Abstract
Patulin, a mycotoxin produced by several molds in fruits, has been frequently detected in apple products. Therefore, regulatory bodies have established recommended maximum permitted patulin concentrations for each type of apple product. Although several analytical methods have been adopted to determine patulin in food, quality control of patulin analysis is not easy, as reliable certified reference materials (CRMs) are not available. In this study, as a part of a project for developing CRMs for patulin analysis, we developed isotope dilution liquid chromatography–tandem mass spectrometry (ID-LC/MS/MS) as a higher-order reference method for the accurate value-assignment of CRMs. 13C7-patulin was used as internal standard. Samples were extracted with ethyl acetate to improve recovery. For further sample cleanup with solid-phase extraction (SPE), the HLB SPE cartridge was chosen after comparing with several other types of SPE cartridges. High-performance liquid chromatography was performed on a multimode column for proper retention and separation of highly polar and water-soluble patulin from sample interferences. Sample extracts were analyzed by LC/MS/MS with electrospray ionization in negative ion mode with selected reaction monitoring of patulin and 13C7-patulin at m/z 153→m/z 109 and m/z 160→m/z 115, respectively. The validity of the method was tested by measuring gravimetrically fortified samples of various apple products. In addition, the repeatability and the reproducibility of the method were tested to evaluate the performance of the method. The method was shown to provide accurate measurements in the 3–40 μg/kg range with a relative expanded uncertainty of around 1 %.

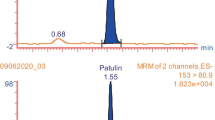
SRM chromatograms of patulin in blank apple concentrate and fortified apple concentrate (10 μg/kg patulin), presenting the retention and separation of patulin from matrix interferences with a multi-mode column (Scherzo SM-C18, 250 mm length, 4.6 mm i.d., 3 μm particle size, from Imtakt) under various LC mobile phase compositions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patulin is an unsaturated heterocyclic lactone (4-hydroxy-4H-furo [3,2c] pyran-2[6H]-one). Patulin is a mycotoxin produced in fruits by several molds belonging to the genera Penicillium, Byssochlamys, and Aspergillus [1]. The acute symptoms of patulin intake include hyperemia, epithelial cell degeneration, intestinal inflammation, and other gastrointestinal and kidney dysfunctions [2]. Exposure to patulin is also known to have chronic health risks associated with genotoxic, immunotoxic, and neurotoxic effects [2, 3]. Patulin has been listed as category 3 human carcinogen by the International Agency for Research on Cancer (IARC) [4]. Patulin has been observed most frequently in apples and apple-based products, though it has been occasionally detected in other fruits such as pears, apricots, peaches, and grapes [2]. The World Health Organization and the Food and Agriculture Organization have provisionally set a maximum tolerable daily intake for patulin of 0.4 μg/kg body weight/day [5]. Based on the tolerance level, the maximum permitted patulin concentration has been set to 50 μg/kg in apple juice and apple concentrates in Korea [6], USA [7], and the European Union [8].
Various analytical methods have been developed to determine patulin in food, especially in apples and apple-based products. Thin-layer chromatography (TLC) was used in early days [9, 10]. TLC is relatively inexpensive and simple, but it is seldom used these days because of its poor accuracy and low sensitivity. High-performance liquid chromatography (HPLC) coupled with ultraviolet (UV) detection has been most widely used [10, 11]. Gas chromatography (GC) has been used, but it requires a cumbersome derivatization step [10, 12, 13]. Recently, mass spectrometry (MS) has been combined with both HPLC and GC for better selectivity and increased sensitivity [10, 14–17], and isotopic-labeled analogues of patulin have been frequently adopted as internal standards [18–23].
Although several methods are widely used to screen patulin in apple products, there is a strong need for quality control and quality assurance, as the measurement of trace amounts of patulin in complex food matrices requires complicated sample preparation processes and is prone to bias. Preparing and using an in-house reference material can be a way to control the quality of daily analytical work of a laboratory. Certified reference materials (CRMs) developed by a qualified CRM producer can be the ultimate solution for validating analytical methods established in laboratories and for daily quality control [24, 25]. This laboratory, as the National Metrology Institute (NMI) of Korea, has planning to develop CRMs for the analysis of patulin in apple products. As a part of this ongoing project, we developed isotope dilution-liquid chromatography/tandem mass spectrometry (ID-LC/MS/MS) as a reference method for the value assignment of patulin in those CRMs. The method should be optimized to fulfill performance requirements as a reference method, which would have a higher-order metrological quality, providing measurement results without bias and with much lower uncertainty than that of methods used in testing laboratories. For NMIs, isotope dilution mass spectrometry (IDMS) is a prior choice as a higher-order reference method as it overcomes difficulties of accurate correction of recovery in sample preparation and shows high repeatability and reproducibility [26–28]. The method developed in this study is based on widely used LC/MS/MS methods for patulin analysis, but is modified to meet the requirements of a higher-order reference method. 13C7-Patulin was used as an internal standard. In the initial stage of this study, we noticed that the highly polar and water-soluble patulin molecule is not retained well with the reverse-phase LC columns (typically C18) used in most LC/UV and LC/MS/MS methods. In reverse-phase columns, patulin tends to elute at the tail part of the solvent front even with mobile phases of high aqueous content [10, 29, 30]. The tail part of the solvent front contains large amounts of ionic and polar species, which cause strong matrix effects (suppression/enhancement of ionization in LC/MS interface). Therefore, a multimode column was adopted for proper retention and separation. Most current analytical methods use either liquid–liquid extraction with ethyl acetate [2, 6, 10, 11, 14, 15, 20, 23, 29, 30] or direct solid phase extraction (SPE) [10, 11, 21, 23, 31] for rapid and simple sample preparation. In this study, a SPE extraction was adopted after the liquid–liquid extraction for further sample cleanup to increase the accuracy of the analytical results. Several types of SPE cartridges were tested to find optimum SPE conditions. The developed method was validated by measuring several types of apple products fortified gravimetrically with various levels of patulin.
Materials and methods
Materials
Primary reference materials for patulin (with a purity of 99.7 % as provided by the manufacturer) were purchased from Biopure (Romer Labs Diagnostic GmbH, Tulln, Austria). The purity of the primary material was determination following protocols maintained in this laboratory (LC/UV analysis of structurally related impurities, Karl-Fischer Coulometry for water content, thermogravimetric analysis for nonvolatile impurities). 13C7-Patulin stock solution (25 μg/mL in acetonitrile) was purchased from Biopure. HPLC-grade organic solvents (methanol, acetonitrile, ethyl acetate, and ethyl ether) were purchased from Burdick and Jackson (Muskegon, MI, USA). Ammonium acetate, sodium carbonate, sodium bicarbonate, and anhydrous sodium sulfate were purchased from Sigma-Aldrich (Gyeonggi-do, Korea). The SPE cartridges tested for sample cleanup were HLB SPE (3 mL, 60 mg, Oasis) from Waters (Seoul, Korea), C18 SPE (3 mL, 500 mg, Supelclean™) from SUPELCO (Gyeonggi-do, Korea), Silica SPE (6 mL, 500 mg, Sep-Pak) from Waters, and EASIMIP™ Patulin molecularly imprinted (MIP) SPE (3 mL) from R-Biopharm (Glasgow, UK). Apple product samples (fresh fruit, concentrates, juices, and jams) were purchased from local markets.
Calibration standard solutions
A calibration standard solution containing 2.5 mg/kg of patulin was prepared by gravimetrically mixing 0.5 mg of the primary reference material with 200 mL of a solvent consisting of 80 % water (containing 5 mmol/L ammonium acetate and 3.5 mmol/L acetic acid) and 20 % methanol. An internal standard solution containing approximately 200 μg/kg 13C7-patulin was prepared by diluting the 13C7-patulin stock solution (25 μg/mL in acetonitrile) with the same solvent used for the patulin standard solution. For IDMS analysis of samples with exact matching calibration, an isotope ratio standard with a 1:1 isotope ratio was prepared by gravimetrically mixing weighed aliquots of the patulin standard solution and the internal standard solution. For preparation of fortified samples, a working standard solution (200 μg/kg) was prepared by gravimetrically diluting the patulin standard solution.
Sample preparation and cleanup
Sample preparation was composed of liquid–liquid extraction with ethyl acetate and further cleanup with a SPE cartridge. Five grams of an apple product sample (ground fresh fruit, concentrates, juices, and jams) was weighed in a 50-mL conical tube. The sample was spiked with an appropriate amount of the internal standard solution so that patulin and spiked 13C7- patulin were present in an approximately 1:1 ratio. The conical tube was vortexed for 10 min to mix and equilibrate the contents. Ethyl acetate was chosen as an extraction solvent after comparing the extraction yields of several solvents. Ten milliliters of ethyl acetate was added into the tube and vortexed for 10 min. The tube was centrifuged at 1800×g for 10 min, and the supernatant (ethyl acetate layer) was collected and transferred to a new 50-mL conical tube. The sample was extracted with another 10 mL of ethyl acetate, and the supernatant was combined with the first extract. The extract was washed with 2 mL of 1.5 % sodium carbonate solution, and residual water was removed with 5 g of sodium sulfate. The extract was dried at 40 °C under nitrogen gas flow. The dried extract was reconstituted with 2.5 mL of acetic acid/acetate buffer solution (pH 4). After testing several types of SPE cartridges (see “Optimizing sample preparation conditions” for test results), HLB SPE (3 mL, 60 mg, Oasis) was chosen in this study for further cleanup of sample extracts. The reconstituted extract was loaded drop-by-drop into a HLB SPE cartridge prewashed with 3 mL of water, 2 mL of methanol, and 3 mL of water again. The cartridge was then washed with 2 mL of 1.0 % sodium bicarbonate solution and 2 mL of 1.0 % acetic acid solution. Patulin was then eluted with 3 mL of 10 % ethyl acetate and 90 % ethyl ether. The eluent was dried under nitrogen and reconstituted with 0.2 mL of the initial LC mobile phase.
For the preparation of fortified samples, apple products (ground fresh fruit, concentrates, juices, and jams) obtained from local markets were used as blank samples after testing for nonexistence of patulin. Five grams of each blank sample was gravimetrically spiked with an appropriate amount of the working standard solution prepared as described in “Calibration standard solutions.” After homogenization by a mechanical shaker for 10 min, fortified samples were subject to the same sample preparation processes as described above.
LC-MS/MS analysis
The LC/MS system consists of a ThermoElectron (San Jose, CA, USA) TSQ Quantum mass spectrometer connected to an Acquity UPLC system (Manchester, UK) through its electrospray ionization interface. A Scherzo SM-C18 column (250 mm length, 4.6 mm i.d., 3 μm particle size) from Imtakt (Kyoto, Japan) was used. The injection volume for sample extracts and the isotope ratio standard solution was 10 μL per LC run. The mobile phase was isocratic with 80 % A (5 mmol/L ammonium acetate and 3.5 mmol/L acetic acid in water) and 20 % B (methanol). After each run for 15 min, the column was washed with 100 % B for 2 min and then 100 % A for 2 min. The mobile phase changed to the initial isocratic mobile phase and kept for 10 min to equilibrate the column for the next run. The flow rate of the mobile phase was 0.5 mL/min. LC eluent was connected to the electrospray ionization (ESI) interface of the mass spectrometer without splitting. The mass spectrometer was operated in negative ion mode. The ESI probe voltage was −4.0 kV. The capillary temperature was set to 350 °C. The flow rates of the nitrogen sheath and auxiliary gas were set to 40 and 35 (in arbitrary units of the mass spectrometer), respectively. Vaporizer temperature was set to 150 °C. For the LC-MS/MS analysis, the mass spectrometer was operated in a selected reaction monitoring (SRM) mode. The collisionally induced dissociation (CID) of [M–H]− to [M–H–CO2]− at m/z 153→m/z 109 was selected as the primary SRM channel for the quantitative analysis of patulin. The corresponding primary channel for 13C7-patulin was the CID of [M–H]− to [M–H–13CO2]− at m/z 160→m/z 115. The CID channel of patulin at m/z 153→m/z 81 for [M–H]− to [M–H–CO2–CO]− and the corresponding channel of 13C7-patulin at m/z 160→m/z 86 for [M–H]− ion to [M–H–13CO2–13CO]− were used for the confirmatory quantitative analysis. The pressure in the collision cell was set to 1.5 mTorr, and the collision energy was set to 10 V.
Postcolumn infusion system
A postcolumn infusion system [26, 32] was used to test sample cleanup efficiencies of various SPE cartridges. In this system, sample extracts were subject to normal LC runs. The postcolumn LC eluent was infused with a constant flow (5 μL/min) of a patulin standard solution (500 μg/kg) via a T-connector installed in front of the ESI interface. MS was operated in the normal SRM mode to obtain matrix effect profiles, which show the influence of sample matrix on the ionization efficiency of constantly infused patulin.
Results and discussion
Optimization of LC/MS conditions
Patulin was ionized to the [M–H]− form in the negative ion mode. The negative ionization mode was chosen in this study, as was done in several other published studies [17, 20–22], as it showed a much higher ionization efficiency for patulin than the positive ion mode. The CID mass spectrum of the [M–H]− ion of patulin is shown in Fig. 1a. The spectrum was dominated by a peak at m/z 109, and minor peaks were observed at m/z 135, m/z 125, and m/z 81. We assigned those peaks by referencing the fragmentation patterns assigned by Zhou et al. [33] for the [M–H]− ion and by Rychlik et al. [34] for the M+. ion of patulin. The peaks at m/z 135, m/z 125, and m/z 81 can be unambiguously assigned to [M–H–H2O]−, [M–H–CO]−, and [M–H–CO2–CO]−, respectively. Although the peak at m/z 109 had been assigned to [M–H–CO2]− and [M–H–C2H4O]− by Zhou et al., our data further clarify the dissociation pattern for the ion at m/z 109 as following. The CID mass spectrum of the [M–H]− ion of 13C7-patulin is shown in Fig. 1b. The peaks at m/z 142, m/z 131, and m/z 86 were attributed to [M–H–H2O]−, [M–H–13CO]−, and [M–H–13CO2–13CO]−, respectively. The spectrum has a dominant peak for [M–H–13CO2]− at m/z 115, but the peak for [M–H–13C2H4O]− at m/z 114 is very weak. Therefore, our results indicate that the peak at m/z 109 in the CID mass spectrum of patulin (Fig. 1a) is mainly attributed to [M–H–CO2]−, not to [M–H–C2H4O]−. In this study, the most dominant CID pathway of patulin, [M–H]− to [M–H–CO2]− at m/z 153→m/z 109 and the corresponding pathway for 13C7-patulin were chosen as the primary SRM channels for quantitative analysis. The CID pathway of [M–H]− to [M–H–CO2–CO]− at m/z 153→m/z 81 for patulin and the corresponding pathway for 13C7-patulin were used as confirmatory channels as they are less sensitive compared to the primary channels and provide measurement results with larger uncertainty.
Most of LC or LC/MS methods reported in the literature and officially standardized methods adopt nonpolar reverse phase C18 columns [10]. In the initial stage of this study, several analytical C18 columns were tested for the retention and separation of patulin. It was noted that patulin, a highly polar and water-soluble acidic compound, was not well retained with those C18 columns. It is presumed that several official methods adopted fully end-capped C18 columns with high carbon loading instead of conventional C18 columns to improve the retention of patulin. In this study, we adopted a multimode column (Scherzo SM-C18) that contains cation and anion ligands in addition to octadecylsilane. While it behaves similarly to conventional C18 columns for nonionic compounds, its ion-exchange ligands allows normal phase mode for the retention of highly polar/ionic compounds without using ion-pairing reagents, which are usually not compatible with LC/MS. Figure 2 shows SRM chromatograms of blank apple concentrate and the same sample fortified with patulin (after sample extraction and cleanup with the optimized method described in “Sample preparation and cleanup”). The mobile phase for Fig. 2a was isocratic with 50 % A (5 mmol/L ammonium acetate and 3.5 mmol/L acetic acid in water) and 50 % B (methanol) with the flow rate of 0.5 mL/min. In the chromatogram of the fortified sample, patulin was well retained and has a good peak shape. The chromatogram of the blank sample showed a peak at the same retention time as that of patulin, indicating that the patulin peak in the chromatogram of the fortified sample overlapped with matrix interference peak(s). Figure 2b and c is chromatograms obtained by increasing the proportion of aqueous buffer (A) composition of the isocratic mobile phase to 60 and 70 %, respectively. With increasing the proportion of aqueous buffer, the interfering peak from sample matrix can be resolved from the patulin peak and can also be separated to two peaks. The chromatogram in Fig. 2d, obtained with an isocratic mobile phase of 80 % A and 20 % B, shows that the patulin peak is well separated from the two interfering peaks. In conjunction with results obtained during the optimization of sample preparation described in the next section, the isocratic mobile phase of 80 % A and 20 % B was chosen for sample analysis. The results prove that the multimode column chosen in this study allows the flexibility of changing the mobile phase composition to control the retention time of patulin to separate it from sample matrix interferences.
SRM chromatograms of patulin in blank apple concentrate and fortified apple concentrate (10 μg/kg patulin), presenting the retention and separation of patulin from matrix interferences with a multi-mode column (Scherzo SM-C18, 250 mm length, 4.6 mm i.d., 3 μm particle size, from Imtakt) under various LC mobile phase compositions. a Isocratic with 50 % A (5 mmol/L ammonium acetate and 3.5 mmol/L acetic acid in water) and 50 % B (methanol). b Isocratic with 60 % A and 40 % B. c Isocratic with 70 % A and 30 % B. d Isocratic with 80 % A and 20 % B. The flow rate of the mobile phase was set to 0.5 mL/min for a–d
Optimizing sample preparation conditions
Ethyl acetate was used as an extraction solvent in most published methods [2, 6, 10, 11, 14, 15, 20, 23, 29, 30]. An ethyl acetate/n-hexane mixture and acetonitrile were also adopted as extraction solvents in a few methods published in the literature [33, 35]. We tested ethyl acetate, ethyl acetate/n-hexane mixtures (96/4 and 60/40, v/v), and acetonitrile as candidate extraction solvents by comparing patulin recoveries obtained with those solvents. Using ethyl acetate resulted in higher than 95 % recovery, the highest extraction efficiency obtained from any of the tested extraction solvents. Therefore, we chose to use ethyl acetate as an extraction solvent.
We used the postcolumn infusion system to test the sample cleanup efficiency of various sample preparation methods. A blank apple concentrate and the same sample fortified with patulin were used as test samples. Figure 3a shows the matrix effect profile of the blank sample after extraction with ethyl acetate. The overlaid SRM chromatogram shown in Fig. 3a was obtained from a normal LC/MS run of the fortified sample after ethyl acetate extraction. No significant interfering matrix can be observed in the SRM chromatogram of patulin from the fortified sample. However, the matrix effect profile of the blank sample shows that the ionization efficiency of patulin fluctuates due to the elution of sample matrices co-extracted with ethyl acetate. In particular, the matrix effect profile showed a broad and deep ionization suppression zone at the retention time of patulin, indicating that further sample cleanup with a proper SPE cartridge is required for the accurate determination of patulin in samples. Therefore, we tested various SPE methods adopted from the published literature. The SPE cartridges tested in this study were described in “Materials.” Figure 3b–d shows the matrix effect profiles of the blank sample after further SPE cleanup with the C18 cartridge [35], with the SiO2 cartridge [36], and with the HLB cartridge [37], respectively. The SRM chromatograms overlaid were obtained from the fortified sample after the same SPE cleanup. The C18 SPE cleanup method successfully removed interferences that caused the broad deep ionization suppression zone at the retention time of patulin, but the ionization efficiency still fluctuates throughout the LC runs because of improper removal of sample interferences. The SiO2 SPE cleanup methods improved matrix effect profiles of the blank sample, which showed a stable and flat ionization efficiency for patulin. However, the profile showed two noticeable interfering peaks near the retention time of patulin. The MIP SPE cartridge specifically designed for patulin was also tested according to the manufacturer’s protocol. Although it provided fast and simple sample cleanup, the sample cleanup efficiency of the MIP SPE method was not satisfactory, as the matrix effect profile fluctuated near the patulin retention time and throughout the LC run (Fig. 3e). As shown in Fig. 3d, the HLB SPE cartridge showed the most effective cleanup as the matrix effect profile was stable and flat near the patulin retention time and throughout the LC run. Therefore, the HLB SPE method was chosen for sample cleanup in this method.
SRM chromatograms (showing matrix effects profiles) from a post-column infusion of a patulin standard solution at the level of 500 μg/kg at a rate of 5 μL/min and from an LC run of a blank apple concentrate after various sample preparation processes. a After liquid–liquid extraction (LLE) with ethyl acetate. b After LLE and SPE cleanup with a C18 cartridge. c After LLE and SPE cleanup with a SiO2 cartridge. d After LLE and SPE cleanup with an HLB cartridge. e After LLE and SPE cleanup with a molecularly imprinted (MIP) cartridge. Overlaid are typical SRM chromatograms of fortified apple concentrate after the same sample preparation processes
Method validation
The ID-LC-MS/MS method was evaluated to test the potential of the current method as a reference method with a higher-order metrological quality. To the best of our knowledge, no matrix CRM with a certified value for patulin is yet available. Therefore, gravimetrically fortified samples were prepared and measured by the method. In addition, performance parameters of the method including repeatability, reproducibility, and sources of uncertainty were evaluated. The following experimental results were obtained during the validation of the method.
A commercial brand of apple concentrate obtained at local markets was tested by the method. Its patulin content was found to be below the detection limit of the method, and it was subsequently used as a blank sample. Five-gram portions of blank sample were gravimetrically fortified with appropriate amounts of the working standard solution. For each of 5, 10, and 30 μg/kg patulin levels, three fortified samples were prepared. Fortified samples were then measured by the analytical method. Table 1 summarizes experimental results obtained over a period of time. For multiple (usually three) samples for each of the three levels, measurement results by the ID-LC/MS method with the primary SRM channels agree with the gravimetrically fortified values within their uncertainties (typically 1–2 % of relative expanded uncertainty with a 95 % confidence level). The same samples were analyzed using the confirmatory SRM channels. Results are listed in Table 1. Although they have slightly larger uncertainties, measurement results with the confirmatory channels were in good agreement with the gravimetrically fortified values and with the corresponding results from using the primary channels. As three samples in each level were tested, the repeatability of the analytical method is demonstrated within their estimated measurement uncertainties. We carried out the same kind of validation test with fortified samples over a different period of time to test the reproducibility of the analytical method, and the agreement of measurement results with gravimetrically fortified values was confirmed [see Electronic Supplementary Material (ESM) Table S1]. These results show that the current method provides reliable analytical results for patulin in apple concentrate in the 3–40 μg/kg range.
We tested the applicability of the method to other apple products such as apple juice, ground fruit, and jam. Table 2 shows the measurement results of apple juice, ground fruit, and jam gravimetrically fortified to the level of 10 μg/kg. The measurement results with using the primary channels and the confirmatory channels were in good agreement with the fortified values within their uncertainties. Therefore, these results shows that the current method can be applied to those apple products.
The limit of detection (LOD) and the limit of quantification (LOQ) were estimated by analyzing fortified samples in the range of 0.5–1 μg/kg. The LOD and LOQ were the levels of patulin estimated to show signal to noise ratios of 3 and 10, respectively, or higher in SRM chromatograms of patulin in samples for both primary and confirmatory SRM channels. The LOD and LOQ of each apple products are listed in Table 3.
Measurement uncertainty
Our laboratory, the National Metrology Institute of Korea, has established and maintained a protocol to evaluate uncertainty in measurement results by IDMS. Details of this protocol have been described in our previous articles [38–40]. A brief description for the evaluation of measurement uncertainty is given here. Uncertainties in weighing sample taken for analysis and the 13C7-patulin internal standard solution spiked to the sample were estimated from the specification of the balance used. For the patulin standard solution and the isotope ratio standard solution, uncertainties in their gravimetric preparation were estimated from self-consistency test by cross-checking multiply prepared sets as describe in our previous articles. Uncertainties in the LC-MS/MS measurements of sample and the isotope ratio standard were estimated from the standard deviation for multiple LC-MS/MS measurements of the observed area ratios (patulin/13C7-patulin). Those uncertainties were combined following the IDMS master equation [40] to calculate the uncertainty of the patulin content in each sample. The measurement uncertainties listed in Tables 1, 2, and 3 were evaluated according to the protocol. The relative expanded uncertainty (with a 95 % confidence level) of the ID-LC/MS method using the primary SRM channels was estimated to be around 1 % for the measurement of patulin in the 3–40 μg/kg range in apple concentrate, as shown in Table 1. The parameters that contribute most to the uncertainty of measurement results were the LC-MS/MS measurement of peak area ratios for patulin and 13C7-patulin in sample extracts and isotope ratio standard solutions (ranging from 0.3 to 0.6 %). The relative expanded uncertainty using the confirmatory SRM channel was estimated to be around 2 % in the same range for patulin. A larger uncertainty in measurement with the confirmatory channels (compared to the measurement with the primary SRM channels) was attributed to higher background noise in its SRM chromatogram.
The measurement uncertainty obtained with the current method is much less than that obtained from published methods, which were usually more than a few percent [10, 11, 20–22]. Furthermore, the measurement uncertainty is much less than one third of the expected relative standard deviation among testing laboratories estimated by the Horwitz equation (26–38 % for the 40–3 μg/kg range), indicating that our method is of adequate metrological quality, as required for a higher-order reference method [41].
Analysis of commercial products
The ID-LC/MS/MS method was applied to analyze commercial apple products obtained from local markets as part of an effort to find a raw material for preparing certified reference materials. Measurement results are summarized in Table 4. Patulin was detected above the LOQ of the analytical method in several apple products. Although patulin levels in all those products except one were below the regulatory level (50 μg/kg) set by several governmental bodies or international organizations, the frequent occurrence of patulin in apple products emphasizes the importance of screening those commercial products with reliable analytical methods. In this context, the ID-LC/MS/MS method we developed as a higher-order reference method can be used to produce reliable CRMs and can therefore play an important role in ensuring the reliability of analytical results obtained by testing laboratories.
Conclusion
Isotope dilution-liquid chromatography/tandem mass spectrometry (ID-LC-MS/MS) has been established and optimized as a higher-order reference method for the accurate determination of patulin in apple products. Sample preparation processes were optimized by adopting liquid–liquid extraction with ethyl acetate and further SPE cleanup with an HLB SPE cartridge. For proper retention and separation of highly polar and water soluble patulin, a multimode column was used instead of C18 reverse phase columns, which have been used in most of methods published in the literature. The multimode column provided more flexibility in adjusting the mobile phase composition to control the retention time of patulin to separate it from interfering peaks originating from sample matrices. The method was validated with gravimetrically fortified apple concentrate, juice, puree, and jam. The relative expanded uncertainty of the method was evaluated to be around 1 % for samples with patulin in the 3–40 μg/kg range. Furthermore, the repeatability and the reproducibility of the method were confirmed within the measurement uncertainty. Those performance parameters showed that the ID-LC-MS/MS method has a higher-order metrological quality suitable as a reference method to be used in national metrology institutes. The candidate reference method will be used in our laboratory for the value assignment of patulin in certified reference materials of apple products.
References
Draughon FA, Ayres JC (1980) Insecticide inhibition of growth and patulin production in Penicillium expansum, Penicillium urticae, Aspergillus clavatus, Aspergillus terreus, and Byssochlamys nivea. J Agric Food Chem 28:1115–1117
Moake MM, Padilla-Zakour OI, Worobo RW (2005) Comprehensive review of patulin control methods in foods. Compr Rev Food Sci Food Saf 4:8–21
Puel O, Galtier P, Oswald IP (2010) Biosynthesis and toxicological effects of patulin. Toxins 2:613–631
International Agency for Research on Cancer (1986) IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans. IARC, Lyon, pp 83–98
Leggott NL, Shephard GS (2001) Patulin in South African commercial apple products. Food Control 12:73–76
Cho MK, Kim K, Seo E, Kassim N, Mtenga AB, Shim WB, Lee SH, Chung DW (2010) Occurrence of patulin in various fruit juices from South Korea: an exposure assessment. Food Sci Biotechnol 19:1–5
Tournas VH, Memon SU (2009) Internal contamination and spoilage of harvested apples by patulin-producing and other toxigenic fungi. Int J Food Microbiol 133:206–209
European Commission (2003) Commission regulation (EC) no. 1425/2003 of 11 August 2003 amending regulation (EC) no. 466/2001 as regards patulin. Off J L203:1–3
Ough CS, Corison CA (1980) Measurement of patulin in grapes and wines. J Food Sci 45:476–478
Shephard GS, Leggott NL (2000) Chromatographic determination of the mycotoxin patulin in fruit and fruit juices. J Chromatogr A 882:17–22
Li JK, Wu RN, Hu QH, Wang JH (2007) Solid-phase extraction and HPLC determination of patulin in apple juice concentrate. Food Control 18:530–534
Suzuki T, Fujimoto Y, Hoshino Y, Tanaka A (1974) Simultaneous determination of patulin and penicillic acid in grains by gas chromatographic method. Agric Biol Chem 38:1259–1260
Tarter EJ, Scott PM (1991) Determination of patulin by capillary gas chromatography of the heptafluorobutyrate derivative. J Chromatogr A 538:441–446
Moukas A, Panagiotopoulou V, Markaki P (2008) Determination of patulin in fruit juices using HPLC-DAD and GC-MSD techniques. Food Chem 109:860–867
Sewram V, Nair JJ, Nieuwoudt TW, Leggott NL, Shephard GS (2000) Determination of patulin in apple juice by high-performance liquid chromatography–atmospheric pressure chemical ionization mass spectrometry. J Chromatogr A 897:365–374
Takino M, Daishima S, Nakahara T (2003) Liquid chromatography/mass spectrometric determination of patulin in apple juice using atmospheric pressure photoionization. Rapid Commun Mass Spectrom 17:1695–1972
Tamura M, Takahashi A, Uyama A, Mochizuki N (2012) A method for multiple mycotoxin analysis in wines by solid phase extraction and multifunctional cartridge purification, and ultra-high-performance liquid chromatography coupled to tandem mass spectrometry. Toxins 4:476–486
Cunha SC, Faria MA, Fernandes JO (2009) Determination of patulin in apple and quince products by GC–MS using 13C5–7 patulin as internal standard. Food Chem 115:352–359
Price KR (1979) A comparison of two quantitative mass spectrometric methods for the analysis of patulin in apple juice. Biomed Mass Spectrom 6:573–574
Rychlik M, Schieberle P (1999) Quantification of the mycotoxin patulin by a stable isotope dilution assay. J Agric Food Chem 47:3749–3755
Kataoka H, Itano M, Ishizaki A, Saito K (2009) Determination of patulin in fruit juice and dried fruit samples by in-tube solid-phase microextraction coupled with liquid chromatography–mass spectrometry. J Chromatogr A 1216:3746–3750
Desmarchelier A, Mujahid C, Racault L, Perring L, Lancova K (2011) Analysis of patulin in pear-and apple-based foodstuffs by liquid chromatography electrospray ionization tandem mass spectrometry. J Agric Food Chem 59:7659–7665
Rychlik M, Asam S (2008) Stable isotope dilution assays in mycotoxin analysis. Anal Bioanal Chem 390:617–628
Ahn S, Kim B, Hwang E (2011) Stability monitoring of pesticide residues in a chinese cabbage certified reference material. Bull Kor Chem Soc 32:1365–1367
Food and Agriculture Organization of the United Nations (1997) Validation of analytical methods for food control, FAO Food and Nutrition Paper 68; ftp://ftp.fao.org/docrep/fao/007/w8420e/w8420e00.pdf
Lee S, Kim B, Kim J (2013) Development of isotope dilution-liquid chromatography tandem mass spectrometry for the accurate determination of fluoroquinolones in animal meat products: optimization of chromatographic separation for eliminating matrix effects on isotope ratio measurements. J Chromatogr A 1277:35–41
De Leenheer AP, Thienpont LM (1992) Applications of isotope dilution-mass spectrometry in clinical chemistry, pharmacokinetics, and toxicology. Mass Spectrom Rev 11:249–307
De Bievre P (1993) SAC 92. Isotope dilution mass spectrometry as a primary method of analysis. Anal Proc 30:328–333
Gökmen V, Acar J (1999) Simultaneous determination of 5-hydroxymethylfurfural and patulin in apple juice by reversed-phase liquid chromatography. J Chromatogr A 847:69–74
Gökmen V, Acar J (1996) Rapid reversed-phase liquid chromatographic determination of patulin in apple juice. J Chromatogr A 730:53–58
Boonzaaijer G, Bobeldijk I, Van Osenbruggen WA (2005) Analysis of patulin in Dutch food, an evaluation of a SPE based method. Food Control 16:587–591
Stahnke H, Reemtsm T, Alder L (2009) Compensation of matrix effects by postcolumn infusion of a monitor substance in multiresidue analysis with LC- MS/MS. Anal Chem 81:2185–2192
Zhou Y, Kong W, Li Y, Logrieco AF, Xu J, Yang M (2012) A new solid-phase extraction and HPLC method for determination of patulin in apple products and hawthorn juice in China. J Sep Sci 35:641–649
Rychlik M, Schieberle P (1998) Synthesis of 13C-labeled patulin [4-hydroxy-4H-furo [3, 2-c] pyran-2(6H)-one] to be used as internal standard in a stable isotope dilution assay. J Agric Food Chem 46:5163–5169
Valle-Algarra FM, Mateo EM, Gimeno-Adelantado JV, Mateo-Castro R, Jiménez M (2009) Optimization of clean-up procedure for patulin determination in apple juice and apple purees by liquid chromatography. Talanta 80:636–642
Rovira R, Ribera F, Sanchis V, Canela R (1993) Improvements in the quantitation of patulin in apple juice by high-performance liquid chromatography. J Agric Food Chem 41:214–216
Dombrink-Kurtzman MA, Blackburn JA (2005) Evaluation of several culture media for production of patulin by Penicillium species. Int J Food Microbiol 98:241–248
Choi J, Hwang E, So HY, Kim B (2003) An uncertainty evaluation for multiple measurements by GUM. Accred Qual Assur 8:13–15
Choi J, Kim DH, Hwang E, So HY (2003) An uncertainty evaluation for multiple measurements by GUM, II. Accred Qual Assur 8:205–207
Kim B, Hwang E, So HY, Son EK, Kim Y (2010) Development of a model system of uncertainty evaluations for multiple measurements by isotope dilution mass spectrometry: determination of folic acid in infant formula. Bull Kor Chem Soc 31:3139–3144
Thompson M, Ellison SL, Wood R (2006) The international harmonized protocol for the proficiency testing of analytical chemistry laboratories (IUPAC Technical Report). Pure Appl Chem 78:145–196
Acknowledgments
This work was supported by the Korea Research Institute of Standards and Science under the project “Establishment of standard system for organic analysis,” grant 15011030.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
(PDF 25.1 kb)
Rights and permissions
About this article
Cite this article
Seo, M., Kim, B. & Baek, SY. An optimized method for the accurate determination of patulin in apple products by isotope dilution-liquid chromatography/mass spectrometry. Anal Bioanal Chem 407, 5433–5442 (2015). https://doi.org/10.1007/s00216-015-8705-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8705-3