Abstract
Protein glycosylation is one of the most important post-translational modifications. Also, efficient enrichment and separation of glycopeptides from complex samples are crucial for the thorough analysis of glycosylation. Developing novel hydrophilic materials with facile and easily popularized synthesis is an urgent need in large-scale glycoproteomics research. Herein, for the first time, a one-step functionalization strategy based on metal-organic coordination was proposed and Fe3O4 nanoparticles were directly functionalized with zwitterionic hydrophilic l-cysteine (L-Cys), greatly simplifying the synthetic procedure. The easily synthesized Fe3O4/L-Cys possessed excellent hydrophilicity and brief composition, contributing to affinity for glycopeptides and reduction in nonspecific interaction. Thus, Fe3O4/L-Cys nanoparticles showed outstanding sensitivity (25 amol/μL), high selectivity (mixture of bovine serum albumin and horseradish peroxidase tryptic digests at a mass ratio of 100:1), good reusability (five repeated times), and stability (room temperature storage of 1 month). Encouragingly, in the glycosylation analysis of human serum, a total of 376 glycopeptides with 393 N-glycosylation sites corresponding to 118 glycoproteins were identified after enrichment with Fe3O4/L-Cys, which was superior to ever reported L-Cys modified magnetic materials. Furthermore, applying the one-step functionalization strategy, cysteamine and glutathione respectively direct-functionalized Fe3O4 nanoparticles were successfully synthesized and also achieved efficient glycopeptide enrichment in human serum. The results indicated that we have presented an efficient and easily popularized strategy in glycoproteomics as well as in the synthesis of novel materials.

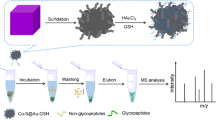
Fe3O4/L-Cys nanoparticles based on one-step functionalization for highly efficient enrichment of glycopeptides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As one of the most predominant post-translational modifications, protein glycosylation carries abundant biological information and regulates complicated functions, such as signal transduction, immune response, cell growth and differentiation [1, 2]. Importantly, many clinical biomarkers and therapeutic targets are glycoproteins [3]. Therefore, a thorough analysis of protein glycosylation is of great significance. However, owing to low abundance of glycoproteins and strong ion suppression of non-glycosylated species, direct glycoproteome analysis by powerful mass spectrometry (MS) technology still remains challenging [4]. Hence, efficient separation and enrichment of glycopeptides from complex samples prior to MS analysis is an indispensable step.
To date, several strategies have been applied to enrich glycopeptides, including boronate affinity, hydrazide chemistry, metal coordination, lectin affinity, and hydrophilic interaction chromatography (HILIC) [5]. Thereinto, HILIC approach, separating the analyte by hydrophilicity differences, is widely adopted because of broad glycan adaptability, simple enrichment procedure, excellent reproducibility, and good MS compatibility [6]. In addition to conventional HILIC materials, zwitterionic-HILIC (ZIC-HILIC) materials, possessing both positive and negative charges, demonstrate improved hydrophilicity and better interaction with glycopeptides [7]. Recently, Cao et al. developed poly(amidoamine) dendrimer for the synthesis of zwitterionically functionalized materials and achieved highly selective enrichment of glycopeptides from biological samples [8]. Magnetic zwitterionic hydrophilic nanoparticles Fe3O4@SiO2@PMSA were also reported for efficient glycopeptide enrichment [9]. Notwithstanding the successful cases, the preparation of most ZIC-HILIC materials usually required a complicated multistep procedure, even suffering from harsh conditions, expensive reagents, and unexpected side reactions [10]. Moreover, the complex structure of composite could probably cause more uncertainties in analysis procedure [11]. All these severely restricted the operability and widespread application. Thus, there is an urgent need to explore a facile and generally applicable synthesis strategy to develop ZIC-HILIC materials, maintaining better glycopeptide affinity simultaneously.
Magnetic materials, especially Fe3O4 nanoparticles, have aroused much attention in biochemistry, cytology, and many other fields due to their fast magnetic response, biocompatibility, easy preparation, and flexible functionalization [12, 13]. Therefore, functionalized Fe3O4 nanoparticles with excellent hydrophilicity were considered to be a fine candidate for glycopeptide enrichment. In previous research, Fe3O4 nanoparticles were usually functionalized via multistep reactions, with surface anchor involved [14, 15]. Thus, it is significative to explore novel approaches to functionalize Fe3O4 nanoparticles to enhance hydrophilicity and simplify the process.
There have been reports that thiol reagents were directly assembled on an iron surface via metal-organic group coordination, supposed to be Fe–S interaction [16, 17]. Besides, in our unpublished research, Fe3O4 nanoparticles were successfully used to couple sulfydryl peptides for N-terminal peptide isolation. Inspired by these works, we have proposed a one-step functionalization strategy in the synthesis of ZIC-HILIC materials that Fe3O4 nanoparticles are functionalized directly with hydrophilic molecules containing thiol groups under mild conditions. With thiol, carboxyl, and amino groups, zwitterionic hydrophilic l-cysteine (L-Cys) could be a wonderful choice for one-step functionalization. Herein, to the best of our knowledge, for the first time, L-Cys one-step functionalized Fe3O4 nanoparticles were synthesized as ZIC-HILIC materials for glycopeptide enrichment. With excellent hydrophilicity and brief composition, the easily synthesized Fe3O4/L-Cys contributed to affinity for glycopeptides and reduction in nonspecific interaction, thus exhibiting outstanding sensitivity, selectivity, reusability, and stability in the enrichment of tryptic digests of standard glycoprotein HRP. Meanwhile, Fe3O4/L-Cys achieved efficient enrichment and analysis in complex human serum. In addition, applying the one-step functionalization strategy, cysteamine and glutathione respectively direct-functionalized Fe3O4 nanoparticles were successfully synthesized and also displayed excellent performance of glycopeptide enrichment in human serum, indicating an efficient and easily popularized strategy in glycoproteomics as well as in the synthesis of novel materials.
Materials and methods
Chemicals and materials
Horseradish peroxidase (HRP), bovine serum albumin (BSA), formic acid (FA), trifluoroacetic acid (TFA), dithiothreitol (DTT), iodoacetamide (IAA), ammonium bicarbonate (NH4HCO3), 2,5-dihydroxy-benzoic acid (DHB), l-cysteine (L-Cys), and sequence-grade trypsin were purchased from Sigma-Aldrich (St. Louis, MO, USA). PNGase F was from New England Biolabs (Ipswich, MA, USA). Acetonitrile (ACN) was obtained from Merck (Darmstadt, Germany). Phosphate buffered solution (PBS) was purchased from Solarbio (Beijing, China). FeCl3·6H2O, sodium acetate, and ethanediol were from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). BCA Protein Assay Kit was produced by Beyotime (Shanghai, China). Human serum was provided by Zhongshan Hospital (Shanghai). All used ultrapure water was purified by Milli-Q system (Millipore, Bedford, MA). All other reagents were of analytical grade.
Synthesis of Fe3O4/L-Cys nanoparticles
Magnetic particles Fe3O4 were prepared via hydrothermal process according to a previous report [18]. As-prepared Fe3O4 particles (22 mg) and l-cysteine (68 mg) were ultrasonic-dispersed into 60 mL PBS buffer (0.01 M). With stirring for 3 h at 60 °C, the functionalization was completed. The synthesized Fe3O4/L-Cys nanoparticles were isolated by a magnet, washed with ultrapure water, and dried in vacuum. The same procedure was conducted for the synthesis of cysteamine functionalized Fe3O4/CS nanoparticles and glutathione functionalized Fe3O4/GSH nanoparticles.
Tryptic digestion of the standard proteins and human serum
HRP and BSA were dissolved in 25 mM NH4HCO3 solution to a final concentration of 1 μg/μL and denatured in boiling water for 5 min. Sequence-grade trypsin was added into the solution at an enzyme/protein mass ratio of 1:40 and incubated at 37 °C overnight. The digestion was stored at − 20 °C for further use.
The total protein content of human serum (2 μL) was determined by BCA method and diluted with a solution containing 25 mM NH4HCO3. After denaturing in boiling water for 5 min, DTT was added with a final concentration of 5 mM. Following reduction of 1 h at 60 °C, alkylation was conducted by adding IAA with a final concentration of 12.5 mM in the dark at 37 °C for 1 h. Finally, the mixture was treated with trypsin (enzyme/protein mass ratio of 1:40) at 37 °C overnight. The digestion was lyophilized and stored at − 20 °C for further use.
Enrichment process of glycopeptides
Fe3O4/L-Cys nanoparticles (50 μg) were added into 50 μL loading buffer (85% ACN/0.1% TFA aqueous solution, v/v) containing tryptic digests, and the mixture was incubated at 37 °C for 15 min to make the nanoparticles capture glycopeptides. The nanoparticles were isolated by a magnet and washed with loading buffer (at least three times) to remove nonspecifically adsorbed non-glycopeptides. Then, the captured glycopeptides were released from the particles with 5 μL eluting buffer (40% ACN/0.1% FA aqueous solution, v/v) at 37 °C for 30 min. The eluent was directly identified with MALDI-TOF MS. As for the optimization of conditions and the investigation of enrichment performance, the as-mentioned procedure was conducted with specific modification.
For the enrichment of glycopeptides from human serum, the lyophilized tryptic digests were dissolved in 500 μL loading buffer, followed by adding 800 μg Fe3O4/L-Cys nanoparticles. After incubating at 37 °C for 30 min, the nanoparticles capturing glycopeptides were separated, rinsed four times with loading buffer, and eluted twice with 30 μL eluting buffer. The eluent was lyophilized and redissolved in 60 μL 25 mM NH4HCO3 and then incubated with 500 U of PNGase F at 37 °C overnight to remove glycan moieties. The resulting deglycosylated peptides were further analyzed by Nano-HPLC-MS/MS.
MALDI-TOF MS analysis
A sample eluent (1.5 μL) was loaded on MALDI plate (MDS SCIEX; Applied Biosystems, USA) and dried at room temperature. And then 1 μL matrix solution (60% ACN/0.1% TFA aqueous solution, v/v) containing 20 mg/mL DHB was added on the sample spot and dried for MS analysis. All the MALDI-TOF MS spectra were determined by 5800 MALDI-TOF MS instrument (Applied Biosystems, USA), which was performed in the positive reflector mode. The laser was at 366 nm, the repetition rate was 200 Hz, and the acceleration voltage was 20 kV.
Nano-HPLC-MS/MS analysis
The analysis was conducted on an EASY-nLC 1000 system (Thermo Fisher Scientific, Waltham, MA) connected to an Orbitrap Fusion mass spectrometer (Thermo Fisher Scientific, San Jose, CA) equipped with an online nano-electrospray ion source. The deglycosylated samples were resuspended with 10 μL mobile phase A (99.9% H2O/0.1% FA, v/v), and 5 μL sample solution was injected and loaded on the trap column (Thermo Scientific Acclaim PepMap C18, 100 μm × 2 cm). The separation was carried out on the analytical column (Acclaim PepMap C18, 75 μm × 25 cm) with a linear gradient, from 2% B (99.9% ACN/0.1% FA, v/v) to 40% B in 105 min and held at initial conditions for 15 min. The flow rate was 300 nl/min.
The Orbitrap Fusion mass spectrometer was operated in the data-dependent mode to switch automatically between MS and MS/MS acquisition. Full-scan MS spectra (m/z 400–1600) were acquired in Orbitrap with a resolution of 120,000 at m/z 200. MS/MS spectra were obtained in Orbitrap with a cycle time of 3 s and resolution of 15,000 at m/z 200. The intensity threshold was 50,000, and the maximum injection time was 100 ms. Ions with charge states 2+, 3+, and 4+ were sequentially fragmented by higher energy collisional dissociation with a normalized collision energy of 35%.
Database searching
Tandem mass spectra were extracted by Proteome Discoverer software (Thermo Fisher Scientific, version 1.4.0.288). All MS/MS data were analyzed via Mascot (Matrix Science, London, UK; version 2.3), searching Uniprot–SwissProt database (taxonomy—Homo sapiens, 20,199 entries), and assuming the digestion of enzyme trypsin. The parameters were set as follows: fragment ion mass tolerance of 0.05 Da, parent ion tolerance of 10.0 ppm, fixed modification of carbamidomethyl on cysteine, variable modifications of oxidation on methionine, and deamidation on asparagine. The peptide level false discovery rates were lower than 1%. Since N-glycosylation generally occurred at sequences of N-X-S/T/C (X ≠ P), the remaining peptides were further filtered to remove non-motif-containing peptides.
Characterizations and measurements
Scanning electronic microscope (SEM) images were obtained by Phenom ProX scanning electronic microscope (Netherlands). Transmission electron microscopy (TEM) images were collected on a JEM2011 transmission electron microscope (Japan). Elemental analysis was performed on Phenom energy-dispersive X-ray (EDX) spectrometer (Netherlands). Fourier-transform infrared (FT-IR) spectra were analyzed on a Nicolet spectrophotometer using KBr pellets (Thermo Fisher, USA). Thermogravimetric analysis (TGA) was carried out on TGA8000 thermogravimetric analyzer (USA) under a nitrogen atmosphere at a heating rate of 10 °C/min from 30 to 900 °C. Zeta potential analysis was conducted on a Malvern Zetasizer Nano instrument (UK).
Results and discussion
Synthesis and characterization of Fe3O4/L-Cys nanoparticles
The procedure for preparing Fe3O4/L-Cys nanoparticles is shown in Scheme 1. Briefly, Fe3O4 nanoparticles synthesized by well-developed solvothermal reaction were directly functionalized with zwitterionic l-cysteine through metal-organic group coordination between Fe and thiol groups. Benefiting from the favorable features of Fe3O4 and L-Cys, the obtained nanoparticles exhibited remarkable characteristics, including simple composition, rapid magnetic response, and outstanding hydrophilicity, thus ensuring the potential in effective enrichment of glycopeptides. Furthermore, with simple operation, mild reaction conditions, and easily available reagents, the concise synthesis route of the magnetic ZIC-HILIC material was commonly applicable.
The morphology of the resulting Fe3O4/L-Cys nanoparticles was analyzed by TEM and SEM. As shown in Fig. 1a and b, the particles were spherical, monodisperse, well distributed, and about 200 nm in diameter. Compared with bare Fe3O4 particles (see Electronic Supplementary Material (ESM) Fig. S1), there was no discernable change after the modification of L-Cys. To further investigate the immobilization of L-Cys, FT-IR spectroscopy was used for characterization (Fig. 1c). The spectra of Fe3O4 and Fe3O4/L-Cys showed a strong absorption peak at 570 cm−1 which was the characteristic peak of Fe–O, indicating the fabrication of Fe3O4 [19]. In the fingerprint region (dotted-line box marked), the peak at 1667, 1585, and 1340 cm−1 were assigned to the group of COOH, NH2, and CH, and many other significant fingerprint peaks of Fe3O4/L-Cys were consistent with that of pure L-Cys while Fe3O4 did not exhibit these features, demonstrating that L-Cys was successfully immobilized on the surface of Fe3O4 particles. Moreover, EDX analysis (ESM Fig. S2) proved the existence of C, N, and S elements, also confirming the successful synthesis of Fe3O4/L-Cys. Meanwhile, the surface functionalization led to the change of the surface charge of nanoparticles in aqueous solution, which made the zeta potential change from 20.7 to − 0.907 mV (ESM Fig. S3).
In order to evaluate the content of immobilized L-Cys, thermogravimetric analysis (TGA) was conducted. As seen from TGA curves (Fig. 1d), Fe3O4 particles displayed a slight mass loss with increasing temperature while Fe3O4/L-Cys showed a larger mass loss, which was attributed to the loss of L-Cys. Calculating from TGA data at 900 °C, the L-Cys content of Fe3O4/L-Cys was approximately 9.33%, larger than other reported L-Cys modified materials [20,21,22]. The abundantly immobilized L-Cys highly enhanced the zwitterionic hydrophilicity of the nanoparticles. It was worth noting that the brief structure and composition of Fe3O4/L-Cys, cutting down possible hydrophobic impacts, could reduce the nonspecific interaction with non-glycopeptides and other non-target components. All these would contribute to efficient enrichment and analysis of glycopeptides from complex samples.
Optimization of conditions
The procedure for enriching glycopeptides by Fe3O4/L-Cys nanoparticles is illustrated in Scheme 1. When incubated with Fe3O4/L-Cys in loading buffer, the relatively hydrophilic glycopeptides tend to bind on the particles while non-glycopeptides remained in the solution [23]. After separated from the solution and washing out non-glycopeptides by magnetic response, the captured glycopeptides were eluted from particles and identified by MS.
In order to achieve the best performance, several conditions, including loading buffer, eluting buffer, mass ratio, and incubation time, were optimized with tryptic digests of standard glycoprotein HRP [24]. By comparing the number of matched glycopeptides and peak intensity of four parallel experiments, 85% ACN/0.1% TFA (v/v) aqueous solution was chosen to be the loading buffer (ESM Fig. S4a). For the purpose of obtaining the full elution of captured glycopeptides, five different eluting buffers were examined and 40% ACN/0.1% FA (v/v) aqueous solution was selected as optimized eluting buffer (ESM Fig. S4b). In the investigation of incubation time (ESM Fig. S4c), there was no significant difference in the enrichment performance from 1 to 30 min, suggesting Fe3O4/L-Cys’ rapid affinity for glycopeptides. In accordance to Fig. S4d (see ESM), the optimal material amount for 0.25 μg HRP digests was 50 μg, indicating the best mass ratio of materials to peptides as 200:1. So far, with magnetic separation and rapid enrichment, the ZIC-HILIC approach of glycopeptide enrichment was established based on Fe3O4/L-Cys nanoparticles.
Selective enrichment of glycopeptides from standard protein
Applying the optimal approach mentioned above, the glycopeptide enrichment performance of Fe3O4/L-Cys and Fe3O4 was evaluated. As shown in Fig. 2a, without any pre-treatment, when 125 fmol/μL HRP tryptic digests were directly analyzed by MALDI-TOF MS, only four glycopeptides with low signal intensity were observed, owing to low abundance and ionization efficiency of glycopeptides. After enrichment with Fe3O4/L-Cys, 21 glycopeptides with enhanced signal intensity were identified (Fig. 2b and ESM Table S1), exhibiting better enrichment performance than previous materials modified with L-Cys [20]. Two identified glycopeptides (GLIQSDQELFSSPN#ATDTIPLVR and LHFHDCFVNGCDASILLDN#TTSFR) were confirmed by MALDI-MS/MS, validating the successful enrichment (ESM Fig. S5). In comparison, after the same treatment of Fe3O4, six glycopeptides with marked non-glycopeptide signals were determined (Fig. 2c), presenting little enrichment effect. The results made it clear that the outstanding enrichment ability of Fe3O4/L-Cys nanoparticles benefited from the one-step immobilized L-Cys. Moreover, the control experiment of a reversed phase enrichment by ZipTip C18 tip showed that the dominant hydrophobic non-glycopeptides with a few hydrophilic glycopeptides of weak signal intensity were captured (ESM Fig. S6), indicating the superior hydrophilicity of Fe3O4/L-Cys nanoparticles.
In order to evaluate the sensitivity of the enrichment approach, HRP tryptic digests with lower concentration were enriched by Fe3O4/L-Cys. When the concentration of HRP digestion decreased to 0.0625 fmol/μL and no signal could be detected in direct MS analysis, four glycopeptides could be identified after enrichment (Fig. 3a). Even at the ultralow concentration of 25 amol/μL, three glycopeptides were still detected after treatment of Fe3O4/L-Cys (Fig. 3b). Compared with ever reported results, such as metal-organic frameworks functionalized magnetic graphene (0.8 fmol/μL) [25] and maltose-functionalized Fe3O4 (1.25 fmol/μL) [14], Fe3O4/L-Cys nanoparticles provided superior sensitivity at present, indicating great potential in trace analysis of protein glycosylation.
To further investigate the selectivity of Fe3O4/L-Cys nanoparticles for glycopeptide enrichment, tryptic digests of non-glycoprotein BSA were introduced as the interference and the mixtures of BSA and HRP tryptic digests at different mass ratios were used as the samples. As shown in Fig. 3c, in the direct MS analysis of the digests’ mixture of BSA and HRP at a mass ratio of 10:1, non-glycopeptide peaks occupied a dominant position in MS spectra and only four glycopeptides from HRP were detected with weak signal intensity due to strong ion suppression of non-glycopeptides. After being enriched by Fe3O4/L-Cys, a large number of non-glycopeptides were effectively removed and 18 glycopeptides were clearly observed with enhanced signal intensity (Fig. 3d), which was contrary to the performance of reversed phase enrichment by ZipTip C18 tip (ESM Fig. S7). Even when the mass ratio was further increased to 100:1 (BSA/HRP), although there were some peaks of non-glycopeptides after enrichment, 13 glycopeptides from HRP were still enriched and identified (ESM Fig. S8). The results confirmed that highly selective enrichment and separation of glycopeptides from complex samples could be achieved by Fe3O4/L-Cys nanoparticles.
Reusability and stability of Fe3O4/L-Cys nanoparticles
In addition, the reusability and stability of Fe3O4/L-Cys nanoparticles were also evaluated. With full eluting until no glycopeptides remained, the particles were applied to enrich the HRP digests again. As Fig. 4a–c shows, the MS spectra were similar from the first to the fifth enrichment, indicating that the particles could be reused at least five times. Moreover, after being stored for 1 month at room temperature, Fe3O4/L-Cys offered almost the same enrichment performance as the freshly prepared ones (Fig. 4d). These robust features counted for a great deal for practical and large-scale application.
Selective enrichment of glycopeptides from human serum
With comprehensive advantages in the enrichment of HRP digests, Fe3O4/L-Cys nanoparticles were further applied for glycosylation analysis of human serum, a real sample with glycosylation of clinical significance and sheer complexity. Without any pre-treatment, according to the same process with HRP enrichment, tryptic digests of 2 μL human serum were enriched by Fe3O4/L-Cys. Also, the captured glycopeptides were deglycosylated and analyzed by nano-LC-MS/MS. Encouragingly, a total of 376 glycopeptides with 393 N-glycosylation sites corresponding to 118 glycoproteins were identified after three replicated analyses (ESM Table S2). The LC-MS/MS spectra of three identified glycopeptides are shown in Fig. S9 (see ESM) as examples. The reproducibility of identified glycopeptides and glycoproteins in three replicated analysis are shown in Fig. 5. It could be seen that about 70.2% glycopeptides (264 out 376) and 84.7% glycoproteins (100 out of 118) were detected at least in two replicated analysis, displaying the effectiveness and advantage of Fe3O4/L-Cys nanoparticles for comprehensive glycosylation research in biological samples. Moreover, the enrichment performance from even a micro-amount of human serum was also investigated. A total of 186 glycopeptides with 197 N-glycosylation sites mapped to 87 glycoproteins were finally identified from 0.5 μL human serum sample within only one LC-MS/MS analysis (ESM Table S3), showing superior sensitivity for practical application. In addition, compared with previously reported L-Cys modified magnetic materials [20, 26], Fe3O4/L-Cys exhibited superior performance for the enrichment and analysis of glycopeptides in human serum (Table 1), thanks to the excellent hydrophilicity and brief composition.
It should be also emphasized that the preparation process of Fe3O4/L-Cys just involved a one-step functionalization. Applying the same one-step strategy, cysteamine functionalized Fe3O4/CS nanoparticles and glutathione functionalized Fe3O4/GSH nanoparticles were also successfully synthesized. With excellent hydrophilicity, the two nanoparticles also showed very prominent performance in the glycosylation analysis of human serum (Table 1, ESM Figs. S10 and S11). It can be seen that an economical, efficient, and easily replicable way to enrich and analyze glycosylation has been presented.
Conclusion
In summary, based on metal-organic coordination, novel ZIC-HILIC nanoparticles Fe3O4/L-Cys were easily synthesized via one-step functionalization. Owing to superior hydrophilicity, brief composition, and rapid magnetic response, the prepared nanoparticles exhibited high sensitivity, selectivity, and efficiency for the enrichment of glycopeptides. Furthermore, the nanoparticles achieved a remarkable result in the enrichment and analysis of complex human serum. In addition, one-step functionalization was successfully applied in the synthesis of other two HILIC materials Fe3O4/CS and Fe3O4/GSH, also achieving efficient glycopeptide enrichment in human serum. With easy synthesis, simple operation, and excellent reusability and stability, an economical and easily popularized strategy has been presented, showing great potential in glycoproteomics as well as in the synthesis of novel materials.
References
Mechref Y, Muddiman DC. Recent advances in glycomics, glycoproteomics and allied topics. Anal Bioanal Chem. 2016;409(2):355–7.
Palaniappan KK, Bertozzi CR. Chemical glycoproteomics. Chem Rev. 2016;116(23):14277–306.
Kailemia MJ, Park D, Lebrilla CB. Glycans and glycoproteins as specific biomarkers for cancer. Anal Bioanal Chem. 2016;409(2):395–410.
Ding W, Hill JJ, Kelly J. Selective enrichment of glycopeptides from glycoprotein digests using ion-pairing normal-phase liquid chromatography. Anal Chem. 2007;79(23):8891–9.
Chen CC, Su WC, Huang BY, Chen YJ, Tai HC, Obena RP. Interaction modes and approaches to glycopeptide and glycoprotein enrichment. Analyst. 2014;139(4):688–704.
Lin H, Ou J, Zhang Z, Dong J, Wu M, Zou H. Facile preparation of zwitterionic organic-silica hybrid monolithic capillary column with an improved "one-pot" approach for hydrophilic-interaction liquid chromatography (HILIC). Anal Chem. 2012;84(6):2721–8.
Shen A, Guo Z, Yu L, Cao L, Liang X. A novel zwitterionic HILIC stationary phase based on "thiol-ene" click chemistry between cysteine and vinyl silica. Chem Commun. 2011;47(15):4550–2.
Cao W, Huang J, Jiang B, Xing G, Yang P. Highly selective enrichment of glycopeptides based on zwitterionically functionalized soluble nanopolymers. Sci Rep. 2016;6:29776.
Chen Y, Xiong Z, Zhang L, Zhao J, Zhang Q, Peng L, et al. Facile synthesis of zwitterionic polymer-coated core-shell magnetic nanoparticles for highly specific capture of N-linked glycopeptides. Nano. 2015;7(7):3100–8.
Yeh CH, Chen SH, Li DT, Lin HP, Huang HJ, Chang CI, et al. Magnetic bead-based hydrophilic interaction liquid chromatography for glycopeptide enrichments. J Chromatogr A. 2012;1224:70–8.
Jiang B, Wu Q, Deng N, Chen Y, Zhang L, Liang Z, et al. Hydrophilic GO/Fe3O4/Au/PEG nanocomposites for highly selective enrichment of glycopeptides. Nano. 2016;8(9):4894–7.
Sun S, Yang G, Wang T, Wang Q, Chen C, Li Z. Isolation of N-linked glycopeptides by hydrazine-functionalized magnetic particles. Anal Bioanal Chem. 2010;396(8):3071–8.
Fratila RM, Moros M, de la Fuente JM. Recent advances in biosensing using magnetic glyconanoparticles. Anal Bioanal Chem. 2016;408(7):1783–803.
Bi C, Zhao Y, Shen L, Zhang K, He X, Chen L, et al. Click synthesis of hydrophilic maltose-functionalized iron oxide magnetic nanoparticles based on dopamine anchors for highly selective enrichment of glycopeptides. ACS Appl Mater Interfaces. 2015;7(44):24670–8.
Zhang S, He X, Chen L, Zhang Y. Boronic acid functionalized magnetic nanoparticles via thiol-ene click chemistry for selective enrichment of glycoproteins. New J Chem. 2014;38:4212–8.
Uehara J, Aramaki K. A surface-enhanced Raman spectroscopy study on adsorption of some sulfur-containing corrosion inhibitors on iron in hydrochloric acid solutions. J Electrochem Soc. 1991;138(11):3245–51.
Wang G, Harrison A, Li X, Whittaker G, Shi J, Wang X, et al. Study of the adsorption of benzimidazole and 2-mercaptobenzothiazole on an iron surface by confocal micro-raman spectroscopy. J Raman Spectrosc. 2004;35:1016–22.
Deng H, Li X, Peng Q, Wang X, Chen J, Li Y. Monodisperse magnetic single-crystal ferrite microspheres. Angew Chem Int Ed. 2005;44(18):2782–5.
Wang J, Wang Y, Gao M, Zhang X, Yang P. Versatile metal-organic framework-functionalized magnetic graphene nanoporous composites: as deft matrix for high-effective extraction and purification of the N-linked glycans. Anal Chim Acta. 2016;932:41–8.
Wu R, Li L, Deng C. Highly efficient and selective enrichment of glycopeptides using easily synthesized magG/PDA/Au/L-Cys composites. Proteomics. 2016;16(9):1311–20.
Sun X, Zhang L, Zhang W. Preparation of cysteine-click maltose modified silica as a hydrophilic interaction liquid chromatography material for the enrichment of glycopeptides. Chin J Chromatogr. 2017;35(7):696–702.
Sun X, Dong J, Li J, Ye M, Ou J, Zhang L, et al. Au-cysteine modified macroporous adsorption resin: preparation and highly selective enrichment and identification of N-linked glycopeptides from the complex biological sample. RSC Adv. 2016;6:113058–65.
Zheng J, Xiao Y, Wang L, Lin Z, Yang H, Zhang L, et al. Click synthesis of glucose-functionalized hydrophilic magnetic mesoporous nanoparticles for highly selective enrichment of glycopeptides and glycans. J Chromatogr A. 2014;1358:29–38.
Kuo CW, Wu IL, Hsiao HH, Khoo KH. Rapid glycopeptide enrichment and N-glycosylation site mapping strategies based on amine-functionalized magnetic nanoparticles. Anal Bioanal Chem. 2012;402(9):2765–76.
Wang J, Li J, Wang Y, Gao M, Zhang X, Yang P. Development of versatile metal-organic framework functionalized magnetic graphene core-shell biocomposite for highly specific recognition of glycopeptides. ACS Appl Mater Interfaces. 2016;8(41):27482–9.
Wu R, Xie Y, Deng C. Thiol-ene click synthesis of L-cysteine-bonded zwitterionic hydrophilic magnetic nanoparticles for selective and efficient enrichment of glycopeptides. Talanta. 2016;160:461–9.
Acknowledgements
This work was supported by China State Key Research Grant (Project 2016YFA0501402) and National High-Tech R&D Program of China (Project 2012AA020202).
Authors’ contributions.
All authors have given approval to the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. The research followed the tenets of the Declaration of Helsinki, and the use of the human serum samples for research was approved by the Ethics Committee of Zhongshan Hospital, Fudan University. Informed consent was obtained from all individual participants included in the research.
Additional information
Published in the topical collection celebrating ABCs 16th Anniversary.
Electronic supplementary material
ESM 1
(PDF 2.06 MB)
Rights and permissions
About this article
Cite this article
Feng, X., Deng, C., Gao, M. et al. Facile and easily popularized synthesis of l-cysteine-functionalized magnetic nanoparticles based on one-step functionalization for highly efficient enrichment of glycopeptides. Anal Bioanal Chem 410, 989–998 (2018). https://doi.org/10.1007/s00216-017-0602-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0602-5