Abstract
Endocrine disrupting compounds (EDCs) are suspected to be responsible for many disorders of the human reproductive system. To establish a causality relationship between exposure to endocrine disruptors and disease, experiments on animals must be performed with improved or new analytical tools. Therefore, a simple, rapid, and effective multi-residue method was developed for the determination of four steroid hormones (i.e., testosterone, androstenedione, estrone, and estradiol), glucuronide and sulfate conjugates of estrone and estradiol and four endocrine disruptors in rat testis (i.e., bisphenol A, atrazine, and active metabolites of methoxychlor and vinclozolin). The sample preparation procedure was based on the Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) approach. An analytical method was then developed to quantify these compounds at ultra-trace levels by liquid chromatography coupled to tandem mass spectrometry. The QuEChERS extraction was optimized with regard to the acetonitrile/water ratio used in the extraction step, the choice of the cleanup method and the acetonitrile/hexane ratio used in the cleanup step. The optimized extraction method exhibited recoveries between 89% and 108% for all tested compounds except the conjugates (31% to 58%). The detection limits of all compounds were below 20 ng g−1 of wet weight of testis. The method was subsequently applied to determine the levels of hormones and EDCs in seven rat testis samples.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the last two decades, numerous studies have been dedicated to a special class of exogenous chemical substances called endocrine disrupting compounds (EDCs) [1–4]. EDCs are believed to have harmful effects on the health of organisms and their descendants [5, 6]. In fact, several studies linked EDCs to the presence of behavioral disorders [7, 8], decreased fertility [9, 10], birth malformations [11, 12], or alteration of the sex ratio [13, 14]. These studies also showed that due to their capacity to mimic or block the action of endogenous hormones, EDCs interfere with important functions of the hormonal system, including transport, binding, action, and elimination of natural hormones [2, 15].
The exact mechanisms leading to the earlier mentioned pathologies are extremely complex and far from being completely understood. Hormonal balance is highly dependent on different enzymatic activities. Among the enzymes, aromatase, 17-beta-hydrosteroid dehydrogenase, and conjugating/deconjugating enzymes are responsible for the balance of androgens (free and conjugated) and estrogens (free and conjugated). These enzymatic activities may be disrupted by EDCs.
Until recently, most of these investigations were limited to the analysis of free hormones despite the fact that both conjugated and free hormones are necessary to monitor the effects of EDCs. Thus, to better understand the impact of EDCs on the hormonal system, it is necessary to develop highly sensitive analytical methods able to simultaneously detect and quantify endogenous hormones, their conjugates and EDCs [16–18]. The quantitation of steroid hormones is fairly common in urine [19–21], bovine hair [22], serum [23, 24], water [25], milk [26], and mammal tissues [27–29] but only rarely in mammal gonads [18]. This can be explained by the fact that the gonad is an extremely complex biological matrix and contains a high level of lipids and, therefore, demands intensive pre-treatment.
Two major methods are reported in literature for the extraction of hormones from animal tissues. The first is solid/liquid extraction followed by solid-phase purification [18, 27], while the second is based on the more recent Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) approach [28–30]. The latter is already a reference method for the extraction and purification in multiclass multiresidue analysis of pesticides in food matrices [30]. The main advantages of the QuEChERS approach are its economy, simplicity, and rapidity. Moreover, this approach leads to high recoveries of very polar and volatile compounds. The original QuEChERS method has been continuously adapted with regard to sample comminution and the extraction of pH-dependent molecules. Therefore, it can be successfully applied to other types of matrices, such as milk [31], sugarcane juice [32], soil samples [33], animal tissues [28, 29, 34], and banana leaves [35]. The QuEChERS sample preparation has rarely been used for the extraction of hormones [28, 36].
Regarding analytical techniques, two methods are mainly used for the analysis of hormones and EDCs: GC-MS or GC-MS/MS [37, 38] and LC-MS/MS [23–25]. However, most of the investigations using GC required a derivatization or hydrolysis step. These steps can lead to many limitations or/and exclude information about phase II metabolism [21, 39, 40]. Phase II metabolism is nevertheless important because it is also responsible for sulfo- and glucuro-conjugated hormones. Our strategy permits the conservation and the analysis of all molecules (sulfo- and glucuro-conjugated hormones included) as intact compounds without derivatization or hydrolysis.
In this context, the aim of this project was to develop a multi-residue methodology for the simultaneous and direct quantitation of four hormones (testosterone (T), androstenedione (A), estrone (E1), and estradiol (E2)), four of their sulfo- and glucuro-conjugates (E1S, E2S, E1G and E2G) and four EDCs (bisphenol A (BPA), atrazine (ATZ), the vinclozolin second metabolite (VM2), and the methoxychlor metabolite 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane (HPTE)) present at ultra-trace levels in rat testes. One metabolite of methoxychlor was studied because methoxychlor is rapidly metabolized in the liver to HPTE. The toxicity of methoxychlor through this metabolite has already been studied [41]. To follow vinclozolin, one metabolite of vinclozolin must be used because this analyte is known for its instability. Different metabolites could be followed [42]. Consequently, the second metabolite of vinclozolin was used.
This method consisted of two steps: the extraction of the analytes using the QuEChERS method, and the analysis of the extracts by LC-MS/MS without derivatization or hydrolysis, avoiding complex and time-consuming sample preparation. This paper describes the optimization of these two steps (QuEChERS extraction and LC-MS/MS analysis) as well as the validation strategy. Next, the validated QuEChERS-LC-MS/MS method was applied to real samples. To the best of our knowledge, this is the first time that QuEChERS sample preparation was applied to testes with ng g−1 reported limits, allowing the simultaneous analysis of four hormones, four hormone conjugates, and EDCs.
Experimental section
Materials and reagents
Testosterone (T), estrone (E1), and 17β-estradiol (E2) were purchased from Sigma at greater than 98% purity; 4-androsten-3,17-dione (A) was obtained from Fluka (Vetranal-quality). Conjugated standard sodium salts [estrone-3-β, d-glucuronide (E1G), estradiol-3-β, d-glucuronide (E2G), estrone-3-sulfate (E1S), and estradiol-3-sulfate (E2S)] were obtained from Sigma (99% purity). Atrazine (ATZ) was purchased from Fluka (Pestanal-quality), and the endocrine disruptors bisphenol A (BPA) and 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane (HPTE) were obtained from Sigma-Aldrich (99% purity). Vinclozolin second metabolite (VM2, N-(3,5-dichlorophenyl)-2-hydroxy-2-methyl-3-butenamide) was purchased from Cayman Chemical.
All deuterated standards used as internal standards (IS) were of 98% purity (or higher) and were obtained from CDN Isotopes: 17β-estradiol-2,4-d2 (E2-d2); estrone-2,4-d2 (E1-d2); testosterone-1,2-d2 (T-d2); 4-androsten-3,17-dione-2,2,4,6,6,16,16-d7 (A-d7); 17β-estradiol-2,4,16,16-d4 3-sulfate sodium salt (E2S-d4); bisphenol A-2,2′,6,6′-d4 (BPA-d4); and atrazine-d5 (ethyl-d5) (ATZ-d5). Table 1 indicates the use of each internal standard for quantitation.
Phenacetin-ethoxy-1-13C (PhC13) used as injection control was purchased from ISOTECH. Methanol (MeOH) and hexane (HEX) were of HPLC grade and acetonitrile (ACN) was of LC-MS grade and obtained from Sigma-Aldrich. Ultra-pure water was obtained from a MilliQ device (Millipore). Standard solutions were prepared in ACN at 1 mg mL−1 and stored at −18 °C.
QuEChERS citrate buffers (Agilent SampliQ QuEChERS Kits) were purchased from Agilent Technologies. Each buffer contains 4 g of anhydrous magnesium sulfate, 1 g of sodium chloride, 1 g of trisodium citrate dehydrate, and 0.5 g of disodium hydrogen citrate sesquihydrate. Dispersive SPE (dSPE) kits were purchased from Carlo Erba. Two different dSPE kits were tested: one containing 150 mg of MgSO4 and 25 mg of primary and secondary amine-bonded silica (PSA), and the other containing 150 mg of MgSO4, 25 mg of PSA, and 25 mg of C18-bonded silica (PSA/C18).
Sample collection and comminution
Adult Sprague–Dawley rats (certified virus free, Hla: (SD) CVF) were purchased from Janvier, Le Genest-Saint-Isle, France. The rats were subjected to a 12-h light and 12-h dark cycle and received food and water ad libitum. All of the procedures were reviewed and approved by the Institutional Animal Care and Use Committee of INERIS. The rats were sacrificed by lethal intraperitoneal pentobarbital injection after a 2-week daily gavage administration of atrazine, bisphenol A, methoxychlor, vinclozolin, or vehicle. The rat testes were harvested and weighed, then homogenized and conditioned in 1 μL of PBS-buffered water per mg of organ to avoid sample degradation. Finally, the testes were ground using an ultra-turax and stored at −20 °C until extraction and analysis.
Sample extraction
A QuEChERS extraction procedure was developed for testis having a weight between 1 and 3 g. Briefly, homogenized testis was transferred to a 15-mL polypropylene centrifuge tube and spiked with 30 ng mL−1 of the IS solution and vortexed for 30 s. Then, a volume of ACN was added to reach a ratio of VACN/Vwater of 2.6 and the mixture was shaken for 30 s. A volume of hexane leading to a VACN/Vhexane ratio of 2 was added. The mixture was then vortexed for an additional 30 s. Next, 3 g of QuEChERS citrate buffer was added directly to the tube; the mixture was then immediately manually shaken to avoid agglomeration of salts and vigorously shaken using a vortex (20 s). The sample was next centrifuged at 5,000 rpm for 5 min. Then, a 350-μL aliquot of the ACN phase was transferred into an autosampler vial and evaporated under a nitrogen stream at 40 °C. The sample was reconstituted with 105 μL of an 80/20 water/ACN solution spiked at 500 ng mL−1 with phenacetin-C13. Finally, the sample was capped and vortexed thoroughly for LC-MS/MS analysis.
LC-MS/MS analysis
An ABSciex API-3200 QTRAP triple quadrupole MS/MS with electrospray ionization (ESI) coupled to an Agilent 1200 LC (binary pump) was used. ABSciex Analyst 1.5 software was used for data collection and instrument control. The analytical column was an Agilent ZORBAX Eclipse Plus C18 (1.8-μm particles, 50-mm long, and 2.1-mm i.d.). An Agilent pre-filter (0.2 μm) was also installed. The injection volume was 5 μL, and the flow rate was 300 μL min−1. Mobile phase (A) was 0.01 mM ammonium acetate in water and mobile phase (B) was acetonitrile. In the negative mode, the gradient program started at 90% (A), decreased linearly to 33.5% within 5 min and then ramped to 100% (B) within 1 min and was held for 10 min at 100% (B). In the positive mode, the gradient profile started at 75% of (A), decreased linearly to 33.5% within 4 min, then ramped to 100% of (B) within 1 min, and was held for 10 min at 100% (B). Re-equilibration before every run was performed for 10 min. Total run times of 26 min in the negative mode and 25 min in the positive mode were obtained. It was necessary to add an equilibration and a cleaning step to avoid clogging of the column.
The MS/MS conditions were optimized using direct infusion into the ESI source in both modes to provide the highest detection and sensitivity for the quantitation ion of each analyte (1 mg L−1 solution at 7 μL min−1). A second MS/MS transition was selected for confirmation. The source temperature was 400 °C and 600 °C for negative and positive modes, respectively, and the ion spray potential was set to −4.5 and 5.5 kV, respectively. The operating pressures of the nitrogen flow for the nebulizer gas (GS1) and for the turbo gas (GS2) were equal to 275.76 and 344.70 kPa for the negative mode and 344.70 and 413.64 kPa for the positive mode. Table 2 presents the MS/MS conditions, retention times and product ion ratios used for each compound.
Validation
Instrument performance
To determine the instrumental detection limit (inst LOD) and to evaluate the linearity of the instrumental method, 9 solutions (targeted compounds in solvent) with concentrations ranging from 1 to 50 ng mL−1 were injected. The instrumental detection limit (inst LOD) was determined for the confirmation transition and corresponded to the analyte concentration that produced a chromatographic peak signal of 3 times the background noise.
Method performance
The method combining the sample preparation protocol and the LC-MS/MS analysis was validated based on an ICH directive [43]. The limits of detection and quantification (LOD and LOQ) were determined for the confirmation transition and corresponded to the analyte concentration that produced a chromatographic peak signal of 3 and 10 times the background noise, respectively. Each value of the estimated limit was checked by extracting and analyzing testes spiked before extraction at different concentrations.
For each compound, linearity was evaluated using a range of 5 concentrations. Each concentration point corresponded to an extracted sample spiked with the compounds of interest. For each concentration range, the 5 points corresponded to 1*LOQ, 3*LOQ, 10*LOQ, 25*LOQ, and 50*LOQ of the method. Linearity is considered validated if the correlation coefficient of the concentration range is greater than 0.99.
Recovery and repeatability were evaluated on three levels of concentration: 3*LOQ, 10*LOQ, and 50*LOQ. The repeatability is associated with the intra-day precision. To estimate intra-day precision, the samples must be spiked, extracted and analyzed under the same conditions by the same manipulator and on the same day. Therefore, each level of concentration was repeated three times. The intra-day precision is expressed as the relative standard deviation (RSD) of a series of measurements [40].
Intermediate precision or inter-day precision was evaluated at the same levels of concentration as used for repeatability. Intermediate precision can be determined if variations, such as different conditions, different manipulators, and different days, are introduced in the process. In our case, two analysts executed the process, different bottles of solvents were used, and the procedures were realized over a period of 3 days. The inter-day precision is also expressed as the relative standard deviation. Consequently, the standard ICH [43] was ensured because more than nine evaluations of precision (intra and inter-day; 3 concentration levels/3 replicate/3 days) were performed.
To complete the study of the limits of our method, the accuracy of the measurement was determined for each concentration level. This term refers to the degree of closeness between the value obtained using the method and the value considered as the true value [43]. It was finally concluded that internal calibration curves were necessary for the quantitation of target analytes in rat testes.
Results and discussion
LC-ESI-MS/MS optimization
The optimization of the ESI-MS/MS parameters was performed in a specific order: direct infusion into the electrospray source of the compound of interest followed by flow injection analysis (FIA). The first step enables the determination of the optimum detection parameters for each analyte through an optimization of the following MS/MS parameters: the choice of the ionization mode, the identification of the parent and product ions, and the selection of the declustering potential (DP) and collision energy (CE) (Table 2). Direct infusion consists of direct and continuous injection of a 1 mg L−1 solution of a pure analyte at 7 μL min−1. Two Multiple Reaction Monitoring (MRM) transitions were monitored for each compound: the more sensitive ion was used for quantitation and the second for confirmation. As shown in Table 2, two ionization modes were necessary to detect all the analytes. We associated the best ionization mode with the mode that provided the best selectivity of the transitions and the smallest noise for the product ion. Three of the 12 selected analytes, atrazine, testosterone, and androstenedione, showed more efficient ionization in the positive mode, whereas the remaining nine compounds showed preferential ionization in the negative mode.
The second step was optimization of the ESI parameters through injection of the sample (0.5 mg L−1 solution of a pure analyte at 300 μL min−1) into the chromatographic system. The optimization of the ESI parameters leads to an optimal spray for all the analytes. Three parameters must be optimized during this process: the operating pressure of the nitrogen flow of the nebulizer gas (GS1), the operating pressure of the nitrogen flow of the turbo gas (GS2), and the temperature of the heating resistors. These parameters should be determined for each ionization mode. These flows are used to focus the beam in a rectilinear direction. GS1 and GS2 were fixed at 275.76 and 344.70 kPa, respectively, for the negative mode, and 344.70 and 413.64 kPa, respectively, for the positive mode.
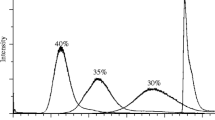
The desolvation temperature was also optimized for each ionization mode. Seven temperatures were tested in the positive and negative mode and ranged from 0 °C to 600 °C. For the positive mode, the maximum responses of all the analytes (T, A, and ATZ) were obtained at 600 °C. For the negative mode, some analytes were sensitive to temperature desorption (i.e., BPA, HPTE, E1, and E2). These latter analytes were degraded at temperatures higher than 400 °C (Fig. 1). E1G, E2G, and E2S were not sensitive to the desolvation temperature. Furthermore, the responses of E1S and VM2 increased with temperature. Considering the different responses of the targeted analytes to the applied temperature, a compromise was necessary. Therefore, the source temperature was set to 400 °C and 600 °C for the negative and positive modes, respectively.
After optimizing the ESI-MS/MS, the liquid chromatography conditions were optimized by searching for good separation of the target analytes when injected as a mixture. An Agilent ZORBAX Eclipse Plus C18 column was used for the separation of the 12 compounds. The nature of the mobile phases, the flow, and the gradient were varied and led to the following results: the compounds were separated within 6.5 min and within 8 min in the positive and negative modes, respectively.
The composition of the solvent used to reconstitute the samples after extraction also had to be optimized. This solvent influenced the quality of the separation, the peaks shapes and the response factor. Thus, this solvent should allow the best response for all of the analytes. Five compositions of solvent were tested: 90/10, 80/20, 70/30, 60/40, and 50/50 water/ACN). To compare the influence of the composition, a mixture of standards at 0.5 mg L−1 was analyzed with the developed LC-MS/MS method. For each analyte, the optimum composition of solvent is reported in Table 3. Considering the different responses of the targeted analytes to the solvent composition, a compromise was again necessary. Therefore, the samples were reconstituted with an 80/20 water/ACN solution because most of the compounds produced their best responses with this composition.
The injection volume was optimized at different concentrations: 0.5, 1, 4, 400, and 1,400 μg L−1 of standard analytes in 80/20 water/ACN. For each concentration, three injection volumes were tested: 1, 2, and 5 μL. Injection volumes larger than 5 μL were not tested, as the volume of ACN would have compromised the separation. Thus, for each concentration and for each injection volume, the responses were measured. A linear response for injection volume was observed in all cases without loss in the quality of chromatographic separation. Thus, an injection volume of 5 μL was chosen to obtain the best limits of detection and quantification.
Extraction
The QuEChERS method consisted of two major steps: a liquid/liquid buffered extraction followed by a dispersive SPE cleanup. In the first step, an organic phase is used to extract the sample from the aqueous phase in which the sample is conditioned. Several organic solvents, such as acetone, ethyl acetate or acetonitrile, can be used, but acetonitrile is known to be the most efficient [44]. Because water and acetonitrile are miscible, a buffered mixture of salts and MgSO4 must also be added. In so doing, phase separation is induced, and the passage of the analytes into the organic phase is improved. Moreover, this procedure allows drying of the sample and improves the pH-dependent stability of the compounds obtained. Consequently, this mixture led to an increase in the recoveries of the analytes [44]. Due to a limited number of available organs, some extraction parameters were fixed: the quantity of salts (3 g), the nature of the buffer salts according to the Standard Method EN 15662 [45] and the nature of the extraction solvent (acetonitrile).
The goal of the sample preparation is to allow an optimal extraction of all the targeted analytes. This is even a more difficult task in the case of gonads with various weights. Thus, the extraction procedure developed was based on a weight-to-volume ratio. To respect these ratios, it was necessary to adapt the proportion of the organic solvent to the weight of each organ. Consequently, optimization of the QuEChERS extraction was achieved by assessing three experiments: (a) the acetonitrile/water volume ratio (VACN/Vwater) for the extraction step, (b) the purification step, and (c) the acetonitrile/hexane volume ratio (VACN/Vhexane). To compare each optimization step, the samples were spiked at the same concentration of 500 μg L−1.
VACN/Vwater ratio for extraction
The liquid–liquid extraction is based on the affinity of the analyte for each phase. This affinity is first dependent on the compound, second on the nature of the solvents and finally on the volume of solvents used for extraction. Thus, the VACN/Vwater ratio was optimized first. The VACN/Vwater ratios of 2.3, 2.6, 3, and 3.5 were subsequently tested. The choice of these values was based on a previous study conducted in our laboratory. The results were normalized with respect to the maximum response and are presented in Fig. 2. As observed, glucuronide conjugates were the most sensitive to the VACN/Vwater ratio, whereas HPTE was poorly extracted with ratios higher than 3. After testing, the best ratio was found to be 2.6, which was used for all future extraction of targeted analytes from testes.
Purification
In the QuEChERS method, the cleanup is performed by dispersive SPE (dSPE) [44]. The dSPE sorbent should retain the matrix co-extractants but not the targeted analytes. To selectively trap the matrix impurities, different dSPE sorbents exist, such as PSA, PSA/C18 and Graphite Carbon Black. The PSA sorbent is used to eliminate polar organic acids, sugars, and lipids, whereas the PSA/C18 removes non-polar compounds [46]. If the dSPE cleanup retains the compounds of interest, this second step is omitted [31] and replaced with another type of cleanup, such as the addition of hexane. Due to its non-polar nature, this solvent ensures the elimination of apolar compounds [47].
To evaluate the efficiency of the dSPE cleanup, two different types of dSPE sorbents were tested: PSA and PSA/C18. In addition, one sample was extracted without the cleanup step. Figure 3 presents the results of this comparison. Compared with the PSA cleanup and extraction without dSPE, the use of PSA/C18 decreased the responses by a factor of two (Fig. 3a, b). This sorbent was therefore not adapted for the analytes under study. The results obtained using the PSA sorbent and no dSPE were equivalent for all the compounds except glucuronide conjugates (E1G and E2G). For these last analytes, the maximal responses were obtained when no dispersive SPE was used. This test revealed that neither the PSA/C18 nor the PSA sorbents were suitable for the dispersive SPE cleanup step proposed in the original QuEChERS method.
Cleanup using hexane
For a cleaning step on the biological matrix, a cleanup was performed using hexane. It has been shown that this solvent allows the elimination of co-extracted non-polar and fatty compounds [47]. Thus, to investigate the impact of hexane on our samples, several VACN/Vhexane ratios ranging from 0 to 4 were tested (Fig. 4). The benefit of adding hexane had already been observed during the experiment, as the samples cleaned with hexane were clearer, less viscous, and evaporated faster. This solvent efficiently eliminated lipids and denatured proteins present in the testes, as these biomolecules had more affinity for hexane than ACN. Thus, hexane ensured the delipidation and defatting of the samples. For the ratios ranging from 2 to 4, equivalent responses were observed (Fig. 4). However, it was also noted that it was easier for the manipulator to recover the cleaned extract for the ratio of 2, as the partition between the ACN and hexane phases was more visible. For this reason, the VACN/Vhexane ratio of 2 was chosen for the cleanup step.
Matrix effects
Matrix effects, such as enhancement or suppression of analytical signals, are frequently observed in the chemical analysis field. This phenomenon either positively or negatively influences the ionization of the analytes, as matrix compounds can be eluted at the same retention times as the compounds of interest [19]. Matrix effects are dependent on the nature of the matrix and the efficiency of the sample preparation step. Therefore, the sample preparation step should eliminate interfering compounds while sparing the analytes of interest. As testes are complex matrices, it was important to thoroughly study this phenomenon. The samples were spiked after extraction, and the corresponding signal areas, Aextract, were compared with those obtained with standards at the same concentration in solvent (Asolvent). The percentage of matrix effect was then calculated according to the following equation:
Matrix effects (%) are presented in Fig. 5. The results indicate that ion suppression was observed for all of the compounds except E1S, for which no matrix effect was determined (0.1%). The maximum ion suppressions of 25%, 40%, and 23% were for ATZ, A and T, respectively. These three compounds are analyzed in the ESI+ mode, while the others were analyzed in the ESI− mode. This observation confirmed that the ESI+ mode was less selective than the ESI− mode to matrix effects. To overcome the ion suppression, deuterated internal standards of compounds were used. As the internal standard undergoes the same interferences as that of the analyte, the phenomenon of ion suppression is similar for the analyte and the internal standard [48, 49]. In fact, internal calibration is considered an effective method to eliminate matrix effects on the accuracy and reproducibility of data [19, 50]. An internal calibration curve was therefore performed for each molecule in our study.
Method validation and method performance
The validation step was crucial, as it proved the consistency of the developed QuEChERS-LC-MS/MS method with the actual validation criteria. The criteria used for validation were the limit of detection (LOD), limit of quantification (LOQ), linearity, extraction recovery, repeatability, intermediate precision, and accuracy (Table 4). The implemented validation plan was performed on five concentration points and extended over a period of 3 days. Each point corresponded to a sample spiked with a standard solution of the targeted analytes. This strategy allowed the assessment of all the criteria required for validation. Moreover, considering that the validation plan was conducted with adult rat testes, specific hormones were naturally present in the matrix at different concentrations, and thus, it was impossible to obtain a blank matrix. Therefore, to obtain a homogenous concentration of hormones in samples for validation, a pool of 33 testes was used. To determine the amount of each naturally present hormone, a non-spiked sample was extracted on each day of the validation. Finally, all the spiked and non-spiked samples were obtained from the same matrix (i.e., sample pool).
LOD represents the lowest concentration of the analyte that can be detected but not quantified, and LOQ is the smallest concentration that can be quantified. LOD and LOQ were determined for signal-to-noise ratios of 3 and 10, respectively, and are reported in Table 4. Regarding hormones, all LOQs were below 15 ng g−1. According to the literature [51], steroids are present in the gonads at low nanogram per gram concentrations; thus, the method developed here will be able to detect steroids at this concentration level. Considering EDCs except for HPTE, LOQs between 0.15 and 1.10 ng g−1 were obtained. Due to weak electrospray ionization, the LOD of HPTE was 20 ng g−1. This level was sufficient for detection, but not quantification, of HPTE in rats that were treated with methoxychlor.
Depending on the compound, LOQs ranged from 0.15 to 51.4 ng g−1. Consequently, it was necessary to establish a range of concentrations (Table 4) for linearity, taking into account the sensitivity of each compound. The calibration curve for each analyte was based on five concentration points. The peak areas of the compounds of interest were corrected by the peak areas of the internal standards vs. concentration using the least squares method. The response of each compound was linear over the entire concentration range, and all correlation coefficients were greater than 0.99.
Recoveries and RSDs calculated on three replicates are given in Table 4. Recoveries were above 50%, except for the sulfate conjugates. Recoveries of E1S and E2S were in the range of 30%, but the RSDs were below 10%, which meant that the developed method was also reliable for these compounds. It is important to underline the excellent recoveries obtained by this method for sexual hormones compared with the literature values [18]. The QuEChERS approach reported here was therefore effective for EDC and hormone extraction.
Intermediate precision was evaluated at the same levels as repeatability on 3 days. RSDs are presented in Table 4 and were determined on the concentrations obtained by internal calibration. RSDs were below 30%, except for bisphenol A, glucuronide conjugates, and testosterone at the lowest concentration. High RSDs for glucuronide conjugates could be explained by the use of the deuterated E2S internal standard. A deuterated glucuronide conjugate would have been more suited but the standard was too expensive. The high RSD for testosterone might be attributed to an underestimation of the natural amount present in testis. Finally, the 40% RSD of bisphenol A was due to instrumental pollution.
Accuracy was assessed using nine measurements (3 concentrations/3 replicates), and the results are presented in Table 4. Accuracy was determined as the bias between the theoretical and calculated concentrations, expressed as a percentage. As observed, the bias values were higher at the 3*LOQ level, but still acceptable at less than 25% (except for E2G). For this last compound, the bias could be explained by the choice of internal standard. Finally, the developed QuEChERS-LC-MS/MS method was validated for all compounds of interest, according to the International Conference on Harmonization (ICH). The presented method simultaneously detected and quantified hormones and EDCs.
Application to real samples
The QuEChERS-LC-MS/MS method developed in this study was applied to rat testes obtained from seven adult animals (Table 5). Three animals were not subjected to any stress (testes 1 to 3). One animal was exposed to ATZ (testis 4), one animal was exposed to BPA (testis 5), one animal was exposed to methoxychlor (testis 6), and the last animal was exposed to VM2 (testis 7). ATZ was detected in testis 4, and BPA was detected in the testis of the animal exposed to this analyte, but HPTE (the metabolite of methoxychlor) and VM2 were not detected. Although it was possible to detect analytes, no concentrations are reported for some of these analytes (i.e., E1, E2, E1G, E2G, E1S, and E2S), as they were inferior to the LOQs. Two analytes were successfully quantified (T and A) (Table 5). The maximum values were obtained for testosterone (between 31 and 205 ng g−1). The levels of this hormone in the analyzed samples corresponded to levels naturally present in adult rat testes [52, 53].
Conclusion
A simple, rapid, selective, and effective multi-residue method was implemented for the determination of four steroid hormones, four hormone conjugates and endocrine disruptors in rat testis. The multi-residue method consisted of a sample preparation protocol based on the Quick, Easy, Cheap, Effective, Rugged, and Safe approach followed by an LC-MS/MS method analysis. The sample preparation was qualified as simple because it was comprised of a liquid–liquid extraction and a cleaning step using hexane. The method was rapid because the extraction could be performed in less than 15 min and required little solvent. The LC-MS/MS method allowed the simultaneous detection and quantification of two classes of molecules in the rat testes. Finally, the multi-residue method was validated according to the criteria of ICH and applied to real samples. This methodology therefore offers the ability to assess the impact of EDCs on hormonal balance in rats.
References
Clementi M, Tiboni GM, Causin R, La Rocca C, Maranghi F, Raffagnato F, Tenconi R (2008) Pesticides and fertility: an epidemiological study in Northeast Italy and review of the literature. Reprod Toxicol 26(1):13–18
Chang H-S, Choo K-H, Lee B, Choi S-J (2009) The methods of identification, analysis, and removal of endocrine disrupting compounds (EDCs) in water. J Hazard Mater 172(1):1–12
Ottinger MA, Abdelnabi M, Quinn M, Golden N, Wu J, Thompson N (2002) Reproductive consequences of EDCs in birds: what do laboratory effects mean in field species? Neurotoxicol Teratol 24(1):17–28
Sonnenschein C, Soto AM (1998) An updated review of environmental estrogen and androgen mimics and antagonists. J Steroid Biochem Mol Biol 65(1–6):143–150
Jobling S, Tyler CR (2006) Introduction: the ecological relevance of chemically induced endocrine disruption in wildlife. Environ Health Perspect 114 (S-1)
Sharpe RM, Irvine DS (2004) How strong is the evidence of a link between environmental chemicals and adverse effects on human reproductive health? Br Med J 328(7437):447–451
Kim HS, Lee BM, Jerome ON (2011) Endocrine disrupting chemicals and human cancer. In: Encyclopedia of Environmental Health. Elsevier, Burlington, pp 296–305
Crain DA, Janssen SJ, Edwards TM, Heindel J, Ho S-m, Hunt P, Iguchi T, Juul A, McLachlan JA, Schwartz J, Skakkebaek N, Soto AM, Swan S, Walker C, Woodruff TK, Woodruff TJ, Giudice LC, Guillette LJ Jr (2008) Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril 90(4):911–940
Petro EML, Covaci A, Leroy JLMR, Dirtu AC, De Coen W, Bols PEJ (2010) Occurrence of endocrine disrupting compounds in tissues and body fluids of Belgian dairy cows and its implications for the use of the cow as a model to study endocrine disruption. Sci Total Environ 408(22):5423–5428
Evans TJ, Ramesh CG, Dvm, Mvsc, Phd, Dabt, Fact (2007) Reproductive toxicity and endocrine disruption. In: Veterinary Toxicology. Academic Press, Oxford, pp 206–244
Milnes MR, Bermudez DS, Bryan TA, Edwards TM, Gunderson MP, Larkin ILV, Moore BC, Guillette JLJ (2006) Contaminant-induced feminization and demasculinization of nonmammalian vertebrate males in aquatic environments. Environ Res 100(1):3–17
Coppock RW (2011) Endocrine disruption in wildlife species. In: Reproductive and Developmental Toxicology. Academic Press, San Diego, pp 1117–1126
Tyler CR, Jobling S, Sumpter JP (1998) Endocrine disruption in wildlife: a critical review of the evidence. Crit Rev Toxicol 28(4):319–361
Rodríguez EM, Medesani DA, Fingerman M (2007) Endocrine disruption in crustaceans due to pollutants: a review. Comp Biochem Physiol-Part A Mol Integr Physiol 146(4):661–671
Ottinger MA, Dean K, Michael DB, Janice M (2010) Vertebrate endocrine disruption. In: Encyclopedia in animal behavior. Academic Press, Oxford, pp 475–484
Labadie P, Budzinski H (2006) Alteration of steroid hormone balance in juvenile turbot exposed to nonylphenol, bisphenol A, tetrabromodiphenyl ether 47, diallylphthalate, oil, and oil spiked with alkylphenols. Arch Environ Contam Toxicol 50(4):552–561
Labadie P, Budzinski H (2006) Alteration of steroid hormone profile in juvenile turbot (Psetta maxima) as a consequence of short-term exposure to 17[alpha]-ethynylestradiol. Chemosphere 64(8):1274–1286
Flores-Valverde AM, Hill EM (2008) Methodology for profiling the steroid metabolome in animal tissues using ultraperformance liquid chromatography-electrospray-time-of-flight mass spectrometry. Anal Chem 80(22):8771–8779
Saudan C, Entenza JM, Baume N, Mangin P, Saugy M (2006) Short-term stability of testosterone and epitestosterone conjugates in urine samples: quantification by liquid chromatography-linear ion trap mass spectrometry. J Chromatogr B 844(1):168–174
Mazzarino M, Rossi F, Giacomelli L, Botrè F (2006) Effect of the systemic versus inhalatory administration of synthetic glucocorticoids on the urinary steroid profile as studied by gas chromatography-mass spectrometry. Anal Chim Acta 559(1):30–36
Strahm E, Kohler I, Rudaz S, Martel S, Carrupt P-A, Veuthey J-L, Saugy M, Saudan C (2008) Isolation and quantification by high-performance liquid chromatography-ion-trap mass spectrometry of androgen sulfoconjugates in human urine. J Chromatogr A 1196–1197:153–160
Marcos V, Perogordo E, Espinosa P, De Pozuelo MM, Hooghuis H (2004) Multiresidue analysis of anabolic compounds in bovine hair by gas chromatography-tandem mass spectrometry. Anal Chim Acta 507(2):219–227
Harwood DT, Handelsman DJ (2009) Development and validation of a sensitive liquid chromatography-tandem mass spectrometry assay to simultaneously measure androgens and estrogens in serum without derivatization. Clin Chim Acta 409(1–2):78–84
Regal P, Vázquez BI, Franco CM, Cepeda A, Fente C (2009) Quantitative LC-MS/MS method for the sensitive and simultaneous determination of natural hormones in bovine serum. J Chromatogr B 877(24):2457–2464
Vulliet E, Wiest L, Baudot R, Grenier-Loustalot M-F (2008) Multi-residue analysis of steroids at sub-ng/L levels in surface and ground-waters using liquid chromatography coupled to tandem mass spectrometry. J Chromatogr A 1210(1):84–91
Tso J, Aga DS (2010) A systematic investigation to optimize simultaneous extraction and liquid chromatography tandem mass spectrometry analysis of estrogens and their conjugated metabolites in milk. J Chromatogr 1217(29):4784–4795
Jantti SE, Tammimaki A, Raattamaa H, Piepponen P, Kostiainen R, Ketola RA (2010) Determination of steroids and their intact glucuronide conjugates in mouse brain by capillary liquid chromatography-tandem mass spectrometry. Anal Chem 82(8):3168–3175
Costain RM, Fesser ACE, McKenzie D, Mizuno M, MacNeil JD (2008) Identification of hormone esters in injection site in muscle tissues by LC/MS/MS. Food Addit Contam 25(12):1520–1529
Stubbings G, Bigwood T (2009) The development and validation of a multiclass liquid chromatography tandem mass spectrometry (LC-MS/MS) procedure for the determination of veterinary drug residues in animal tissue using a QuEChERS (Quick, Easy, Cheap, Effective, Rugged and Safe) approach. Anal Chim Acta 637(1–2):68–78
Anastassiades M, Lehotay S, Stajnbaher D, Schenck F (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86(2):412–431
Martínez Vidal J, Frenich A, Aguilera-Luiz M, Romero-González R (2010) Development of fast screening methods for the analysis of veterinary drug residues in milk by liquid chromatography-triple quadrupole mass spectrometry. Anal Bioanal Chem 397(7):2777–2790
Furlani RPZ, Marcilio KM, Leme FM, Tfouni SAV (2010) Analysis of pesticide residues in sugarcane juice using QuEChERS sample preparation and gas chromatography with electron capture detection. Food Chem 126(3):1283–1287
Pinto CG, Laespada MEF, Martín SH, Ferreira AMC, Pavón JLP, Cordero BM (2009) Simplified QuEChERS approach for the extraction of chlorinated compounds from soil samples. Talanta 81(1–2):385–391
Zhao L, Stevens J (2010) Determination of quinolone antibiotics in bovine liver using Agilent SampliQ QuEChERS kits by LC/MS/MS. Agilent Technologies, Inc. 2010 5990-5085EN
González-Curbelo MÁ, Hernández-Borges J, Ravelo-Pérez LM, Rodríguez-Delgado MÁ (2010) Insecticides extraction from banana leaves using a modified QuEChERS method. Food Chem 125(3):1083–1090
Fu RJ, Zhai A (2010) Determination of Hormones in Shrimp by Agilent 1290 Infinity LC, Poroshell 120 LC Column and QuEChERS Sample Prep. LC GC Eur: 11–12
Hernando MD, Mezcua M, Gomez MJ, Malato O, Aguera A, Fernandez-Alba AR (2004) Comparative study of analytical methods involving gas chromatography-mass spectrometry after derivatization and gas chromatography-tandem mass spectrometry for the determination of selected endocrine disrupting compounds in wastewaters. J Chromatogr A 1047(1):129–135
Xu J, Wu L, Chen W, Chang AC (2008) Simultaneous determination of pharmaceuticals, endocrine disrupting compounds and hormone in soils by gas chromatography-mass spectrometry. J Chromatogr A 1202(2):189–195
Bowers LD, Sanaullah (1996) Direct measurement of steroid sulfate and glucuronide conjugates with high-performance liquid chromatography-mass spectrometry. J Chromatogr B Biomed Sci Appl 687(1):61–68
Mansilha C, Melo A, Rebelo H, Ferreira I, Pinho O, Domingues V, Pinho C, Gameiro P (2010) Quantification of endocrine disruptors and pesticides in water by gas chromatography-tandem mass spectrometry. Method validation using weighted linear regression schemes. J Chromatogr A 1217:6681–6691
Harvey CN, Esmail M, Wang Q, Brooks AI, Zachow R, Uzumcu M (2009) Effect of the methoxychlor metabolite HPTE on the rat ovarian granulosa cell transcriptome in vitro. Toxicol Sci 110(1):95–106
Sierra-Santoyo A, Barton HA, Hughes MF (2004) Liquid chromatography determination of the anti-androgen vinclozolin and its metabolites in rat serum. J Chromatogr B 809(1):105–110
ICH (2005) International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use. Paper presented at the ICH harmonised tripartite guideline, validation of analytical procedures: text and methodology Q2(R1), ICH Geneva
Majors RE (2008) QuEChERS—a new technique for multi-residue analysis of pesticides in foods and agricultural samples. Lc Gc Asia Pac 11(1)
EN 15662: 2009-01-01-Foods of plant origin—Determination of pesticide residues using GC-MS and/or LC-MS/MS following acetonitrile extraction/partitioning and clean-up by dispersive SPE-QuEChERS method (2009). Austrian Standards Institute,Österreichisches Normungsinstitut (ON) Heinestraße 38, 1020 Wien,
Agilent T (2009) Agilient SampliQ QuEChERS Kits. Agilent Technologies, Inc 2009 5990-3562EN
Przybylski C, Segard C (2009) Method for routine screening of pesticides and metabolites in meat based baby-food using extraction and gas chromatography-mass spectrometry. J Sep Sci 32(11):1858–1867
Borts DJ, Bowers LD (2000) Direct measurement of urinary testosterone and epitestosterone conjugates using high-performance liquid chromatography/tandem mass spectrometry. J Mass Spectrom 35(1):50–61
Annesley TM (2003) Ion suppression in mass spectrometry. Clin Chem 49(7):1041–1044. doi:10.1373/49.7.1041
Hewavitharana AK (2011) Matrix matching in liquid chromatography–mass spectrometry with stable isotope labelled internal standards—is it necessary? J Chromatogr A 1218:359–361
McNamara KM, Harwood DT, Simanainen U, Walters KA, Jimenez M, Handelsman DJ (2010) Measurement of sex steroids in murine blood and reproductive tissues by liquid chromatography-tandem mass spectrometry. J Steroid Biochem Mol Biol 121(3–5):611–618
Resko JA, Feder HH, Goy RW (1968) Androgen concentrations in plasma and testis of developing rats. J Endocrinol 40(4):485–491. doi:10.1677/joe.0.0400485
Corpechot C, Baulieu E-E, Robel P (1981) Testosterone, dihydrotestosterone and androstanediols in plasma, testes and prostates of rats during development. Acta Endocrinol 96(1):127–135. doi:10.1530/acta.0.0960127
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pouech, C., Tournier, M., Quignot, N. et al. Multi-residue analysis of free and conjugated hormones and endocrine disruptors in rat testis by QuEChERS-based extraction and LC-MS/MS. Anal Bioanal Chem 402, 2777–2788 (2012). https://doi.org/10.1007/s00216-012-5723-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-5723-2