Abstract
Highly selective glycopeptide enrichment is important before mass spectrometry analysis because of the ultra-low abundance of glycopeptides in the peptide mixtures. Herein, a UiO-66-NH2-based magnetic composite was prepared and used for the hydrophilic enrichment of glycopeptides. The composite was modified with phytic acid (PA) molecules by partially replacing 2-aminoterephthalic acid ligands in UiO-66-NH2, with electrostatic interactions also promoting this modification process. Based on the hydrophilicity of both the PA molecules and the UiO-66-NH2 skeleton, the resulting material, denoted as MUiO-66-NH2/PA, showed excellent dual hydrophilicity towards glycopeptide enrichment. Compared with pure UiO-66-NH2, the specific surface area and hydrophilicity of the prepared material were increased, and MUiO-66-NH2/PA exhibited good magnetic responsiveness to facilitate a convenient enrichment procedure. HRP and IgG were used as standard proteins to evaluate the glycopeptide enrichment properties, with 21 and 34 glycopeptides enriched from their tryptic digests. Furthermore, MUiO-66-NH2/PA showed outstanding sensitivity (1 fmol/μL) and selectivity (HRP/BSA = 1:1000), and achieved remarkable glycopeptide enrichment performance for practical human serum samples. Notably, MUiO-66-NH2/PA showed perfect reusability and stability, achieving enrichment performance after five cycles similar to that of the first use. This material can be used for glycopeptide enrichment to obtain further glycosylation information, providing the possibility for cancer treatment.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protein glycosylation is among the most widespread post-translational modifications in biological entities, influencing many vital activities, including cellular growth, recognition, adhesion, spread, and immune response [1,2,3]. Abnormal glycosylation might be related to many human neurodegenerative diseases and cancers, such as Alzheimer’s disease and breast cancer [4]. Analyzing glycosylation information can help identify new disease biomarkers or drug targets. The current research on glycoproteomics is based mainly on biological mass spectrometry (MS) [5, 6]. However, owing to their high molecular weight and complex physical and chemical properties, enriching whole glycoproteins is more difficult, with the identification of whole glycoproteins showing low reliability [7, 8]. Therefore, the strategy of digesting glycoproteins into peptides is being more widely employed, facilitating the acquisition of detailed glycosylation information for comparison and supplementation with protein databases. However, the glycopeptide content accounts for only about 2% of peptide mixtures, with mass spectrum signals largely masked by non-glycopeptides [9]. Therefore, enriching glycopeptides before MS analysis is important.
At present, there are several methods for glycopeptide enrichment, including lectin affinity, hydrazide chemistry, boric acid affinity, and hydrophilic interaction [10,11,12,13,14]. Among these methods, hydrophilic interaction exhibits significant advantages. For example, the enrichment performance is not affected by glycoforms and the enrichment process does not destroy carbohydrate chain structures [15]. Metal organic frameworks (MOFs) possess abundant pores and high specific surface area, providing great potential for glycopeptide enrichment [16]. Glycopeptide enrichment using hydrophilic MOFs is dependent mainly on their own hydrophilic interactions. However, interactions between the MOFs and glycopeptides are limited. MOF modification can significantly modify the surface properties to enhance hydrophilicity [17]. Two main strategies are used to modify MOFs. The first is direct synthesis modification (DSM), in which the MOF ligands are replaced during synthesis [18]. However, most related synthesis processes are relatively harsh, and the available ligands and synthetic methods are limited. These factors impact the application of DSM. The second strategy is post-synthetic modification (PSM). As more modification materials and methods are available for this strategy, the use of PSM has been increasing [19, 20]. Most hydrophilic PSM are based on chemical bond formation between the modified materials and amino or carboxyl groups in the MOF ligands. This can improve the hydrophilicity of the MOF surface, but sacrifices partial hydrophilic groups, to give limited hydrophilic sites. This strategy also ignores the MOF characteristics of large specific surface area, abundant pores, and chelation ability of the metal sites [21, 22].
In the present study, phytic acid (PA)-modified magnetic dual-hydrophilic composite MUiO-66-NH2/PA was designed for glycopeptide enrichment. Owing to the abundant hydrophilic groups, porous structure, and relatively strong framework, UiO-66-NH2, formed by coordination of a zirconium cluster and 2-aminoterephthalic acid, was used as a model MOF. PA, an organic acid containing six phosphate groups with ultra-high hydrophilicity, was used to partially replace ligands of UiO-66-NH2 through interaction between Zr4+ and the phosphate groups, further enhancing the hydrophilicity of the material. Interestingly, the electrostatic interaction between PA and UiO-66-NH2 promoted this modification. The resulting composite MUiO-66-NH2/PA exhibited high magnetic responsiveness to facilitate rapid separation and very high dual hydrophilicity due to the hydrophilicity of the PA molecules and UiO-66-NH2 skeleton. This material was used to enrich glycopeptides with high selectivity, good sensitivity, outstanding adsorption capacity, decent stability, and favorable reusability, and exhibited remarkable glycopeptide enrichment performance for mixed human serum samples. Based on this excellent enrichment performance and simple synthesis strategy, we believe that MUiO-66-NH2/PA could be applied to glycopeptide enrichment in numerous complex biological samples. The obtained glycosylation information might reflect the relevant properties of the corresponding glycoproteins, providing the possibility of use in cancer treatment.
Experimental
The details of the instruments and chemicals used in the experiment are shown in the Supplementary Electronic Material (ESM).
Preparation of MUiO-66-NH2
Firstly, Fe3O4 was synthesized as described previously, and Fe3O4@SiO2 was synthesized by coating with a layer of hydroxylated silica for further modification [23, 24]. Next, ZrCl4 (0.489 g) and Fe3O4@SiO2 (0.2 g) were evenly dispersed in 50 mL N,N-dimethylformamide (DMF) by ultrasonic method for 10 min. After adding 2-amino terephthalic acid (0.38 g), the resulting solution was stirred at 120 °C for 6 h. The reaction products were washed three times with DMF in ultrasound, and then three times with absolute ethanol. The obtained MUiO-66-NH2 was dried in vacuum at 60 °C.
Preparation of MUiO-66-NH2/PA
Briefly, 0.15 g MUiO-66-NH2 and 0.2 g polyvinylpyrrolidone (PVP) were evenly dispersed in ethanol (30 mL) followed by mechanical stirring for 60 min. Thereafter, the mixture of phytic acid (PA) and ethanol (20 mL, v/v = 1/40) was gently dropped into the reaction solution. The whole mixture was stirred at 25 °C for 6 h. The obtained MUiO-66-NH2/PA was washed six times alternately with deionized water and absolute ethanol, and finally dried under vacuum conditions.
Sample preparation
The tryptic digest process for standard proteins was as follows [25, 26]: 1 mg horseradish peroxidase (HRP; IgG, bovine serum albumin [BSA]) was dissolved in denaturing buffer (400 μL, 50 mM NH4HCO3, with 8 M urea). Dithiothreitol (10 μL, 200 mM) was added and heated at 56 °C for 50 min, and then iodoacetamide (90 μL, 400 mM) was added to alkylate at 30 °C for 50 min in the dark. Subsequently, the reaction mixture was diluted to 5 mL with NH4HCO3 aqueous solution (50 mM). Trypsin (w/w = 1:25) was added to incubate for 18 h at 37 °C. After the enzymolysis, the peptide mixture was cryopreserved at −20 °C.
The digestion of human serum was similar to that of standard proteins. Ten microliters of human serum was diluted with denaturing buffer (90 μL, 50 mM NH4HCO3, with 8 M urea), and the resulting solution was treated with dithiothreitol (5 μL, 200 mM) and iodoacetamide (20 μL, 400 mM). Subsequently, the reaction mixture was diluted to 1 mL with NH4HCO3 aqueous solution (50 mM), and then trypsin (w/w = 1/25) was added to incubate at 37 °C for 18 h. The obtained peptide mixture was cryopreserved at −20 °C for later use.
Procedure for glycopeptide enrichment from biological samples
MUiO-66-NH2/PA (1 mg) was incubated in loading buffer (200 μL, ACN/TFA = 99:1, v/v) containing 20 μL tryptic digest of HRP (IgG or human serum), and then the mixture was vortexed for 35 min vigorously. After magnetic separation, the precipitations were washed three times with washing buffer (200 μL, ACN/H2O/TFA = 90:9:1, v/v/v) repeatedly. The MUiO-66-NH2/PA with glycopeptides was eluted by eluent (20 μL, H2O/TFA = 99:1, v/v) for 10 min. The collected solution was detected by matrix-assisted laser desorption/ionization–time-of-flight mass spectroscopy (MALDI-TOF MS) or treated with PNGase F.
Deglycosylation of glycopeptides
After lyophilizing the eluent from protein standard samples or human serum enriched with MUiO-66-NH2/PA, 17 μL deionized water was added to the residual substances. Thereafter, 2 μL 10× GlycoBuffer 2 and 1 μL PNGase F were added to deglycosylate the glycopeptides. The reaction mixture was incubated at 37 °C for 18 h, and the obtained enzymatic hydrolysate was detected by MALDI-TOF MS or liquid chromatography–tandem mass spectrometry (LC-MS/MS) [27].
MALDI-TOF MS analysis
After the glycopeptide sample (1 μL) and matrix (1 μL) were evenly mixed, the mixture (1 μL) was dropped on the target plate to form a crystalline film, and then the target plate was subjected to analysis. Positive reflection mode was used for all samples. In MALDI-TOF MS mode, DHB solution (25 mg/mL, ACN/H2O/H3PO4 = 70:29:1, v/v/v) was selected as the matrix; in the MS/MS mode, HCCA saturated solution (ACN/H2O = 30/70, v/v, with 0.1% TFA) was selected as the matrix.
Results and discussion
Characterization of MUiO-66-NH2/PA
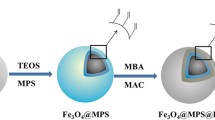
The synthetic route to MUiO-66-NH2/PA is shown in Scheme 1. First, Fe3O4 magnetic particles were prepared and the surface was modified with hydroxylated silica. The magnetic precursor material was then modified with UiO-66-NH2. Due to the presence of Zr4+, 2-aminoterephthalic acid ligands in UiO-66-NH2 were partially replaced by PA molecules owing to the stronger coordination of Zr4+ with the phosphate group than with the carboxyl group in 2-aminoterephthalic acid. Electrostatic interactions between the MOF and PA assisted this synthesis. The dual hydrophilicity of the as-prepared materials was attributed to the remaining 2-aminoterephthalic acid ligands in UiO-66-NH2 and the modified PA molecules. Similar to a jigsaw puzzle, which is formed by the connection of adjacent elements (Scheme 2), the dual-hydrophilic interaction between glycopeptides and MUiO-66-NH2/PA can be used to enrich glycopeptides.
The morphology of MUiO-66-NH2/PA was characterized by scanning electron microscopy (SEM). Fe3O4@SiO2 comprised smooth and spherical particles about 400 nm in size (Fig. 1a). After coating with UiO-66-NH2 (Fig. 1b), the Fe3O4@SiO2 surface was modified with polyhedral crystals, confirming the successful preparation of MUiO-66-NH2. When the prepared materials were treated with PA, the surface morphology changed significantly (Fig. 1c). Energy-dispersive spectroscopy (EDX) elemental mapping (Fig. 1d) showed strong signals for Fe, Zr, and P, indicating even distribution in the as-prepared materials. These results indicated that MUiO-66-NH2/PA had been successfully prepared.
To further confirm the successful synthesis of MUiO-66-NH2/PA, Fourier transform infrared (FT-IR) spectroscopy, X-ray powder diffraction (XRD) analysis, thermo gravimetric analyzer (TGA), X-ray photoelectron spectroscopy (XPS), Brunauer–Emmett–Teller (BET) theory, and a vibrating-sample magnetometer (VSM) were applied to characterize the as-prepared composites. As shown in Fig. 2a, absorption signals at 576 cm−1 were ascribed to the Fe–O vibration in Fe3O4, while the signal at 1085 cm−1 was attributed to the Si–O–Si stretching vibration. These peaks confirmed the presence of Fe3O4@SiO2. After modification with UiO-66-NH2, new adsorption signals appeared at 1617, 1495, and 1438 cm−1, which were attributed to benzene skeleton vibrations of the 2-aminoterephthalic acid ligands in UiO-66-NH2, while the signal at 1657 cm−1 was attributed to the C=O bond stretching vibration in 2-aminoterephthalic acid, confirming successful modification with UiO-66-NH2. FT-IR analysis of MUiO-66-NH2/PA showed a peak at around 1072 cm−1, which was traced to the presence of phosphate and SiO2, while the notable increase in peak intensity compared with Fe3O4@SiO2 confirmed the successful functionalization with PA. Meanwhile, the signals at 1657, 1617, 1495, and 1438 cm−1 were significantly reduced. This trend was attributed to the increase in PA molecules and decrease in the original 2-aminoterephthalic acid ligands in UiO-66-NH2. The XRD diagram is shown in Fig. 2b. A series of peaks at 29.9°, 35.3°, 42.9°, 53.2°, 56.8°, and 62.4° appeared in all three materials, and were attributed to Fe3O4 [28, 29]. Strong diffraction peaks at 7.02° and 8.28° in MUiO-66-NH2 confirmed the presence of UiO-66-NH2 [30]. After modification with PA, the intensity of the peaks at 7.02° and 8.28° was significantly decreased, indicating that some of the MOF crystal structures were decomposed such that long-range order was lost. XPS analysis was performed to confirm the phosphorus oxidation state of the composite after modification with PA. Compared with the spectra of UiO-66-NH2 (ESM Fig. S1a) and UiO-66-NH2/PA (ESM Fig. S1b), a new peak at 133.68 eV, assigned as the characteristic P2p peak, was observed in UiO-66-NH2/PA. The fitted subpeaks of P2p (ESM Fig. S1c) were attributed to the contributions of Zr–O–P (134.2 eV) and R–PO3H2 (133.3 eV), which indicated the presence of two chemical environments for phosphorus atoms [31, 32]. XPS analysis clearly confirmed the coordination interaction between PA molecules and Zr4+. Combined with FT-IR and XRD analysis, these results showed that PA molecules had partially replaced the original ligands in UiO-66-NH2. According to TGA analysis (Fig. 2c), Fe3O4@SiO2 showed about 6% weight loss as the temperature was gradually increased to 1000 °C, while MUiO-66-NH2 and MUiO-66-NH2/PA showed weight loss of 34% and 25%, respectively. The lower weight loss of MUiO-66-NH2/PA might be related to some unstable UiO-66-NH2 crystals being decomposed by PA, which might improve the stability of MUiO-66-NH2/PA. Zeta potential characterization indicated that Fe3O4@SiO2, MUiO-66-NH2, and MUiO-66-NH2/PA (Fig. 3a) were negatively, positively, and negatively charged, respectively, in agreement with the surface hydroxyl, amino, and phosphoric acid groups present after each modification step. This trend confirmed the successful synthesis of the composite, and also showed that the electrostatic interaction between PA and MUiO-66-NH2 aided material synthesis. The BET adsorption–desorption curve was also used to evaluate changes in the material surface properties (Fig. 3b). After modification with PA, the specific surface area of the as-prepared material was increased by 34.47%, from 235.01 to 329.48 m2/g. As shown by VSM analysis (Fig. 2d), the saturation magnetization value of Fe3O4@SiO2 was 49.59 emu/g, while that of MUiO-66-NH2/PA was 20.38 emu/g. This decrease might be due to modification of the composite with UiO-66-NH2 and PA. However, MUiO-66-NH2/PA still showed outstanding magnetic responsiveness.
Optimization of MUiO-66-NH2/PA synthesis conditions
The hydrophilicity of MUiO-66-NH2/PA was derived from UiO-66-NH2 and PA. PA molecules partially replaced some ligands in UiO-66-NH2 to enhance the hydrophilicity of the material. Therefore, the amount of PA was particularly important for the synthesis of MUiO-66-NH2/PA. As shown in ESM Fig. S2, when the amount of PA solution (PA/ethanol = 1:40, v/v) added was increased from 5 to 20 mL, the types of glycopeptides enriched increased accordingly, with 21 glycopeptides enriched when 20 mL of PA was used. However, when the amount of PA added was 25 mL, the characteristic diffraction peaks (7.02° and 8.28°) of UiO-66-NH2 showed significantly reduced intensity by XRD analysis (ESM Fig. S3). This trend might be related to excessive replacement of 2-aminoterephthalic acid ligands in UiO-66-NH2 with PA molecules, causing a collapse of the MOF structure. This phenomenon may have led to a decrease in the number of enriched glycopeptides. To confirm this, materials modified with 25 mL of PA were used for glycopeptide enrichment (ESM Fig. S2e). The types of glycopeptides enriched were found to decrease, while the signals of non-glycopeptide peaks were enhanced (such as signals at m/z 2998.9 and 3141.2). Therefore, under the optimal synthesis conditions, the amount of PA solution added was 20 mL.
Optimization of enrichment conditions
In the glycopeptide enrichment procedure, the ratios of the loading buffer (ACN/H2O, 99:1, v/v), washing buffer (ACN/H2O/TFA, 90:9:1, v/v/v), and eluent (H2O/TFA, 99:1, v/v) can significantly influence the enrichment performance. Glycopeptides are more inclined to be adsorbed on highly hydrophilic materials or dissolved in water, rather than dissolved in ACN. Therefore, the most suitable enrichment conditions can be identified mainly by adjusting the ratio of ACN to deionized water. HRP, a glycoprotein containing many glycopeptides in the peptide mixture after enzymolysis, was selected as the standard glycoprotein to obtain appropriate glycopeptide enrichment conditions. First, different loading buffers (ACN/H2O/TFA, 99:0:1, 95:4:1, 90:9:1, 85:14:1, and 80:19:1, v/v/v; 180 μL) and HRP tryptic digest (20 μL) were added to MUiO-66-NH2/PA (1 mg). After vigorous shaking for 30 min at 25 °C, the supernatants were detected by MALDI-TOF MS (ESM Fig. S4). As the proportion of H2O in the loading buffer increased, the glycopeptide signals in the supernatant showed regular enhancement, meaning that the glycopeptide components enriched on MUiO-66-NH2/PA decreased. Therefore, ACN/H2O (99:1, v/v) was selected as the optimal loading condition. Furthermore, the washing buffer must be able to remove non-glycopeptides and inorganic salts adsorbed on the surface of MUiO-66-NH2/PA. In the enrichment process, different washing buffers (ACN/H2O/TFA, 99:0:1, 95:4:1, 90:9:1, 85:14:1, 80:19:1, v/v/v) were applied. When the washing buffer component was 90:9:1 (v/v/v) ACN/H2O/TFA, MUiO-66-NH2/PA enriched the most types of glycopeptides, and the signal intensity of non-glycopeptides from MALDI-TOF MS analysis was much lower (ESM Fig. S5). Finally, a suitable eluent will elute the maximum amount of glycopeptides enriched on MUiO-66-NH2/PA. Different elution conditions (H2O/ACN/TFA, 99:0:1, 90:9:1, 80:19:1, 70:29:1, v/v/v) were investigated, showing that higher content of ACN resulted in less elution (ESM Fig. S6). Therefore, 99:1 (v/v) H2O/TFA was selected as the optimal eluent.
Enrichment performance of glycopeptides
As shown in Scheme 2, the eluent containing glycopeptides was directly analyzed by MALDI-TOF MS after enrichment. Figure 4 shows the glycopeptide enrichment performance of MUiO-66-NH2/PA in the HRP tryptic digest. When the tryptic digest was directly detected by MALDI-TOF MS, the glycopeptide signals were very limited and essentially covered by the non-glycopeptide signals (Fig. 4a). Using different enrichment materials, 12 glycopeptides were enriched by MUiO-66-NH2, while 21 glycopeptides were enriched by MUiO-66-NH2/PA (Fig. 4b and c). The types and signal intensity of the enriched glycopeptides were greatly improved after PA modification. This indicated that PA functionalization enhanced the hydrophilicity of the composite, which improved the glycopeptide enrichment performance. To further confirm that the enriched peptides were glycopeptides, the glycopeptide at m/z 3671.19 was analyzed by MALDI-TOF MS/MS (Fig. 4d), with the peptide sequence found to be GLIQSDQELFSSPN#ATDTIPLVR (m/z 2502.48). The signals of MH−16.95 (m/z 2485.53), MH+83.24 (m/z 2585.72), and MH+119.70 (m/z 2705.42) were features of glycopeptides, demonstrating that the enriched peptides were glycopeptides. These results confirmed that MUiO-66-NH2/PA exhibited excellent enrichment performance and selectivity for glycopeptides.
MALDI-TOF MS spectrum of HRP tryptic digest (a) without enrichment, (b) enriched by MUiO-66-NH2, and (c) enriched by MUiO-66-NH2/PA; and (d) MALDI-MS/MS spectra of m/z 3671.19. Marked letters indicate the following: F, fucose; H, hexose (mannose or galactose); N, N-acetylglucosamine. Glycopeptide signals were marked with red dots (a, b, c); The signals of MH−16.95 (m/z 2485.53), MH+83.24 (m/z 2585.72), and MH+119.70 (m/z 2705.42) were characteristic signals of glycopeptides (d)
As the most abundant immunoglobulin in human serum, IgG was used as another standard protein to evaluate the glycopeptide enrichment ability of MUiO-66-NH2/PA, and 34 glycopeptides were enriched from the IgG tryptic digest (ESM Fig. S7). Specific glycopeptide information is shown in ESM Table S4. Two signals at m/z 2602.3 and 2634.4 were detected by MALDI-TOF MS/MS, with corresponding peptide sequences of EEQFN#STFR (m/z 1156.94) and EEQFN#STYR (m/z 1188.90), respectively. These results confirmed that MUiO-66-NH2/PA showed high selectivity and enrichment performance for IgG tryptic digest.
Practical biological samples contain numerous components, such as proteins, lipids, carbohydrates, and inorganic salts. This complex matrix background causes considerable interference with glycopeptide enrichment. The presence of non-glycopeptides also has a significant impact owing to the low proportion of glycopeptides. Therefore, high selectivity for glycopeptide enrichment is necessary. The selectivity of MUiO-66-NH2/PA was assessed by enriching glycopeptides in a mixture of HRP and BSA tryptic digests. When the proportion of HRP and BSA tryptic digests was 1:10 (w/w), 21 types of glycopeptides were enriched (Fig. 5a), which was equivalent to the effect of directly enriching glycopeptides in HRP tryptic digest. When the ratio was 1:1000 (w/w), although some non-glycopeptide signals were enhanced, the types of glycopeptides enriched remained the same (Fig. 5c). These results indicate that as-prepared MUiO-66-NH2/PA exhibited excellent selectivity.
To evaluate the binding capacity of MUiO-66-NH2/PA for glycopeptides, different masses of MUiO-66-NH2/PA (60, 80, and 100 μg) were added to the mixture of HRP tryptic digest (4 μg, 0.2 mg/mL, 20 μL) and loading buffer (ACN/TFA = 99:1, 180 μL); after incubation for 35 min under vortex conditions, the obtained supernatants were directly detected by MALDI-TOF MS. Figure S8 (see ESM) shows that when the mass of MUiO-66-NH2/PA reached 100 μg, glycopeptides in the HRP tryptic digest were completely enriched. Thus we assumed that 4 μg of glycopeptides could be completely enriched by 100 μg of MUiO-66-NH2/PA. Therefore, the binding capacity of MUiO-66-NH2/PA was about 40 μg/mg.
In actual complex biological samples, the glycopeptide concentration is generally low, accounting for only 2–5% of the peptide mixture. Therefore, only a lower detection limit can meet the actual analysis demand. During the glycopeptide enrichment process in the present study, when the HRP tryptic digest concentrations were 50 and 5 fmol/μL, 13 and 7 types of glycopeptide were detected, respectively (Fig. 6). Meanwhile, when the HRP concentration was as low as 1 fmol/μL, glycopeptide signals were still detected at m/z 3354.0, 3378.2, 3673.2, and 4985.9. The experimental results showed that even at very low concentrations, MUiO-66-NH2/PA demonstrated excellent glycopeptide enrichment performance. This remarkable detection sensitivity was attributed to the modification with PA molecules, greatly improving the hydrophilicity and selectivity of the material, resulting in ultra-high enrichment performance toward glycopeptides. Compared with some reported studies, MUiO-66-NH2/PA exhibited excellent glycopeptide enrichment performance (ESM Table S5).
Owing to the remarkable magnetic responsiveness, reuse of MUiO-66-NH2/PA would be convenient. HRP tryptic digest was selected to evaluate the reusability and stability of the as-prepared materials. MUiO-66-NH2/PA was stored at room temperature for 8 months. After each cycle, MUiO-66-NH2/PA was washed with eluent (TFA/ACN = 99:1, 200 μL) and loading buffer (ACN/TFA = 99:1, 200 μL) for 10 min, respectively, before proceeding to the next cycle. Long-term storage had essentially no influence on the enrichment performance toward glycopeptides. After five cycles, the enrichment performance remained similar to that achieved in the first use (ESM Fig. S9). By monitoring the signal-to-noise ratios of five glycopeptide signals with strong intensities (m/z 1843.2, 3354.0, 3378.2, 3673.2, and 4985.9) in five cycles, the average signal-to-noise ratio from the fifth use was found to be 90.08% of that from the first use (Fig. 7). Notably, the reusability was better than most other materials (such as ZIF-L-Co-S-Au-Cys and magnetic mesoporous phenolic resin) [21, 33]. This was attributed to interactions between PA molecules and Zr4+, making MUiO-66-NH2/PA more stable, and simultaneously endowing the material with ultra-high hydrophilicity.
To evaluate the glycopeptide enrichment ability of MUiO-66-NH2/PA for actual complex biological samples, mixed human serum was selected as the experimental sample. After mixed human serum tryptic digest (10 μL) was enriched using MUiO-66-NH2/PA, the obtained mixture was treated with PNGase F for 18 h, and then detected by LC-MS/MS. The glycopeptide information obtained was searched using MASCOT software, and 101 glycopeptides were identified, corresponding to 48 glycoproteins. These results were better than some reported studies [34, 35]. The specific glycosylation information is recorded in ESM Table S6. Therefore, we concluded that MUiO-66-NH2/PA can be used to enrich glycopeptides in practical complex biological systems.
Conclusions
In short, a novel UiO-66-NH2-based dual-hydrophilic magnetic composite was successfully synthesized for glycopeptide enrichment. Phytic acid (PA) with abundant hydrophilic groups and good coordination ability was selected to partially replace ligands of UiO-66-NH2 on the magnetic composites, with electrostatic interactions promoting the modification process. This synthesis strategy significantly enhanced the hydrophilicity of UiO-66-NH2, with as-prepared MUiO-66-NH2/PA exhibiting excellent selectivity, good sensitivity, outstanding adsorption capacity, high stability, favorable reusability, and good magnetic response for glycopeptide enrichment. Meanwhile, the as-prepared material was successfully applied to glycopeptide assay in actual human serum samples, with 101 glycopeptides identified, corresponding to 48 glycoproteins. Based on the high-efficiency enrichment performance, we believe that MUiO-66-NH2/PA has great potential for glycoprotein pretreatment, providing much more glycosylation information and promoting the search for new tumor markers and drug carriers.
References
Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2001;291(5512):2370–6.
Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15(9):540–55.
Cao L, Diedrich JK, Ma Y, Wang N, Pauthner M, Park S, et al. Global site-specific analysis of glycoprotein N-glycan processing. Nat Protoc. 2018;13(6):1196–212.
Reily C, Stewart TJ, Renfrow MB, Novak J. Glycosylation in health and disease. Nat Rev Nephrol. 2019;15(6):346–66.
Schjoldager KT, Narimatsu Y, Joshi HJ, Clausen H. Global view of human protein glycosylation pathways and functions. Nat Rev Mol Cell Bio. 2020;21(12):729–49.
Shajahan A, Heiss C, Ishihara M, Azadi P. Glycomic and glycoproteomic analysis of glycoproteins—a tutorial. Anal Bioanal Chem. 2017;409(19):4483–505.
Yates J, Kelleher N. Top down proteomics. Anal Chem. 2013;85(13):6151.
Xiao H, Suttapitugsakul S, Sun F, Wu R. Mass spectrometry-based chemical and enzymatic methods for global analysis of protein glycosylation. Acc Chem Res. 2018;51(8):1796–806.
Novotny MV, Alley WR. Recent trends in analytical and structural glycobiology. Curr Opin Chem Biol. 2013;17(5):832–40.
Ruiz-May E, Hucko S, Howe KJ, Zhang S, Sherwood RW, Thannhauser TW, et al. A comparative study of lectin affinity based plant N-glycoproteome profiling using tomato fruit as a model. Mol Cell Proteomics. 2014;13(2):566–79.
Peng J, Hu Y, Zhang H, Wan L, Wang L, Liang Z, et al. High anti-interfering profiling of endogenous glycopeptides for human plasma by the dual-hydrophilic metal–organic framework. Anal Chem. 2019;91(7):4852–9.
Sajid MS, Jabeen F, Hussain D, Ashiq MN, Najam-ul-Haq M. Hydrazide-functionalized affinity on conventional support materials for glycopeptide enrichment. Anal Bioanal Chem. 2017;409(12):3135–43.
Shao W, Liu J, Liang Y, Yang K, Min Y, Zhang X, et al. “Thiol-ene” grafting of silica particles with three-dimensional branched copolymer for HILIC/cation-exchange chromatographic separation and N-glycopeptide enrichment. Anal Bioanal Chem. 2018;410(3):1019–27.
Chen Z, Huang J, Li L. Recent advances in mass spectrometry (MS)-based glycoproteomics in complex biological samples. TrAC Trends Anal Chem. 2019;118:880–92.
Tang F, Yu Q-W, Yuan B-F, Feng Y-Q. Hydrophilic materials in sample pretreatment. TrAC Trends Anal Chem. 2017;86:172–84.
Zhao X, Wang Y, Li D-S, Bu X, Feng P. Metal–organic frameworks for separation. Adv Mater. 2018;30(37):1705189.
Mandal S, Natarajan S, Mani P, Pankajakshan A. Post-synthetic modification of metal–organic frameworks toward applications. Adv Funct Mater. 2021;31(4):2006291.
Masoomi MY, Morsali A, Dhakshinamoorthy A, Garcia H. Mixed-metal MOFs: unique opportunities in metal–organic framework (MOF) functionality and design. Angew Chem Int Ed. 2019;58(43):15188–205.
Kalaj M, Cohen SM. Postsynthetic modification: an enabling technology for the advancement of metal–organic frameworks. ACS Central Sci. 2020;6(7):1046–57.
Wang X, Na Z, Yin D, Wang C, Wu Y, Huang G, et al. Phytic acid-assisted formation of hierarchical porous CoP/C nanoboxes for enhanced lithium storage and hydrogen generation. ACS Nano. 2018;12(12):12238–46.
Zhou Y, Xu Y, Zhang C, Emmer Å, Zheng H. Amino acid-functionalized two-dimensional hollow cobalt sulfide nanoleaves for the highly selective enrichment of N-linked glycopeptides. Anal Chem. 2020;92(2):2151–8.
Hu X, Liu Q, Wu Y, Deng Z, Long J, Deng C. Magnetic metal-organic frameworks containing abundant carboxylic groups for highly effective enrichment of glycopeptides in breast cancer serum. Talanta. 2019;204:446–54.
Zhang R, Wang Z, Wang T, Su P, Yang Y. Boronic acid-decorated metal-organic frameworks modified via a mixed-ligand strategy for the selective enrichment of cis-diol containing nucleosides. Anal Chim Acta. 2020;1106:42–51.
Zhang R, Wang Z, Zhou Z, Li D, Wang T, Su P, et al. Highly effective removal of pharmaceutical compounds from aqueous solution by magnetic Zr-based MOFs composites. Ind Eng Chem Res. 2019;58(9):3876–84.
Alagesan K, Khilji SK, Kolarich D. It is all about the solvent: on the importance of the mobile phase for ZIC-HILIC glycopeptide enrichment. Anal Bioanal Chem. 2017;409(2):529–38.
Xia C, Jiao F, Gao F, Wang H, Lv Y, Shen Y, et al. Two-dimensional MoS2-based zwitterionic hydrophilic interaction liquid chromatography material for the specific enrichment of glycopeptides. Anal Chem. 2018;90(11):6651–9.
Ma Y, Yuan F, Zhang X, Zhou Y, Zhang X. Highly efficient enrichment of N-linked glycopeptides using a hydrophilic covalent-organic framework. Analyst. 2017;142(17):3212–8.
Zhou Z, Gao Z, Shen H, Li M, He W, Su P, et al. Metal–organic framework in situ post-encapsulating DNA–enzyme composites on a magnetic carrier with high stability and reusability. ACS Appl Mater Interfaces. 2020;12(6):7510–7.
Song J, He W, Shen H, Zhou Z, Li M, Su P, et al. Self-assembly of a magnetic DNA hydrogel as a new biomaterial for enzyme encapsulation with enhanced activity and stability. Chem Commun. 2019;55(17):2449–52.
Sarker M, Song JY, Jhung SH. Carboxylic-acid-functionalized UiO-66-NH2: a promising adsorbent for both aqueous- and non-aqueous-phase adsorptions. Chem Eng J. 2018;331:124–31.
Pu C, Zhao H, Hong Y, Zhan Q, Lan M. Facile preparation of hydrophilic mesoporous metal–organic framework via synergistic etching and surface functionalization for glycopeptides analysis. Anal Chem. 2020;92(2):1940–7.
Contreras-Ramirez A, Tao S, Day GS, Bakhmutov VI, Billinge SJL, Zhou H-C. Zirconium phosphate: the pathway from Turbostratic disorder to crystallinity. Inorg Chem. 2019;58(20):14260–74.
Ma W, Xu L, Li X, Shen S, Wu M, Bai Y, et al. Cysteine-functionalized metal–organic framework: facile synthesis and high efficient enrichment of N-linked glycopeptides in cell lysate. ACS Appl Mater Interfaces. 2017;9(23):19562–8.
Chen Y, Sheng Q, Hong Y, Lan M. Hydrophilic nanocomposite functionalized by carrageenan for the specific enrichment of glycopeptides. Anal Chem. 2019;91(6):4047–54.
Yang S, Wang C, Yu X, Shang W, Chen DDY, Gu Z. A hydrophilic two-dimensional titanium-based metal-organic framework nanosheets for specific enrichment of glycopeptides. Anal Chim Acta. 2020;1119:60–7.
Funding
This work was supported by Beijing Natural Science Foundation (2202037), National Natural Science Foundation of China (21675008), China Postdoctoral Science Foundation (2020 M680313), and Fundamental Research Funds for the Central Universities (ZY2015).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1.68 mb)
Rights and permissions
About this article
Cite this article
Su, P., Wang, Z., Li, X. et al. Fabrication of magnetic dual-hydrophilic metal organic framework for highly efficient glycopeptide enrichment. Anal Bioanal Chem 413, 5267–5278 (2021). https://doi.org/10.1007/s00216-021-03535-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03535-w