Abstract
Five novel ionic liquids (ILs), 1,3-dibutylimidazolium bromide [BBMIm][Br], 1-pentyl-3-butylimidazolium bromide [BPMIm][Br], 1-hexyl-3-butylimidazolium bromide [BHMIm][Br], 1,1'-(butane-1,4-diyl)bis(3-butylimidazolium) bromide [C4(BMIm)2][Br2], and 1,1'-(butane-1,4-diyl)bis(3-methylimidazolium) bromide [C4(MIm)2][Br2], were prepared and used in situ to react with bis(trifluoromethane)sulfonamide lithium salt to extract the myclobutanil, tebuconazole, cyproconazole, and prothioconazole from water samples. The results showed that mono-cationic ILs had much better recovery than dicationic ILs, and mono-imidazolium IL bearing butyl groups at N-1 and N-3 sites had the best recovery. When the length of the alkyl substituent group was more than four carbons at N-3 site, the recovery decreased with increase of alkyl chain length of 1-butylimidazolium IL. The extraction efficiency order of triazoles from high to low was [BBMIm][Br], [BPMIm][Br], [BHMIm][Br], [BMIm][Br] (1-butyl-3-methylimidazolium bromide), [C4(BMIm)2]Br2, [C4(MIm)2]Br2. An in situ ionic liquid dispersive liquid–liquid microextraction combined with ultrasmall superparamagnetic Fe3O4 was established as a pretreatment method for enrichment of triazole fungicides in water samples by using the synthetic [BBMIm][Br] as the cationic IL and used to detect analytes followed by high-performance liquid chromatography. Under the optimized conditions, the proposed method showed a good linearity within a range of 5–250 μg L−1, with the determination coefficient (r2) varying from 0.998 to 0.999. High mean enrichment factors were achieved ranging from 187 to 323, and the recoveries of the target analytes from real water samples at spiking levels of 10.0, 20.0, and 50.0 μg L−1 were between 70.1% and 115.0%. The limits of detection for the analytes were 0.74–1.44 μg L−1, and the intra-day relative standard deviations varied from 5.23% to 8.65%. The proposed method can be further applied to analyze and monitor pesticides in other related samples.

The scheme of the in-situ DLLME method for the determination of triazoles using the imidazolium-based ionic liquids
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Triazole fungicides are the most commonly used fungicides in the world because of their high efficiency and broad spectrum activity [1]. In the UK, triazoles account for up to 20% of fungicides used, among which epoxiconazole, prothioconazole, and tebuconazole are the three most common [2]. Besides, triazole fungicides have high chemical and photochemical stability, low biodegradability, and are easily transported in the environment [3]. All these properties can contribute to triazole fungicides persisting in the soil and water for a long time [4]. Thus triazoles will further contaminate drinking water, agricultural products, and by-products [5,6,7]; eventually, they will impact human health. After double screening by the Environmental Protection Agency Endocrine Disruptor Screening Program, epoxiconazole, myclobutanil, and tebuconazole were all suspected as endocrine disruptors [8, 9]. According to another study, tebuconazole increased nipple retention in male offspring, and also increased the gestation period in pregnant rat dams [10]. Therefore, most countries and regions have published relevant standards and regulations for triazole residues. For example, in the European Union, maximum residue limits (MRLs) of triazole pesticides in food and fodder are regulated. The MRLs of cyproconazole, prothioconazole, and tebuconazole are 0.05, 0.01, and 0.02 mg kg−1, respectively [11]. The development of effective methods and instrumentation for detection of trace triazoles in the environment is critical for the achievement of environmentally viable and safe technologies.
Many instruments can be employed to detect triazoles such as gas chromatography–nitrogen phosphorus detector (GC-NPD) [12], gas chromatography–flame ionization detection (GC-FID) [13], and high-performance liquid chromatography with UV–vis detector (HPLC-UVD) [14]. To achieve an accurate determination result, mass spectrometry was also used by coupling with HPLC or GC [15]. During the processes of monitoring trace pesticide residues in environmental water, the pretreatment method is the most important procedure. In recent years, the determination of pesticides in water samples by different pretreatment methods has been reported [16,17,18]. The traditional techniques, such as liquid–liquid extraction and solid-phase extraction, are tedious, time-consuming, and use large quantities of solvent. Therefore these methods are gradually being replaced by new ones. Within these approaches, there is an important shift towards the development of microextraction procedures, such as solid-phase microextraction (SPME) and liquid-phase microextraction (LPME). Both of these aim to eliminate or minimize the consumption of organic solvents [7]. In order to save time and cost, dispersive liquid–liquid microextraction (DLLME) was employed [19]. In this method, the appropriate mixture of extraction solvent and disperser solvent is injected into the aqueous sample, and it generates a cloudy solution immediately. After centrifugation, the particles of extraction solvent are precipitated in the bottom of the conical tube [20]. DLLME provides high recovery and enrichment factor within a very short time (a few seconds). DLLME involves fine particles of extraction solvent which are entirely dispersed into the aqueous phase.
Ionic liquids (ILs) are very simply molten salts which are made of cations and anions. The structure of ILs can be designed and the physicochemical properties are therefore modifiable. ILs have unique solvent properties, i.e., they are oleophilic and hydrophilic, so they are able to substitute for traditional organic solvents. As an accepted “green solvent”, ILs have no detectable vapor pressure, ignorable environmental toxicity, and can also be recycled [21, 22]. Besides, the majority of ILs have good surface activity; consequently, they can also replace organic solvent in DLLME. DLLME coupled with ILs is a very popular pretreatment method in trace contaminant detection, such as triazole, silver(I), carbamate, pyrethroid, benzoylurea, and so on [6, 23,24,25,26].
Centrifugation is a common process to separate in situ hydrophobic IL from water samples; however, the emulsion formed is difficult to isolate absolutely. To overcome this problem, magnetic particles are used for phase separation to take the place of centrifugation. In our previous work, Fe3O4 ultrasmall magnetic nanoparticles were used to detect pyrethroid pesticides in water samples [26]. The results showed that Fe3O4 can quickly retrieve ILs by physisorption and electrostatic interaction. Fe3O4 ultrasmall magnetic nanoparticles have large surface area to volume ratio to adsorb in situ hydrophobic IL; owing to their superparamagnetic property, they can be easily isolated from sample solutions by application of an external magnetic field [27]. Some studies involving DLLME have applied Fe3O4 ultrasmall magnetic particles and proved the unexceptionable separation ability of Fe3O4 [28].
In this work, five novel imidazolium-based ILs were prepared and used to react in situ with bis(trifluoromethane)sulfonamide lithium salt (LiNTf2), which was combined with ultrasmall superparamagnetic Fe3O4 to extract the triazole fungicides from water samples. In order to find the relationship between the chemical structure of the imidazolium ILs and the extraction recovery of the triazoles, the influence of factors on extraction efficiency, such as the amount of IL, the ratio of IL/LiNTf2, the kind of diluent, the pH of water samples, and the extraction time, were investigated to get the optimized pretreatment method.
Experimental
Apparatus
The HPLC system used for the analysis and separation consisted of two LC-20ATvp pumps and an SPD-M20Avp, UV–vis photodiode array detector (Shimadzu, Japan). A reversed-phase Kromasil ODS C18 column (250 mm × 4.6 mm, 5 μm, Sigma–Aldrich, St. Louis, MO, USA) for LC system and LC solution Lite workstation were employed to obtain and process chromatographic data. A Vortex-Genie Mixer (Scientific Industries, USA) was used for the elution of target compounds. An FA1004 electronic balance was purchased from Beijing Electronic Balance Factory.
Chemical and reagents
Myclobutanil, tebuconazole (≥ 98%), LiNTf2, 1-butylimidazole, 1-methylimidazole, Fe3O4 (20 nm), and [BMIm][Br] were purchased from Aladdin Chemical Reagent Corporation (Shanghai, China). Cyproconazole and prothioconazole with purity ≥ 98% were purchased from Sigma–Aldrich (St. Louis, MO, USA). 1,4-Dibromobutane, 1-bromobutane, 1-bromopentane, and 1-bromohexane were obtained from Tianjin Heowns Biochemical Technology Co. Ltd. (Tianjin, China). Other chemicals and reagents were obtained from Sinopharm Chemical Reagent Beijing Co., Ltd. (Beijing, China). HPLC-grade methanol and acetonitrile were from Fisher Scientific Company (UK). Ultrapure water was generated using a Milli-Q water purification system (Millipore, Co. USA).
Synthesis of ILs
Mono-cationic ILs
Acetonitrile (50 mL) as solvent was added into a 100-mL flask, and then mixed 1-butylimidazole (26 mmol) and 20 mmol of the corresponding 1-bromoalkane. The mixture was heated at reflux for 24 h. After the reaction was finished, the mixture was cooled to room temperature. The acetonitrile was evaporated off under reduced pressure and the residue was dissolved in pure water. The water layer was washed with diethyl ether and concentrated. The residue was dried under vacuum at 35 °C and a colorless liquid was obtained (Fig. 1, Table 1). The data from the NMR spectra are shown in the Electronic Supplementary Material (ESM, Figs. S1, S2, and S3).
Dicationic ILs
[C4(BMIm)2][Br2]: The synthesis of [C4(BMIm)2][Br2] was similar to that of mono-cationic ILs except that a different ratio of 1-butylimidazole (50 mmol) and 1,4-dibromoalkane (20.5 mmol) was used.
[C4(MIm)2][Br2]: A mixture containing 1,4-dibromoethane (20.5 mmol), 1-methylimidazole (50 mmol), and acetonitrile (50 mL) was added into a 100-mL flask and heated at reflux for 24 h. After the reaction was finished, the mixture was cooled to room temperature. The precipitate was filtered and washed with acetonitrile. The residue was dried under vacuum at 35 °C and a white solid was obtained. (Fig. 1, Table 1). The data from the NMR spectra are shown in the ESM (Figs. S4 and S5).
Sample preparation
Stock standard solutions of the four triazole pesticides were prepared in acetonitrile at a concentration of 1000 mg L−1. A certain amount was removed and diluted to different concentrations with acetonitrile to generated spiked samples. The working solution (each analyte at 100 μg L−1) was diluted with pure water and used to optimize the method. The working solutions of cationic ILs and LiNTf2 had the same concentration (0.20 M). Pond, river, and lake water samples were obtained from a local village, Nanchang He branch, and Cuihu national urban wetland park (Haidian District, Beijing), respectively. All water samples were stored at −18 °C in a freezer and filtered through 0.45-μm membranes before use.
Pretreatment procedures
In this study, five kinds of newly synthesized ILs and a commercial IL were tested in the in situ IL-DLLME method. First, 500 μL of IL solution was added into a 15-mL conical centrifuge tube containing 10 mL of sample solution and then gently shaken to disperse the IL uniformly. Next, 500 μL of LiNTf2 aqueous solution was added and a turbid solution formed immediately. Then the tube was vortexed for 30 s to strengthen the enrichment effect. Thereafter, 30 mg Fe3O4 was added into the tube. The tube was shaken thoroughly and a magnet was positioned to adsorb hydrophobic IL, which contained the target analytes, at the bottom of the tube. After the supernatant solution was decanted, the adsorbed analytes were eluted with 40 μL of acetonitrile under intense vortex for 30 s. Finally, 20 μL of analyte solution was injected into the HPLC instrument for analysis. All the experiments were performed in at least triplicate and the means of the results were used for optimization.
Analysis of triazole fungicides
The analysis of four kinds of triazoles was accomplished by HPLC. The mobile phase was a gradient of methanol/water (0.2% acetic acid) in methanol: 0–10 min, 60–90%; 10–15 min, 90%. The flow rate was 1.0 mL min−1 and the injection volume was 20 μL. The target compounds were monitored at 230 nm by a photodiode array detector.
Method validation
Because an exact quantitative analysis was needed in this study, the external standard calibration method was used. The four involved triazole fungicides in this study were used as the external standard substances.
In accordance with previous studies [25, 26], limits of detection (LODs) were calculated on the basis of the concentration of the target compounds, whose peak area was three times the area of the noise of the blank (S/N ≥ 3) after the optimized produce.
Enrichment factor, recovery, and LODs were calculated using the formulas below:
C1 is the concentration of the target component in the elution phase; C0 is the original concentration of the target component in the water sample; V1 is the volume of the elution phase (40 μL); V0 is the volume of the water sample (10 mL). Sb is the standard deviation of the blank signal and m is the slope of the calibration curve after extraction.
The intra-day precisions were obtained by six replicates within a day following the optimized produce, and the inter-day precisions were prepared by two replicates in each of 3 days at the same optimized conditions. The standard deviation error bars of all data were calculated by SPSS statistics software (version 20.0).
Results and discussion
Factors affecting extraction efficiency
Effect of IL
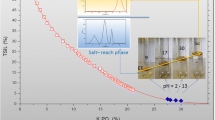
ILs were used in this study to examine their selectivity towards triazole fungicides. The IL should be soluble in water; after addition of the ion-exchanger, the formed IL should have low solubility in water so that the whole extraction system turns into an emulsion instantly [29]. The hydrophobic ILs [BBMIm][N(Tf)2], [BPMIm][N(Tf)2], [BHMIm][N(Tf)2], [BMIm][N(Tf)2], [C4(BMIm)2][N(Tf)2]2, and [C4(MIm)2][N(Tf)2]2 were synthesized through metathesis reaction between bis(trifluoromethane)sulfonamide ion (N(Tf)2−) and bromine ion (Br−). During the optimization step, [C4(MIm)2][N(Tf)2]2 did not show any enrichment capacity for triazoles at all. As shown in Fig. 2a, the extraction efficiency of triazoles was the highest when using [BBMIm][N(Tf)2], followed by [BPMIm][N(Tf)2], [BHMIm][N(Tf)2], [BMIm][N(Tf)2], and [C4(BMIm)2][N(Tf)2]2. [BMIm][Br] is a commonly used extraction agent in the published articles which involved IL-DLLME [30,31,32]. In this work, however, it showed low extraction efficiency for triazole fungicides because [BMIm][N(Tf)2] could not form a stable microemulsion system, which was easily able to revert to a pellucid solution at room temperature. The two tested dicationic ILs also could not form a stable microemulsion system; only [C4(BMIm)2][N(Tf)2]2 showed a low recovery. When equimolar IL was added, [C4(MIm)2][N(Tf)2]2 formed the largest amount of hydrophobic IL, and made the whole system more turbid than the other ILs did; after a vigorous oscillation, hydrophobic IL was easily separated from the water. A possible reason is that the stronger hydrophobicity of the cationic molecule favors the aggregation of the ILs. Therefore, more monomers are included in the IL aggregates, which could reduce the enrichment efficiency because of partial extraction of the analyte [26]. On account of the good concentration effect, [BBMIm][Br] was used to extract triazole fungicides in water samples in the next processes. According to the results, some relationships between the chemical structure of the imidazolium ILs and the extraction recovery of the triazoles can be deduced: mono-cationic ILs had much better recovery than dicationic ILs, and mono-imidazolium IL bearing butyl groups at N-1 and N-3 sites had the best recovery. When the length of the alkyl substituent group was more than four carbons at the N-3 site, the recovery decreased with increase of alkyl chain length of 1-butylimidazolium IL.
Factors affecting extraction efficiency. All working solutions were 100 μg L−1 of each triazole fungicide, and each spiked water sample was 10 mL. MNPs (magnetic nanoparticles) 30 mg. a Effect of different ILs. Conditions: each kind of IL 500 μg L−1, aq. solution (0.20 M), LiNTf2 aq. solution 500 μg L−1 (0.20 M), diluent with acetonitrile 40 μL, pH not adjusted, extraction time 1 min. b Effect of diluent. Conditions: [BB(MIm)][Br] aq. solution (0.20 M), LiNTf2 aq. solution 500 μg L−1 (0.20 M), each diluent added 40 μL, pH not adjusted, extraction time 1 min. c Effect of amount of [BBMIm][Br]. Conditions: equimolar ratio of [BBMIm][Br]/LiNTf2, elution with 40 μL acetonitrile. d Effect of the ratio of hydrophilic IL/anion-exchange reagent. Conditions: [BBMIm][Br] aq. solution (0.20 M), various volumes of LiNTf2 aq. solution (0.20 M), elution with 40 μL acetonitrile. e Effect of pH. [BBMIm][Br] aq. solution (0.20 M), LiNTf2 aq. solution 500 μg L−1 (0.20 M), acetonitrile 40 μL, pH previously adjusted with HCl and NaOH, extraction time 1 min. f Effect of extraction time. [BBMIm][Br] aq. solution (0.20 M), LiNTf2 aq. solution 500 μg L−1 (0.20 M), acetonitrile 40 μL, pH not adjusted. The bars in the figure represent standard deviation (±SD)
Effect of diluent
In DLLME, the diluent must be rather miscible with both hydrophilic and hydrophobic ILs. It is therefore expected that the presence of a diluent promotes desorption of hydrophobic ILs which contained analytes out from the Fe3O4. For this purpose, methanol, isopropanol, dimethyl sulfoxide (DMSO), and acetonitrile were tested as diluents. The volume of each diluent added was 40 μL and the results are shown in Fig. 2b. When acetonitrile was used as the diluent, all four triazoles had the best recoveries.
Effects of amount of IL and molar ratio of IL/LiNTf2
The effect of the amount of [BBMIm][Br] on recovery was studied in the solution (0.20 M) with volume range of 200–700 μL when keeping the molar ratio of [BBMIm][Br] to LiNTf2 at 1:1. As can be seen from Fig. 2c, the optimal volume of [BBMIm][Br] was 500 μL corresponding to adding 0.0001 mol of [BBMIm][Br]. Higher amounts of [BBMIm][Br] caused a decrease of extraction efficiency, which could be due to the limited sample volume.
Different molar ratios ranging from 0.5:1 to 2:1 were tested when the volume of [BBMIm][Br] aqueous solution (0.20 M) was set to 500 μL (Fig. 2d). In the range of 0.5:1 to 1:1, the extraction efficiency of triazoles was increased. Higher ratios of [BBMIm][Br] to LiNTf2 showed a declining trend. Therefore, 0.0001 mol of [BBMIm][Br] and 500 μL of LiNTf2 aqueous solution (0.20 M) were used in next experiments.
Effects of pH and extraction time
The pKa values of triazole fungicides are 1.21–5.39 [33]. Hence, the pH of water samples can affect the solubility and hydrolytic degradation of pesticides. The pH also can influence the solubility of in situ hydrophobic ILs and the stability of the formed microemulsion system. In natural conditions, the pH value of water is 6.5–9.0; the maximum change permitted as a result of a waste discharge must not exceed 0.5 pH units [34]. Effects of pH from 3 to 8 on the recoveries of triazoles were investigated as shown in Fig. 2e. Higher recoveries of triazoles can be achieved at pH 4. Therefore, samples solutions were adjusted to pH 4 before enrichment by adding HCl solution.
LLE involves exploiting the difference of solubility of the analytes in aqueous phase and in immiscible organic phase, and it needs time to achieve the extraction balance [35]. DLLME is carried out between the sample and a formed cloud of fine extractant drops when the mixture of extraction and disperser solvents is injected into the aqueous sample [36]. The contact surface between phases is widely increased and leads to reduction of extraction time and improvement of enrichment factor. Because the in situ hydrophobic ILs have abilities both as a disperser and extractor, in situ IL-DLLME saves more time than the original DLLME method does. In this research, extraction time was from the point of adding the LiNTf2 to the point of using the magnet to adsorb the IL. Figure 2f confirms the expected result that in situ IL-DLLME does not need much time, and 30 s can afford a good recovery.
Method validation
Under the optimized conditions, the proposed in situ IL-DLLME was validated for LODs, linear range, determination coefficients (r2), and enrichment factor, and the results are shown in Table 2. Cyproconazole, myclobutanil, tebuconazole, and prothioconazole exhibited good linearity with r2 = 0.999 in the range of 5–250 μg L−1. The LODs ranged from 0.74 to 1.44 μg L−1 and enrichment factors were in the range of 187–323. The intra-day relative standard deviations (RSDs) ranged from 5.23% to 8.65% (n = 6). The inter-day RSDs ranged from 5.81% to 8.94% (n = 6).
Table S1 (see ESM) shows the comparison of the proposed in situ IL-DLLME methodology with several reported methods for extraction of triazoles in water samples followed by HPLC [6, 7, 14, 16, 37]. It can be observed that the entire process time is rather short (less than 3 min). There may be two reasons or this: one is the excellent stability of the microemulsion system formed by [BBMIm][N(Tf)2]2, and the another is the utilization of ultrasmall superparamagnetic Fe3O4, which requires less time for separation of phases. The developed in situ IL-DLLME method presented similar LODs to other studies when used in combination with HPLC-UV. Moreover, the enrichment factors, between 187 and 323, are relatively high.
Application to real water samples
The validated method was finally applied to the analysis of four real water samples. The analytes detected were quantified by the standard addition method. All four triazoles were not detected in this method. Figure 3 shows the typical chromatograms of pond water with in situ IL-DLLME optimized in this study. As is presented in Table 3, the recoveries were more than 70% for all target analytes, with RSDs ranging from 0.47% to 11.32% in tap, pond, river, and lake water samples.
HPLC analysis of triazoles in water sample. a Standards, b pond water sample without pretreatment, c pond water sample after pretreatment, d spiked pond water sample (10 μg L−1) after pretreatment; 1/1' cyproconazole, 2/2' myclobutanil, 3/3' tebuconazole, 4/4' prothioconazole. Conditions: 10 mL sample, 500 μL [BBMIm][Br] solution (0.20 M), 500 μL LiNTf2 solution (0.20 M), 30 mg MNPs, elution with 40 μL acetonitrile. HPLC conditions: gradient of methanol/water (0.2% acetic acid) in methanol: 0–10 min, 60–90%; 10–15 min, 90%; flow rate 1.0 mL min−1, injection volume 20 μL, photodiode array detector 230 nm
Conclusions
In this work, five novel synthetic imidazolium-based ILs and a commercial IL ([BMIM][Br]) were used to enrich triazoles in water. Thus, [BBMIm][Br] (500 μL, 0.20 M) reacted in situ with LiNTf2 (500 μL, 0.20 M) forming hydrophobic IL as the extraction agent, and 30 mg of Fe3O4 was added to water samples whose pH had previously been adjusted to 4 with HCl; finally 40 μL acetonitrile was injected as the diluent. Under these optimized conditions, high recoveries in pure water (87.4–101.5%) and real water (70.1–115%), short pretreatment time (<3 min), and low extraction solvent consumption were achieved. This method is promising and can be further applied to analyze and monitor many kinds of pesticides in other related samples.
References
Kahle M, Buerge IJ, Hauser A, Muller MD, Poiger T. Azole fungicides: occurrence and fate in wastewater and surface waters. Environ Sci Technol. 2008;42(19):7193–200.
Price CL, Parker JE, Warrilow AG, Kelly DE, Kelly SL. Azole fungicides—understanding resistance mechanisms in agricultural fungal pathogens. Pest Manag Sci. 2015;71(8):1054–8.
Farajzadeh MA, Khoshmaram L. Air-assisted liquid-liquid microextraction-gas chromatography-flame ionisation detection: a fast and simple method for the assessment of triazole pesticides residues in surface water, cucumber, tomato and grape juices samples. Food Chem. 2013;141(3):1881–7.
Cui T, Zhang Y, Han W, Li J, Sun X, Shen J, et al. Advanced treatment of triazole fungicides discharged water in pilot scale by integrated system: Enhanced electrochemical oxidation, upflow biological aerated filter and electrodialysis. Chem Eng J. 2017;315:335–44.
Hu Y, Li J, Li G. Synthesis and application of a novel molecularly imprinted polymer-coated stir bar for microextraction of triazole fungicides in soil. J Sep Sci. 2011;34(10):1190–7.
Li Y, Zhang J, Peng B, Li S, Gao H, Zhou W. Determination of triazole pesticides in rat blood by the combination of ultrasound-enhanced temperature-controlled ionic liquid dispersive liquid–liquid microextraction coupled to high-performance liquid chromatography. Anal Methods. 2013;5(9):2241.
Wang H, Yang X, Hu L, Gao H, Lu R, Zhang S, et al. Detection of triazole pesticides in environmental water and juice samples using dispersive liquid-liquid microextraction with solidified sedimentary ionic liquids. New J Chem. 2016;40(5):4696–704.
Taxvig C, Hass U, Axelstad M, Dalgaard M, Boberg J, Andeasen HR, et al. Endocrine-disrupting activities in vivo of the fungicides tebuconazole and epoxiconazole. Toxicol Sci. 2007;100(2):464–73.
Paul Friedman K, Papineni S, Marty MS, Yi KD, Goetz AK, Rasoulpour RJ, et al. A predictive data-driven framework for endocrine prioritization: a triazole fungicide case study. Crit Rev Toxicol. 2016;46(9):785–833.
Jensen BH, Petersen A, Christiansen S, Boberg J, Axelstad M, Herrmann SS, et al. Probabilistic assessment of the cumulative dietary exposure of the population of Denmark to endocrine disrupting pesticides. Food Chem Toxicol. 2013;55:113–20.
EU. EU pesticide database. http://ec.europa.eu/food/plant/pesticides/max_residu_levels_en. Accessed 2016.
Güdücü HE, İnam R, Aboul-Enein HY. Determination of organophosphorus and triazole pesticides by gas chromatography and application to vegetable and commercial samples. J Liq Chromatogr Relat Technol. 2011;34(19):2473–83.
Farajzadeh MA, Mogaddam MR, Aghdam AA. Comparison of air-agitated liquid-liquid microextraction technique and conventional dispersive liquid-liquid micro-extraction for determination of triazole pesticides in aqueous samples by gas chromatography with flame ionization detection. J Chromatogr A. 2013;1300:70–8.
Wang C, Wu Q, Wu C, Wang Z. Application of dispersion-solidification liquid-liquid microextraction for the determination of triazole fungicides in environmental water samples by high-performance liquid chromatography. J Hazard Mater. 2011;185(1):71–6.
Wei Q, Song Z, Nie J, Xia H, Chen F, Li Z, et al. Tablet-effervescence-assisted dissolved carbon flotation for the extraction of four triazole fungicides in water by gas chromatography with mass spectrometry. J Sep Sci. 2016;39(23):4603–9.
Tang T, Qian K, Shi T, Wang F, Li J, Cao Y. Determination of triazole fungicides in environmental water samples by high performance liquid chromatography with cloud point extraction using polyethylene glycol 600 monooleate. Anal Chim Acta. 2010;680(1-2):26–31.
Farajzadeh MA, Sorouraddin SM, Mogaddam MRA. Liquid phase microextraction of pesticides: a review on current methods. Microchim Acta. 2014;181(9-10):829–51.
Yang G, He Z, Liu X, Liu C, Zhan J, Liu D, et al. Polymer-coated magnetic nanospheres for preconcentration of organochlorine and pyrethroid pesticides prior to their determination by gas chromatography with electron capture detection. Microchim Acta. 2016;183(3):1187–94.
Yan H, Cheng X, Yan K. Rapid screening of five phthalate esters from beverages by ultrasound-assisted surfactant-enhanced emulsification microextraction coupled with gas chromatography. Analyst. 2012;137(20):4860–6.
Rezaee M, Assadi Y, Milani Hosseini MR, Aghaee E, Ahmadi F, Berijani S. Determination of organic compounds in water using dispersive liquid-liquid microextraction. J Chromatogr A. 2006;1116(1-2):1–9.
Rogers RD, Seddon KR. Chemistry. Ionic liquids–solvents of the future? Science. 2003;302(5646):792–3.
Jungnickel C, Łuczak J, Ranke J, Fernández JF, Müller A, Thöming J. Micelle formation of imidazolium ionic liquids in aqueous solution. Colloids Surf A Physicochem Eng Asp. 2008;316(1-3):278–84.
Reyna-Gonzalez JM, Torriero AA, Siriwardana AI, Burgar IM, Bond AM. Extraction of silver(I) from aqueous solutions in the absence and presence of copper(II) with a methimazole-based ionic liquid. Analyst. 2011;136(16):3314–22.
Zhou Q, Pang L, Xiao J. Ultratrace determination of carbamate pesticides in water samples by temperature controlled ionic liquid dispersive liquid phase microextraction combined with high performance liquid phase chromatography. Microchim Acta. 2011;173(3-4):477–83.
Wang H, Hu L, Li W, Yang X, Lu R, Zhang S, et al. In-syringe dispersive liquid-liquid microextraction based on the solidification of ionic liquids for the determination of benzoylurea insecticides in water and tea beverage samples. Talanta. 2017;162:625–33.
Fan C, Liang Y, Dong H, Ding G, Zhang W, Tang G, et al. In-situ ionic liquid dispersive liquid-liquid microextraction using a new anion-exchange reagent combined Fe3O4 magnetic nanoparticles for determination of pyrethroid pesticides in water samples. Anal Chim Acta. 2017;975:20–9.
Shokri M, Beiraghi A, Seidi S. situ emulsification microextraction using a dicationic ionic liquid followed by magnetic assisted physisorption for determination of lead prior to micro-sampling flame atomic absorption spectrometry. Anal Chim Acta. 2015;889:123–9.
Lasarte-Aragones G, Lucena R, Cardenas S, Valcarcel M. Effervescence assisted dispersive liquid-liquid microextraction with extractant removal by magnetic nanoparticles. Anal Chim Acta. 2014;807:61–6.
Fan C, Li N, Cao X. Determination of chlorophenols in honey samples using in-situ ionic liquid-dispersive liquid-liquid microextraction as a pretreatment method followed by high-performance liquid chromatography. Food Chem. 2015;174:446–51.
Lopez-Darias J, Pino V, Ayala JH, Afonso AM. In-situ ionic liquid-dispersive liquid-liquid microextraction method to determine endocrine disrupting phenols in seawaters and industrial effluents. Microchim Acta. 2011;174(3-4):213–22.
Yao C, Li T, Twu P, Pitner WR, Anderson JL. Selective extraction of emerging contaminants from water samples by dispersive liquid-liquid microextraction using functionalized ionic liquids. J Chromatogr A. 2011;1218(12):1556–66.
Zhang C, Cagliero C, Pierson SA, Anderson JL. Rapid and sensitive analysis of polychlorinated biphenyls and acrylamide in food samples using ionic liquid-based in situ dispersive liquid-liquid microextraction coupled to headspace gas chromatography. J Chromatogr A. 2017;1481:1–11.
Konasova R, Dytrtova JJ, Kasicka V. Determination of acid dissociation constants of triazole fungicides by pressure assisted capillary electrophoresis. J Chromatogr A. 2015;1408:243–9.
EPA. Environmental Protection. Environmental quality standard of surface water: EPA; 2017. Chap. 61, p.11.
Turner NW, Subrahmanyam S, Piletsky SA. Analytical methods for determination of mycotoxins: a review. Anal Chim Acta. 2009;632(2):168–80.
Ojeda CB, Rojas FS. Separation and preconcentration by dispersive liquid–liquid microextraction procedure recent applications. Chromatographia. 2011;74(9-10):651–79.
Zhang Q, Tian M, Wang M, Shi H, Wang M. Simultaneous enantioselective determination of triazole fungicide flutriafol in vegetables, fruits, wheat, soil, and water by reversed-phase high-performance liquid chromatography. J Agric Food Chem. 2014;62(13):2809–15.
Acknowledgements
The authors acknowledge financial support of this work by the National Key Research and Development Program of China (2016YFF0203802) and the National Natural Science Foundation of China (31672067).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors of this article declare no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 455 kb)
Rights and permissions
About this article
Cite this article
Yang, J., Fan, C., Kong, D. et al. Synthesis and application of imidazolium-based ionic liquids as extraction solvent for pretreatment of triazole fungicides in water samples. Anal Bioanal Chem 410, 1647–1656 (2018). https://doi.org/10.1007/s00216-017-0820-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0820-x