Abstract
Measuring both progestagens, androgens, corticosteroids as well as estrogens with a single method makes it possible to investigate the effects of endocrine-disrupting chemicals (EDCs) on the main pathways in the mammalian steroidogenesis. This paper presents two simple methods for the determination of the major steroid hormones in biological matrixes using liquid chromatography tandem mass spectrometry (LC-MS2). A novel method was developed for the determination of 14 steroids in the H295R in vitro assay without the need for solid phase extraction (SPE) purification prior to LC-MS2 analysis. The in vitro assay was validated by exposing H295R cells to prochloraz for inhibiting steroid hormone secretion and by exposing cells to forskolin for inducing steroid hormone secretion. The developed method fulfills the recommendations for the H295R assay suggested by the OECD. Furthermore, a simple off-line SPE methodology was developed for the necessary clean-up of in vivo assays. Samples, such as gonad tissue, plasma and serum, are complex biological matrixes, and the SPE methodology was optimized to remove salts and proteins prior to elution of target analytes. At the same time, lipophilic compounds were retained on the SPE cartridge during elution. This, combined with the multi-steroid LC-MS2 method, made it possible to determine 10 steroids in male Sprague-Dawley rat gonad tissue. Furthermore, it was possible to quantify 6 steroids in the plasma. In general, the observed concentration of steroid hormones in plasma, testes, and H295R cell medium corresponded well with previous studies. The off-line SPE method was validated using spiked charcoal-stripped serum. Method recovery, accuracy, precision and robustness were all good. Instrument sensitivity was in the range of 55–530 pg/mL (LLOQ).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
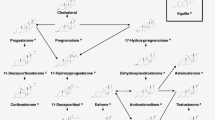
The vertebrate steroidogenic pathway produces steroid hormones essential for the regulation of sex differentiation, reproduction, growth, metabolism and immune functions [1]. A reliable, highly sensitive and time-efficient method for quantifying steroid hormones is essential in clinical settings to diagnose endocrine disorders as well as in research settings to investigate fundamental endocrinology and effects of endocrine-disrupting chemicals (EDC). Endocrine disruptors are increasingly recognized as a health threat. The growing prevalence of hormone-dependent cancers and obesity, declining sperm quality, rising frequency of undescended testis and hypospadias among newborns and the increased need for assisted conception are concerning long-term health effects, which may be coupled to EDC [2–9].
As EDCs may interfere at several points in the steroidogenic pathway [10], a simultaneous determination of a greater number of steroid hormones allows for a better mechanistic understanding as to how chemicals may disturb the pathway [10, 11]. Due to the OECD guideline [12], the in vitro H295R assay is widely used as an effective screening tool for investigating possible EDCs. H295R cells express all the key enzymes in the mammalian steroidogenesis and produce all the major steroids including androgens, corticosteroids, estrogens and progestagens [12]. Thus, multi-steroid hormone (≥8) methods have been developed for determination of steroid concentrations in H295R cell medium. However, these methods [13–16] were not sensitive enough to include analysis of estrogens that are produced in low quantities in this cell line. Other methods include estrogen analysis, but they all rely on either off-line cleanup, derivatisation or multimethods for obtaining the required sensitivity [17–19]. However, reliable estrogen measurements are one of the main criteria in the OECD guideline [12]. Thus, the main purpose of this study was to develop a novel method which simultaneously determines several androgen, corticosteroid, estrogen and progestagen steroid hormones secreted by H295R cells without the need of either derivatization, off-line solid phase extraction (SPE) or separate LC-MS2 methods. The developed method reduces manpower and financial costs and enables high throughput of samples which would make large scale screenings of possible EDCs more feasible.
The H295R assay is, however, only an approximation to a much more complex endocrine system of multicellular organisms [12]. Consequently, when a chemical tests positive for endocrine disrupting activity, in the H295R screening assay, it is often necessary to validate this outcome in in vivo studies, e.g. by analyzing blood and endocrine tissues from experimental animals [20]. Therefore, we also developed, validated and applied an off-line SPE method for simultaneous determination of 19 steroid hormones in samples from serum, plasma and gonads. These 19 steroids constitute the most important steroids in vertebrates, in particular in mammals. We also included 11-ketotestosterone since this steroid is the dominant androgen in many fish species [21, 22].
Experimental
Chemicals and materials
Androstenedione (AN), pregnenolone (PREG), progesterone (PROG), dehydroepiandrosterone (DHEA), testosterone (TS), dihydrotestosterone (DHT), estrone (E1), 17α-estradiol (αE2), 17β-estradiol (βE2), aldosterone (ALDO), cortisol (COR), corticosterone (COS),17α-hydroxyprogesterone (17-OHPROG), 17α-hydroxypregnenolone (17-OHPREG), 11-deoxycorticosterone (11-deoxyCOS), 11-deoxycortisol (11-deoxyCOR), cortisone (CORNE), 11-ketotestosterone (11-ketoTS) and cholesterol (CHOL) were purchased from Sigma-Aldrich, Glostrup, Denmark with a purity >96 %. Androstenediol (ADIOL) was purchased from Toronto Research Chemicals (North York, ON, Canada) with a purity >98 %. Deuterated analogues were applied as internal standards (IS); d7-androstenedione (ANd7), d4-estrone (E1d4), d5-17β-estradiol (βE2d5), d8-corticosterone (COSd8) and d8-11-deoxycorticosterone (11-deoxyCOSd8) were obtained from CDN isotopes (Pointe-Claire, QC, Canada), while d9-progesterone (PROGd9), d3-testosterone (TSd3) and d3-dihydrotestosterone (DHTd3), d7-aldosterone (ALDOd7) and d4-cortisol (CORd4) were purchased from TRC, all with a deuterated purity above 98 %. d5-11-deoxycortisol (11-deoxyCORd5) and d6-dehydroepiandrosterone (DHEAd6) were obtained from Sigma-Aldrich with a deuterated purity >98 %. All utilized solvents were analytical grade. Methanol was obtained from Fisher Scientific (Leics, UK), acetone and n-heptane were obtained from Fisher Scientific (Slangerup, Denmark). Formic acid 98–100 % was purchased from Merck (Merck KGaA, Darmstadt, Germany). All H2O used was ultrapure water produced by a Milli-Q system (Millipak 40). The H295R human adrenocortical carcinoma cell line was obtained from American Type Culture Collection (ATCC, #CRL-2128, Manassas, VA, USA). Cells were cultured in 75 cm2 flasks from Sigma-Aldrich (Brøndby, Denmark), Trypsin and Dulbecco’s Modified Eagle’s Medium and Ham’s F-12 Nutrient mixture (DMEM/F12) medium (GibcoBRL Life Technologies, Nærum, Denmark) supplemented with 10 mL/L of ITS+premix and 25 mL/L Nu-serum from BD Bioscience (Brøndby, Denmark). Phosphate buffered saline from OXOID Dulbecco A (Hampshire, UK). Prochloraz (PRO) and forskolin (FOR) were obtained from Sigma-Aldrich (Glostrup, Denmark). Heparin Sodium Salt A3004 from AppliChem (Germany).
Standard solutions
Individual stock solutions of 10.0 μg/mL in methanol were prepared as working solutions during method development and optimization. A mixed stock solution was prepared in methanol containing 20 μg/mL of each analyte. From this, a dilution series was made ranging from 0.0001 to 1 μg/mL in methanol to be used during validation and application. The internal standard (IS) mixture, containing 12 deuterated analogues, was prepared with a concentration of 0.1 μg/mL in methanol. To each sample, 50 μL of this IS mixture was applied, corresponding to 5 ng for each IS.
Sample preparation
H295R cell medium
The H295R steroid hormone synthesis assay was performed according to the OECD validation guideline [12]. In brief, cells were cultured in 75 cm2 flasks with 30 mL DMEM/F12 media supplemented with 1 % ITS-premix and 2.5 % Nu-serum at 37 °C with a 5 % CO2 atmosphere. When cells reached 75–95 % confluence, the cells were trypsinated. The cells were only used for experiments between passage 4 and 12°. For the exposure experiments, the cells were grown in 24 well plates with a density of 3 × 105 cells/mL. The cells were allowed to settle for 24 h, after which the medium was changed and the test compounds (PRO – inhibitor and FOR – inducer) was added. PRO in the concentration range 0.001–1 μM and FOR in the range 0.01–10 μM. To avoid interference from low levels of steroid hormones present in the Nu serum, plating was conducted with serum-free media. Each compound was tested in seven concentration levels in triplicates, and the experiment was repeated on two different days (n = 6). On each test plate, a solvent control (SC) (medium with 0.1 % DMSO) was included in triplicates. In accordance with the OECD guideline [12], the maximal concentration of DMSO in the cell medium was 0.1 %. The cells were incubated in the presence of the test compounds for 48 h. Hereafter, 950 μL medium was carefully removed and stored at −20 °C for later hormone analysis. Cell viability was confirmed with the resazurin assay, as described by Nielsen et al. [10]. All tested concentrations confirmed viable cells.
Gonad tissue
Gonads were collected from male Sprague-Dawley rats, aged between 12 and 20 weeks. The animals were housed in type IV cages with ASTP wooden bricks, a shelter and ad libitum access to food (Altromin 1314F) and tap water, at the University of Copenhagen. The stable was maintained at a constant temperature of 22 ± 1 °C, a humidity of 60 ± 10 % and a 12:12 h light/dark regime. Dissection was performed immediately after euthanization. Organs were immediately frozen in liquid nitrogen after dissection and hereafter stored in −80 °C until use.
Serum and plasma
The method was developed and optimized on charcoal-stripped foetal bovine serum (cat. no. 04-201-1A, Biological Industries, Israel) free of steroid hormones. The application was conducted using 12-week-old male Sprague-Dawley rat plasma, housed as described above. Immediately after euthanization, the blood was collected using heparin-coated centrifuge tubes. The blood was kept on ice and centrifuged (5 min, 4 °C and ̴7500 G) to separate plasma from the red blood cells. Plasma samples were stored at −80 °C until further analysis.
Standard procedure
Steroid extraction from H295R cell medium and precipitation
From each well, 950 μL cell medium was transferred to a 2-mL Eppendorf tube and 50 μL 0.1 μg/mL IS solution was added. Protein precipitation was conducted by adding 900 μL ice-cold acetonitrile, vortexing the samples, a 10-min wait to complete precipitation and centrifuging at ∼9500 G for 10 min. Hereafter, the supernatant was collected and evaporated under a gentle stream of nitrogen at 60 °C to ∼1 mL. Hereafter, a second protein precipitation was conducted using 900 μL ice-cold methanol. Again, samples were vortexed, left for 10 min and centrifuged at ∼1500 G for 10 min. Finally, the supernatant was collected, transferred to a 1.5-mL LC vial and evaporated to 1 mL under a gentle stream of nitrogen at 60 °C.
Steroid extraction from gonads and off-line SPE procedure
Each SPE column (500 mg Bond elute C18 solid phase extraction cartridges with 10 mL reservoir, Agilent, USA) was placed on a vacuum manifold (IST Vacmaster, Biotage, Uppsala, Sweden) and pre-conditioned with 3 mL n-heptane, 3 mL acetone, 3 mL methanol and finally with 5 mL H2O with a flow of ∼1 mL/min.
One hundred milligram tissue was transferred to a 2-mL Eppendorf tube. Fifty microliters 0.1 μg/mL IS solution was added on top of tissue followed by 1 mL extraction solvent (H2O/methanol, 25:75, v/v). Steroids were extracted from the tissue using a Tissue Tearor (Model 985370, BioSpec Products, Inc., Bartlesville, UK) operating at highest speed to completely homogenize the sample. Hereafter, the sample was vortexed and left for 10 min before being centrifuged at ∼9500 G for 6 min. The supernatant was transferred to a 10-mL glass vial, and an additional 1 mL extraction solvent was added to the Eppendorf tube containing the tissue. Homogenization and centrifugation were repeated, and the second supernatant was transferred to the 10-mL glass vial. In total, three extraction cycles were conducted.
Hereafter, the extract was diluted to a total volume of 9 mL using H2O and transferred to the pre-conditioned SPE cartridge. Enrichment was performed at a flow of approx. 1 mL/min. After enrichment, the SPE cartridge was washed with 9 mL H2O at a flow of approx. 10 mL/min followed by an additional wash with 9 mL H2O/methanol 75:25 (v/v) solution at a flow of 10 mL/min. The high flow was generated by applying vacuum. Subsequently, analytes were eluted from the SPE cartridges using 5 mL H2O/methanol 20:80 (v/v) at a flow of approx. 1 mL/min. Finally, the collected extract was evaporated to 1 mL under a gentle stream of nitrogen at 60 °C and transferred to a 1.5-mL LC vial.
Steroid extraction from serum and plasma and off-line SPE procedure
SPE cartridges were conditioned as described above. Collected plasma samples were thawed, and 50 μL 0.1 μg/mL IS solution was added to each 400 μL plasma sample (Sprague-Dawley rat). Then samples were diluted with 4 mL H2O and loaded to the pre-conditioned SPE at a flow of approx. 1 mL/min. After enrichment, SPE cartridges were washed and steroids were extracted as described above for gonads.
Liquid chromatography including online clean-up
For online cleanup and chromatographic separation of steroids, a binary 1290 Agilent Infinity Series system and a binary 1100 Agilent HPLC pump were used in combination (Agilent Technologies, Palo Alto, CA, USA). For online cleanup, a C18 enrichment column (μbondapak® C18, 3.9 × 20 mm, 10 μm, Waters) was used. The enrichment column was connected to the autosampler through the TTC switching valve (two positions, 6 ports). Between the autosampler and the TTC switching valve, a 0.3-μm in-line filter (1290 infinity in-line filter, Agilent) was installed.
Separation of the steroid hormones was performed using a C18 analytical column (Kinetex, 2.6 μm C18 100 A, 75 × 2.1 mm, Phenomenex, USA) with a guard column placed in front of the analytical column (C18, 2.1 mm, Phenomenex, USA). The guard and analytical columns were connected to the TTC switching valve and the MS switching valve. An isocratic flow of 1 mL/min H2O/methanol/formic acid 90:10:0.1 (v/v/v) was generated by the 1100 pump which was connected to the autosampler. The 1290 pump performed a gradient elution with a flow rate of 0.3 mL which was connected to the TTC switching valve.
The gradient mobile phase A and B composed of H2O with 0.1 % formic acid (v/v) and pure methanol, respectively. The elution gradient was maintained at 10 % B for the first 2 min, 10.0–30.0 % B from 2.0 to 2.2 min, 30.0–60.0 % B from 2.2 to 8.0 min, maintained at 60.0 % B from 8.0 to 10.0 min, 60.0–85.0 % from 10.0 to 12.30 min, 85.0–99.5 % B from 12.3 to 12.5 min and held at 99.5 % B from 12.5 to 14.8 min, before re-equilibrating the column unto 16 min. The TTC switching valve was positioned left from 0 to 2.0 min, right from 2.0 to 15.2 min and left 15.2 to 16.0 min. The MS switching valve directed the flow to waste from 0 to 5.5 min and from 12.5 to 16 min. The thermostated autosampler was set at 7 °C and the thermostated column oven set at 40 °C. Electronic Supplementary Material (ESM) Fig. S1 shows the described LC setup. An injection volume of 100 μL was achieved by installing an 80-μL needle seat and ejecting four times 20 μL into it before injecting the entire sample with the last 20 μL. The large injected volume did not have an effect on peak shape or retention time since the sample was focused on the enrichment column. Chromatograms for individual steroids and their deuterated analogues can be seen in ESM Fig. S2. The chromatograms were acquired by spiking carbon-striped serum with 50 μL 0.1 μg/mL IS solution and 10 μL 0.1 μg/mL working solution. Hereafter, the protocol for steroid extraction from serum and plasma and off-line SPE procedure was executed.
Mass spectrometry
For detection, an AB SCIEX 4500 QTRAP mass spectrometer (Applied Biosystems, Foster City, CA, USA) equipped with an atmospheric pressure chemical ionization (APCI) Turbo V source was used. Multiple reaction monitoring (MRM) was performed in positive mode during analysis with target scan time of 0.8 s. The nebulizer current was set at 3 mA with a source temperature of 550 °C. Nitrogen was applied as curtain, collision and ion source gases with settings of 40 psi, high and 40 psi, respectively. Individually optimized MRM parameters can be found in ESM Table S1. The obtained ions of each analyte are listed in Table 1. The table also shows selected internal standards, molecular formulas, molar masses and retention times.
LC and MS optimization was conducted using Analyst v. 1.6.2 software package (AB SCIEX) and obtained data was processed in MultiQuant v. 3.0 software (AB SCIEX). Calculations and graphics were performed using Microsoft Office Excel 2010 and GraphPad Prism v. 6.03 (GraphPad Software, San Diego, CA, USA).
Results and discussion
MS2 development and optimization
In flow injection analysis (FIA)-MS and in MS2 experiments, precursor, quantifier, qualifier ions and source and compound parameters were selected and optimized. This was conducted at a flow of 0.3 mL/min. Depending on the specific analyte, a mobile phase B content was chosen in the FIA. For example, cortisol had a RT of 7.07 min, and at this time, the mobile phase composition was ∼55 % B. Using a T-piece prior to the ion source, analytes were infused with a flow of 20 μL/min at a concentration of 0.1 μg/mL in a H2O/methanol (1:1, v/v) solution.
All target analytes could be ionized in positive mode using an APCI source, which is in accordance with Carvalho et al. [23], Ceglarek et al. [24] and Koren et al. [25]. In-source water losses from E2, PREG, 17-OHPREG and DHEA were observed, which have also been observed using electrospray ionization [14]. To the authors’ knowledge, ionization mode and ion formation of ADIOL and 11-ketoTS have not previously been reported in the literature when using an APCI interface. As precursor ion 255.2 m/z was chosen for ADIOL, which is the M +1 minus the loss of two water molecules (ESM Fig. S3) and for 11-ketoTS M + 1, 303.4 m/z, was chosen as precursor ion.
HPLC development and optimization
Previously, off-line C18 SPE has often been applied prior to GC-MS/MS and LC-MS/MS analysis of steroid hormones in H295R cell medium [10, 18, 19, 26–30]. The present HPLC method was developed for the purpose of online cleanup to reduce manpower and the use of SPE cartridges when processing samples from aqueous cell medium. The online clean-up aim was to retain the target steroids on the enrichment column (C18 bond) while washing salts, proteins, etc. directly into waste in the beginning of the HPLC run (ESM Fig. S1). It was found that the steroids did not elute from the enrichment column when keeping the methanol content ≤20 % in an aqueous solution with a flow 1 mL/min for 2 min (a methanol content of 10 % is used in the present method). After a 2-min rinse, the TTC valve position was shifted and the steroids were backflushed onto the analytical column when a gradient was applied. The majority of the steroids were separated with a mobile phase B (methanol) gradient rice of 5.2 %/min. ADIOL and DHT were not separated using this gradient, and it was necessary to maintain mobile phase B at 60 % from 8 to 10 min to achieve enough peak separation. In general, the analytical C18 Kinetex column provided nice peak separation and peak tailing was reduces by applying 0.1 % formic acid to mobile phase A (H2O). The retention times of the analytes were between 5.98 (ALDOd7) and 12.28 (PREG) min.
Optimization of off-line SPE methodology
Initially, in vivo samples were applied directly to the LC-MS2 method without performing off-line SPE. Unfortunately, this approach resulted in a rapid buildup of column back pressure and a frequently need for guard and enrichment column replacement, even though an on-line clean-up step was integrated in the LC methodology. To accommodate these problems, it was decided to apply off-line SPE prior to LC-MS2 analysis to achieve a robust LC method.
C18 bond off-line SPE have been widely used as a clean-up step prior HPLC-MS/MS analysis when determining steroid hormones in serum [31, 32, 34]. In this present study, a fast off-line C18 bond SPE was developed, as a cleanup prior to HPLC-MS2 analysis when determining steroids in serum/plasma and gonad samples. As mentioned above, the cleanup of the complex biological matrixes, rich in lipophilic components, was conducted off-line to avoid contamination of the analytical column, but also to minimize contamination of the ion source and quadropoles as well as to reduce possible matrix effects. The off-line SPE methodology was an optimization and expansion of the method developed by Abdel-Khalik et al. [30], where TS, PREG, COR, ALDO, 17OH-PROG and cholesterol were determined in H295R incubation medium using off-line C18 SPE and LC-MS2. In the study of Abdel-Khalik et al. [30], the aqueous medium was loaded on the SPE cartridges followed by a 2 × 3 mL tap water wash. Hereafter, air-drying was applied for 30 min using a vacuum manifold. Finally, the target analytes were eluted using 7 mL H2O/methanol (1:99 v/v) solution containing 2.5 mM ammonium acetate. In the present study, CHOL was not a target analyte, and as demonstrated by Abdel-Khalik et al. [30], it was possible to elute TS, PREG, COR, ALDO, 17OH-PROG while cholesterol was retained on the cartridge. This was done by using an extraction solvent with a weaker elution strength (H2O/methanol, 25:75 v/v) which still yielded a high recovery of 102, 72.7, 176.2, 122.9 and 93.1 % for the five compounds respectively.
Thus, the rationale behind the present study was to include all the steroids listed in Table 1 and their deuterated analogues (ISs) in a similar SPE methodology. The intent was to develop a SPE method where the elution strength of the rinsing solution was as high as possible to wash out salts, polar and semi-polar compounds, without losing any target analytes. Furthermore, it was an objective to find an eluent with eluation strength as weak as possible in order to retain lipophilic compounds on the SPE and still yield a maximum recovery of the target analytes.
To investigate eluation strengths of different solvents, an experiment was setup where an Agilent 1100 pump was connected to a SPE cartridge (C18 bond, 500 mg, 3 mL reservoir, Varian Inc., Palo Alto, Agilent, CA, USA) using a customized adaptor. The bottom of the cartridge was connected to the Qtrap 4500 MS instrument through a peek t-piece using another customized adaptor. The setup can be seen in ESM Fig. S4.
The deuterated aldosterone analogue (ALDOd7) was the most polar steroid used in this study (ALDO log p = 1.06 [33]) and was therefore monitored when determining the strength of the washing solution. First cartridges were pre-conditioned as previously described. Then 100 ng ALDOd7 (10 μL 10 μg/mL in methanol) was added on top of the cartridges which were then sealed with the customized adaptor. Four different solvent strengths were tested using different compositions of H2O and methanol. A flow of 1 mL/min was applied to simulate the flow being used when loading samples on cartridges using a manifold. The temperature was ambient. The acquired ALDOd7 chromatograms are shown in Fig. 1. Results showed that with higher methanol content, less solvent is needed for ALDOd7 to completely elute from the C18 cartridge. Using a methanol content of 40, 35 and 30 %, ALDOd7 was eluted from the SPE cartridge using 10, 15 and 25 mL, respectively. Using 22 mL of a 25 % methanol solution, the baseline signal remained constant, suggesting that the elution strength was too weak to extract ALDOd7 from the SPE. However, when the methanol content of the solvent was changed to 75 % (marked with an arrow on the figure), complete breakthrough of ALDOd7 occurred immediately. The 30 and 25 % methanol solutions were both potential rinsing choices, but the conservative choice of 25 % methanol solution was chosen as rinsing solution.
Elution of ALDOd7 on a 500 mg C18 bond SPE cartridge. Four solutions with different elution strength were tested. The elution strength affected peak width and volume needed for complete elution. Using aqueous solutions containing 40, 35 and 30 %, methanol (v/v), ALDOd7 was eluted from the SPE using 10, 15 and 25 mL, respectively. When using 22 mL of a 25 % methanol solution, the intensity baseline remained constant, indicating that ALDOd7 was retained on the SPE cartridge. However, when applying 22 mL of a 75 % methanol solution (marked with an arrow on the figure), complete breakthrough of ALDOd7 occurred immediately. These results show that it was possible to wash samples attached to SPE cartridges with a 25 % methanol solution without elution target analytes, prior to eluting with 75 % methanol
Optimization of the elution solvent was likewise conducted as shown in ESM Fig. S4. Being the compound with the lowest polarity (Log p = 4.22, [33]), PREG was monitored to determine the solvent strength needed to elute all the compounds. SPE cartridges were pre-conditioned as previously described. Fifty microliters 10 μg/mL of PREG was spiked into a 100-mL 25 % methanol solution. Nine milliliters of this solution was added to the cartridges and loaded with a flow of ∼1 mL/min. Finally, still wet, the adaptors were connected to the cartridges and five chromatograms were acquired with mobile phase contents of 100, 95, 90, 80 and 75 % methanol respectively with a LC flow of 1 mL/min. The chromatograms are displayed in Fig. 2. Using 100, 95 and 90 % methanol, PREG was eluted using less than 3 mL eluent. Approx. 5 mL was needed when using 80 % and approx and 8 mL when using 75 % methanol. These results are consistent with the data acquired in Abdel-Khalik et al. [30]. Here, a pre- and post-spike approach was used to determine the absolute recovery of PREG, which yielded a recovery of 73 % using 7 mL of a 74.5 % methanol solution. Both 75 and 80 % solutions were considered as possible eluents, but the 5-mL 80 % methanol solution was chosen due to the lower H2O content (1 mL vs. 2 mL H2O), which reduces the time consuming and costly evaporation of the eluate.
Elution of PREG on a 500-mg C18 bond SPE cartridge using five solutions with different elution strength. Nine milliliters of an aqueous solution (25 % methanol, v/v) containing PREG was loaded onto pre-conditioned cartridges with a flow of ∼1 mL/min. Hereafter, the cartridges were connected to a LC and a MS2 and a flow of 1 mL/min was applied. Aqueous solutions with 100, 95, 90, 80 and 75 % methanol (v/v) was tested. Using 100, 95 and 90 % methanol PREG was eluted using less than 3 mL eluate. Approx. 5 mL was needed when using 80 % and approx. 8 mL when using 75 % methanol
The effect of applying turbo-flow SPE rinsing was tested to speed up the working process. One milliliter carbon-stripped bovine serum was spiked with 10 μL 10 μg/mL of a COR, ALDOd7, βE2, PREG and TS solution and diluted in 4 mL H2O. Samples were loaded to pre-conditioned cartridges at a flow of ∼1 mL/min. All cartridges were rinsed with 9 mL H2O using a turbo flow of 10 mL/min. Hereafter, the cartridges were rinsed with a 9 mL H2O/methanol (75:25, v/v) solution using three different flow rates. First, 9 mL with a 10 mL/min flow was tested. Secondly, 7 mL with 10 mL/min followed by 2 mL with a flow of 1 mL/min. Finally, a 9-mL rinse with a flow rate of 1 mL/min was tested. The 10 mL/min flow was generated using vacuum on a manifold. Subsequently, the three cartridges were connected to the LC adaptors and an H2O/methanol (20:80, v/v) solution was applied with a flow of 1 mL/min. The acquired chromatograms were similar regardless of the applied rinsing flow prior to connecting the cartridges to the LC system (data not shown). This shows that the rinsing flow rate has no significant influence on target analyte recovery.
In the study of Hansen et al. [34], column drying was conducted for samples applied to C18 SPE cartridges to remove H2O prior to extraction of steroid hormones. Primarily, this was done to avoid incomplete derivatization of hydroxy groups prior to GC-MS/MS analysis, but column drying can also be a practical approach in terms of storage and transport of samples. The effect of drying was tested in an experiment similar to the above mentioned. Bovine serum was spiked with the analytes, diluted, loaded and rinsed with H2O using vacuum. Hereafter, still wet, one sample was rinsed with 9 mL 25 % methanol solution and subsequently connected to the LC adaptor and run on the online setup. Simultaneously, one sample was air-dried for 30 min using the vacuum manifold followed by a rewetting using 9 mL 25 % methanol solution before it was connected to the LC adaptor. ESM Fig. S5 shows the acquired chromatograms. A notable difference between the two samples was that additional extraction solvent was needed to recover the analytes after drying. As a result, two extraction protocols were applied to the methodology, one using 5 mL extraction solvent when cartridges are still wet and one using 6 mL when cartridges are air-dried. The recovery of samples applied to SPE cartridges was determined conducting both scenarios. The recovery experiment is described below and the results can be seen in Table 2.
Validation and application
For each analyte, a ten-point calibration curve was made, containing a zero sample and nine concentration levels. The calibration curves ranged from 0.25 ng/mL to 0.50 μg/mL with six replicates for each concentration level. Slope, intercept and coefficient of determination (R 2) can be seen in Table 2. No weighting was applied to the data.
Lower limit of detection (LLOD) and lower limit of quantification (LLOQ) were determined as recommended by the ICH guideline from 2005 [35] (Eqs. 1 and 2). σ is the standard deviation of the area ratio obtained from the least concentrated mixture of each analyte above LLOQ. S is the slope of the calibration curve for each analyte.
Estimated LLOD and LLOQ values can be found in Table 2. Herein, LLOQ values ranged from 55 to 530 pg/mL. In other studies, where androgens, corticosteroids, estrogens and progestagens are included in the analytical methodology, LLOQ levels are within the same range when using similar instruments (e.g. API 4000 [24] or AB Sciex 5500 [25] with APCI interface). Carry-over was determined by injecting a blank sample after injection of the highest concentration when running the calibration curve. No carry-over was detected (<20 % of LLOQ). Within-run precision was determined by injecting 0.1 ng/mL, 5.0 ng/mL and 100 ng/mL solutions six times each. Between-run precision was determined by injecting a 1.0 ng/mL solution one time a day for six days in a row. Method accuracy was determined using spiked carbon-stripped bovine serum in six concentrations levels with six replicates on each level. The spiking levels of analytes can be seen in Table 2 and 50 μL 0.1 μg/mL IS solution was spiked into each sample. The experiment was conducted as described in “Standard procedure.”
Recovery of target analytes was determined on the off-line SPE. Two recovery studies were conducted. The first experiment was conducted with SPE cartridges kept wet and analytes extracted immediately after loading and washing. In the second experiment, SPE cartridges were air-dried for 30 min using a vacuum manifold. Hereafter, the cartridges were stored at −18 °C for 6 weeks before rewetting and extraction. As mentioned above, 6 mL H2O/methanol (80:20, v/v) was used to extract the analytes from the air-dried SPE cartridges which differ from the standard protocol for “wet” SPE columns. The spiking level was 25 ng for the target analytes and 5 ng for IS. Recoveries were determined with a pre- and post-spiked approach, where pre-spiking was conducted in serum and post-spiking was conducted in the SPE eluate. Recoveries were calculated as described in Eq. 3.
The developed method was applied to 400 μL plasma samples and 100 mg gonad tissue samples collected from male Sprague-Dawley rats. Furthermore, the method was applied to the H295R assay where steroid hormone synthesis was inhibited using forskolin and induced using prochloraz. Detected background levels from negative QC samples were deducted unknown samples, and concentrations were estimated using the slope from the linear regression determined from the calibration curves. Validation and application results are reported in Table 2 and in Fig. 3.
Steroid production in the H295R cell line following the OECD [12] guideline. Steroids were extracted, cleaned up and analysed using the developed method. Effects of the OECD guideline inducer FOR (n = 6) are shown in green whereas the guideline inhibitor PRO (n = 6) is shown in red. Error bars are standard deviations. Note the difference in y-axis scale
According to FDA guidelines [36], precision, expressed in %RSD, should not exceed 15 % except for LLOQ samples where 20 % variance is accepted. In the present study, variances for all concentrations were determined to be within 0.7–15.6 % which is acceptable. In general, the largest variation was determined at the 0.1 ng/mL concentration level. Method accuracy describes the closeness of the obtained concentrations to the known nominal value. Accuracy is reported as the percent of the nominal value. The mean obtained concentration, calculated using the calibration curves, should be within 15 % of the nominal values except for LLOQ levels where 20 % is accepted. For a majority of the obtained data, these criteria are fulfilled and show good accuracy of the SPE method. For 11-deoxycortisol, 11-ketoTS and βE2 over- and under-estimation occurs. The SPE accuracy was tested using six concentration levels with six replicates in each concentration and can thereby be used as calibration curve for 11-deoxycortisol, 11-ketoTS and βE2. For 11-deoxycortisol, 11-ketoTS and βE2 the linear equations are y = 0.3013x, R 2 = 0.9999, range 0.1–100 ng/mL, y = 0.091x + 0.0202, R 2 = 0.9999, range 0.1–100 ng/mL and y = 0.254x + 0.0579, R 2 = 1, range 0.1–100 ng/mL, respectively.
Determination of method recovery was conducted to check if the obtained data from the online experiment also would apply when working off-line with true samples. Both wet and air-dried SPE cartridges were tested. Both experiments showed a complete recovery yield. The washing and extraction solutions were optimized for the investigated steroid hormones, which not only gave a high recovery yield but also an effective sample cleanup. When SPE eluate was evaporated to dryness and reconstituted in 200 μL H2O/methanol (80:20, v/v), no particles were observed, no precipitation occurred and extracts were clear as a blank sample (even when working with biological matrixes such as gonads and adrenals).
Even though samples were pure and online cleanup was applied in the HPLC methodology, an over estimation of ALDO, with a factor 4-5, was observed using the ALDO/ALDOd7 ratio in the accuracy experiment. These results suggest either a lower recovery of ALDOd7 or a greater suppression of the ALDOd7 ions (RT 5.98 min) in comparison to ALDO (RT 6.40 min). Based on the experiments described above, loss of ALDOd7 in comparison to ALDO is most unlikely since a 25 % methanol aqueous solution was a conservative choice and the difference in polarity between the two compounds is small. Matrix effects and ion suppression were not investigated herein, but, in house, ion suppression of corticosteroids has been observed when target analytes are co-eluting in the beginning of a HPLC run. This resulted in a greater ion suppression of analytes with a short RT (data not published, but presumably due to low mass compounds eluting early). Values closer to the nominal concentration of ALDO improved when using ALDO/CORd4 ratios (the accuracy results are given in Table 2). These results suggest that the intensity of ALDOd7 is more suppressed in comparison to ALDO and therefore CORd4 was used as IS for ALDO.
An application was conducted using 400 μL plasma and 100 mg gonad tissue from male Sprague-Dawley rats. Applying these sample sizes, it was possible to simultaneously quantify 6 steroids in plasma and 10 steroids in testis. In Table 3, steroid levels in plasma and gonad tissue from this present study are shown along with steroid levels found in other studies. For testis, we identified a single study [37] which report levels of AN and TS in the range 5.5–41 ng/g (AN) and 31–205 ng/g (TS) which is also in good accordance with the results presented in the present paper. For plasma, the measured concentrations were generally in agreement with other studies using male rats of same age [20, 34, 37–41]. The progestagens PREG and PROG are, however, exceptions. Previous studies using RIA found these progestagens in levels around 2.7–4 ng/mL [38, 39], whereas the present study found a PROG concentration of 0.29 ng/mL and PREG to be below LLOD. In a previous study using GC-MS/MS, PROG was below limit of detection (0.36 ng/mL) and PREG levels were lower than in studies using RIA [34]. This indicates different results between RIA and chromatographic techniques, which may be due to cross-reactivity for the progestagens using RIA assays.
The H295R assay application was conducted following the OECD [12] protocol, inhibiting and inducing steroid hormone synthesis using procloraz and forskolin. This was done to evaluate if the present HPLC-MS2 methodology could be used to detect and quantify steroids in this important assay. The results are shown in Fig. 3. We were able to detect 14 steroids excreted by the H295R cells, only ALDO, ADIOL, αE2, DHT and 11-ketoTS could not be detected. As expected, FOR induced all steroids in the steroidogenesis except for CORNE. The performance criteria for the OECD [12] guideline suggest that the basal steroid production should be at least 2.5 to 5 times higher than the estimated LOQ. This criteria is met for the majority of the steroids, except for COS and βE2. In both cases, however, it was possible to detect and quantify the two steroids, indicating the rather conservative approach used to estimate LLOD and LLOQ in the present study.
It is worth noticing that the OECD [12] guideline inhibitor PRO is not inhibiting the first and rate-limiting step converting CHOL to PREG. This can be seen from the clear accumulation of PREG and PROG, indicating that the CYP11A1 is able to convert CHOL to PREG which is then converted to PROG by the 3β-HSD. Instead, PRO seems to inhibit the CYP17-hydroxylase converting progestagens into hydroxy-progestagens and androgens and CYP21 converting progestagens into corticosteroids, thereby shutting down the steroidogenesis.
To the author’s knowledge, the present method is the first method to analyse steroids from all 4 major steroid classes in H295R cells using on-line cleanup. In some of the previously developed multi-methods [13–16], it was possible to quantify androgens, corticosteroids and progestagens but the analytical methods were not sensitive enough to include any of the estrogens which are important criteria in the OECD guideline [12]. In [19], off-line SPE was conducted prior to analysis of androgens, corticosteroids, estrogens and progestagens whereas in [18], βE2 was successfully included by conducting off-line SPE and derivatization prior to analysis. In [17], derivatization of estrogens was applied and three separate LC-MS2 methods were used to quantify steroids from the four groups. In the present paper, we also reported the concentrations of 10 steroids in testis of male rats. Information on steroids in soft tissues from experimental model animals is scarce, as is also evident from Table 3. Further development of these methods will ensure fast, cheap and reliable tools for screening compounds with endocrine-disrupting properties in in vitro and in vivo assays.
Conclusion
A novel method for extraction, cleanup and quantification of steroid hormones in both in vivo and in intro samples was developed and validated. In total, 4 progestagens, 6 corticosteroids, 6 androgens and 3 estrogens were analysed. With low effort and costs, high recovery, good accuracy and efficient cleanup were achieved using the off-line SPE method for in vivo samples. For in vitro samples, only online cleanup was needed for high quality analysis. The LC-MS2 method proved robust with good precision. The instrument sensitivity was within the expected range (LLOQ 55–530 pg/mL) when analyzing multiple steroid hormones (>8) in plasma and serum using APCI [42]. The methodology was successfully applied to plasma and gonad tissue samples from male Sprague-Dawley rats. Using a multi-steroid LC-MS2 method, it was possible to quantify 10 steroid hormones in rat gonad tissue. To the knowledge of the author, this has not previously been published and especially dihydrotestosterone, which is converted from testosterone by 5α-reductase, is important as it is a very potent androgen with higher affinity to the androgen receptor than testosterone and is considered an end-product hormone in the mammalian steroidogenesis [12].
Furthermore, we validated the method using the H295R assay and demonstrated that the developed method fulfills the OECD recommendations for steroid analysis. This novel method makes it possible to investigate the effects of EDCs on the main pathways in the mammalian steroidogenesis using one LC-MS2 method without the need of derivatization or off-line SPE prior to analysis, which can contribute to large-scale screenings of possible EDCs. Compared to other approaches such as RIA and ELISA, the major advantage of the developed method is that it allows for simultaneous analysis of several steroids in single samples. Also, cross-reactivity, which is sometimes observed with other methods such as RIA and ELISA, is avoided.
References
Miller WL, Auchus RJ. Endocr Rev. 2011;32:81–151.
Colborn T, Saal FS, Soto AM. Environ Impact Assess Rev. 1994;14:469–89.
Giwercman A, Carlsen E, Keiding N, Skakkebak NE. Environ Health Perspect. 1993;101:65–71.
Grün F, Blumberg B. Endocrinology. 2006;147:50–5.
Grün F, Blumberg B. Mol Cell Endocrinol. 2009;304:19–29.
Joensen UN, Bossi R, Leffers H, Jensen AA, Skakkebaek NE, Jørgensen N. Environ Health Perspect. 2009;117:923–7.
Jørgensen N, Asklund C, Carlsen E, Skakkebaek NE. Int J Androl. 2006;29:54–61.
Priskorn L, Holmboe SA, Jacobsen R, Jensen TK, Lassen TH, Skakkebaek NE. Int J Androl. 2012;35:449–55.
Skakkebæk NE, Meyts ER-D, Main KM. Hum Reprod. 2001;16:972–8.
Nielsen FK, Hansen CH, Fey JA, Hansen M, Jacobsen NW, Halling-Sørensen B, et al. Toxicol in Vitro. 2012;26:343–50.
Hecker M, Newsted JL, Murphy MB, Higley EB, Jones PD, Wu R, et al. Appl Pharmacol. 2006;217:114–24.
OECD. OECD guidelines for the testing of chemicals http://www.oecdilibrary.org/docserver/download/9745601e.pdf?expires=1449586480&id=id&accname=guest&checksum=D5920268C409B7FC8578D7AB262A2C97. 2011.
Schloms L, Storbeck KH, Swart P, Gelderblom WCA, Swart AC. J Steroid Biochem Mol Biol. 2012;128:128–38.
Tonoli D, Fürstenberger C, Boccard J, Hochstrasser D, Jeanneret F, Odermatt A, et al. Chem Res Toxicol. 2015;28:955–66.
van der Pas R, Hofland LJ, Hofland J, Taylor AE, Arlt W, Steenbergen J, et al. J Endocrinol. 2012;215:403–12.
Xing Y, Edwards MA, Ahlem C, Kennedy M, Cohen A, Gomez-Sanches CE, et al. J Endocrinol. 2011;209:327–35.
Zhang X, Chang H, Wiseman S, He Y, Higley E, Jones P, et al. Toxicol Sci. 2011;121:320–7.
Iwaoka Y, Hashimoto R, Koizumi H, Yu J, Okabe T. Life Sci. 2010;86:894–8.
Rosenmai AK, Nielsen FK, Pedersen M, Hadrup N, Trier X, Christensen JH, et al. Toxicol Appl Pharmacol. 2013;266:132–42.
Quignot N, Arnaud M, Robidel F, Lecomte A, Tournier M, Cren-Olivé C, et al. Reprod Toxicol. 2012;33:339–52.
Matty AJ. Fish endocrinology, Matty AJ (ED.). 1985; 138-173.
Kindler PM, Philipp DP, Gross MR, Bahr JM. Gen Comp Endocrinol. 1989;75:446–53.
Carvalho VM, Nakamura OH, Vieira JGH. J Chromatogr B. 2008;872:154–61.
Ceglarek U, Kortz L, Leichtle A, Fiedler GM, Kratzsch J, Thiery J. Clin Chim Acta. 2009;401:14–118.
Koren L, Ng ESM, Soma KK, Wynne-Edwards KE. PLoS ONE. 2012;7:e32496.
Winther CS, Nielsen FK, Hansen M, Styrishave B. Int J Toxicol. 2013;32:219–27.
Jacobsen NW, Hansen CH, Nellemann C, Styrishave B, Halling-Sørensen B. Toxicol in Vitro. 2015;29:1729–35.
Guldvang A, Hansen CH, Weisser JJ, Halling-Sørensen B, Styrishave B. Reprod Toxicol. 2015;58:174–83.
Sørensen AM, Hansen CH, Bonomo S, Olsen L, Jørgensen FS, Weisser JJ, Kretschmann AC, Styrishave B. Toxicol in Vitro. 2016;34:71–80.
Abdel-Khalik J, Björklund E, Hansen M. J Chromatogr B. 2013;935:61–9.
Yamashitaa K, Miyashirob Y, Maekubob H, Okuyamab M, Honmab S, Takahashia M, et al. Steroids. 2011;74:920–6.
Fanellia F, Belluomoa I, Lalloa VDD, Cuomoa G, Iasiob RD, Baccinia M, et al. Steroids. 2011;76:244–53.
US National Library of Medicine. ChemIDplus advanced http://chem.sis.nlm.nih.gov/chemidplus/. 2010.
Hansen M, Jacobsen NW, Nielsen FK, Björklund E, Styrishave B, Halling-Sørensen B. Anal Bioanal Chem. 2011;400:3409–17.
ICH. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Validation of Analytical Procedure: Text and Methodology Q2(R1) 1-17 http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf. 2005.
U.S. FDA, Guidance for Industry – Bioanalytical Method Validation, May 2001 http://www.fda.gov/downloads/Drugs/.%20.%20./Guidances/ucm070107.pdf.
Pouech C, Tournier M, Quignot N, Kiss A, Wiest L, Lafay F, et al. Anal Bioanal Chem. 2012;402:2777–88.
Concas A, Popcu P, Sogliano C, Serra M, Purdy RH, Biggio G. Pharmacol Biochem Behav. 2000;66:39–45.
Serra M, Pisu MG, Muggironi M, Parodo V, Papi G, Sari R, et al. Psychopharmacology (Berlin). 2001;158:48–54.
Zorrilla LM, Gibson EK, Jeffay SC, Crofton KM, Setzer WR, Cooper RL, et al. Toxicol Sci. 2009;107:56–64.
Pollard I. J Endocrinol. 1988;119:275–80.
Abdel-Khalik J, Björklund E, Hansen M. J Chromatogr B. 2013;928:58–77.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experimental protocols for collecting plasma and testis samples from male rats were approved by the Danish Animal Experimentation Council (24th of July 2014, license holder: Anne-Marie Heegaard, case no. 2014-15-0201-00031).
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 536 kb)
Rights and permissions
About this article
Cite this article
Weisser, J.J., Hansen, C.H., Poulsen, R. et al. Two simple cleanup methods combined with LC-MS/MS for quantification of steroid hormones in in vivo and in vitro assays. Anal Bioanal Chem 408, 4883–4895 (2016). https://doi.org/10.1007/s00216-016-9575-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9575-z