Abstract
The incidence of patulin (PAT) in fruit products is a worldwide concern due to its acute and chronic toxic effects. Therefore, accurate and reliable PAT measurements are important for preventing consumer health risks. Our previous method, which was based on a common technique that uses ethyl acetate extraction and liquid chromatography–tandem mass spectrometry with isotope dilution (ID–LC–MS/MS), has shown great performance for the determination of PAT in apple products. However, prolonged extraction times and multistep clean-up processes were required to sufficiently eliminate the matrix interferences. Herein, a feasible alternative ID–LC–MS/MS method was successfully established, employing simplified and reliable sample preparation steps. The clean-up process was performed using molecularly imprinted polymer–solid-phase extraction (MIP–SPE) cartridges, which eliminated matrix interferences and facilitated the trace quantification. While the previous method used a multimode LC column for the retention of polar patulin, the current method used a UPLC HSS T3 column, which further improved the peak sharpness and reduced the run time. The method was validated by measuring fortified samples in the concentration range of 5‒100 µg/kg. The accuracy varied between 97.8 and 102.0%, with relative standard deviation for interday and intraday precision being below 3%. The measurement uncertainty was lower than 4% (at a 95% level of confidence). Therefore, this method demonstrated adequate metrological quality with greatly enhanced performance over various reported methods. Additional key benefits of this method are easy manipulation, short preparation time, and lower consumption of hazardous solvents. Finally, the method was successfully applied to commercially available apple-based products.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agro-food industries are consistently threatened by fungal contamination and subsequent production of mycotoxins, which pose serious risks to food safety and security around the world [1]. The mycotoxins that cause the most concern globally are aflatoxins, ochratoxin A, patulin (PAT), type A trichothecenes, type B trichothecenes, citrinin, zearalenone, and fumonisins [2,3,4,5,6,7]. PAT, a naturally occurring heterocyclic lactone (see Fig. 1), is produced by at least 60 fungal species, with Byssochlamys, Penicillium, and Aspergillus as the most frequent PAT-producing fungi [1]. The main dietary sources of PAT are apple-based products which are often processed from partially rotten raw fruits initially contaminated with molds [8]. Therefore, the occurrence of PAT in apple-based products has been suggested as an essential indicator for the quality of raw fruits used in their manufacturing [8, 9]. PAT was first discovered in the 1940s as an antibiotic, but it was soon found to be toxic to livestock [1, 10]. Different studies have shown its acute and chronic toxic symptoms such as intestinal inflammation, pulmonary congestion, convulsions, agitation, neurotoxicity, immunotoxicity, genotoxicity, hepatulinotoxicity, vomiting, ulceration, hyperemia, edema, and epithelial cell degeneration [11, 12]. Due to its considerably serious adverse impacts on human health, many countries, including Korea, have adopted maximum permissible values of PAT, which are 25 µg/kg in solid apple products, 50 µg/kg in apple juice, and 10 µg/kg in apple products intended for young children and infants [10, 13, 14].
Different surveillance studies have revealed that the PAT incidence in food is a worldwide problem [9, 15]. Therefore, monitoring and controlling PAT contamination using sensitive, simple, and robust analytical methods are of imperative concern for avoiding consumer health risks and potential economic losses. To date, the most frequently applied analytical methods for the detection of PAT in food are based on chromatographic analysis using gas chromatography–mass spectrometry (GC–MS) [16], high-performance liquid chromatography with ultraviolet detection (HPLC–UV) [12, 17], and high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS) [1, 14, 18]. However, GC–MS methods often necessitate an inevitable time-consuming derivatization step to make PAT volatile so that it can be analyzed [9]. Although HPLC–UV has been commonly used for PAT analysis due to the strong absorption of UV light at 276 nm, the shortcomings of this method are also obvious [19]. Compounds such as 5-hydroxymethylfurfural (HMF) and other phenolic compounds can interfere with the UV/Vis absorbances of PAT and generally hinder chromatographic resolution, thereby making the accurate determination of PAT more laborious [9, 19]. On the other hand, UPLC–MS/MS-based methods have undoubtedly become the most preferred analytical approaches for mycotoxin analysis and can effectively overcome the drawbacks of other conventional techniques owing to their high level of chromatographic separation, and their enhanced sensitivity and selectivity [15, 20]. Solid-phase extraction (SPE), liquid–liquid extraction (LLE) with organic solvent (i.e., ethyl acetate), and QuEChERS (quick, easy, cheap, effective, rugged, and safe) are the main strategies of sample preparation for PAT analysis. It is noteworthy that SPE-based methods have become increasingly common with broad acceptability, and many methods using various adsorbent materials have been reported for PAT analysis [9, 14, 19]. However, these traditional SPE cartridges are occasionally nonspecific for the target analyte and complicated sample pretreatment steps, and prolonged time are often required to sufficiently eliminate the co-extracted interferences [21]. In recent years, molecularly imprinted polymers (MIPs) have become a promising pretreatment technology, and an increasing number of MIP–SPEs have been developed for the analysis of different mycotoxins [1, 22, 23]. MIPs are synthetic polymers with specific cavities designed to provide strong affinity toward a certain molecule [1, 10]. Therefore, these cartridges can successfully separate and enrich a target molecule from a complex sample matrix with excellent selectivity and good enrichment capability, thereby overcoming the limitations of conventional SPE cartridges [21, 24, 25].
While various analytical methods have been established for the detection and quantification of PAT in liquid apple products (i.e., apple juice), the availability of reliable analytical methods for solid apple products (i.e., apple powder) is still rather limited [9, 26]. On the other hand, verifying the accuracy of PAT measurements in food is of critical importance, particularly for government authorities responsible for establishing meaningful regulations to protect human health. Matrix certified reference materials (CRMs) constitute key tools for laboratories to fulfill this purpose. The application of CRMs in validating analytical methods, proficiency testing, and establishing traceability of analytical results to the International System of Units (SI) are also related benefits [27,28,29].
Our laboratory at the National Metrology Institute (NMI) of Korea previously established an ID–LC–MS/MS method for the accurate determination of PAT in apple juice [14] and has also developed an apple juice CRM that was certified with this method. Although this method has shown great performance for the accurate determination of PAT in apple products, prolonged extraction time and multistep clean-up processes were required for sufficiently eliminating interferences from co-extracted substances. To properly retain this highly polar analyte, a multimode LC column was used, but it also required long LC runs. The method was successfully applied to the certification of an apple juice CRM. However, the liquid-form CRM was found to have inadequate stability for more than 2 years of long-term storage at − 20 °C due to the degradation of patulin, particularly in neutral and basic media. Therefore, our focus was directed to the development of apple powder CRMs.
In this research work, we attempted to improve the existing ID–LC–MS/MS method by simplifying the multistep sample preparation procedures and adopting a recently improved reversed-phase UPLC column (particle size < 2 µm) instead of a multimode LC column to reduce the overall analysis time. The developed method still uses 13C7-PAT (see Fig. 1) as an internal standard, which permitted the accurate correction of analyte recovery during sample pretreatment steps, as well as the appropriate compensation of instrumental fluctuation during LC–MS/MS detection. Since the composition of the apple powder matrix is complex, the performance of different SPE cartridges was comparatively investigated to efficiently eliminate matrix interferences. Unlike various existing methodologies, including the method previously established in this laboratory [9, 11, 14, 17], the proposed analytical method offers a simpler, more selective strategy, which involves quicker sample preparation. This strategy relies on MIP–SPE purification, which significantly maximizes the analytical throughput. The method was adequately validated and successfully applied to different commercially available apple-related foods (i.e., apple powder, apple juice, and apple puree) to confirm its feasibility.
Experimental
Chemicals and materials
All organic solvents with HPLC grade, including methanol, ethyl ether, ethyl acetate, and acetonitrile, were supplied by Burdick & Jackson (Muskegon, MI, USA). Ammonium acetate, sodium bicarbonate, and acetic acid (all of MS grade) were obtained from Sigma–Aldrich (Gyeonggi-do, Korea). Neat patulin (5 mg/bottle) was obtained from Romer Labs (Tulln, Austria). The purity of the neat PAT was assigned in this laboratory following previously established protocols with the mass balance approach and was used as the primary reference material of PAT in this study [30]. The assigned purity of PAT was 99.57 ± 0.17%. 13C7-PAT (25 μg/mL in acetonitrile) was also purchased from Romer Labs and used as a stock solution. Pure water (18.2 MΩ.cm resistivity) was produced in-house by using a Milli-Q purification system (Millipore, USA). For sample clean-up using solid-phase extraction, SupelMIP cartridges (Sigma–Aldrich, Gyeonggi-do, Korea), 200 mg Bond Elut Plexa PAX cartridges (Agilent Technologies, Palo Alto, CA, USA), 60 mg Oasis HLB™ cartridges (Waters, Milford, MA, USA), and MycoSep 228 AflaPat cartridges (Romer Labs, Tulln, Austria) were investigated. Sample extracts were filtered through PVDF syringe filters provided by Whatman (Clifton, NJ, USA) with pore sizes of 0.22 and 0.45 µm. The neat PAT material was weighed using a XP2U ultra-micro balance (Mettler Toledo, USA). Several commercially available apple-related foods (i.e., apple powder, apple juice, and apple puree) were purchased from local retail markets in Korea.
Preparation of standard solutions
A stock solution of PAT at a concentration of 5 mg/kg was gravimetrically prepared by dissolving 0.1 mg of solid reference material in 20.27 mL of a solvent composed of 95% 2 mmol/L ammonium acetate in water (containing 1 mmol/L acetic acid) and 5% acetonitrile. The stock solution was kept in the dark at − 20 °C and renewed every 2 months. A working solution containing 200 µg/kg PAT was gravimetrically prepared by diluting an appropriate aliquot of the stock solution in the same solvent. A working internal standard solution (200 µg/kg) of 13C7-PAT was prepared in the same manner from its stock solution (25 μg/mL in acetonitrile). All working solutions were stored in the dark at − 20 °C and renewed weekly. For exact matching calibration in IDMS measurements, an isotope ratio standard solution (1:1 isotope ratio) was prepared by gravimetrically mixing an appropriate aliquot of the PAT working standard solution and its respective 13C7-PAT working internal standard solution at levels approximately mimicking analyte levels in the tested samples (based on the preliminary test results).
Sample extraction and clean-up
First, 0.5 g of homogenized apple-based samples (apple powder, apple juice, and apple puree) was gravimetrically placed into a 50-mL polypropylene centrifuge tube and quantitatively spiked with a working internal standard solution so that the final sample solution contained approximately a 1:1 isotope ratio of PAT to 13C7-PAT. Ten milliliters of water/acetic acid (99:1; v/v) was added and mixed by vortexing for 20 min for extraction and equilibration of PAT and its internal standard. Following 6 min of centrifugation at 2800 g, the sample extract was filtered through a 0.45-µm PVDF syringe filter and loaded onto the SupelMIP SPE cartridge, previously conditioned with 2 mL of acetonitrile and 1 mL of water. The analyte-containing cartridge was sequentially washed with 3 mL of sodium bicarbonate, 3 mL of water, and 0.5 mL of diethyl ether, and the analyte was eluted using 3 mL of ethyl acetate. After adding approximately 0.1 mL of acetic acid, the resulting eluent was dried under a gentle nitrogen stream. The sample residue was immediately reconstituted with 0.3 mL of the initial mobile phase and filtered into an amber glass vial for LC–MS/MS analysis.
In the initial development of this method, three further conventional SPE cartridges, a MycoSep 228 AflaPat SPE cartridge, a Bond Elut Plexa PAX cartridge, and an Oasis HLB™ cartridge, were also investigated for the clean-up of sample extracts. The MycoSep 228 AflaPat SPE cartridge was used after extraction with an acetonitrile–water (84:16; v/v) mixture following a slightly modified method recently described by Przybylska et al. [31]. Meanwhile, the Bond Elut Plexa PAX and Oasis HLB™ SPE cartridges were applied following double extraction with ethyl acetate, which is the most common extraction solvent used for PAT extraction from apple products [14, 32].
UPLC–MS/MS analysis
PAT determination was carried out on a Waters Acquity UPLC™ system (Milford, MA, USA) interfaced with a Triple Quad™ 6500Q tandem mass spectrometer (AB Sciex, Foster City, CA, USA) through an ESI source. LC separation was achieved by an Acquity UPLC HSS T3 column (100 mm length × 2.1 mm i.d., 1.8 µm particle size) with a mobile phase composed of water containing 1 mmol/L acetic acid and 2 mmol/L ammonium acetate (phase A) and acetonitrile (phase B) at a flow rate of 0.2 mL/min. The sample injection volume was 10 µL. A gradient elution program was applied to PAT separation as follows: 0–1.5 min, B 5–20%; 1.5‒4.5 min, B 20–35%; 4.5–6 min, B 35–70%; and 6–8 min, B 70‒5%, resulting in a total chromatographic run time of 8 min.
The mass spectrometer was run in negative electrospray ionization (ESI) mode with the optimal ionization source conditions set as follows: desolvation temperature, 600 °C; capillary voltage, 4000 V; sheath gas pressure (N2), 35 psi; desolvation gas pressure (N2), 25 psi; collision gas pressure (N2), 7 psi; and curtain gas pressure (N2), 30 psi. MS detection was conducted in normal multiple reaction monitoring (MRM) mode by setting two MRM transitions for each of PAT and 13C7-PAT. Of the two transitions, the highly sensitive transition was used for quantification, and the other transition was used for identification purposes. The MRM transitions and corresponding MS/MS parameters, including decluttering potentials, collision energies, entrance potentials, etc., are listed in Table 1. Data acquisition, analysis, and instrument control were processed by Analyst software 1.6.3 (AB Sciex).
Post-column infusion system
Matrix effects are often observed in LC–MS/MS analysis of complex sample matrices. To investigate the effect of co-extracted matrix interferences on the ionization of the target analyte and its isotope-labeled analog, the post-column infusion technique was used [4, 33, 34]. In this approach, a blank apple powder sample was extracted, cleaned up following the sample preparation procedure described in “Sample extraction and clean-up,” and finally submitted to a normal LC run. The LC eluent was infused with a continuous flow (10 mL/min) of a standard solution containing 5 mg/kg PAT and its isotope-labeled analog using a t-union positioned between the LC column and the ESI source. MS analysis was conducted in normal MRM mode to provide MRM chromatograms that showed matrix effect profiles.
Quantification of PAT
The mass fraction (µg/kg) of PAT in the samples was quantified by comparing the isotope ratios of PAT/[13C7]-PAT observed from the analysis of the sample solution with those of the isotope ratio standard solution using the following master equation of IDMS. A single-point calibration strategy with exact matching of isotope ratios was applied [35,36,37].
where Csample is the mass fraction (µg/kg) of PAT in the sample; Cs-sol is the concentration of the PAT standard solution; Msample is the mass of the sample; Mis-sol, spiked is the mass of the [13C7]-PAT solution spiked into the sample aliquot; Ms-sol.,std.mix. and Mis-sol,std.mix. are the masses of the PAT standard solution and [13C7]-PAT solution, respectively, which were mixed into the isotope ratio standard solution; ARstd.mix. and ARsample are the peak area ratios of PAT/[13C7]-PAT observed by LC–MS/MS for the isotope ratio standard solution and the sample solution, respectively.
Method validation
The ID–LC–MS/MS method for the quantitative analysis of PAT in apple products was validated to evaluate its performance characteristics. Several analytical parameters, including validity, repeatability, reproducibility, selectivity, matrix effect, detection limit (LOD), quantification limit (LOQ), and sources of uncertainty were carefully evaluated under the optimal experimental conditions to test the potential of this method to exhibit a higher-order metrological quality as a candidate reference method.
Results and discussion
Optimization of MS/MS conditions
The parameters for ESI and MS/MS were carefully optimized to obtain ideal analytical conditions for the detection of PAT and 13C7-PAT (see Table 1). The mass spectrometer was operated in the negative electrospray ionization (ESI) mode, as this mode revealed a higher signal intensity compared with the positive electrospray ionization mode. PAT was ionized to the deprotonated molecular ion [M − H]− at m/z 153, which was selected as the precursor ion due to its high intensity (Fig. S1a). In the MS/MS spectrum (see Fig. S2a), two dominant and characteristic fragment ions were observed at m/z 109 and m/z 81; these were assigned as [M − H − CO2]− and [M − H − CO2 − CO]−, respectively. Because the fragment peak observed at m/z 109 was the most abundant, the collision-induced dissociation (CID) pathway from m/z 153 to m/z 109 was selected as the primary MRM quantitation channel, while the CID pathway of m/z 153 to m/z 81 was selected for confirmatory purposes. Concerning 13C7-patulin, the MS and MS/MS spectra are displayed in Fig. S2. Similarly, the transition channel from m/z 160 to m/z 115 was selected as the primary MRM quantitation channel, while the channel from m/z 160 to m/z 86 was selected as the secondary qualifier channel. This result is in accordance with those of other previously reported studies [1, 9, 14, 38].
Optimization of UPLC conditions
Various combinations of mobile phase solvents were tested for chromatographic optimization. Notably, the solvent combination consisting of acetonitrile as phase B was favorable to the ionization process, which led to a rise in the observed signal (peak area) with better resolution compared to using methanol. This finding is consistent with those of other previous works [1, 15, 38]. Additionally, different concentrations of ammonium acetate and acetic acid ranging from 1 to 10 mmol/L were tested with mobile phase A. Acetic acid (1 mmol/L) and ammonium acetate (2 mmol/L) were found to be suitable and yielded more efficient ionization as well as a better peak shape. Thus, the mobile phase employed herein was composed of water containing 1 mmol/L acetic acid and 2 mmol/L ammonium acetate (phase A) and acetonitrile (phase B).
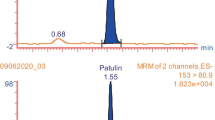
Since PAT is a highly polar compound, its analysis poses challenging issues in terms of chromatographic retention. As described in “Introduction,” our existing method uses a multimode analytical LC column that requires a long LC run time. Recently, separation science has been revolutionized with the introduction of UPLC systems, and highly improved reversed-phase UPLC columns (particle size < 2 µm) with advanced end-capping and bonding techniques are widely available. We attempted to adopt those systems to reduce LC run time, which is critical when the method is used for CRM certification because such certification process requires tens of consecutive LC runs without interruption between the runs. In this study, two candidate UPLC columns, namely an Acquity BEH C18 column (100 mm length × 2.1 mm i.d., 1.7 µm particle size) and an Acquity UPLC HSS T3 column (100 mm length × 2.1 mm i.d., 1.8 µm particle size), were investigated based on their chromatographic retention for PAT by reversed-phase LC. The latter was chosen in this study because it provided more appropriate retention of the target analyte with a satisfactory resolution. In the early stages of this study, LC elution with an isocratic mobile phase (80% A and 20% B) at a flow rate of 0.2 mL/min was applied (Fig. S3). However, due to peak broadening and relatively lower detection sensitivity, different gradient profiles were investigated to further enhance the peak resolution and intensity. The gradient elution program described in “UPLC–MS/MS analysis” allowed separation of PAT within 4 min with a total chromatographic run time of 8 min, including column re-equilibration, which is considered a feasible time to provide high sample throughput in view of method application in the routine monitoring of PAT and lengthy CRM certifications, including homogeneity tests. Figure 2 depicts typical MRM chromatograms of PAT and its corresponding [13C]-isotopic analog in a blank LC run, a blank apple powder sample, a working standard solution (25 μg/kg level), a fortified apple powder sample (25 μg/kg level), and a naturally contaminated apple powder sample under the optimal chromatographic conditions, along with those of a blank LC run. The chromatograms showed that the retention time of PAT was approximately 3.37 ± 0.02 min.
Typical MRM chromatograms of patulin and its internal standard in a (a) blank LC run, (b) blank apple powder sample, (c) working standard solution (25 µg/kg), (d) fortified blank apple powder sample (25 µg/kg), and (e) naturally contaminated apple powder sample after reconstitution into apple puree by adding the proper amount of water (the patulin level was 12.21 µg/kg after reconstitution)
Sample extraction and clean-up
Apple is a complex sample matrix, so serious matrix interferences are often encountered during PAT determination. Due to the high polarity and low retention time of PAT, it is commonly eluted from a reverse-phase LC system simultaneously with other polar matrix substances [9, 15]. Therefore, the development of an efficient sample preparation procedure with a reduced amount of co-extracted sample matrix is of critical importance, especially for highly complex matrices [19, 21, 39, 40]. Our existing method and other reported methods mostly adopt extraction with organic solvents (ethyl acetate) and complex clean-up processes that require prolonged time [9, 11, 14, 15, 17]. In an attempt to simplify sample preparation steps and to eliminate potential co-eluted matrix interferences, the performances of four different SPE cartridges with different functionalities and specificities were comparatively evaluated in this study. The candidate SPE cartridges were (i) Oasis HLB™, (ii) MycoSep 228 AflaPat, (iii) Bond Elut Plexa PAX, and (iv) SupelMIP. Apple powder samples were collected from retail markets and screened for the absence of PAT within the LOD of the method. A blank sample (0.5 g) containing nondetectable levels of PAT was used to test the purification efficiency of the different SPE cartridges.
The clean-up efficiency for each SPE cartridge was evaluated using post-column infusion techniques (“Post-column infusion system”) by observing matrix effect profiles that can clearly indicate the effect of the co-extracted species on PAT detection. Figure 3(a-e) shows the instrumental configuration of the post-column infusion technique along with the MRM chromatograms of the obtained matrix effect profiles. Notably, the Oasis HLB™ SPE (Fig. 3(b)) and MycoSep 228 AflaPat SPE (Fig. 3(c)) cartridges could not completely remove co-eluting matrix interferences, which caused considerable ion suppression at the retention time of PAT. In contrast, the Bond Elut Plexa PAX SPE (Fig. 3(d)) and SupelMIP SPE cartridges (Fig. 3(e)) showed excellent ionization profiles without significant ion suppression or enhancement zones in the MRM chromatograms. However, the ethyl acetate–based sample preparation steps prior to Bond Elut Plexa PAX SPE were burdensome and time-consuming [32, 41]. Furthermore, the use of sodium carbonate for washing ethyl acetate solution can cause PAT degradation because this compound (a lactone) is more stable in an acidic medium [11]. Therefore, the SupelMIP SPE–based clean-up method involving simpler and more rapid sample extraction steps was selected in this study to increase the sample throughput.
Schematic diagram showing the instrumental configuration of the post-column infusion technique (a) and matrix effect profiles obtained from post-column infusion following clean-up of the apple powder sample extract with the Oasis HLB™ SPE (b), MycoSep 228 SPE (c), Bond Elut Plexa PAX SPE (d), and SupelMIP SPE (e) cartridges, respectively. An MRM chromatogram acquired from a typical LC–MS/MS run of blank apple powder sample spiked with patulin and its isotopic internal standards at 200 µg/kg is overlaid
To eliminate apple matrix components such as phenolic compounds (i.e., HMF) attached nonspecifically to the MIP, a washing step was essential. Following the loading of the sample extract, two different solvents (1% acetic acid or 1% sodium bicarbonate) were tested at the washing step. As shown in Fig. S4, an MRM chromatogram with little matrix effects or background noise was obtained when 3 mL of 1% sodium bicarbonate was used as the washing solvent due to the great capacity of this solution to eliminate interfering phenolic compounds from the cartridges [42]. The addition of acetic acid (one drop) to the eluate was mandatory to avoid PAT degradation during the evaporation step. It is also worth noting that the apparent extraction recovery of PAT following sample extraction and clean-up using the candidate SPE cartridges varied between 60 and 68%. However, further investigations to improve the recovery were not our priority in this study since the use of an isotope-labeled internal standard (13C7-PAT) allowed for the accurate correction of recovery during the overall sample preparation steps.
Method validation
Analysis of gravimetrically fortified samples
To the best of our knowledge, no matrix CRM with a certified value for PAT in apple powder is currently available for accuracy evaluation. Therefore, several apple powder products were purchased from local retail markets in Korea. After screening for the absence of PAT within the limit of detection (LOD) of the proposed method, one product was chosen as a blank sample. Approximately 0.5-g portions of the blank apple powder sample were gravimetrically fortified at four different contamination levels (5, 10, 25, and 50 µg/kg). These fortified samples were subjected to analysis using the proposed ID–LC–MS/MS method to test its validity. The applicability of the ID–LC–MS/MS method to apple juice and apple puree was similarly tested by fortification of blank samples of these apple matrices at three different contamination levels (20, 50, and 100 µg/kg). At each concentration of each type of sample matrix, four fortified samples were also analyzed. The overall measurement results are listed in Table S1, while only representative results are given in Table 2. For all types of apple products, the measurement results are in good agreement with the respective fortified values within their uncertainties (typically ranging from ~ 1 to 3.8%), thereby demonstrating the validity of the method. The accuracy (estimated as measured analyte concentration/fortified concentration × 100) varied between 97.8 and 102%, which, importantly, is better than the reported accuracy of previous studies [15, 43] and comparable to other earlier studies, including our existing method [14, 44].
Precision
The intraday and interday precision of the candidate ID–LC–MS/MS method was determined by measuring fortified apple powder samples. To evaluate the intraday precision of the method, four portions of blank apple powder (0.5 g for each) were gravimetrically fortified with 5 µg/kg PAT, and another four portions were fortified to 10 µg/kg. The eight fortified samples were analyzed on the same day by the ID–LC–MS/MS method. The same intraday precision experiments were carried out at three different periods to evaluate the interday precision of this method. The obtained results are listed in Table S2. At each validation level, the measurement results agreed well with the respective fortified values within their uncertainties (typically ranging from ~ 1 to 3.8%), which indicates that the current method exhibits good intraday precision and can be reproduced at different periods.
Further assessment of the method precision was carried out through the analysis of a naturally contaminated sample, a homogenized apple puree (10 g of apple powder obtained from a local market was reconstituted by adding 40 mL of water and homogenized by mixing). Figure 2(e) is the MRM chromatogram of this apple puree sample. In each of the three different time periods, four subsamples were measured with the ID–LC/MS/MS method. As summarized in Table 3, the relative standard deviations (RSDs) of the interday and intraday precisions were less than 3% and 2%, respectively, and the measurement results (the mean of the four subsamples) at all periods agreed with each other within their uncertainties. These results indicate that the proposed method features very good repeatability and reproducibility at levels considerably less than the maximum regulatory limit of PAT in apple puree (25 µg/kg), thereby revealing the high analytical performance of this method.
Selectivity toward PAT
The selectivity of the method was ascertained via the analysis of both blank apple powder and fortified apple powder based on monitoring the characteristic MRM transitions at the specified retention time of PAT. The LC–MS/MS chromatograms of blank, spiked, and naturally contaminated apple powder samples displayed in Fig. 2(b-e) show the absence of interference peaks in the elution time window of PAT. Additionally, the agreement of the measurement results with the corresponding fortified values ensures the absence of any significant matrix interferences that may overlap with PAT or 13C7-PAT in their MRM chromatograms. The results from the post-column infusion study further supported this observation, which demonstrated that there were no significant ion enhancement or suppression zones at PAT elution time, as shown in the matrix effect profiles. This result confirms that the ID–LC–MS/MS method proposed here is highly selective.
Limit of detection (LOD) and limit of quantification (LOQ)
The sensitivity of the developed method was estimated in terms of LOD and LOQ following the EU Commission Decision 2002/657/EC as the minimum PAT concentrations that generate chromatogram peaks with signal-to-noise ratios of 3/1 and 10/1, respectively [45]. Noncontaminated samples were fortified with a series of decreasing PAT concentrations in the range of 2.0–0.2 μg/kg until an equivalent response was observed. The samples were analyzed in triplicate using the proposed method. The LODs for apple powder, apple juice, and apple puree were 0.45, 0.33, and 0.40 µg/kg, respectively. The LOQs were 1.6, 1.10, and 1.30 µg/kg, respectively. The LODs and LOQs obtained in this study are lower than those reported by other authors [15, 43] and slightly higher than those obtained in our previous method [14]. The higher LODs and LOQs were attributed to background noise observed in the primary MRM channel (m/z 153.0 to 109.0) of PAT, even though no significant matrix effect was encountered during PAT determination by LC–MS/MS. We also noted that this PAT MRM channel showed slightly higher background noise even for a blank LC run (see Fig. 2(a)). Therefore, we attribute this effect to some unknown trace organic impurities that are persistently present in the aqueous mobile phase. Although the background noise of the PAT MRM channel led to slightly elevated LODs and LOQs of the current method, these LODs and LOQs were far below the maximum residue limits adopted by Korea, the EU, and the FDA for various foods [13, 46], indicating the good sensitivity of this method to verify trace levels of PAT in apple products within legal tolerance.
Evaluation of measurement uncertainty
To ensure that the ID–LC–MS/MS method can be used as a higher-order reference method, the measurement uncertainties must be evaluated and must be considerably smaller than those of the methods typically used in regular testing laboratories. The measurement uncertainty for the quantitation of PAT in apple products by the current ID–LC–MS/MS method was estimated based on a protocol previously developed and maintained in this laboratory [47, 48]. Uncertainty sources are from weighing the sample, weighing the internal standard solution spiked into the sample, and the gravimetric preparation of isotope ratio standard (weighing calibration standard solution and internal standard solution). Uncertainties of the area ratios (PAT/13C7-PAT) observed from LC–MS/MS analysis of isotope ratio standards and samples were also included. These uncertainties were combined to calculate the overall expanded uncertainty of each measurement value. The relative expanded uncertainties of the measurement results listed in Tables 2, 3, and 4 were typically less than 4%, which are also comparable to our previous ID–LC–MS/MS method [14]. Notably, the uncertainty due to the area ratios obtained by LC–MS/MS measurements (RSD of multiple measurements ranging from 1 to 2%) has mainly contributed to the overall expanded uncertainty. Importantly, the measurement uncertainties reported in this study were one-fifth smaller than the expected RSDs (22.4–35%) calculated from the Horwitz equation in the concentration range of 100–5 μg/kg [49]. Hence, these results indicate that our method can exhibit a higher-order metrological quality to provide highly accurate and precise measurement results. Furthermore, this method has great potential for producing reliable CRMs as quality control materials that can be used to ensure the reliability of measurement results acquired in testing laboratories.
Application of the ID–MS/MS method to real samples
The proposed analytical method was successfully applied to analyze PAT in 13 commercial apple-based products, including apple powder (n = 4), apple juice (n = 5), and apple puree (n = 4). The samples were acquired from local retail markets in Korea and analyzed following the procedure described in “Experimental.” Detailed results on these samples are given in Table 4, which also represents the occurrence rate, average, and contamination range in the total samples. As presented in this table, PAT was detected in 8 out of 13 tested samples with an incidence rate of 61.53%. The contamination levels ranged from 2.38 to 59.96 µg/kg, and the average PAT concentration was 23.79 µg/kg. Apple juice samples presented the highest incidence of PAT contamination (83.33%), with an average contamination level of 39.07 µg/kg. PAT was found in 2 out of 4 apple powder and apple puree samples (50%), and the average contamination levels were 7.76 and 9.28 µg/kg, respectively. Among all the investigated apple products, only two apple juice samples were positive for PAT at levels exceeding the maximum regulatory limit legally set for apple juice (50 µg/kg). It is worth mentioning that the samples collected here were utilized for method development and for testing its applicability to real samples but not for conducting official surveys on PAT occurrence for risk assessment purposes. Therefore, these results cannot be interpreted as representative of the domestic processed apple market.
Conclusion
In summary, a new ID–LC–MS/MS method for the accurate determination of PAT in apple products was developed and optimized. The method included reliable and easy-to-operate sample preparation processes that allowed quicker and more sufficient elimination of the co-extracted interferences compared with our existing ID–LC–MS/MS method and other reported methods. Chromatographic and mass spectrometric conditions were optimized in negative ESI mode to achieve sensitive determination. Unlike conventional analytical LC columns, the use of the UPLC column allowed PAT separation with a shorter chromatographic run time (8 min), which was a feasible time to provide high analytical throughput. In addition, the observed LOQ of 1.60 μg/kg was far below the maximum residue limits approved for apple powder by various regulatory authorities. Under the optimum conditions, the results of method validation (i.e., accuracy, precision, measurement uncertainty, etc.) demonstrated the higher-order metrological quality of the current method with analytical performance nearly comparable with that of our previous ID–LC–MS/MS method. The feasibility of this method was also confirmed through the analysis of 13 commercially available apple-based products, revealing a PAT incidence of 61.53% in the total number of samples, with the highest contamination level reaching 59.96 µg/kg in apple juice. Ultimately, the results of this study have shown that the newly designed method of broad applicability has great potential to provide a promising and convenient way to reliably monitor PAT contamination in food samples. Furthermore, this method will be applied to the development of CRMs to assist the quality control of analytical methods commonly applied in testing laboratories.
References
Zhao M, Shao H, He Y, Li H, Yan M, Jiang Z, et al. The determination of patulin from food samples using dual-dummy molecularly imprinted solid-phase extraction coupled with LC-MS/MS. J Chromatogr B. 2019;1125:121714. https://doi.org/10.1016/j.jchromb.2019.121714.
Yang Y, Li G, Wu D, Liu J, Li X, Luo P, et al. Recent advances on toxicity and determination methods of mycotoxins in foodstuffs. Trends food Sci Tech. 2020;96:233–52. https://doi.org/10.1016/j.tifs.2019.12.021.
Turner NW, Bramhmbhatt H, Szabo-Vezse M, Poma A, Coker R, Piletsky SA. Analytical methods for determination of mycotoxins: an update (2009–2014). Anal Chim Acta. 2015;901:12–33. https://doi.org/10.1016/j.aca.2015.10.013.
Gab-Allah MA, Choi K, Kim B. Accurate determination of type B trichothecenes and conjugated deoxynivalenol in grains by isotope dilution–liquid chromatography tandem mass spectrometry. Food Control. 2021;121:107557. https://doi.org/10.1016/j.foodcont.2020.107557.
Gab-Allah MA, Choi K, Kim B. Development of isotope dilution-liquid chromatography/tandem mass spectrometry for the accurate determination of type-A trichothecenes in grains. Food Chem. 2021;344:128698. https://doi.org/10.1016/j.foodchem.2020.128698.
Tahoun IF, Gab-Allah MA, Yamani RN, Shehata AB. Development and validation of a reliable LC-MS/MS method for simultaneous determination of deoxynivalenol and T-2 toxin in maize and oats. Microchem J. 2021:106599. https://doi.org/10.1016/j.microc.2021.106599.
Li X, Ma W, Ma Z, Zhang Q, Li H. Recent progress in determination of ochratoxin a in foods by chromatographic and mass spectrometry methods. Crit Rev Food Sci Nutr. 2021:1–32. https://doi.org/10.1080/10408398.2021.1885340.
Funes G, Resnik S. Determination of patulin in solid and semisolid apple and pear products marketed in Argentina. Food Control. 2009;20(3):277–80. https://doi.org/10.1016/j.foodcont.2008.05.010.
Vaclavikova M, Dzuman Z, Lacina O, Fenclova M, Veprikova Z, Zachariasova M, et al. Monitoring survey of patulin in a variety of fruit-based products using a sensitive UHPLC–MS/MS analytical procedure. Food Control. 2015;47:577–84. https://doi.org/10.1016/j.foodcont.2014.07.064.
Guo W, Pi F, Zhang H, Sun J, Zhang Y, Sun X. A novel molecularly imprinted electrochemical sensor modified with carbon dots, chitosan, gold nanoparticles for the determination of patulin. Biosens Bioelectron. 2017;98:299–304. https://doi.org/10.1016/j.bios.2017.06.036.
Valle-Algarra FM, Mateo EM, Gimeno-Adelantado JV, Mateo-Castro R, Jiménez M. Optimization of clean-up procedure for patulin determination in apple juice and apple purees by liquid chromatography. Talanta. 2009;80(2):636–42. https://doi.org/10.1016/j.talanta.2009.07.040.
Lee TP, Sakai R, Manaf NA, Rodhi AM, Saad B. High performance liquid chromatography method for the determination of patulin and 5-hydroxymethylfurfural in fruit juices marketed in Malaysia. Food Control. 2014;38:142–9. https://doi.org/10.1016/j.foodcont.2013.10.018.
No E. Commission regulation (EC) No. 1881/2006 of 19 December 2006. Setting maximum levels for certain contaminants in foodstuffs (text with EEA relevance). Official Journal of European Commission. 1881;364(20.12):2006.
Seo M, Kim B, Baek S-Y. An optimized method for the accurate determination of patulin in apple products by isotope dilution-liquid chromatography/mass spectrometry. Anal Bioanal Chem. 2015;407(18):5433–42. https://doi.org/10.1007/s00216-015-8705-3.
Li X, Li H, Ma W, Guo Z, Li X, Li X, et al. Determination of patulin in apple juice by single-drop liquid-liquid-liquid microextraction coupled with liquid chromatography-mass spectrometry. Food Chem. 2018;257:1–6. https://doi.org/10.1016/j.foodchem.2018.02.077.
Kharandi N, Babri M, Azad J. A novel method for determination of patulin in apple juices by GC–MS. Food Chem. 2013;141(3):1619–23. https://doi.org/10.1016/j.foodchem.2013.05.080.
Sadok I, Szmagara A, Staniszewska MM. The validated and sensitive HPLC-DAD method for determination of patulin in strawberries. Food Chem. 2018;245:364–70. https://doi.org/10.1016/j.foodchem.2017.10.093.
Gab-Allah MA, Mekete KG, Choi K, Kim B. Occurrence of major type-B trichothecenes and deoxynivalenol-3-glucoside in cereal-based products from Korea. J Food Compos Anal. 2021;99:103851. https://doi.org/10.1016/j.jfca.2021.103851.
Wang Y, Wen Y, Ling Y-C. Graphene oxide-based magnetic solid phase extraction combined with high performance liquid chromatography for determination of patulin in apple juice. Food Anal Methods. 2017;10(1):210–8. https://doi.org/10.1007/s12161-016-0570-y.
Rodríguez-Carrasco Y, Berrada H, Font G, Mañes J. Multi-mycotoxin analysis in wheat semolina using an acetonitrile-based extraction procedure and gas chromatography–tandem mass spectrometry. J Chromatogr A. 2012;1270:28–40. https://doi.org/10.1016/j.chroma.2012.10.061.
Yang Y, Li Q, Fang G, Wang S. Preparation and evaluation of novel surface molecularly imprinted polymers by sol–gel process for online solid-phase extraction coupled with high performance liquid chromatography to detect trace patulin in fruit derived products. RSC Adv. 2016;6(59):54510–7. https://doi.org/10.1039/C6RA08736A.
Song L, He J, Chen N, Huang Z. Combined biocompatible medium with molecularly imprinted polymers for determination of aflatoxins B1 in real sample. J Sep Sci. 2019;42(24):3679–87. https://doi.org/10.1002/jssc.201900564.
Urraca J, Marazuela M, Merino E, Orellana G, Moreno-Bondi M. Molecularly imprinted polymers with a streamlined mimic for zearalenone analysis. J Chromatogr A. 2006;1116(1–2):127–34. https://doi.org/10.1016/j.chroma.2006.03.032.
Goda ES, Gab-Allah M, Singu BS, Yoon KR. Halloysite nanotubes based electrochemical sensors: a review. Microchem J. 2019;147:1083–96. https://doi.org/10.1016/j.microc.2019.04.011.
Gab-Allah M, Goda ES, Shehata A, Gamal H. Critical review on the analytical methods for the determination of sulfur and trace elements in crude oil. Crit Rev Anal Chem. 2020;50(2):161–78. https://doi.org/10.1080/10408347.2019.1599278.
Li X, Li H, Li X, Zhang Q. Determination of trace patulin in apple-based food matrices. Food Chem. 2017;233:290–301. https://doi.org/10.1016/j.foodchem.2017.04.117.
Olivares I, Souza G, Nogueira A, Toledo G, Marcki D. Trends in developments of certified reference materials for chemical analysis-focus on food, water, soil, and sediment matrices. TrAC Trends Anal Chem. 2018;100:53–64. https://doi.org/10.1016/j.trac.2017.12.013.
Shehata A, Mohamed G, Gab-Allah M. Development of crude oil reference material certified for the concentrations of sulfur, iron, nickel, vanadium and magnesium. Mapan. 2017;32(2):101–12. https://doi.org/10.1007/s12647-017-0205-9.
Tahoun IF, Rend EA, Gab-Allah MA. Preparation and value assignment of parabens and phenoxyethanol in cosmetic cream certified reference material. J Chem Metrol. 2021;15(1). https://doi.org/10.25135/jcm10.25135/15.110.25135/jcm.59.21.04.2049.
Lee J, Kim B. Mass balance method for purity assessment of organic reference materials: for thermolabile materials with LC-UV method. Bull Korean Chem Soc. 2014;35(11):3275–9. https://doi.org/10.5012/bkcs.2014.35.11.3275.
Przybylska A, Bazylak G, Kosicki R, Altyn I, Twaruzek M, Grajewski J, et al. Advantageous extraction, cleanup, and UHPLC-MS/MS detection of patulin mycotoxin in dietary supplements and herbal blends containing hawberry from Crataegus spp. J Anal Methods Chem. 2019;2019. https://doi.org/10.1155/2019/2159097.
Iqbal SZ, Malik S, Asi MR, Selamat J, Malik N. Natural occurrence of patulin in different fruits, juices and smoothies and evaluation of dietary intake in Punjab. Pakistan Food Control. 2018;84:370–4. https://doi.org/10.1016/j.foodcont.2017.08.024.
Lee S, Kim B, Kim J. Development of isotope dilution-liquid chromatography tandem mass spectrometry for the accurate determination of fluoroquinolones in animal meat products: optimization of chromatographic separation for eliminating matrix effects on isotope ratio measurements. J Chromatogr A. 2013;1277:35–41. https://doi.org/10.1016/j.chroma.2012.12.047.
Ahn S, Lee S, Lee J, Kim B. Accurate determination of ochratoxin A in Korean fermented soybean paste by isotope dilution-liquid chromatography tandem mass spectrometry. Food Chem. 2016;190:368–73. https://doi.org/10.1016/j.foodchem.2015.05.114.
Shin H, Kim B, Lee J. Investigation of isotope dilution mass spectrometric (ID-MS) method to determine niacin in infant formula, breakfast cereals and multivitamins. Food Chem. 2013;138(2–3):1109–15. https://doi.org/10.1016/j.foodchem.2012.11.046.
Lim YO, Kim B, Ahn S, Kim J. Improvement of accuracy for the determination of vitamin A in infant formula by isotope dilution-liquid chromatography/tandem mass spectrometry. Food Chem. 2011;126(3):1393–8. https://doi.org/10.1016/j.foodchem.2010.11.118.
Vogl J, Pritzkow W. Isotope dilution mass spectrometry—a primary method of measurement and its role for RM certification. Mapan. 2010;25(3):135–64. https://doi.org/10.1007/s12647-010-0017-7.
Yang Y, Yang Y, Shao B, Zhang J. A simple and rapid method for determination of patulin in juice by high performance liquid chromatography tandem mass spectrometry. Food Anal Methods. 2017;10(9):2913–8. https://doi.org/10.1007/s12161-017-0859-5.
Gab-Allah MA, Shehata AB. Determination of iron, nickel, and vanadium in crude oil by inductively coupled plasma optical emission spectrometry following microwave-assisted wet digestion. Chem Pap. 2021. https://doi.org/10.1007/s11696-021-01633-8.
Shehata A, Mohamed G, Gab-Allah M. Simple spectrophotometric method for determination of iron in crude oil. Pet Chem. 2017;57(12):1007–11. https://doi.org/10.1134/S096554411712012X.
Rodríguez-Bencomo JJ, Sanchis V, Viñas I, Martín-Belloso O, Soliva-Fortuny R. Formation of patulin-glutathione conjugates induced by pulsed light: a tentative strategy for patulin degradation in apple juices. Food Chem. 2020;315:126283. https://doi.org/10.1016/j.foodchem.2020.126283.
Khorrami AR, Taherkhani M. Synthesis and evaluation of a molecularly imprinted polymer for pre-concentration of patulin from apple juice. Chromatographia. 2011;73(1):151–6. https://doi.org/10.1007/s10337-010-1892-3.
Zhang K, Wong JW, Mai H, Trucksess MW. Dopant-assisted atmospheric pressure photoionization of patulin in apple juice and apple-based food with liquid chromatography–tandem mass spectrometry. J Agric Food Chem. 2014;62(18):4112–8. https://doi.org/10.1021/jf5006726.
Li X, Ma W, Zhang Q, Li H, Liu H. Determination of patulin in apple juice by amine-functionalized solid-phase extraction coupled with isotope dilution liquid chromatography tandem mass spectrometry. J Sci Food Agric. 2021;101(5):1767–71. https://doi.org/10.1002/jsfa.10790.
Commission E. Commission Decision 2002/657/EC of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off J Eur Communities. 2002;50:8–36.
Harris KL, Bobe G, Bourquin LD. Patulin surveillance in apple cider and juice marketed in Michigan. J Food Prot. 2009;72(6):1255–61. https://doi.org/10.4315/0362-028X-72.6.1255.
Choi J, Hwang E, So H-Y, Kim B. An uncertainty evaluation for multiple measurements by GUM. Accredit Qual Assur. 2003;8(1):13–5. https://doi.org/10.1007/s00769-002-0520-9.
Kim B-J, Hwang E-J, So H-Y, Son E-K, Kim Y-S. Development of a model system of uncertainty evaluations for multiple measurements by isotope dilution mass spectrometry: determination of folic acid in infant formula. Bull Korean Chem Soc. 2010;31(11):3139–44. https://doi.org/10.5012/bkcs.2010.31.11.3139.
Thompson M, Ellison SL, Wood R. The International Harmonized Protocol for the proficiency testing of analytical chemistry laboratories (IUPAC Technical Report). Pure Appl Chem. 2006;78(1):145–96. https://doi.org/10.1351/pac200678010145.
Funding
This study was supported by the Korea Research Institute of Standards and Science under the project “Establishing measurement standards for organic analysis” (Grant no. 20011034). The funding source had no further detailed involvement in this study.
Author information
Authors and Affiliations
Contributions
Mohamed A. Gab-Allah: methodology, investigation, validation, and writing—original draft; Kihwan Choi: conceptualization, resources, supervision, and writing—review and editing; Byungjoo Kim: conceptualization, project administration, supervision, and writing—review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gab-Allah, M.A., Choi, K. & Kim, B. Development of isotope dilution–liquid chromatography/tandem mass spectrometry as a candidate reference method for the accurate determination of patulin in apple products. Anal Bioanal Chem 414, 1867–1879 (2022). https://doi.org/10.1007/s00216-021-03817-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03817-3