Abstract
Meliaceae plants have been extensively used in agriculture, folklore, and traditional medicine. They are the major storehouses for structurally diverse limonoids (meliacins) possessing various bioactivities like antifeedant, insecticidal, antimicrobial, etc. However accurate detection of these tetranortriterpenes from the vast pool of metabolites in plant tissue extracts or biological sample is a crucial challenge. Though the mass spectrum (MS) provides the molecular mass and the corresponding elemental composition, it cannot be relied precisely. The exact identification of a specific metabolite demands the MS/MS spectrum containing the signature product ions. In the present study, we have developed the UHPLC Q-Orbitrap–based method for identification, quantification, and characterization of limonoids in different plant tissue extracts requiring minimum plant material. Using this method, we carried out the limonoid profiling in different tissue extracts of sixteen Meliaceae plants and the identification of limonoids was performed by comparing the retention time (RT), ESI-( +)-MS spectrum, and HCD-MS/MS of the purified fifteen limonoids used as reference standards. Our results revealed that early intermediates of the limonoid biosynthetic pathway such as azadiradione, epoxyazadiradione, and gedunin occurred more commonly in Meliaceae plants. The MS/MS spectrum library of the fifteen limonoids generated in this study can be utilized for identification of these limonoids in other plant tissue extracts, botanical fertilizers, agrochemical formulations, and bio pesticides.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Limonoids are plant specialized metabolites exhibiting a wide spectrum of biological activities important for animal, plant, and human welfare. They belong to a class of tetracyclic triterpenoids which are formed by loss of four terminal carbons of the side chain in the apotirucallane (C30) skeleton and then cyclized to form the 17α-furan ring, which is also known as tetranortriterpenoids (C26). Limonoids are majorly present in Meliaceae and Rutaceae family plants [1, 2]. Among different members of Meliaceae, neem/Azadirachta indica (AZI) has been the subject of intense investigation throughout these years [3, 4] and utilized for its eco-friendly biopesticide containing azadirachtin A (a key limonoid). It almost took 22 years to complete the chemical synthesis of azadirachtin involving 71 steps by Steven Ley and group [5, 6]. The high structural complexity of azadirachtin and low yield make the chemical synthesis not feasible for large-scale production.
Limonoids exhibit significant insecticidal activity, of which azadirachtin is the main component responsible for potent antifeedant effects on insects. It exists in different forms as A, B, D, E, F, G, H, I, K, M, N, etc. of which azadirachtin A is the most abundant. Limonoids have also been reported to possess growth-regulating activity [1, 7]. Neem limonoid–based biopesticides are widely formulated by the agricultural industry. Some of the commercial neem products (seed kernel extract of A. indica) such as Margosan-O, Turplex, and Align have been granted approval for pest control usage in the USA by the environmental protection agency [8]. At 1 ppm, 17β-hydroxyazadiradione, salannin, 6-deacetylnimbin, gedunin, and 7-deacetylgedunin showed 100% larval mortality against Helicoverpa armigera [9]. Apart from insecticidal activity, limonoids are known to possess a broad bioactivity spectrum including anti-inflammatory, anti-cancer, anti-malarial, anti-ulcer, anti-microbial, hypoglycemic activity, etc. [10,11,12,13,14,15].
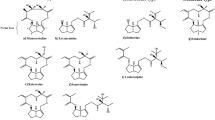
The chemical structures of limonoids used as reference standards in this study include azadirachtin A, azadirachtin B, salannin, 3-deacetylsalanin, nimbin, 6-deacetylnimbin, nimbinene, 6-deacetylnimbinene, nimbanal, salanol acetate, azadiradione, epoxyazadiradione, azadirone, gedunin, and nimocinol (1–15) which are represented in Fig. 1. These limonoids belong to six different limonoid skeletons such as azadirachtin, salanin, nimbin, nimbinene, azadirone, and gedunin. The accurate identification of these limonoids in a huge pool of metabolites such as plant tissue extracts, organic fertilizers, agrochemical formulations and bio pesticides, etc. is a major challenge. The functional group decoration on these limonoids affects their solubility. The high structural resemblance of these limonoids results in low chromatographic resolution. The high complexity and structural similarity in bio analytical samples complicate their routine analysis. Therefore, a precise and versatile analytical technique is needed for identification of selected limonoids. Several analytical methods were developed for analysis of limonoids which focused mainly on azadirachtins but not other skeletal structures of limonoids [16,17,18]. In some HPLC–MS-MS- or LC–MS-based methods, only for 4–8 different limonoids of different skeletal structures were analyzed [19, 20]. The previously described high-performance liquid chromatography (HPLC)–coupled UV detection method lacks selectivity and sensitivity required for their quantification, and efficient separation cannot be achieved [21]. Though a good separation was achieved by HPLC/tandem mass spectrometry or ultra-high performance liquid chromatography (UHPLC)/tandem mass spectrometry with improved selectivity, sensitivity, and detection limit up to one or two decimal units, these methods still lacked accuracy [22,23,24,25]. In few other reports with detection limit of up to four decimals, a clean separation of limonoids using the liquid chromatography program was achieved up to a maximum of eight limonoids only [26, 27]. This is the first time that a UHPLC method for a single shot separation of fifteen limonoids in a time frame of 35 min is reported. Another distinctive component of our method is the high-energy C-trap dissociation (HCD) with Orbitrap detection mass spectrometry along with detection sensitivity up to four decimal units. The method reported in this paper is reliable and easily reproducible. This method was validated by identifying the limonoids in different tissue extracts such as leaf, fruit, bark, and the flower of sixteen Meliaceae plants. In this study, the MS/MS product ion library of the fifteen limonoids generated was utilized for the identification of limonoids in plant tissue crude extracts. Also, the abundance of the identified limonoids was quantified. The different quantitative parameters such as linear range, limit of detection (LOD), limit of quantification (LOQ), recovery, repeatability, and reproducibility were determined. This study generated not only a coherent, time-efficient HCD-MS/MS (Orbitrap detection) method to identify and quantitate the limonoids but also a tissue-specific limonoid profile of sixteen Meliaceae plants. This limonoid profiling will aid researchers to harness the biosynthetic potential to produce limonoids on the industrial scale.
Materials and methods
Chemicals
For all HCD-MS/MS experiments, LC–MS-grade solvents were procured from Merck, Darmstadt, Germany.
Plant material
Various plant parts such as fruit, flower, leaf, and bark of sixteen plant species were collected from Karnataka state in India. Aglaia lawii, Aglaia barberi Gamble, Aglaia simplicifolia, Dysoxylum binectariferum, and Walsura trifolia were collected on the way to Bisale forest, Hassan district, Karnataka. Aphanamixis polystachya near Balupete, Sakaleshpur Taluka, Hassan district; Cipadessa baccifera from Kattaya forest, Hassan district; Chukrasia tabularis, Kemmangundi, Chikmagalur district; Melia azedarach near Belvathally forest, Arasikere, Hassan district; Melia dubia-Dudda, Hassan district; Reinwardtiodendron anamalaiense, Bisale forest, Hassan district; Soymida febrifuga, on the way to Lakkinakoppa junction, Shivamogga district; Toona ciliata, Bababudan hills, Chikkamagaluru district; Trichilia connaroides-Sakaleshpur, Hassan district; Dysoxylum malabaricum, Agumbe, Shivamogga district, Karnataka; Swietenia macrophylla, Hassan (Fig. S1). AZI tissues were collected from an identified plant at CSIR-NCL campus.
Extraction of limonoids
Fresh tissues (0.5 g) were extracted with methanol (10 mL) thrice, by continuous stirring for 3 h. The pooled methanol layer after concentration under reduced pressure at 50 °C was partitioned between 20 mL each of ethyl acetate and water. The organic layer was separated, passed through anhydrous sodium sulfate, and concentrated under similar conditions to obtain the triterpenoid extract. Extraction of individual tissues was performed in triplicates.
UHPLC Q-Orbitrap MS/MS profiling of limonoids
HCD-MS/MS analysis was performed on a Q Exactive Orbitrap equipped with an Accela 1250 pump (Thermo Scientific). Five microliters of sample dissolved in methanol (concentration 0.2 mg/mL) was injected. The chromatographic separation of the limonoid mixture was resolved through a Waters Acquity BEH C18 UPLC column (2.1 × 100 mm; particle size, 1.7 μm) using a methanol–water gradient solvent program of 35 min. The gradient program started with 40% methanol which was linearly increased to 50% at 5 min. From 5 to 10 min, the amount of methanol was increased to 60%, from 10 to 25 min to 65%, and from 25 to 30 min to 90% which was maintained until 32 min. Following this, the amount of methanol was decreased to 40% at 34 min and held up to 35 min. The flow rate used in the gradient program was 0.3 mL/min. The water used in the solvent system contained 0.1% MS–grade formic acid. The tune parameters for MS and MS/MS analysis are as follows: sheath gas (nitrogen) flow rate of 45 units, auxiliary gas (nitrogen) flow rate of 10 units, sweep gas (nitrogen) flow rate of 2 units, spray voltage set at 3.60 |KV|, spray current of 3.70 μA, capillary temperature set at 320 °C, s-lens RF level of 50, heater temperature set at 350 °C, mass resolving power of 70,000 full width at half maximum (FWHM), automatic gain control (AGC) target set at 1 × 106 ions, and maximum injection time of 250 ms. The ESI–MS and MS/MS data were recorded within the mass range m/z 100 to 1000 in positive ion mode. The MS analysis of limonoids was carried out in full-scan mode, while the MS/MS analysis was performed in parallel reaction monitoring (PRM) mode with a mass tolerance window of 5 ppm. The NCE of 20% and 25% was applied for fragmentation of limonoids 1–10 and 11–15 respectively. The inclusion parameters such as mass (m/z) and retention time for each limonoid are listed in Table 1. In this study, MS/MS analysis was investigated by employing high-energy C-trap dissociation (HCD) with Orbitrap detection. Data was analyzed through Thermo Scientific Xcalibur software.
Method validation and quantification of limonoids in different tissues
For each limonoid used as reference standard, the linearity of the optimized method ranged between 0.04 and 40 ng/µl by weighed least squares regression expressed as determination coefficient (R2) (Fig. S2). The limit of detection (LOD) and LOQ values were calculated by the formula LOD = 3.3*(SD/S) and LOQ = 10*(SD/S) where “SD” is the standard deviation and “S” is the slope. The method recoveries for each limonoid were evaluated at three different concentrations of 0.08 ng/µl, 1.6 ng/µl, and 30 ng/µl (n = 3) by spiking in neem fruit tissue after extraction and then calculated by comparing the difference between unspiked and spiked samples. The method precision was evaluated in terms of repeatability (intra-day) and reproducibility (inter-day) and expressed as the relative standard deviation (RSD %, n = 3). In the post-extraction addition method, a known amount of each limonoid or a structural analog (nimbidinol) which does not occur naturally in plants was added to four different matrices (fruit/flower/leaf/bark) of AZI and the matrix effect was calculated by dividing the analyte response in matrix (after subtracting the background endogenous levels of limonoids) with the analyte response in solvent at the same concentration. The matrix effect percentage was calculated using following equation:
where “A/A′” is the analyte response in spiked matrix/solvent, respectively, at the same concentration and “B” is the analyte response in the original (unspiked) matrix. Accordingly, the matrix effect percent higher or lower than zero indicates the ion enhancement or suppression, respectively, whereas more or less equal to zero indicates no significant matrix effect.
Results and discussion
Isolation of neem limonoids and method development
To obtain the neem limonoids, the solvent partition technique was employed in which the methanolic tissue extract of AZI (fruit and leaf) containing all the metabolites was prepared. To separate the triterpenoids from this pool, the methanolic extract was partitioned in equal volumes of ethyl acetate and water (1:1). The organic fraction enriched with the triterpenoids was then subjected to column chromatography which yielded fifteen pure limonoids (1–15, Fig. 1). The effect of different solvents on the total limonoid content per gram of tissue is shown in Fig. 2. The total limonoid content in neem fruit tissue was increased by 6% when extracted with ethanol and 15% with methanolic extract partitioned between ethyl acetate and water when compared to the methanolic extract. However, the total limonoid content was decreased by 14% and 15% in ethyl acetate and acetone extract, respectively, when compared to methanolic fruit extract. These limonoids were characterized by nuclear magnetic resonance spectroscopy and high-resolution mass spectrometry (Fig. S3-S62). And each purified limonoid was dissolved in LC–MS-grade methanol to yield a stock solution of 0.02 mg/mL. Now, 50 µL each of this stock solution was pooled together and utilized for method development. This limonoid mixture (reference standards) was injected into the column (Waters Acquity BEH C18 UPLC column, 2.1 × 100 mm) with a particle size of 1.7 μm. The gradient solvent program was developed using water and methanol as solvent system. After several trial runs and optimizations of various parameters, we finally established the UHPLC-MS protocol which could clearly resolve all the fifteen limonoids in a time frame of 35 min. The total ion chromatogram of the limonoid mixture is shown in Fig. 3. The mass spectrum for each of the limonoid in the reference standard mixture was obtained in positive ion mode within the mass range m/z 100 to 1000 Da. The molecular ion for all the thirteen limonoids (3–15) was detected in the mass spectra which appeared as protonated molecule (M + H)+ and sodium adduct (M + Na)+ except for azadirachtin A (1) and B (2) (Fig. S6, S10). Due to dehydration in azadirachtin A and B, their molecular ion (precursor ion) peak was detected in the mass spectra as (M-H2O + H)+ instead of protonated molecular ion. The detection sensitivity of these molecular ions (m/z values) was obtained to the fourth decimal point with high accuracy of less than 5 ppm error (Table 1) along with their elemental composition.
Tandem mass spectrometry of neem limonoids
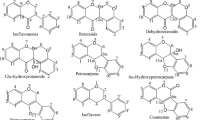
High-energy C-trap/collisional dissociation (HCD) is a contemporary type of fragmentation with high-resolution detection of fragment or product ions in the Orbitrap mass analyzer (HCD-MS/MS). In HCD, the molecular ion is fragmented in a collision cell generating the product ions which are then transferred back in C-trap to reach Orbitrap for analysis. In comparison with the traditional collision-induced dissociation (CID), HCD fragmentation generates increased product ion fragments, thus yielding high-quality MS/MS spectra [28]. Additionally, HCD fragmentation offers no low-mass cut-off, thus generating a broader range of fragmentation pathways. The MS/MS spectra of the reference limonoids were recorded in parallel reaction monitoring (PRM) mode with a specified mass range using HCD-MS/MS. The normalized collision energy (NCE) was optimized for each limonoid so as to generate a MS/MS spectrum with both low and high mass product ions. The NCE of 20% and 25% was found to be optimal for limonoids 1–10 and 11–15, respectively. We identified the product ions with detection sensitivity up to the fourth decimal point with high accuracy of less than 5 ppm error (Table S1) along with their elemental composition. From our previous studies of structure fragment relationships (SFRs), we identified the key mass product ions [26, 29, 30] of limonoids (1–15). The structure of skeleton specific key mass product ions along with the m/z value and elemental composition are represented in Fig. 4. The identified product ions are the signature ions to identify the skeleton type in a complex metabolite pool of plant tissue extract or any biological samples or pharmaceutical formulations. This information was used to validate the above-developed method by identifying the limonoids in tissue crude extracts of Meliaceae plants. Apart from this, our study also identified the limonoid-specific product ions, which is attributed to our high confidence in identifying and authenticating the presence of specific limonoids in tissue crude extracts of Meliaceae plants. For instance, the structures of product ions for gedunin-type skeleton represented in Fig. 4 will appear for any limonoids belonging to the gedunin skeleton. Though gedunin also forms product ions whose structure and m/z value are exactly similar to those of the azadirone skeleton type (signature product ions), the gedunin skeleton–specific product ions act as markers to distinguish from that of azadirone skeleton–type limonoids in the metabolite pool.
Method validation and limonoid analysis in Meliaceae plants
The above-developed method was validated by studying the different analytical parameters such as linearity, limit of detection (LOD), limit of quantification (LOQ), accuracy through recovery studies, precision (repeatability and reproducibility), and matrix effects for identification and quantification. The linearity of the developed method for all the fifteen reference limonoids ranged between 0.9961 and 0.9993 indicating the reliability of the method for quantitative analysis. The LOD and LOQ were calculated for each of the reference limonoids and are listed in Table 1. The recoveries in the low spiking level (0.08 ng/µL) ranged between 100.1 and 104.8% with RSD of 1.3–3.5% for intraday and 2.8–4.8% for interday; those in the medium spiking level (1.6 ng/µL) ranged between 101.1 and 105.7% with RSD of 0.3–3.8% for intraday and 1.1–5% for interday; and those in the high spiking level (30 ng/µL) ranged between 103.3 and 108.4% with RSD of 1–4.8% for intraday and 2.3–6.2% for interday for all the reference limonoids. The stable isotope–labeled internal standards for quantification of limonoids are not commercially available; therefore, we evaluated the matrix effect by post-extraction standard addition method, in which a known amount of each limonoid or a structural analog which does not occur naturally in plants such as nimbidinol was added to four different matrices (fruit/flower/leaf/bark) of AZI to study the matrix effect. We did not observe any significant ion enhancement or suppression in any matrix for the limonoids as well as the structural analog confirming no matrix effects (Table S2). This may be attributed to the selective limonoid extraction method and also the use of diluted (50-fold) tissue extract for analysis. The identification and profiling of AZI limonoids in the crude extract of fruit (F), flower (FW), leaf (L), and bark (B) tissues from Meliaceae plants are based on the comparison of the HCD-MS/MS product ion profile. A total of sixteen Meliaceae plants were selected for the study, namely Aglaia barberi Gamble (AGB), Aglaia lawii (AGL), Aglaia simplicifolia (AGS), Aphanamixis polystachya (APP), Chukrasia tabularis (CHT), Cipadessa baccifera (CIB), Dysoxylum binectariferum (DYB), Dysoxylum malabaricum (DYM), Melia azedarach (MEA), Melia dubia (MED), Reinwardtiodendron anamalaiense (REA), Soymida febrifuga (SOF), Swietenia macrophylla (SWM), Toona ciliata (TOC), Trichilia connaroides (TRC), and Walsura trifolia (WAT). These plants were authenticated and their collection number is mentioned in Table S3. For limonoid profiling, the methanolic tissue crude extracts of each plant was partitioned between ethyl acetate and water to obtain the limonoid-rich organic layer. For identification of limonoids, 5 µL of the organic extract (0.2 mg/mL) was injected in the above-developed method. The limonoid identification in the tissue crude extracts involved two major steps. In the preliminary step, the retention time and the mass spectrum of the observed peaks were matched with the reference standards. After confirming the exact match of RT and mass spectrum, the peaks were then shortlisted for further analysis. In the secondary step, the selected peaks with their molecular ion were subjected to HCD-MS/MS fragmentation to generate a product ion profile at the optimal NCE. The comparison of product ion profile (MS/MS spectrum) between the reference standards and the crude tissue extracts leads to the identification of limonoids (Fig. S63). Only the exact match of RT, the mass of the molecular ion peak with elemental composition (< 5 ppm error), and the product ion profile with that of reference standards confirms the presence of the limonoid in the tissue extract. If there is no match of the product ion profile, it declines the presence of the limonoid. Also, the error rate (< 5 ppm) is crucial for high reliability for the presence of limonoid in the tissue crude extracts. In our study, we observed that conclusions based only on the exact match of RT and mass of the molecular ion will not confirm the presence of limonoid in the pool of metabolites. For instance, the molecular ion peaks at RT of limonoid 14, 2, 6, and 15 were observed in tissue crude extracts of AGL (fruit), DYB (bark), SWM (bark), and TOC (fruit), respectively (Fig. S64, S65), which had the same mass as compared to reference limonoid. However, when we subjected these for HCD-MS/MS fragmentation, the generated product ion profile did not match with the suspected reference limonoid (Fig. S64, S65). Therefore this clearly indicates the low reliability of the previous methods involving only MS–based limonoid tracings. Although there is a previously reported UHPLC-MS/SRM method using CID-MS/MS analysis for neem metabolites, it lacks reliability and specificity due to the lack of identification of limonoid-specific product ions along with their elemental composition and error value [25]. For instance, a previous study concluded the presence of azadirachtin (1, 2) in the leaf extracts of MEA and MED without mentioning the elemental composition and the error limit [25]. In our investigation, though we found the exact match of RT, mass of molecular ion for 1, 2 in leaf extracts of MEA and MED, but the expected elemental composition for the molecular ion was not generated in any given error limits (Fig. S66, S67). So this confirms the absence of azadirachtin A/B (1/2) in the leaf extracts of MEA and MED consistent with previous investigations [31, 32]. Several comparative studies on UHPLC coupled to triple quadrupole MS/Orbitrap high-resolution MS (HRMS)–based methods are reported previously for myriad molecules, which conclude the higher confirmatory and quantitative capabilities of Orbitrap HRMS [33]. Furthermore, in other crude extracts, even though the mass was found to be the same as that of the reference standard, the MS/MS fragmentation pattern differed greatly under similar conditions (Fig. S64, S65). Therefore, in our study, we have overcome the drawbacks of previous analysis methods for precise identification of fifteen limonoids in a concise time frame.
Application of the developed method in tissue-specific quantitative limonoid profiling of Meliaceae plants
Differential levels of limonoids were detected across different tissues of the Meliaceae plant. Limonoids such as 14, 11, 12, 3 and 5 were the most widely distributed among the investigated plants (Fig. 5). Among the investigated plants, SOF, SWM, TRC, and MED were found to contain high abundance of reference limonoids followed by CHT, WAT and MEA. No limonoids were detected in AGB, AGS and DYB tissue extracts (Fig. 6). We observed that gedunin (14) was widely distributed limonoid among different tissues of Meliaceae plants namely CHT, CIB, MEA, MED, REA SOF, SWM, and TRC (Fig. S68, Table S4). Azadiradione was the second most detected limonoid dispersed across twelve different tissues of CHT, DYM, SOF SWM, TRC and WAT, followed by epoxyazadiradione in eight different tissues of SOF, SWM, TRC and WAT (Fig. S68). The quantitative occurrence of limonoids across different tissues of Meliaceae plants is represented (Table 2). In the quantitative abundance of limonoids, azadirone was highest at 1.9047 mg/g tissue followed by salannin (1.8595 mg/g), 6-deacetlylnimbiene (0.2876 mg/g), salannolacetate (0.1891 mg/g), gedunin (0.1121 mg/g), nimbanal (0.0596 mg/g), azadiradione (0.0470 mg/g), and epoxyazadiradione (0.0319 mg/g) (Fig. 7). However, neem limonoids 1, 2, and 7 were not detected in any tissue extracts of other 16 Meliaceae plants. Profiling studies revealed that the limonoids were mostly found in fruit and bark tissues of Meliaceae plants (Table S4). The limonoids (1–15) quantified from tissue extracts were in concentration ranges between 1.9047 and 0.00004 mg/g tissue.
Tissue-specific abundance of fifteen limonoids in sixteen Meliaceae plants. [*Aglaia lawii (AGL), Aglaia barberi Gamble (AGB), Aglaia simplicifolia (AGS), Aphanamixis polystachya (APP), Chukrasia tabularis (CHT), Cipadessa baccifera (CIB), Dysoxylum binectariferum (DYB), Dysoxylum malabaricum (DYM), Melia azedarach (MEA), Melia dubia (MED), Reinwardtiodendron anamalaiense (REA), Soymida febrifuga (SOF), Swietenia macrophylla (SWM), Toona ciliata (TOC), Trichilia connaroides (TRC), and Walsura trifolia (WAT)]
Tissue-specific comparison of limonoid content between neem and Meliaceae plants
The tissue-specific comparison of limonoid occurrence between AZI and other Meliaceae plants will provide the alternative source for harnessing diverse limonoids and their biosynthetic route. From the quantitative tissue-specific limonoid profile, we observed that limonoids with less functional group modification (11–14) are widely distributed across Meliaceae plants. The other limonoids (1–10) with a wide range of skeletal modification/decoration with an array of functional groups such as tiglate, acetate, and isovalerate are abundant in the AZI fruit. Salannin having a strong antifeedant and growth-inhibiting activity on insects was six fold higher in MED fruit [34] (Fig. S68) when compared to the AZI fruit. Similarly azadirone was three fold higher in the AGL fruit when compared to the AZI fruit. However, limonoids such as 5, 11, and 12 were high in AZI fruits when compared with other Meliaceae fruits (Fig. S68). Gedunin, a notable biological therapeutic limonoid, well known for its heat shock protein (Hsp) inhibition [35], was more than three fold higher in SOF flowers when compared to AZI flowers. Also, a high abundance of nimbanal (nine fold in leaf and one fold in bark) was observed in SOF tissues when compared to AZI tissues. One of the striking observations was the 33-fold high abundance of azadiradione in SOF bark when compared to AZI bark. Also, an eight fold high abundance of epoxyazadiradione in SOF bark was detected when compared to AZI bark. Although limonoids of five different skeleton types were detected in Meliaceae plants, the highly rearranged azadirachtin skeleton-type limonoids were not detected. This tissue-specific limonoid profiling in Meliaceae plants revealed that the high-value azadirachtins are limited to AZI plants only.
Conclusion
In conclusion, we have developed and validated the most specific, reliable HCD-MS/MS Orbitrap method for the rapid identification of limonoids with ease and requiring minimum plant material. The method was validated by identifying and quantifying the limonoids in Meliaceae tissue extracts. A total of fifteen limonoids belonging to six different skeletons such as azadirachtin, salanin, nimbin, nimbinene, azadirone, and gedunin were used, and a skeleton-specific product ion profile was generated. These product ion profiles were used to identify the limonoids and confirm their presence in Meliaceae tissue crude extracts with high confidence. The study of the botanical distribution of limonoids in the Meliaceae family can be utilized to identify the chemotaxonomic markers. These plants have potential to be explored in the future for many pharmacological activities. Most of the putative intermediates of the limonoid biosynthetic pathway like azadirone, azadiradione, epoxyazadiradione, and gedunin were found to be highly distributed in the plants such as TRC, WAT, SWM, SOF, CHT, and MED. Henceforth, the genomic exploitation of these Meliaceae plants will guide the elucidation of the limonoid biosynthetic pathway. The method developed in this study is highly precise to detect limonoids (1–15) in a metabolite pool such as tissue crude extract, organic pesticides, pharmaceutical formulations, etc. in a concise time frame.
References
Roy A, Saraf S. Limonoids: overview of significant bioactive triterpenes distributed in plants kingdom. Biol Pharm Bull. 2006;29:191–201. https://doi.org/10.1248/bpb.29.191.
Dreyer DL, Pickering MV, Cohan P. Distribution of limonoids in the rutaceae. Phytochemistry. 1972;11:705–13. https://doi.org/10.1016/0031-9422(72)80036-0.
Chen J, Fan X, Zhu J, Song L, Li Z, Lin F, Yu R, Xu H, Zi J. Limonoids from seeds of Azadirachta indica A. Juss. and their cytotoxic activity. Acta Pharm Sin B. 2018;8:639–44. https://doi.org/10.1016/j.apsb.2017.12.009.
Gupta SC, Prasad S, Tyagi AK, Kunnumakkara AB, Aggarwal BB. Neem (Azadirachta indica): an Indian traditional panacea with modern molecular basis. Phytomedicine. 2017;34:14–20. https://doi.org/10.1016/j.phymed.2017.07.001.
Veitch GE, Beckmann E, Burke BJ, Boyer A, Maslen SL, Ley SV. Synthesis of azadirachtin: a long but successful journey. Angew Chemie - Int Ed. 2007;46:7629–32. https://doi.org/10.1002/anie.200703027.
Ley SV, Abad-Somovilla A, Anderson JC, Ayats C, Bänteli R, Beckmann E, Boyer A, Brasca MG, Brice A, Broughton HB, Burke BJ, Cleator E, Craig D, Denholm AA, Denton RM, Durand-Reville T, Gobbi LB, Göbel M, Gray BL, Grossmann RB, Gutteridge CE, Hahn N, Harding SL, Jennens DC, Jennens L, Lovell PJ, Lovell HJ, De La Puente ML, Kolb HC, Koot WJ, Maslen SL, McCusker CF, Mattes A, Pape AR, Pinto A, Santafianos D, Scott JS, Smith SC, Somers AQ, Spilling CD, Stelzer F, Toogood PL, Turner RM, Veitch GE, Wood A, Zumbrunn C. The synthesis of azadirachtin: a potent insect antifeedant. Chem - A Eur J. 2008;14:10683–704. https://doi.org/10.1002/chem.200801103.
Tan QG, Luo XD. Meliaceous limonoids: chemistry and biological activities. Chem Rev. 2011;111:7437–522. https://doi.org/10.1021/cr9004023.
Pohanish RP. Sittig’s handbook of pesticides and agricultural chemicals. Elsevier; 2015.
Murugan K, Jeyabalan D, Kumar NS, Babu R, Sivaramakrishnan S, Nathan SS. Antifeedant and growth-inhibitory properties of neem limonoids against the cotton bollworm, Helicoverpa armigera (Hübner). Int J Trop Insect Sci. 1998;18:157–62. https://doi.org/10.1017/S1742758400007803.
Hashmat I, Azad H, Ahmed A. Neem ( Azadirachta indica A. Juss ) - a nature’s drugstore: an overview. Int Res J Biol Sci. 2012;1:76–9.
Alam A, Haldar S, Thulasiram HV, Kumar R, Goyal M, Iqbal MS, Pal C, Dey S, Bindu S, Sarkar S, Pal U, Maiti NC, Bandyopadhyay U. Novel anti-inflammatory activity of epoxyazadiradione against macrophage migration inhibitory factor: inhibition of tautomerase and proinflammatory activities of macrophage migration inhibitory factor. J Biol Chem. 2012;287:24844–61. https://doi.org/10.1074/jbc.M112.341321.
Ilango K, Maharajan G, Narasimhan S. Anti-nociceptive and anti-inflammatory activities of Azadirachta indica fruit skin extract and its isolated constituent azadiradione. Nat Prod Res. 2013;27:1463–7. https://doi.org/10.1080/14786419.2012.717288.
Kumar D, Haldar S, Gorain M, Kumar S, Mulani FA, Yadav AS, Miele L, Thulasiram HV, Kundu GC. Epoxyazadiradione suppresses breast tumor growth through mitochondrial depolarization and caspase-dependent apoptosis by targeting PI3K/Akt pathway. BMC Cancer. 2018;18:1–17. https://doi.org/10.1186/s12885-017-3876-2.
Maity P, Biswas K, Chattopadhyay I, Banerjee RK, Bandyopadhyay U. The use of neem for controlling gastric hyperacidity and ulcer. Phyther Res. 2009;23:747–55. https://doi.org/10.1002/ptr.2721.
Paritala V, Chiruvella KK, Thammineni C, Ghanta RG, Mohammed A. Phytochemicals and antimicrobial potentials of mahogany family. Rev Bras Farmacogn. 2015;25:61–83. https://doi.org/10.1016/j.bjp.2014.11.009.
Forim MR, da Silva MF das GF, Cass QB, Fernandes JB, Vieira PC. Simultaneous quantification of azadirachtin and 3-tigloylazadirachtol in Brazilian seeds and oil of Azadirachta indica: application to quality control and marketing. Anal Methods. 2010;2:860. https://doi.org/10.1039/c0ay00008f.
Song L, Wang J, Gao Q, Ma X, Wang Y, Zhang Y, Xun H, Yao X, Tang F. Simultaneous determination of five azadirachtins in the seed and leaf extracts of Azadirachta indica by automated online solid-phase extraction coupled with LC–Q-TOF–MS. Chem Cent J. 2018;12:85. https://doi.org/10.1186/s13065-018-0453-y.
de Paula JAM, Brito LF, Caetano KLFN, de Morais Rodrigues MC, Borges LL, Da Conceição EC. Ultrasound-assisted extraction of azadirachtin from dried entire fruits of Azadirachta indica A. Juss. (Meliaceae) and its determination by a validated HPLC-PDA method. Talanta. 2016;149:77–84. https://doi.org/10.1016/j.talanta.2015.11.005.
Schaaf O, Jarvis AP, van der Esch SA, Giagnacovo G, Oldham NJ. Rapid and sensitive analysis of azadirachtin and related triterpenoids from Neem (Azadirachta indica) by high-performance liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. J Chromatogr A. 2000;886:89–97. https://doi.org/10.1016/S0021-9673(00)00492-1.
Barrek S, Paisse O, Grenier-Loustalot MF. Analysis of neem oils by LC-MS and degradation kinetics of azadirachtin-A in a controlled environment : characterization of degradation products by HPLC-MS-MS. Anal Bioanal Chem. 2004;378:753–63. https://doi.org/10.1007/s00216-003-2377-0.
Dai J, Yaylayan VA, Vijaya Raghavan GS, Parè JR, Liu Z. Multivariate calibration for the determination of total azadirachtin-related limonoids and simple terpenoids in neem extracts using vanillin assay. J Agric Food Chem. 2001;49:1169–74. https://doi.org/10.1021/jf001141n.
Sarais G, Caboni P, Sarritzu E, Russo M, Cabras P. A simple and selective method for the measurement of azadirachtin and related azadirachtoid levels in fruits and vegetables using liquid chromatography electrospray ionization tandem mass spectrometry. J Agric Food Chem. 2008;56:2939–43. https://doi.org/10.1021/jf7037407.
Grimalt S, Thompson DG, Coppens M, Chartrand DT, Shorney T, Meating J, Scarr T. Analytical study of azadirachtin and 3-tigloylazadirachtol residues in foliage and phloem of hardwood tree species by liquid chromatography–electrospray mass spectrometry. J Agric Food Chem. 2011;59:8070–7. https://doi.org/10.1021/jf2023947.
Drożdżyński D, Kowalska J. Rapid analysis of organic farming insecticides in soil and produce using ultra-performance liquid chromatography/tandem mass spectrometry. Anal Bioanal Chem. 2009;394:2241–7. https://doi.org/10.1007/s00216-009-2931-5.
Rangiah K, Varalaxmi BA, Gowda M. UHPLC-MS/SRM method for quantification of neem metabolites from leaf extracts of Meliaceae family plants. Anal Methods. 2016;8:2020–31. https://doi.org/10.1039/C5AY03065J.
Haldar S, Mulani FA, Aarthy T, Dandekar DS, Thulasiram HV. Expedient preparative isolation and tandem mass spectrometric characterization of C-seco triterpenoids from Neem oil. J Chromatogr A. 2014;1366:1–14. https://doi.org/10.1016/j.chroma.2014.09.006.
Haldar S, Phapale PB, Kolet SP, Thulasiram HV. Expedient preparative isolation, quantification and characterization of limonoids from Neem fruits. Anal Methods. 2013;5:5386–91. https://doi.org/10.1039/c3ay41136b.
Jedrychowski MP, Huttlin EL, Haas W, Sowa ME, Rad R, Gygi SP. Evaluation of HCD- and CID-type fragmentation within their respective detection platforms for murine phosphoproteomics. Mol Cell Proteomics. 2011;10(M111): 009910. https://doi.org/10.1074/mcp.M111.009910.
Aarthy T, Mulani FA, Pandreka A, Kumar A, Nandikol SS, Haldar S, Thulasiram HV. Tracing the biosynthetic origin of limonoids and their functional groups through stable isotope labeling and inhibition in neem tree (Azadirachta indica) cell suspension. BMC Plant Biol. 2018;18:230. https://doi.org/10.1186/s12870-018-1447-6.
Mulani FA, Nandikol SS, Haldar S, Thulasiram HV. Accurate identification of bioactive Meliaceae limonoids by UHPLC–MS/MS based structure–fragment relationships (SFRs). ACS Omega. 2021;6:26454–76. https://doi.org/10.1021/acsomega.1c03697.
Wang Y, Chen X, Wang J, Xun H, Sun J, Tang F. Comparative analysis of the terpenoid biosynthesis pathway in Azadirachta indica and Melia azedarach by RNA-seq. Springerplus. 2016;5:819. https://doi.org/10.1186/s40064-016-2460-6.
Hodgson H, De La Peña R, Stephenson MJ, Thimmappa R, Vincent JL, Sattely ES, Osbourn A. Identification of key enzymes responsible for protolimonoid biosynthesis in plants: opening the door to azadirachtin production. Proc Natl Acad Sci U S A. 2019;116:17096–104. https://doi.org/10.1073/pnas.1906083116.
Herrero P, Cortés-Francisco N, Borrull F, Caixach J, Pocurull E, Marcé RM. Comparison of triple quadrupole mass spectrometry and Orbitrap high-resolution mass spectrometry in ultrahigh performance liquid chromatography for the determination of veterinary drugs in sewage: benefits and drawbacks. J Mass Spectrom. 2014;49:585–96. https://doi.org/10.1002/jms.3377.
Govindachari TR, Narasimhan NS, Suresh G, Partho PD, Gopalakrishnan G. Insect antifeedant and growth-regulating activities of salannin and other c-seco limonoids from neem oil in relation to Azadirachtin. J Chem Ecol. 1996;22:1453–61. https://doi.org/10.1007/BF02027724.
Braga TM, Rocha L, Chung TY, Oliveira RF, Pinho C, Oliveira AI, Morgado J, Cruz A. Biological activities of gedunin—a limonoid from the Meliaceae family. Molecules. 2020;25:493. https://doi.org/10.3390/molecules25030493.
Acknowledgements
FAM and SSN acknowledge the Council of Scientific and Industrial Research (CSIR), New Delhi, and the Department of Science and Technology (DST), New Delhi, for the fellowship, respectively. This work is supported by the Department of Biotechnology, India, and CSIR, New Delhi, sponsored the network project (Project No. MLP01024, HCP0002).
Author information
Authors and Affiliations
Contributions
Fayaj A Mulani: investigation, methodology, and writing—original draft.
Sharvani S. Nandikol: investigation, methodology, and writing—original draft.
Jagadeesh S. Kajjihundi: collected the plants and prepared the herbarium.
Niranjana Pathappa: collected the plants and prepared the herbarium.
Sharanappa Puttappa: collection and identification of the plants.
Hirekodathakallu V. Thulasiram: conceptualization, funding acquisition, supervision of the overall work, checking of the data and manuscript preparation with inputs from FAM and SSN.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary information associated with this article can be found in the online version of the manuscript doi.
Rights and permissions
About this article
Cite this article
Mulani, F.A., Nandikol, S.S., Kajjihundi, J.S. et al. Ultra-high performance liquid chromatography Q-Orbitrap MS/MS–based profiling and quantification of limonoids in Meliaceae plants. Anal Bioanal Chem 414, 6093–6106 (2022). https://doi.org/10.1007/s00216-022-04169-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04169-2