Then, the glycopeptide-loaded Fe3O4-CG@mSiO2 was magnet-separated, and was washed three times in washing buffer (85% ACN/0.5% H3PO4, V/V). A centrifuge tube was then filled with 10 μL of eluent (30% ACN) and vibrated for 30 min at 37 °C. The eluent was then collected and analyzed by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) MS.
The process for enriching glycopeptides from serum exosomes was as follows. In brief, 0.5 mg of Fe3O4-CG@mSiO2 was dissolved in 100 μL of loading buffer containing a predetermined quantity of serum exosomes. The mixed solution was then shaken for 30 min at 37 °C. After magnetic separation, the glycopeptide-loaded Fe3O4-CG@mSiO2 was washed five times in washing buffer. Glycopeptides were collected after elution with 2 × 10 μL of the eluent. After lyophilizing the eluent and resolving it in NH4HCO3 solution (25 mM), PNGase F was added, and the mixture was vibrated for 16 h at 37 °C. The resulting liquid was lyophilized before detection using nanoscale liquid chromatography–tandem mass spectrometry (nano-LC–MS/MS).
Evaluation of the enrichment recovery
Two microliters of light-tagged HRP digestion was first enriched by Fe3O4-CG@mSiO2 nanospheres, and the eluent was collected and combined with 2 µL of heavy-tagged HRP digestion. The resultant solution was then enriched with Fe3O4-CG@mSiO2, and the eluent was used for MALDI-TOF MS analysis. The intensity ratio of light- and heavy-tagged glycopeptides was assessed in three parallel experiments to confirm the enrichment recovery.
Characterization of Fe3O4-CG@mSiO2 nanospheres
The microscopic morphology of Fe3O4-CG@mSiO2 was examined using transmission electron microscopy (TEM) images taken with a JEOL JEM-2100F microscope (Japan). The surface morphology and elemental composition of Fe3O4-CG@mSiO2 were observed using scanning electron microscopy (SEM) images and energy-dispersive X-ray spectroscopy (EDX) obtained using a Hitachi S-4800 microscope (Japan). The crystal structure of Fe3O4-CG@mSiO2 was studied using X-ray diffraction (XRD). GSH and Cys dual-functional Fe3O4-CG@mSiO2 nanospheres were validated using Fourier transform infrared (FT-IR) spectroscopy.
Results and discussion
Preparation and characterization of Fe3O4-CG@mSiO2
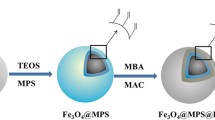
The procedure for the preparation of dual-functional hydrophilic mesoporous nanospheres (Fe3O4-CG@mSiO2) is exhibited in Fig. 2. Two hydrophilic amino acids (Cys and GSH) were added during the preparation of Fe3O4, and the Fe3O4-CG nanospheres were synthesized by a one-step solvothermal method. Next, using a template technique and CTAB as a structure-directing agent, a layer of mesoporous silica was modified on the surface of the Fe3O4-CG. To verify whether the nanospheres were successfully prepared and assess their application potential in terms of enrichment of glycopeptides, we conducted the following characterizations and experiments.
SEM and TEM were used to determine the shape and size of the Fe3O4-CG@mSiO2 nanospheres. The produced nanospheres, which are shown in Fig. 3a, have a nice spherical form and a diameter of between 200 and 300 nm. Additionally, as seen in the SEM image (Fig. 3b and c), the nanospheres are uniform in size and distribution. EDX was employed to observe the elemental composition of Fe3O4-CG@mSiO2. As seen in Fig. S1, the existence of elements N, O, C, Fe, Si, and S demonstrated the successful synthesis of Fe3O4-CG@mSiO2 nanospheres. The crystal structure of the nanospheres was characterized by XRD, and Fig. S2 shows that the locations of the six primary distinctive diffraction peaks are compatible with the earlier studies [34, 35]. FT-IR was used to verify that two hydrophilic amino acids and mesoporous silica were successfully coated on the surface of the Fe3O4 in turn. According to Fig. S3, the Fe–O stretching vibration was assigned to the peak at 575 cm−1, and the Cys and GSH characteristic peaks were attributed to the peaks at 1260 cm−1, 1534 cm−1, and 1639 cm−1. Si–O–Si symmetric and asymmetric stretching vibrations, respectively, were given the vibrational frequencies of 798 cm−1 and 1084 cm−1. The outcomes demonstrated the function groups and layer were successfully modified.
Specific enrichment of glycopeptides from standard proteins with Fe3O4-CG@mSiO2
HRP digestion was utilized as a standard sample to evaluate the enrichment capability of the Fe3O4-CG@mSiO2nanospheres toward glycopeptides. For comparison, Fe3O4-CG was chosen to enrich glycopeptides under the same conditions. In Fig. 4a, before enrichment, only four characteristic peaks of glycopeptides appear in the spectrum. After enrichment by Fe3O4-CG, 16 glycopeptide peaks can be clearly observed, and the intensity and number of glycopeptide peaks are obviously increased. In addition, the spectrum background is clean, which confirmed the relatively lower interference. The effect is better, but still not ideal. After enrichment by Fe3O4-CG@mSiO2, a total of 19 glycopeptide peaks can be observed, and the intensity of the glycopeptide peaks is further increased. This phenomenon confirmed the great enrichment performance of Fe3O4-CG@mSiO2 nanospheres toward glycopeptides. Table S1 of the supplementary material contains the complete information for glycopeptides derived from HRP digestion after enrichment by Fe3O4-CG@mSiO2.
We performed further tests to investigate the detection limit for assessment of the enrichment capability of Fe3O4-CG@mSiO2 nanospheres. The majority of glycopeptides can be observed in Fig. 5a, when the concentration of the HRP digestion is 5 fmol/μL. The number of glycopeptide peaks decreased significantly when the HRP digestion concentration was further diluted to 0.5 fmol/μL; however, nine glycopeptides still remained on the spectrum. (Fig. 5b). After diluting the HRP digestion 10 times to 0.05 fmol/L, six glycopeptide peaks were seen (Fig. 5c). Four glycopeptides were still detectable at concentrations as low as 0.005 fmol/μL, and just a few non-glycopeptides were visible on the spectrum (Fig. 5d).
Meanwhile, the size exclusion property of Fe3O4-CG@mSiO2 nanospheres was demonstrated by adopting various proportions of BSA protein as interference mixed into β-casein digestion. As seen in Fig. 6a, when the ratio of BSA protein to HRP digestion was 1:500, most of the glycopeptide peaks could be observed, and the background of the spectrum remained clean and the peak intensity was still relatively high. When the ratio of BSA protein to HRP digestion increased to 1:1000, 12 glycopeptides could be identified. Further expanding the ratio of BSA protein (Fig. 6b), 11 glycopeptides could be captured with the Fe3O4-CG@mSiO2 nanospheres when the ratio of BSA protein to HRP digestion was 1:5000 (Fig. 6c). Even when the ratio of BSA protein to HRP digestion reached 1:10,000, seven glycopeptides could still be enriched with the Fe3O4-CG@mSiO2 nanospheres, and almost no non-glycopeptide peaks were present in the spectrum (Fig. 6d).
In addition, we designed experiments to investigate the reusability of Fe3O4-CG@mSiO2 nanospheres through a cyclic experiment. As shown in Fig. S4a, after four cycles, 18 glycopeptide peaks could be captured. At the same time, after 7 and 10 cycles, we can find that there was no obvious difference in the peak number or peak intensity (Fig. S4b and Fig. S4c). The result demonstrated that the Fe3O4-CG@mSiO2 nanospheres have superior reusability.
Finally, to assess the enrichment recovery of the Fe3O4-CG@mSiO2 nanospheres toward HRP digestion, we used the stable isotope dimethyl labeling technique. The recovery rate was calculated as the ratio of the peak intensity of the light-labeled glycopeptide to the equivalent peak intensity of the heavy-labeled glycopeptide. The outcomes are displayed in Fig. S5. After three parallel experiments, we measured the average recovery of the Fe3O4-CG@mSiO2 nanospheres as 108.6 ± 5.5%. The experimental results demonstrate that the material has good recovery capability.
Specific enrichment of glycopeptides from serum exosomes with Fe3O4-CG@mSiO2
After the encouraging results of the preceding experiments, we assessed the use of the Fe3O4-CG@mSiO2 nanospheres on actual biological samples. Exosomes have attracted significant research interest due to their widespread application in the clinical field. However, so far, the investigation of glycopeptides in serum exosomes is rare. Therefore, to verify the specific capture ability of the material for glycopeptides, we used the Fe3O4-CG@mSiO2 nanospheres to enrich glycopeptides in serum exosomes. After treatment by Fe3O4-CG@mSiO2 nanospheres, we could detect 156 glycopeptides assigned to 64 proteins with nano-LC–MS/MS. Table S2 of the supplementary material contains the complete information for glycopeptides derived from the human serum exosomes after enrichment by Fe3O4-CG@mSiO2. The superior specific capture capacity of Fe3O4-CG@mSiO2 nanospheres makes it easy to efficiently capture glycopeptides from serum exosomes, which facilitates large-scale glycoproteomic research. Further, the nanospheres are likely to have great application value in clinical diagnosis in the future.
Conclusions
In summary, ultra-hydrophilic mesoporous silica magnetic nanospheres Fe3O4-CG@mSiO2 were prepared via solvothermal and surface functionalization methods. The Fe3O4-CG@mSiO2 nanospheres exhibited good glycopeptide enrichment effects due to the large specific surface area and small steric hindrance. Simultaneously, the Fe3O4-CG@mSiO2 demonstrated superparamagnetic properties for fast and efficient solid–liquid separation. The Fe3O4-CG@mSiO2 nanospheres showed remarkable performance of glycopeptides enrichment, including great sensitivity, the size exclusion effect, recovery rate, and sustained reusability. Moreover, the application of the Fe3O4-CG@mSiO2 nanospheres for the enrichment of glycopeptides in serum exosomes achieved excellent results, with the successful identification of 156 glycopeptides assigned to 64 proteins derived from human serum exosomes. In short, the high capacity of Fe3O4-CG@mSiO2 to specifically capture glycopeptides in standard digestion and serum exosomes will facilitate large-scale glycoproteome research and may have great potential in clinical diagnosis.
References
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. https://doi.org/10.1126/science.aau6977
Chen IH, Xue L, Hsu CC, Paez JS, Pan L, Andaluz H, Wendt MK, Iliuk AB, Zhu JK, Tao WA. Phosphoproteins in extracellular vesicles as candidate markers for breast cancer. Proc Natl Acad Sci USA. 2017;114(12):3175–80. https://doi.org/10.1073/pnas.1618088114
Zhao YM, Jensen ON. Modification-specific proteomics: strategies for characterization of post-translational modifications using enrichment techniques. Proteomics. 2009;9(20):4632–41. https://doi.org/10.1002/pmic.200900398
Smith LM, Kelleher NL. Proteoforms as the next proteomics currency. Science. 2018;359(6380):1106–7. https://doi.org/10.1126/science.aat1884
Qing GY, Yan JY, He XN, Li XL, Liang XM. Recent advances in hydrophilic interaction liquid interaction chromatography materials for glycopeptide enrichment and glycan separation. Trac-trends Anal Chem. 2020;124:115570–82. https://doi.org/10.1016/j.trac.2019.06.020
Chen CC, Su WC, Huang BY, Chen YJ, Tai HC, Obena RP. Interaction modes and approaches to glycopeptide and glycoprotein enrichment. Analyst. 2014;139(4):688–704. https://doi.org/10.1039/c3an01813j
Chen YJ, Xiong ZC, Zhang LY, Zhao JY, Zhang QQ, Peng L, Zhang WB, Ye ML, Zou HF. Facile synthesis of zwitterionic polymer-coated core-shell magnetic nanoparticles for highly specific capture of N-linked glycopeptides. Nanoscale. 2015;7(7):3100–8. https://doi.org/10.1039/c4nr05955g
Zhang YY, Fonslow BR, Shan B, Baek MC, Yates JR. Protein analysis by shotgun/bottom-up proteomics. Chem Rev. 2013;113(4):2343–94. https://doi.org/10.1021/cr3003533
Gao CH, Bai J, He YT, Zheng Q, Ma WD, Lei ZX, Zhang MY, Wu J, Fu FF, Lin Z. Postsynthetic functionalization of Zr4+-immobilized core-shell structured magnetic covalent organic frameworks for selective enrichment of phosphopeptides. ACS Appl Mater Interfaces. 2019;11(14):13735–41. https://doi.org/10.1021/acsami.9b03330
Novotny MV, Alley WR. Recent trends in analytical and structural glycobiology. Curr Opin Chem Biol. 2013;17(5):832–40. https://doi.org/10.1016/j.cbpa.2013.05.029
Wang YL, Liu MB, Xie LQ, Fang CY, Xiong HM, Lu HJ. Highly efficient enrichment method for glycopeptide analyses: using specific and nonspecific nanoparticles synergistically. Anal Chem. 2014;86(4):2057–64. https://doi.org/10.1021/ac403236q
Yao JZ, Wang JW, Sun NR, Deng CH. One-step functionalization of magnetic nanoparticles with 4-mercaptophenylboronic acid for a highly efficient analysis of N-glycopeptides. Nanoscale. 2017;9(41):16024–9. https://doi.org/10.1039/c7nr04206j
Su P, Wang Z, Li X, Li M, Li G, Gong Z, Song JY, Yang Y. Fabrication of magnetic dual-hydrophilic metal organic framework for highly efficient glycopeptide enrichment. Anal Bioanal Chem. 2021;413(21):5267–78. https://doi.org/10.1007/s00216-021-03535-w
Hua SW, Feng QS, Xie ZH, Mao HJ, Zhou YP, Yan YH, Ding CF. Post-synthesis of covalent organic frameworks with dual-hydrophilic groups for specific capture of serum exosomes. J Chromatogr A. 2022;1679:463406. https://doi.org/10.1016/j.chroma.2022.463406
Saleem S, Sajid MS, Hussain D, Jabeen F, Najam-ul-Haq M, Saeed A. Boronic acid functionalized MOFs as HILIC material for N-linked glycopeptide enrichment. Anal Bioanal Chem. 2020;412(7):1509–20. https://doi.org/10.1007/s00216-020-02427-9
Li YL, Deng CH, Sun NR. Hydrophilic probe in mesoporous pore for selective enrichment of endogenous glycopeptides in biological samples. Anal Chim Acta. 2018;1024:84–92. https://doi.org/10.1016/j.aca.2018.04.030
Sun NR, Wu H, Chen HM, Shen XZ, Deng CH. Advances in hydrophilic nanomaterials for glycoproteomics. Chem Commun. 2019;55(70):10359–75. https://doi.org/10.1039/c9cc04124a
Feng XY, Deng CH, Gao MX, Zhang XM. Facile and easily popularized synthesis of l-cysteine-functionalized magnetic nanoparticles based on one-step functionalization for highly efficient enrichment of glycopeptides. Anal Bioanal Chem. 2020;410(3):989–98. https://doi.org/10.1007/s00216-017-0602-5
Yeh CH, Chen SH, Li DT, Lin HP, Huang HJ, Chang CI, Shih WL, Chern CL, Shi FK, Hsu JL. Magnetic bead-based hydrophilic interaction liquid chromatography for glycopeptide enrichments. J Chromatogr A. 2012;1224:70–8. https://doi.org/10.1016/j.chroma.2011.12.057
Zhang Y, Jing HY, Meng B, Qian XH, Ying WT. L-cysteine functionalized straticulate C3N4 for the selective enrichment of glycopeptides. J Chromatogr A. 2020;1610:460545. https://doi.org/10.1016/j.chroma.2019.460545
Wu WR, Tang RZ, Pan L, Wang CY, Zhang JJ, Ma SJ, Shen YH, Ou JJ. Fabrication of hydrophilic zwitterionic microspheres via inverse suspension polymerization for the enrichment of N-glycopeptides. Microchim Acta. 2021;188(10):348. https://doi.org/10.1007/s00604-021-05010-w
Li XW, Zhang HY, Zhang N, Ma SJ, Ou JJ, Ye ML. One-step preparation of zwitterionic-rich hydrophilic hydrothermal carbonaceous materials for enrichment of n-glycopeptides. ACS Sustain Chem Eng. 2019;7(13):11511–20. https://doi.org/10.1021/acssuschemeng.9b01382
Ji YS, Lv RH, Song SH, Huang JF, Zhang LW, Huang G, Li JA, Ou JJ. Facile fabrication of zwitterionic magnetic composites by one-step distillation-precipitation polymerization for highly specific enrichment of glycopeptides. Anal Chim Acta. 2019;1053:43–53. https://doi.org/10.1016/j.aca.2018.12.003
Yi LH, Shao YF, Fu MY, Yan YH, Ding CF, Tang KQ. One-step preparation of magnetic zwitterionic-hydrophilic dual functional nanospheres for in-depth glycopeptides analysis in Alzheimer's disease patients' serum. J Chromatogr A. 2022;1669:462929. https://doi.org/10.1016/j.chroma.2022.462929
Tian Y, Tang RZ, Liu LY, Yu Y, Ma SJ, Gong BL, Ou JJ. Glutathione-modified ordered mesoporous silicas for enrichment of N-linked glycopeptides by hydrophilic interaction chromatography. Talanta. 2020;217:121082. https://doi.org/10.1016/j.talanta.2020.121082
Yan YY, Han RL, Hou YF, Zhang HJ, Yu JC, Gao WQ, Xu L, Tang KQ. Bowl-like mesoporous polydopamine with size exclusion for highly selective recognition of endogenous glycopeptides. Talanta. 2021;233:122468. https://doi.org/10.1016/j.talanta.2021.122468
Zhang QQ, Huang YY, Jiang BY, Hu YJ, Xie JJ, Gao X, Jia B, Shen HL, Zhang WJ, Yang PY. In situ synthesis of magnetic mesoporous phenolic resin for the selective enrichment of glycopeptides. Anal Chem. 2021;90(12):7357–63. https://doi.org/10.1021/acs.analchem.8b00708
Kong SY, Zhang QQ, Yang LJ, Huang YY, Liu MQ, Yan GQ, Zhao HH, Wu MX, Zhang XM, Yang PY, Cao WQ. Effective enrichment strategy using boronic acid-functionalized mesoporous graphene-silica composites for intact n- and o-linked glycopeptide analysis in human serum. Anal Chem. 2021;93(17):6682–91. https://doi.org/10.1021/acs.analchem.0c05482
Zheng Y, Pu CL, Zhao HL, Gu QY, Zhu TY, Lan MB. Hydrophilic arginine-functionalized mesoporous polydopamine-graphene oxide composites for glycopeptides analysis. Anal Chem. 2022;1189:123049. https://doi.org/10.1016/j.jchromb.2021.123049
Wang ZD, Wu RQ, Chen HM, Sun NR, Deng CH. Synthesis of zwitterionic hydrophilic magnetic mesoporous silica materials for endogenous glycopeptide analysis in human saliva. Nanoscale. 2018;10(11):5335–41. https://doi.org/10.1039/c7nr08613j
Chen HM, Li YL, Wu H, Sun NR, Deng CH. Smart hydrophilic modification of magnetic mesoporous silica with zwitterionic l-cysteine for endogenous glycopeptides recognition. ACS Sustain Chem Eng. 2019;7(2):2844–51. https://doi.org/10.1021/acssuschemeng.8b06258
Li DP, Zhang JH, Xie GS, Ji FF, Shao XJ, Zhu L, Cai ZW. A dual-zwitterion functionalized ultra-hydrophilic metal–organic framework with ingenious synergy for enhanced enrichment of glycopeptides. Chem Commun. 2019;55(93):13967–70. https://doi.org/10.1039/c9cc06785j
Sun NR, Wang JW, Yao JZ, Deng CH. Hydrophilic mesoporous silica materials for highly specific enrichment of n-linked glycopeptide. Anal Chem. 2017;89(3):1764–71. https://doi.org/10.1021/acs.analchem.6b04054
Zhang Y, Kuang M, Zhang LJ, Yang PY, Lu HJ. An accessible protocol for solid-phase extraction of N-linked glycopeptides through reductive amination by amine-functionalized magnetic nanoparticles. Anal Chem. 2013;85(11):5535–41. https://doi.org/10.1021/ac400733y
Deng H, Li XL, Peng Q, Wang X, Chen JP, Li YD. Monodisperse magnetic single-crystal ferrite microspheres. Angew Chem Int Ed. 2005;44(18):2782–5. https://doi.org/10.1002/anie.200462551
Acknowledgements
This research was funded by Ningbo major science and technology project (2022Z133), the medical health Project of Health Department of Zhejiang Province (2021KY1045), Ningbo public welfare science and technology project (2021S104).
Ethically approved human serum samples used in this research were collected with the consent of volunteers. The study was conducted with the approval of the experimental ethics committee of Ningbo University and its affiliated hospital.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Consent for publication
All of the authors have given approval for the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yi, L., Wang, B., Feng, Q. et al. Surface functionalization modification of ultra-hydrophilic magnetic spheres with mesoporous silica for specific identification of glycopeptides in serum exosomes. Anal Bioanal Chem 415, 1741–1749 (2023). https://doi.org/10.1007/s00216-023-04575-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-04575-0